Abstract
Background
Dedicator of cytokinesis 8 (DOCK8) deficiency can be cured by allogeneic hematopoietic stem cell transplantation (HSCT). Reports of outcome are still limited.
Objective
To analyze the results of HSCT in patients with DOCK8 deficiency and report whether approaches resulting in mixed chimerism result in clinically relevant immune reconstitution.
Methods
We performed retrospective chart review of 11 patients with DOCK8 deficiency, and measured DOCK8 expression and cytokine production.
Results
Of 11 patients, 7 received HSCT from related and 4 from unrelated donors; nine patients received busulfan-based conditioning regimens. Survival was excellent (10/11 patients alive, 91%), including a patient who had undergone liver transplantation. Patients showed significant improvements in the frequency and severity of infections. While eczema resolved in all, food allergies and high IgE levels persisted in some patients. Lymphopenia, eosinophilia, low numbers of naïve CD8+ T cells and switched memory B cells, and Th1/Th2 cytokine imbalance improved in most patients. While the 8 matched related or unrelated donor recipients had full donor chimerism, all 3 recipients of mismatched unrelated donor HSCT had high levels of donor T cell chimerism, and low B and myeloid chimerism (0–46%). Almost all switched memory B cells were of donor origin. All patients including those with mixed chimerism mounted robust antibody responses to vaccination.
Conclusion
Allogeneic HSCT ameliorated the infectious and atopic symptoms of DOCK8 deficiency. In patients with mixed chimerism, selective advantage for donor-derived T cells and switched memory B cells promoted restoration of cellular and humoral immunity and protection against opportunistic infection.
Keywords: Primary immunodeficiency, DOCK8 deficiency, hematopoietic stem cell transplantation, conditioning, mixed chimerism
INTRODUCTION
Dedicator of cytokinesis 8 (DOCK8) deficiency is a combined immunodeficiency, first described as a major genetic cause of autosomal recessive hyper-IgE syndrome in 2009.1,2 Two recently published large retrospective cohort studies reported eczema, recurrent respiratory infections, persistent viral infections, food allergies and abscesses as the major clinical features of the disease.3,4 Malignancies, typically hematological or epithelial, are reported in 8–17% of DOCK8-deficient patients.3,4 Immunological findings include T cell lymphopenia, hypereosinophilia, elevated IgE, low IgM, low antibody responses, poor memory B cell generation, and a T helper type 1 (Th1)/T helper type 2 (Th2) cytokine imbalance.3–5 DOCK8 deficiency carries high morbidity and mortality, with an overall survival of 50% and an event-free survival of 21% at 20 years.3 Hematopoietic stem cell transplantation (HSCT) is currently the only curative treatment option available.
Published experience with HSCT for DOCK8 deficiency is limited to case reports and small series of DOCK8-deficient patients.6–14 Because the majority of cases reported underwent myeloablative conditioning and had full donor chimerism, whether mixed chimerism is sufficient to improve the symptoms of DOCK8 deficiency is unknown. In this retrospective study of 11 patients, we report survival, complications, lineage specific chimerism, immune reconstitution, and resolution of clinical and laboratory features of DOCK8 deficiency, after both myeloablative and reduced intensity approaches.
METHODS
Patients and clinical characteristics of DOCK8 deficiency
We performed a retrospective chart review of 9 patients at Boston Children’s Hospital (Boston, MA, USA) and 2 patients at Great Ormond Street Hospital (London, UK), with approval by the local ethics committees. Research testing was performed with written informed consent from the parent or guardian and assent when appropriate. Eight patients were recruited from the Kuwait National Primary Immunodeficiency Registry under the research protocol titled Functional and Genetic Studies of Primary Immunodeficiency Syndromes, approved by the Research and Ethics Committee of the Ministry of Health in Kuwait. Clinical and laboratory features pre- and post-HSCT recorded included eczema, recurrent sinopulmonary infections, viral infections (herpes simplex virus, varicella zoster virus, Epstein-Barr virus (EBV), molluscum contagiosum, human papillomavirus and cytomegalovirus), fungal infections (Candida and others), abscesses, autoimmunity, asthma, food allergies, and malignancies. Mutational analysis, and DOCK8 expression by Western blot and flow cytometry were performed as previously described.5,11,13 Mutations for 8 patients have been reported elsewhere.5,11,13
HSCT
We recorded age at HSCT, treatment pre-transplant, conditioning regimen including busulfan exposure as determined by the cumulative area under the curve (AUC; mg*h/L), graft versus host disease (GVHD) prophylaxis, donor type, donor cell source, CD34+ cell dose, day of neutrophil engraftment, complications post-HSCT, acute and/or chronic GVHD, and chimerism in T cell, B cell and myeloid lineages. Chimerism in the various lineages post-HSCT was determined by short tandem repeat of magnetically separated cells.
Immune reconstitution
Lymphocyte subsets were analyzed using FACSCanto, FACSAria or LSR II flow cytometers (BD Biosciences) and FlowJo software (Treestar). Immune reconstitution was assessed by comparing the level of CD3+, CD4+, CD8+, CD19+, CD16/56+, CD4+CD45RA+ (naïve CD4 T cells), CD8+CD45RA+ (naïve CD8 T cells), CD19+CD27+IgD− (switched memory B cells) and CD19+CD27+IgD+ cells (unswitched memory B cells) pre-HSCT and at the last available follow-up post-HSCT. We only included patients that had their last measurement ≥ 12 months post-HSCT. We compared all measurements with age-dependent normal values.15–17
Patients received 3 doses of protein-conjugated pneumococcus and tetanus vaccine at least 4 weeks apart according to standard guidelines after discontinuation of immunosuppression and immunoglobulin substitution. Response was determined by measuring specific antibody titers ≥1 month after the last vaccination.
Cytokine production by peripheral blood mononuclear cells
Culture supernatants of peripheral blood mononuclear cells (PBMC) stimulated with phytohemagglutinin (PHA) were tested by ELISA for levels of IL-2, IL-4, TNFα (Beckman-Coulter, France) and IFN-γ (Immunotech, France) as per the manufacturer’s instructions. Samples were tested in triplicate at 24 h for IL-4 and IL-2 and at 96 h for TNFα and IFN-γ. The sensitivities of cytokine detection were 3 pg/ml for IFN-γ, 10 pg/ml for TNFα, 5 pg/ml for IL-2 and 5 pg/ml for IL-4. At the time of testing, there were no clinical evidence of acute infection or allergic reaction and none of the patients were on systemic immunosuppression. Testing was done for the same patients pre- and post-HSCT.
Total and allergen-specific IgE testing
Total and allergen-specific serum IgE levels were assessed with HYTEC Enzyme Immunoassay (HYCOR Europe Bio Crest B.V. Lohfelden, Germany) according to standard procedures. IgE measurements were compared to age-dependent normal values.
Statistical analysis
Paired t-tests to compare pre- and post-HSCT cell counts were performed using GraphPad Prism version 5.04 for Windows, GraphPad Software (San Diego California, USA). A P-value ≤ 0.05 was considered significant.
RESULTS
Patient characteristics
Of 11 patients, 10 had complete absence of DOCK8 protein expression by Western blot and/or flow cytometry13 (Table 1, Figure E1 of the Online Repository for patients 4 and 7). All patients were born from consanguineous parents and presented with characteristic manifestations of DOCK8 deficiency, including eczema (11/11), viral infections (11/11), food allergies (10/11), recurrent sinopulmonary infections (9/11), and skin abscesses (6/11). None of the patients had symptomatic autoimmunity.
Table I.
Patient Characteristics prior to HSCT
| Patient | Gender | Mutationa | DOCK8 protein expressionb | Clinical manifestations of DOCK8 | IVIG Pre-HSCT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eczema | Food allergies | Infections | Fungal | Other | ||||||||
| Bacterial | Viral | |||||||||||
| Recurrent sinopulmonary infections | Skin abscesses | Cutaneous | Systemic | |||||||||
| 1 | M | +1 IVS44 G>A | Reduced | + | + | + | − | − | EBV | − | − | |
| 2 | F | Del ex 29–36 | − | + | + | + | − | HSV HPV |
− | − | Asthma | + |
| 3 | F | Del ex 1–2 | − | + | − | + | + | MC | EBV | + | Asthma, poor dentition | − |
| 4 | F | Del ex 1–23 | − | + | + | + | + | HSV HPV |
CMV | Asthma, liver failure, cryptosporidium induced sclerosing cholangitis | + | |
| 5c | F | Del ex 1–5 | − | + | + | + | + | HSV | − | − | Poor dentition | + |
| 6c | F | Del ex 1–5 | − | + | + | + | + | HSV | CMV | + | Asthma, poor dentition, severe bronchiectasis s/p lobar resections | + |
| 7 | M | Del ex 10–12 | − | + | + | − | − | HSV | VZV EBV CMV |
− | EBV-driven leiomyosarcoma, MRSA pericarditis, chronic cryptosporidium and Giardia | − |
| 8c | M | Del ex 1–5 | − | + | + | − | + | MC | − | − | + | |
| 9c | F | Del ex 1–5 | − | + | + | + | + | HSV | − | + | Asthma, poor dentition | + |
| 10c | M | Del ex 1–5 | − | + | + | − | − | MC | − | − | + | |
| 11c | F | Del ex 1–5 | − | + | + | + | − | HSV MC |
− | − | Poor dentition | + |
All mutations are homozygous.
DOCK8 protein expression by either Western blot or flow cytometry
Related patients (Patients 9 and 11 are sisters, patients 5, 6, 8, 9, 10 and 11 are cousins)
CMV: cytomegalovirus, Del: deletion, EBV: Epstein-Barr virus, F: female, HSCT: hematopoietic stem cell transplantation, HSV: herpes simplex virus, IVIG: intravenous immunoglobulins, M: male, MC: molluscum contagiosum, MRSA: methicillin-resistant Staphylococcus aureus, VZV: varicella zoster virus.
Patient 4 suffered from end-stage liver disease (cirrhosis, portal hypertension, and ascites) secondary to chronic cryptosporidium-induced sclerosing cholangitis. She received a living-related left lateral segment liver transplantation from her mother, who was 4/6 HLA matched in GVHD direction, 5/6 matched in rejection direction, and ABO blood group compatible. Her liver graft function was excellent. Transplant recovery was complicated by a bile leak that became infected with Candida tropicalis, requiring percutaneous drainage on post-operative day 30 and antifungal therapy. She began conditioning for HSCT on post-operative day 65 with normal liver graft function. The transplanted liver was functioning well without signs of rejection 3.9 years post transplant.
HSCT procedure, survival and transplant related complications
The median age at transplant was 6.2 years (range 2.1 – 11.0 years). Donors included matched sibling donors (MSD, n=7, Table IIA), fully matched (MURD, n=1) or mismatched (mMURD, n=3) unrelated donors (Table IIB). Two of the mMURD recipients received peripheral blood stem cells and the remaining 9 patients received bone marrow. Most patients (9/11) received busulfan-based regimens combined either with cyclophosphamide or fludarabine. Median busulfan cumulative AUC among all patients was 75.7 mg*h/L [range 50.1–90.5]. Patients 9 and 11 also received antithymocyte globulin. Notably, patient 4 had normal busulfan excretion after liver transplantation. The remaining 2 patients received a reduced intensity conditioning regimen, fludarabine, melphalan and alemtuzumab.
Table IIA.
Hematopoietic stem cell transplantation procedure and outcome
| Patient | Age at HSCT (years) | Pre-treatment | Conditioning regimen | Bu AUC (mg* h/L) | GVHD prophylaxis | Donor type | Donor cell source | Cell dose (×106 CD34+ cells/kg) | Day of neutrophil engraftment | Complications | GVHD | Chimerism at last measurement (years post-HSCT) | Status (years post-HSCT) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.2 | Rituximab for EBV viremia | Bu/Cy | 67.2 | CSA/MTX | MSD | BM | 16.57 | 31 | - | T cell: >97% B cell: >97% Myeloid: >95% (3.2) |
A&W (4.6) | |
| 2 | 8.9 | Bu/Cy | 67.1 | CSA/MTX | MSD | BM | 9.39 | 18 | Klebsiella sepsis | - | T cell: 100% B cell: 97% Myeloid: 100% (0.1) |
Patient deceased at day +58 | |
| 3 | 10.2 | Rituximab for EBV viremia | Bu/Flu | 77.3 | CSA/MTX | MSD | BM | 11.75 | 27 | CMV reactivation; persistent and worsening CNS white matter changes (leukoencephalopathy) and neurologic symptoms responsive to corticosteroids; idiopathic pneumonia syndrome (day +91) treated with etanercept | - | T cell: >97% B cell: >97% Myeloid: >97% (2.5) |
A&W (4.2) Otitis treated with tympanostomy tubes |
| 4 | 11.0 | Living related liver transplant for liver failure/chronic sclerosing cholangitis | Bu/Flu | 90.5 | Tacrolimus/MTX | MSD | BM | 13.49 | 28 | Neurologic symptoms of unclear etiology, pericardial effusion, presumed viral meningoencephalitis responsive to Foscarnet | - | T cell: >97% B cell: >97% Myeloid: >97% (2.7) |
A&W (3.7). Pneumonias, asymptomatic EBV viremia |
| 5 | 5.8 | Bu/Flu | 70.8 | CSA/MTX | MSD | BM | 10.38 | 20 | - | T cell: 96% B cell: >97% Myeloid: >97% (0.6) |
A&W (2.0) | ||
| 6 | 7.0 | Lung resections x 2 | Bu/Flu | 87.8 | CSA/MTX | MSD | BM | 12.75 | 27 | Disseminated HSV with Bell’s palsy | - | T cell: >97% B cell: >97% Myeloid: >97% (2.3) |
A&W (2.5) Pneumonias, otitis treated with tympanostomy tubes, abscesses |
| 7 | 8.0 | Rituximab for EBV viremia; resection of leiomyosarcoma | Bu/Flu/rATG | 50.1 | CSA/MTX | MSD | BM | 6.26 | 29 | CMV pneumonitis, EBV viremia, transient pseudotumor cerebri | No acute | T cell: 100% B cell: 100% Myeloid: 100% (0.5) |
A&W (0.6) |
Table IIB.
Hematopoietic stem cell transplantation procedure and outcome
| Patient | Age at HSCT (years) | Pre-treatment | Conditioning regimen | Bu AUC (mg* h/L) | GVHD prophylaxis | Donor type | Donor cell source | Cell dose (×106 CD34+ cells/kg) | Day of neutrophil engraftment | Complications | GVHD | Chimerism at last measurement (years post-HSCT) | Status (years post-HSCT) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 6.2 | Alemtuzumab/Flu/Melphalan | n/a | CSA/MMF | mMURD (8/10) | PB | 10 | n/a | - | T cell: 89% B cell: 11% Myeloid: 0% (2.0) |
A&W (4.4) | ||
| 9 | 3.4 | Bu/Flu/eATG | 85.6 | CSA/MTX/prednisone | MURD | BM | 3.65 | 21 | CMV reactivation | - | T cell: 100% B cell: 100% Myeloid: 100% (3.1) |
A&W (3.5) | |
| 10 | 2.3 | Alemtuzumab/Flu/Melphalan | n/a | CSA/MMF | mMURD (9/10) | PB | 23.72 | 15 | EBV viremia | - | T cell: 82% B cell: 0% Myeloid: 0% (1.8) |
A&W (2.8) | |
| 11 | 2.1 | Bu/Flu/eATG | 80.6 | CSA/MTX/prednisone | mMURD (9/10) | BM | 2.26 | 34 | HSV viremia, ezcema herpeticum, Enterobacter bacteremia | - | T cell: 97% B cell: 46% Myeloid: 7% (2.5) |
A&W (2.8) |
A&W: alive and well, ATG: anti-thymocyte globulin (equine or rabbit), AUC: area under the curve, BM: bone marrow, Bu: busulfan, CMV: cytomegalovirus, CNS: central nervous system, CSA: cyclosporine A, Cy: cyclophosphamide, EBV: Epstein-Barr virus, Flu: fludarabine, GVHD: graft versus host disease, HSCT: hematopoietic stem cell transplantation, HSV: herpes simplex virus, MMF: mycophenolate mofetil, mMURD: mismatched unrelated donor, MSD: matched sibling donor, MTX: methotrexate, MURD: matched unrelated donor, n/a: not available, PB: peripheral blood, RIC: reduced intensity conditioning,
All patients recovered from pancytopenia. Chimerism in the T cell, B cell and myeloid lineages was full in 8 patients, and mixed in 3 patients, measured 0.5–3.2 years post-HSCT (Table II). After a median follow-up of 3.2 years (range 7 months – 5.6 years) survival was 10/11 (91%); one patient died 58 days post-HSCT of bacterial sepsis.11 HSCT was generally well tolerated without veno-occlusive disease, acute or chronic GVHD. Complications post-HSCT occurred in 7/11 patients and were mostly infectious.
Infections and atopy improve or resolve post-HSCT
All patients with chronic or difficult to treat viral infections cleared these infections post-HSCT. Despite full chimerism in all cell lineages, patients 3, 4 and 6 continued to have bacterial infections including pneumonia, otitis media treated with tympanostomy tubes, and skin boils, though at a much reduced frequency compared to pre-HSCT. Patients 4 and 6 have significant residual bronchiectasis.
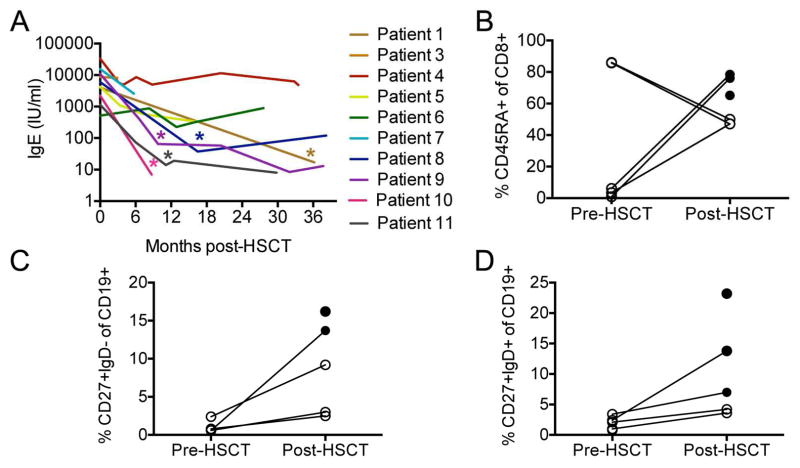
All patients with eczema had resolution post-HSCT. While elevated eosinophil counts consistently declined post-HSCT in all patients (Table E1), total serum IgE normalized in 5 patients, and remained elevated in 3 patients (4, 5, 6), despite full donor chimerism (Fig. 1A).
FIG. 1.
Immune reconstitution after HSCT for DOCK8 deficiency. A, Persistence of IgE elevation post-HSCT. Asterisks mark the first measurement with an IgE value within the normal range for age. B, Naïve CD8 cells. C, Switched memory B cells. D, Unswitched memory B cells. Levels shown are pre-HSCT and at the last follow-up (≥ 12 months) post HSCT. Open circles indicate that the measured value is abnormal for age and closed circles indicate that the value is normal.
Ten patients (91%) presented with food allergy confirmed by elevated food allergen-specific IgE. Clinically, food allergies resolved post-HSCT in the vast majority of patients (8/10), as confirmed by oral challenges. Patient 4 had an anaphylactic reaction to milk at 3.9 months post-HSCT and has not been rechallenged. Patient 6 had an anaphylactic reaction to the H. influenzae type b vaccine, but tolerates all foods. Furthermore, allergen-specific IgE decreased in all patients tested (data not shown).
Immune reconstitution post-HSCT
Of 10 patients who are alive after HSCT, 9 have stopped immunosuppression after a median duration of 7.1 months post-HSCT [range 5.5–15.5] and all are off of immunoglobulin substitution. Patient 5 continues on tacrolimus to prevent rejection of the transplanted liver. We analyzed CD3+, CD4+, CD8+, CD19+, CD16/56+, CD4/CD8 ratio, CD4+CD45RA+ (naïve CD4 T cells), CD8+CD45RA+ (naïve CD8 T cells), CD19+CD27+IgD− (switched memory B cells) and CD19+CD27+IgD+ (unswitched memory B cells) counts and percentages in patients who had both a pre-HSCT and a post-HSCT measurement ≥ 12 months post-HSCT (see Table E2 in this article’s Online Repository). Pre-HSCT, CD3+ cell, CD4+ cell and CD8+ cell counts were abnormally low for age in 6/9, 6/9 and 5/9 patients respectively; all counts normalized post-HSCT except the CD4+ T cell count in patient 5, which remained low for age. The CD4/CD8 ratio was abnormally low in 6/9 patients pre-HSCT. Of these, 3 continued to have an abnormally low ratio post-HSCT (patient 4, 5 and 10). Percentages of naïve CD8 T cells (p=0.002), switched (p=0.007) and unswitched memory B cells (p=0.02) all significantly increased post-HSCT and normalized in the majority of patients (Fig. 1B–1D).
We analyzed antibody response to vaccines in the patients who had pre- and post-vaccination titers available (patients 1, 3, 4, 5, 8, 11). All 6 patients mounted protective titers to pneumococcus to at least 7 of 14 serotypes, and to tetanus, including 2 who had mixed B cell chimerism (patients 8 and 11). The third patient with mixed chimerism, patient 10, did not have a pre-HSCT vaccine pneumococcal titer available, but had protective titers to both pneumococcus and tetanus post-vaccination.
Selective advantage for T cell lineage and switched memory B cells in patients with mixed chimerism
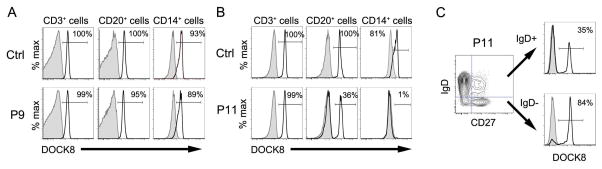
All 3 patients (patients 8, 10 and 11) who had mixed chimerism demonstrated by STR analysis and/or by flow cytometry (Table II, Figure 4) had received mMURD transplants, and 2 of 3 patients received reduced intensity conditioning regimens. Patients with mixed chimerism had 82–97% donor cells in the T cell lineage, whereas chimerism in the B cell and myeloid lineage was 0–46% and 0–7% respectively at 1.8–2.5 years post-HSCT. Patient 8 had high level donor chimerism in the CD3+ T cells, mixed chimerism in CD20+ B lineage and negligible chimerism in CD14+ monocytes indicated by flow cytometry for DOCK8 expression in contrast to patient 6 who had high level donor chimerism in all lineages (Figure 4A–B). We further found that switched memory CD27+ IgD− cells of patient 6 had a higher percentage of cells expressing DOCK8 compared to CD20+ IgD+ cells (86% versus 35%, Figure 4C), suggesting selective advantage for switched memory B cells.
Shift towards Th1 cytokines post-HSCT
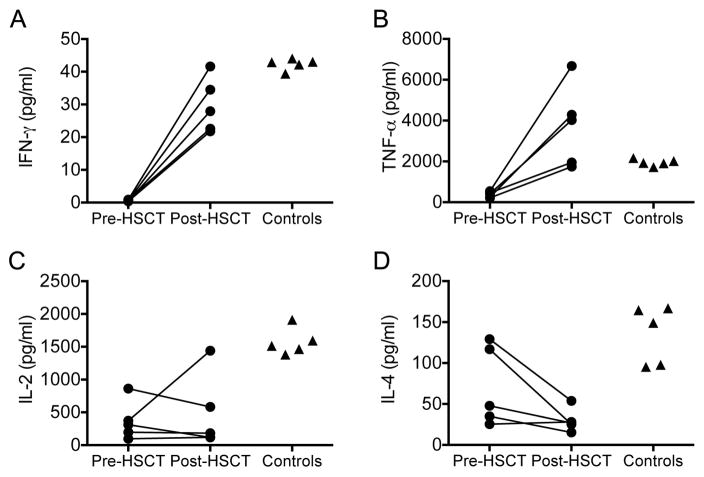
Prior to HSCT, PBMCs of 5 patients tested secreted significantly lower amounts of IL-2, TNF-α and IFN-γ than those from healthy controls in response to stimulation with PHA. Repeat testing post-HSCT showed a significant increase in the Th1 cytokines IFN-γ and TNF-α (p = 0.001 and 0.018 respectively) and a trend towards a decrease in the Th2 cytokine IL-4 (p=0.085) (Fig. 5). Cytokine production by PBMC of untransplanted DOCK8 patients show a Th2 bias.5 We therefore compared the ratios of the mean secreted IL-2, TNF-α and IFN-γ to that of IL-4 in transplanted patients. Compared to the Th2 bias pre-HSCT, the post-HSCT ratios showed a shift toward Th1 cytokines (Table III).
Table III.
Th1/Th2 ratios pre- and post- HSCT in DOCK8 deficient patients
| Pre-HSCT | Post-HSCT | Control | |
|---|---|---|---|
| IL-2:IL-4 | 5.20 | 16.37 | 11.67 |
| IFN-γ:IL-4 | 5.34 | 125 | 14.44 |
| TNF-α:IL-4 | 0.009 | 0.99 | 0.31 |
DISCUSSION
DOCK8 deficiency has high mortality, and HSCT should be considered as early as possible before development of significant organ damage or viral-driven malignancies. In our cohort, survival was very good, 91% (9/11), which we attribute to their young age, early identification of several by family history, and aggressive pre-HSCT management, including liver transplantation in one case. We recommend that even patients with significant comorbidities should undergo HSCT as long as these conditions can be aggressively treated prior to HSCT.
Many patients with DOCK8 deficiency have organ damage necessitating reduced intensity conditioning approaches, which in turn carry a higher risk of mixed donor chimerism. Indeed both patients receiving fludarabine, melphalan, alemtuzumab conditioning had mixed chimerism, while patient 8 developed mixed chimerism despite receiving a myeloablative dose of busulfan (AUC >80 mg*hr/L). As all 3 patients with mixed chimerism received mMURD graft, HLA mismatch may also have played a role. Nevertheless, mixed chimerism was sufficient to improve immunity, due to the strong selective advantage for donor-derived T cells and switched memory B cells that express DOCK8. All patients with mixed chimerism had higher donor chimerism in the T than in B or myeloid lineages, similar to what has been shown in mouse models,18,19 thus protecting the patients against life-threatening viral and opportunistic infection. Effective humoral immunity after vaccination in our patients with mixed chimerism is likely mediated by donor-derived switched memory B cells, which also have strong selective advantage as seen in patient 11 (Fig. 2) and in mouse models.20 Based on the finding that DOCK8-deficient patients may have NK cell revertants, we suspect that donor-derived NK cells may exhibit selective advantage,21 but did not examine NK chimerism in our cohort. Long-term follow-up is needed to determine whether or not immunological reconstitution in these patients will be sustained. Whether the lack of donor-derived myeloid cells has clinical consequences remains unclear.
FIG. 2.
DOCK8 expression by flow cytometry post-HSCT. A, DOCK8 expression in cells from control and patient P9 post-HSCT. B, DOCK8 expression in cells from control and patient P11 post-HSCT. C, DOCK8 expression in CD20+IgD− and CD20+IgD+ cells from patient P11 post-HSCT. Shaded histograms indicate isotype control. Numbers represent the percentage of cells that expressed DOCK8.
DOCK8-deficient patients have been shown to have an imbalance of Th1/Th2 cytokine production, which may impair their ability to combat viruses due to deficiency of IFN-γ production.5 Ex vivo cytokine levels measured pre- and post-transplant in a portion of our cohort showed improved Th1 cytokine production. These findings may explain the improvement in the severity and frequency of viral infections post-HSCT in our cohort. We also saw a trend towards decrease in IL-4 production. Consistent with the improvement in Th1/Th2 balance we observed, and with other reports of clinical outcome,14 our patients showed resolution of eczema, and most showed decline or normalization of eosinophil count, total IgE and allergen-specific IgE levels post HSCT. Interestingly, 3 patients with full donor chimerism continue to have significantly elevated serum IgE, presumably generated from recipient-derived long-lived plasma cells. Why some patients continue to produce high levels of IgE post-HSCT remains unknown. In the case of patient 4, the known predilection to food allergies in liver transplant recipients may play a role.22 We speculate that ongoing infections related to pre-existing bronchiectasis may cause persistent immune activation and dysregulation, as manifested by the reversed CD4 to CD8 ratio, and ongoing IgE production.
In summary, DOCK8 patients in our cohort had excellent survival rates and immune reconstitution, despite differences in transplant technique and post-HSCT donor chimerism. For patients who have a suitable donor, HSCT is the recommended curative option as long as there is aggressive management of infectious sequelae and end-organ damage prior to HSCT. The strong selective advantage we demonstrated in T and switched memory B cells implies that correction of a portion of HSC, such as that which may be achieved with gene therapy, may be sufficient to correct symptoms.
Supplementary Material
FIG. 3.
Cytokine production in response to PHA pre- and post-HSCT in five patients with DOCK8 deficiency and healthy adult controls. A, IFN-γ. B, TNF-α. C, IL-2. D, IL-4.
CLINICAL IMPLICATIONS.
Early allogeneic transplantation should be pursued in patients with DOCK8 deficiency. Approaches resulting in mixed chimerism nevertheless led to clinically significant immunological improvement. Food allergies may persist.
Acknowledgments
This work was funded by a Translational Investigator Service Award (to S.-Y.P.) and the National Institutes of Health (R01 AI100315 to R.S.G., R01 AI085090 to T.C). J.I.C. was supported by a training award from National Heart Lung and Blood Institute (5T32HL007574-33). J. v.d. Spek was supported by a Ter Meulen Grant of the Royal Netherlands Academy of Arts and Sciences. C.M.B was supported by the Ruth L. Kirschstein National Research Service Award.
ABBREVIATIONS
- AUC
area under the curve
- DOCK8
dedicator of cytokinesis 8
- EBV
Epstein-Barr virus
- GVHD
graft versus host disease
- HSCT
hematopoietic stem cell transplantation
- MSD
matched sibling donor
- MURD
matched unrelated donor
- mMURD
mismatched unrelated donor
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Q, Davis J, Lamborn I, et al. Combined Immunodeficiency Associated with DOCK8 Mutations. N Engl J Med. 2009 doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt KR, McGhee S, Winkler S, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–302.e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options - a review of 136 patients. J Clin Immunol. 2015;35:189–98. doi: 10.1007/s10875-014-0126-0. [DOI] [PubMed] [Google Scholar]
- 4.Engelhardt KR, Gertz ME, Keles S, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.12.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Herz W, Ragupathy R, Massaad MJ, et al. Clinical, immunologic and genetic profiles of DOCK8-deficient patients in Kuwait. Clin Immunol. 2012;143:266–72. doi: 10.1016/j.clim.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Mousa H, Hawwari A, Alsum Z. In DOCK8 deficiency donor cell engraftment post-genoidentical hematopoietic stem cell transplantation is possible without conditioning. The Journal of allergy and clinical immunology. 2013;131:1244–5. doi: 10.1016/j.jaci.2012.12.663. [DOI] [PubMed] [Google Scholar]
- 7.Barlogis V, Galambrun C, Chambost H, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011;128:420–22.e2. doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Bittner TC, Pannicke U, Renner ED, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr. 2010;222:351–5. doi: 10.1055/s-0030-1265135. [DOI] [PubMed] [Google Scholar]
- 9.Boztug H, Karitnig-Weiß C, Ausserer B, et al. Clinical and Immunological Correction of DOCK8 Deficiency by Allogeneic Hematopoietic Stem Cell Transplantation Following a Reduced Toxicity Conditioning Regimen. Pediatr Hematol Oncol. 2012 doi: 10.3109/08880018.2012.714844. 120816133521002. [DOI] [PubMed] [Google Scholar]
- 10.Gatz SA, Benninghoff U, Schütz C, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]
- 11.McDonald DR, Massaad MJ, Johnston A, et al. Successful engraftment of donor marrow after allogeneic hematopoietic cell transplantation in autosomal-recessive hyper-IgE syndrome caused by dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2010;126:1304–5.e3. doi: 10.1016/j.jaci.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metin A, Tavil B, Azık F, et al. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. Pediatric Transplantation. 2012;16:398–9. doi: 10.1111/j.1399-3046.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 13.Pai SY, de Boer H, Massaad MJ, et al. Flow cytometry diagnosis of dedicator of cytokinesis 8 (DOCK8) deficiency. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuellar-Rodriguez J, Freeman AF, Grossman J, et al. Matched related and unrelated donor hematopoietic stem cell transplantation for DOCK8 deficiency. Biol Blood Marrow Transplant. 2015:1037–45. doi: 10.1016/j.bbmt.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gent Rv, Tilburg CMv, Nibbelke EE, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133:95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol. 2010:271–9. doi: 10.1111/j.1365-2249.2010.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Lambe T, Crawford G, Johnson AL, et al. DOCK8 is essential for T-cell survival and the maintenance of CD8(+) T-cell memory. Eur J Immunol. 2011;41:3423–35. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randall KL, Chan SS-Y, Ma CS, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–20. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randall KL, Lambe T, Johnson A, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283–91. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing H, Zhang Q, Zhang Y, Hill BJ, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014 Jun;133(6):1667–75. doi: 10.1016/j.jaci.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebel M-J, Chapdelaine H, Paradis L, Roches Des A, Alvarez F. Increase in de novo food allergies after pediatric liver transplantation: tacrolimus vs. cyclosporine immunosuppression. Pediatric Transplantation. 2014 Nov;18(7):733–9. doi: 10.1111/petr.12342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.