Abstract
In clinic, cetuximab, an anti-EGFR antibody, improves treatment outcomes in colorectal cancer (CRC). KRAS-mutant CRC is generally resistant to cetuximab, although difference of the sensitivity among KRAS-mutants has not been studied in detail. We previously developed the cancer tissue-originated spheroid (CTOS) method, a primary culture method for cancer cells. We applied CTOS method to investigate whether ex vivo cetuximab sensitivity assays reflect the difference in sensitivity in the xenografts. Firstly, in vivo cetuximab treatment was performed with xenografts derived from 10 CTOS lines (3 KRAS-wildtype and 7 KRAS mutants). All two CTOS lines which exhibited tumor regression were KRAS-wildtype, meanwhile all KRAS-mutant CTOS lines grew more than the initial size: were resistant to cetuximab according to the clinical evaluation criteria, although the sensitivity was quite diverse. We divided KRAS-mutants into two groups; partially responsive group in which cetuximab had a substantial growth inhibitory effect, and resistant group which exhibited no effect. The ex vivo signaling assay with EGF stimulation revealed that the partially responsive group, but not the resistant group, exhibited suppressed ERK phosphorylation ex vivo. Furthermore, two lines from the partially responsive group, but none of the lines in the resistant group, exhibited a combinatory effect of cetuximab and trametinib, a MEK inhibitor, ex vivo and in vivo. Taken together, the results indicate that ex vivo signaling assay reflects the difference in sensitivity in vivo and stratifies KRAS mutant CTOS lines by sensitivity. Therefore, coupling the in vivo and ex vivo assays with CTOS can be a useful platform for understanding the mechanism of diversity in drug sensitivity.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer prevalence and death worldwide. Treatment of CRC has progressed by the recent development of new drugs, such as oxaliplatin and irinotecan, as well as molecular target drugs, such as cetuximab, panitumumab, bevacizumab, aflibercept, regorafenib, and ramucirumab [1–3]. Anti-EGFR antibodies, cetuximab and panitumumab, have contributed remarkably to the treatment of metastatic CRC, though CRCs with KRAS mutations at codons 12 and 13 are resistant to these antibodies [4–6]. NRAS mutations and mutations at other codons in KRAS also reportedly contribute to cetuximab resistance [7–9]. Currently, expanded RAS wildtype CRCs (KRAS and NRAS wildtype) are candidates for anti-EGFR therapy [1]. To improve treatment outcomes for CRC, it is important to develop effective therapies for RAS mutants.
Established cancer cell lines have contributed to the study of cancer and development of new drugs, though discrepancies are often observed between experimental results and clinical trials, possibly due to changes or severe selection during the establishment and passage of cells in vitro [10, 11]. Inter-patient heterogeneity may not be maintained in established cell lines [12, 13]. On the other hand, patient-derived xenografts (PDXs) better preserve the original characteristics of patient tumors, and the assay results reflect the clinical trials [12–17]. However, the costly and time consuming assay of PDXs is not suitable for testing multiple candidate drugs or studying detailed signaling pathways.
Previously, we developed the cancer tissue-originated spheroid (CTOS) method, a preparation and culture method for primary cancer cells from patient tumors[18]. By maintaining cell-cell contact throughout the process, we can avoid anoikis and prepare pure cancer cells stably and efficiently. We previously reported the successful preparation of CTOSs from various cancers, including colon, lung, bladder, brain, and uterine cancer [18–23]. CTOSs preserve the original characteristics both ex vivo and in vivo [18–20, 22, 23]. CTOSs can also be prepared efficiently from CTOS-derived xenograft tumors and subjected to ex vivo experiments.
As CRC PDX models have been reported to reflect the results of clinical trials for cetuximab [14–17], we investigated whether an ex vivo cetuximab sensitivity assay using CTOSs can reflect the results of an in vivo study using CTOS xenografts. Using the ex vivo platform, we attempted to find biomarkers and the effective drugs to combine with cetuximab for KRAS mutant CRC.
Materials and methods
CTOS preparation, culture, and cryopreservation
The preparation of CTOSs from CRC patients was performed as described previously [18]. Briefly, surgical specimens were obtained from Osaka Medical Center for Cancer and Cardiovascular Diseases after obtaining informed consent. The surgical specimens were mechanically and enzymatically digested into small fragments. Materials retained by 100 μm or 40 μm cell strainers (BD Falcon, Franklin Lakes, NJ) were collected and cultured in suspension in StemPro hESC (Invitrogen, Carlsbad, CA) with 8 ng/ml of bFGF (Invitrogen) to form CTOSs. Frozen stocked CTOSs were thawed and xenograft tumors generated as described above. CTOSs were prepared from the xenografts and subjected to further analysis. Cryopreservation was performed using StemCell Keep (BioVerde, Kyoto, Japan).
Animal studies
Animal studies were approved by the Institutional Animal Care and Use Committee of Osaka Medical Center for Cancer and Cardiovascular Diseases and performed in compliance with the institutional guidelines. For cetuximab mono-therapy, a mixture of CTOSs and Matrigel was transplanted into the flank of NOD/SCID mice (Charles River Laboratories Japan, Yokohama, Japan). When the tumor reached 160 mm3, cetuximab was injected intraperitoneally twice a week at 20 and 60 mg/kg. For the combination therapy, tumors were generated as described above using BALB/cAJcl-nu/nu mice (CLEA Japan, Tokyo, Japan). When the tumor reached 300 mm3, cetuximab was injected intraperitoneally twice a week at 20 mg/kg and trametinib administrated orally every day at 0.3 mg/kg. Trametinib was suspended in 0.5% methyl cellulose with 0.2% Tween80. Tumor size was measured twice a week and the tumor volume calculated as follows: 0.5 x width2 x length. For ethical reasons mice bearing an excessive tumor volume (>2,000 mm3) were euthanized.
Grouping of CTOS lines by sensitivity to cetuximab in vivo
CTOS lines were classified into three groups according to their sensitivity to cetuximab. The regression group consisted of the lines in which the average tumor volume at day 11 was the same or less than the average starting volume. The partially responsive group consisted of lines in which the average tumor volume was suppressed more than 10% compared to the average volume of non-treated tumors but did not show regression. The resistant group consisted of lines in which the average tumor volume at day 11 was suppressed less than 10% compared to the average volume of non-treated tumors.
Mutational analysis
Mutational analysis was performed using Ion AmpliSeq™ Cancer Hotspot Panel v2 (ThermoFisher, Waltham, MA) and next-generation sequencing (TAKARA, Kusatsu, Japan).
Immunohistochemistry
Formalin-fixed paraffin-embedded samples were used for immunohistochemistry as described previously [18]. Antigen retrieval was performed by autoclave incubation in citrate buffer (pH 6.0). Primary antibody specific for the EGFR (clone D38B1) was obtained from Cell Signaling Technologies (Danvers, MA). Images were acquired using the CellSens standard imaging software (Olympus, Tokyo, Japan). Hematoxylin was used for counter staining. The staining intensity was evaluated as low if membranous staining was present in less than 10% of tumor cells, med if membranous staining was present in 10% to 50% of tumor cells, and high if membranous staining was present in more than 50% of tumor cells.
Reagents
Trametinib was purchased from Selleck Chemicals (Houston, TX, USA). Drug screening was performed using SCADS Inhibitor Kit IV. The drugs were dissolved in DMSO and used below 0.1% DMSO.
CTOS-based sensitivity assay
After CTOS preparation, the CTOSs were cultured in suspension overnight in the standard CTOS medium (StemPro hESC plus bFGF). Each CTOS was embedded in a gel droplet of Matrigel GFR (BD Biosciences, Bedford, MA) and cultured for 7 days in the standard CTOS medium or DMEM containing 10% fetal bovine serum (FBS medium) containing the indicated doses of cetuximab. In the case of neuregulin 1 (NRG1) / heregulin (HRG) stimulation, 10 ng/ml of HRG (Peprotech, Rocky Hill, NJ) was added to the FBS medium. The effect was evaluated by comparing the area of the spheroid to that of day 0 as measured using Image J software. (National Institutes of Health, Bethesda, MD).
Reconstituted spheroid-based sensitivity assay
After washing the CTOSs with PBS, they were dissociated into single cells using 0.25% trypsin/EDTA and filtered through a 40 μm cell strainer (BD Falcon). Approximately 1x104 cells/100ul were seeded in poly-HEMA-coated 96-well plates and cultured in the FBS medium and 10 μM of ROCK inhibitor Y27632 (Wako, Osaka, Japan). Each drug was added and the CTOSs cultured for 7 days. ATP content was measured at day 7 by CellTiter-Glo Luminescent Cell Viability Assays (Promega, Madison, WI, USA) and adjusted to the content of the vehicle-treated control.
Western blot
For signaling assays using CTOSs, the medium was changed to the basal medium (DMEM/F12 containing 1.8% BSA, 1x nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin [all from Invitrogen], 0.1 mM β-mercaptoethanol [Wako]), the standard CTOS medium, or the FBS medium the day after CTOS preparation. The CTOSs were cultured overnight, treated with 100 nM cetuximab for 2 hours, and the samples collected. For signaling assays using reconstituted spheroids, the spheroids were treated with the drugs 2 days after re-aggregation and samples collected 2 hours after treatment. In the case of EGF stimulation, 10 ng/ml of EGF (Invitrogen) was added 15 min before sample collection. Immunoblots were performed as described previously [18]. Primary antibodies against EGFR (clone D38B1), pEGFR (Tyr1068) (clone D7A5), pHER2 (Y1221/1222) (clone 6B12), pHER3 (Tyr1289) (clone 21D3), AKT (clone 40D4), pAKT (Ser473) (clone D9E), p44/42 MAPK (clone 3A7), and pp44/42 MAPK (Thr202/Tyr204) (clone D13.14.4E) were obtained from Cell Signaling Technologies, and anti β-actin (clone AC-15) from Sigma-Aldrich (St. Louis, MO).
Statistical analysis
One-way or two-way ANOVA followed by Bonferroni’s post-test was used for comparisons of multiple groups, and correlations were analyzed by Pearson’s correlation using GraphPad Prism 6 software (San Diego, CA). P<0.05 was considered significant.
Results
In vivo cetuximab sensitivity assay using CTOS lines stratified KRAS mutant colorectal cancer
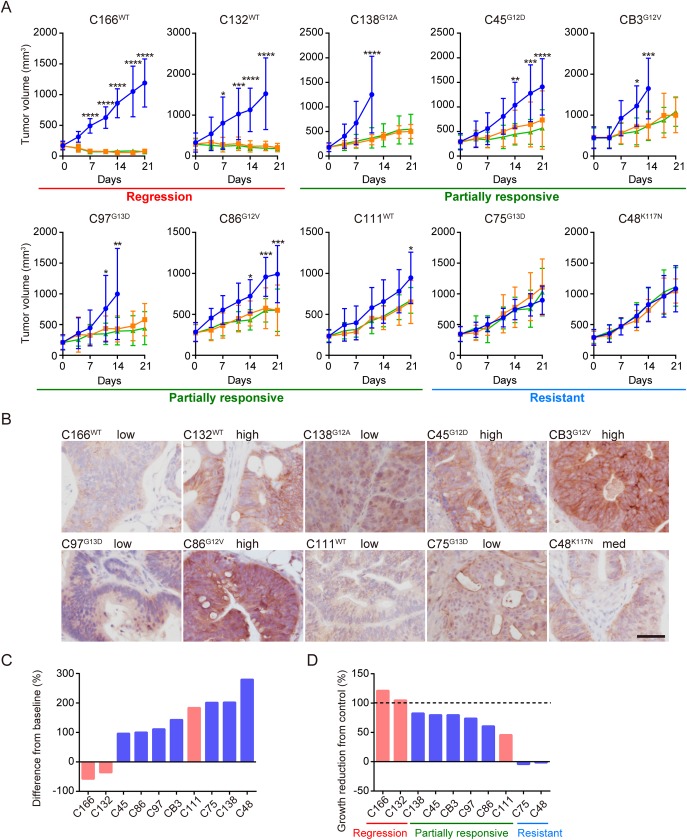
We established 10 CTOS lines from patients with CRC (Table 1). Among these 10 lines, three were KRAS-wildtype and seven were KRAS mutants. CTOSs were injected subcutaneously into NOD-SCID mice to create CTOS-derived xenografts, which were subjected to the cetuximab sensitivity assay in vivo. The sensitivity varied among the lines, some of which exhibited tumor regression (regression group), a partial response (partially responsive group), or resistance (resistant group) (Fig 1A). EGFR staining did not correlate with cetuximab sensitivity in the xenograft tumors (Fig 1B) as previously reported for clinical samples [24]. The clinically used waterfall plot, in which tumor volume is compared to the volume at the starting point, is shown in Fig 1C. Both lines in the regression group were KRAS-wildtype, whereas all of the KRAS mutants grew more than the initial volume and were in the partially responsive or resistant groups. The results were compatible with previous clinical reports and studies using PDX mouse models [5, 15–17].
Table 1. Clinical and genomic profiles of 10 CTOS lines.
| Clinical Information | Mutational status of CTOS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Patient sex | Tumor location | Tumor histology | TNM stage | KRAS | NRAS | BRAF | PIK3CA | AKT1 | APC | TP53 |
| C166 | F | Ra | Mod | ⅢB | WT | WT | WT | WT | WT | WT | WT |
| C132 | F | T | Mod | ⅢB | WT | WT | WT | WT | WT | MT | WT |
| C111 | F | Rs | Mod | ⅣB | WT | WT | WT | WT | WT | MT | MT |
| C138 | F | A | Mod | ⅡA | G12A | WT | WT | WT | WT | MT | WT |
| C45 | M | Ra | Mod | ⅣA | G12D | WT | WT | WT | WT | MT | WT |
| CB3 | F | A | Well | ⅡA | G12V | WT | WT | WT | WT | MT | WT |
| C97 | F | Rb | Mod | ⅢC | G13D | WT | WT | MT | WT | WT | MT |
| C86 | M | S | Mod | ⅣA | G12V | WT | WT | WT | WT | MT | MT |
| C75 | F | Ra | Mod | ⅣA | G13D | WT | WT | WT | MT | MT | WT |
| C48 | M | Rab | Mod | ⅡA | K117N | WT | WT | MT | WT | MT | WT |
Patient sex: M, male; F, female. Tumor location: A, ascending colon; T, transverse colon; S, sigmoid colon; Rs, rectosigmoid; Ra, upper rectum; Rb, lower rectum. Tumor histology: Well, well differentiated adenocarcinoma, Mod, moderately differentiated adenocarcinoma. Mutational status of CTOS: A panel of 50 frequently mutated genes in cancer was annalyzed. No mutations other than those mentioned above were detected. WT, wild type, MT, mutant. Mutations are shaded gray.
Fig 1. In vivo cetuximab sensitivity assay revealed diverse effect on 10 CTOS-derived xenogfrafts.
A, Growth curves of subcutaneous tumors originating from colorectal CTOS lines. Blue, treated with vehicle; orange, cetuximab (20 mg/kg); green, cetuximab (60 mg/kg). Mean±SD is shown. N = 4–6 in each treated group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; 60 mg/kg cetuximab versus control; two-way ANOVA with Bonferroni post-test. Regression, partially responsive, and resistant are explained in the text. The type of KRAS mutant is indicated in superscript to the left of the line name. B, Microscopic images of vehicle-treated xenografts in A stained with EGFR antibody. Scale bar, 50 μm. Grading by staining intensity is shown. C, Waterfall plot of cetuximab-treated tumor growth (differences from baseline). The average sizes of cetuximab (60 mg/kg) treated tumors at day 21 were subtracted by the sizes at day 0 and the ratio compared to the average sizes at day 0 as follows: (VCmab (day 21)—VCmab (day 0))/ VCmab (day 0) x 100. Red bars, wildtype KRAS tumors; blue, KRAS mutants. D, Growth reduction from control (vehicle treated) tumors. The average sizes of vehicle-treated tumors at day 11 were subtracted by the sizes of tumors treated with cetuximab (60 mg/kg) and the ratio compared to the average sizes at day 11 as follows: (Vvehicle(day 11)- VCmab(day 11))/ Vvehicle(day 11) x 100.
Experimental settings allowed growth reduction to be assessed from control (vehicle treated) tumors (Fig 1D). The difference between the partially responsive group and resistant group was clear. Thus, KRAS mutant CTOS lines were divided into two groups by cetuximab sensitivity: the partially responsive group and the resistant group. We assessed hotspot mutations of 50 genes. Neither KRAS mutation type nor any single mutation correlated with the difference (Table 1).
Optimizing conditions for ex vivo cetuximab sensitivity assay for CTOSs
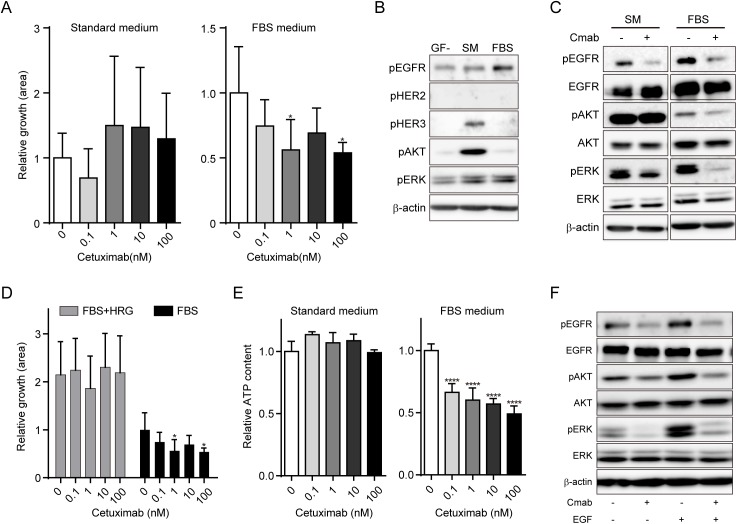
To investigate whether in vivo and ex vivo cetuximab sensitivity assays correlate, we tested the C132 CTOS line from the in vivo regression group. CTOS growth was not suppressed by cetuximab in the standard CTOS medium (Fig 2A). The media contained HRG [19], a ligand of HER3, the activation of which has been reported to be one of the mechanisms of cetuximab resistance [15, 25]. Alternatively, we examined another media containing 10% fetal bovine serum (FBS medium), and found that C132 CTOSs were sensitive to cetuximab (Fig 2A). The basal status of intracellular signaling revealed a remarkable difference between the media. In the standard CTOS medium, basal levels of HER3 and AKT phosphorylation were higher (Fig 2B), and AKT and ERK phosphorylation was suppressed less by cetuximab than in the FBS medium (Fig 2C). Adding HRG to the FBS medium abolished the growth inhibitory effect of cetuximab (Fig 2D). Thus, HER3 activation by HRG in the standard CTOS medium obscured the effect of cetuximab ex vivo. In addition, to reduce the deviation in growth among CTOSs (Fig 2A), we dissociated C132 CTOSs into single cells and reconstituted the spheroids by culturing the cells at high density in a low attachment culture dish. The decrease in deviation was prominent in the assay using reconstituted spheroids compared to CTOSs (Fig 2A and 2E), and the relative ATP levels were confirmed to be remarkably decreased by cetuximab treatment in the FBS medium but not the standard CTOS medium (Fig 2E). Using the reconstituted spheroids, phosphorylation of EGFR, AKT, and ERK was suppressed by cetuximab treatment with or without EGF stimulation (Fig 2F). Therefore, we applied the reconstituted spheroids in FBS medium in further evaluations.
Fig 2. Establishment of ex vivo cetuximab sensitivity assays.
A, Growth of C132 CTOSs cultured with the indicated doses of cetuximab for 7 days. The relative growth at day 7 adjusted by day 0 is shown. Culture media are indicated. Mean±SD is shown. N = 3–6. Significance of the decrease in relative growth. *P < 0.05, versus 0; one-way ANOVA with Bonferroni post-test. B, Western blots of lysates from C132 CTOSs cultured in the indicated media for 24 h. GF-, basal medium without any growth factor; SM, the standard CTOS medium; FBS, the FBS medium. Each antibody is indicated. C, Western blots of lysates from C132 CTOSs treated with or without cetuximab (100 nM) for 2 h. CTOSs were cultured in the indicated media for 24 h before cetuximab treatment. Cmab, cetuximab. D, Growth of C132 CTOSs cultured with the indicated doses of cetuximab for 7 days. CTOSs were cultured in the FBS medium with (gray bars) or without (black bars, same as Fig 2A) 10 ng/ml HRG. The relative growth at day 7 adjusted by day 0 is shown. Mean±SD is shown. N = 5–6. Significance of the decrease in relative growth. *P < 0.05, versus 0; one-way ANOVA with Bonferroni post-test. E, Results of the reconstituted spheroid-based assay. The viable cell number in the spheroids was evaluated by ATP content, and the ratio to that of non-treated spheroids is shown. CTOSs were cultured for 7 days in the indicated media with the indicated doses of cetuximab. Mean±SD is shown. N = 5–6. Significance of the decrease in relative ATP content. ****P < 0.0001, versus 0; one-way ANOVA with Bonferroni post-test. F, Western blot evaluation of intracellular signaling ex vivo. Reconstituted spheroids generated from C132 CTOSs were treated with or without 100 nM cetuximab for 2 h and then stimulated with or without 10 ng/ml of EGF for 15 min.
Suppression of ERK phosphorylation ex vivo paralleled KRAS mutant cetuximab sensitivity in vivo
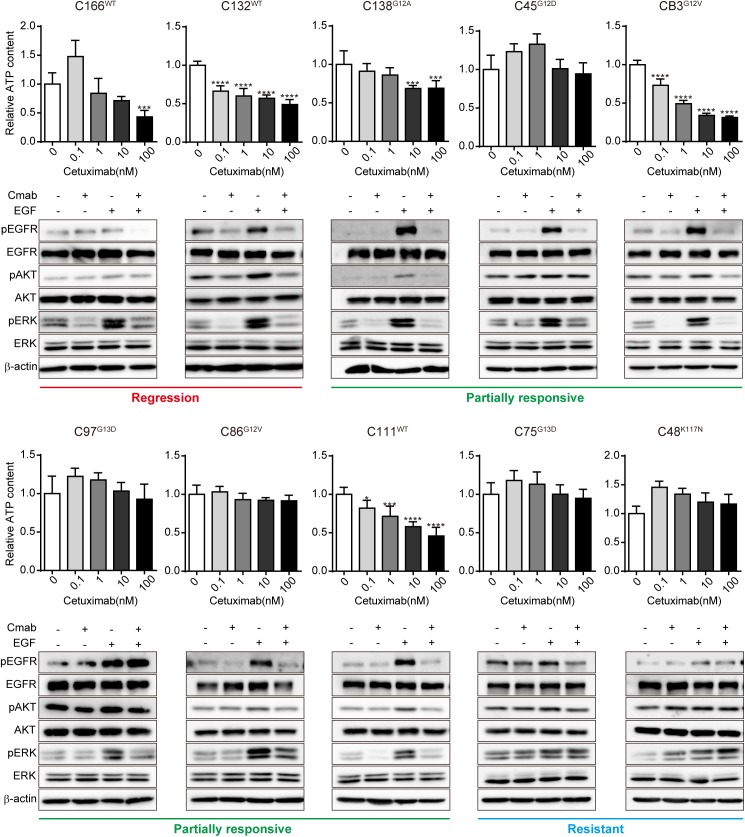
We performed ex vivo growth assays and signaling assays using reconstituted spheroids (Fig 3). Substantial differences were observed among the lines. The growth suppression by cetuximab in vivo was correlated to the ex vivo growth, as well as ERK phosphorylation with or without EGF stimulation (S1 Fig).
Fig 3. Response of ERK phosphorylation to cetuximab treatment stratified KRAS mutants into two groups ex vivo.
The result of the reconstituted spheroid-based assay in each CTOS line is shown. The CTOS lines are ordered according to the results of the in vivo assay and the grouping indicated below. The type of KRAS mutant is indicated in superscript to the left of the line name. Upper panels: relative ATP content of reconstituted spheroids cultured with the indicated doses of cetuximab for 7 days. Mean±SD is shown. N = 3–6. Significance of the decrease in relative ATP content. *P < 0.05, ***P < 0.001, ****P < 0.0001, versus 0; one-way ANOVA with Bonferroni post-test. Lower panels: ex vivo signaling assay by Western blot. Reconstituted spheroids were treated with or without 100 nM cetuximab for 2 h and then stimulated with or without 10 ng/ml EGF for 15 min. Cmab, cetuximab.
The suppression of growth and ERK phosphorylation without EGF stimulation was distinct between the regression and resistant groups (2/2 vs. 0/2), whereas the responses varied among the partially responsive group. For example, C111 exhibited markedly discrepant results between in vivo and ex vivo assays. In contrast, with EGF stimulation, ERK phosphorylation was suppressed not only in the regression group, but also in the partially responsive group. It was still not suppressed in the resistant group. The results indicate that the resistant group has quite distinct characteristics that can be better distinguished by sustained ERK phosphorylation after cetuximab treatment in the presence of EGF stimulation ex vivo. In regards to the mutational status of KRAS, the seven KRAS mutant lines were distributed into two groups: partially responsive (5/7) and resistant (2/7). Thus the KRAS mutants were stratified into two groups based on the effect of cetuximab not only in vivo but also ex vivo.
Combination of cetuximab and trametinib was effective in the partially responsive group ex vivo
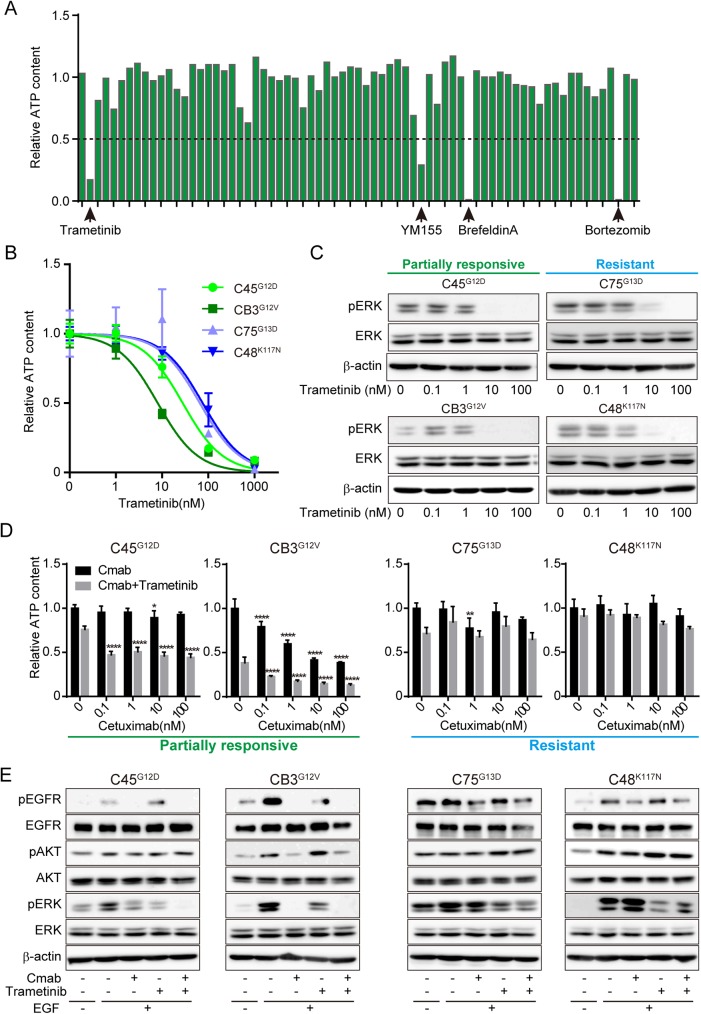
As the KRAS mutant CRC CTOS lines in the partially responsive group exhibited a partial response while those in the resistant group exhibited no response, we expected that combination therapy may improve the effect of cetuximab in the partially responsive group. First, we screened 71 signaling inhibitors as single reagents using reconstituted spheroids derived from C45 CTOSs, a line from the partially responsive group (Fig 4A and S1 Table). Four drugs resulted in growth inhibition in which the relative ATP content was <0.5. One drug was trametinib, a MEK inhibitor. As MEK is a downstream molecule of KRAS, we further studied trametinib.
Fig 4. Combination of cetuximab and trametinib was more effective in the partially responsive group ex vivo.
A, Screening of 71 drugs using reconstituted C45 spheroids. The spheroids were cultured with 100 nM of drug for 7 days. The drugs for which the relative ATP content was less than 0.5 are indicated. Mean values are shown. N = 4. B, Dose-response curve of trametinib in four CTOS lines (the partially responsive group, C45 and CB3; the resistant group, C75 and C48). The type of KRAS mutant is indicated in superscript to the left of the line name. C, Lysates of reconstituted spheroids from four CTOS lines were subjected to Western blotting. The spheroids were treated with the indicated doses of trametinib for 4 h. The antibodies used are indicated. D, Relative ATP content of reconstituted spheroids cultured for 7 days with cetuximab alone at the indicated doses (black bars), or in combination with 10 nM of trametinib (gray bars). Mean±SD is shown. N = 6. Significance of the decrease in relative ATP content. *P < 0.05, **P < 0.01, ****P < 0.0001, versus 0; one-way ANOVA with Bonferroni post-test. E, Western blots of lysates from the reconstituted spheroids treated with or without 100 nM cetuximab, 5 nM trametinib, or a combination of 100 nM cetuximab and 5 nM trametinib for 2 h with or without stimulation with 10 ng/ml EGF for 15 min.
To determine the dose of trametinib for the combination treatment assays, we examined mono-treatment with trametinib at various doses (Fig 4B and 4C). The C75 and C48 lines in the resistant group were relatively resistant in the growth assay, although ERK phosphorylation was remarkably inhibited in all lines examined when the doses were more than 10 nM (Fig 4B and 4C). Therefore, we chose 10 nM for the growth assay (Fig 4D). Combination treatment in the partially responsive group (C45, CB3) effected growth to a greater extent than trametinib mono-treatment, but this was not observed in the resistant group (C75, C48) (Fig 4D). For the signaling assay, we chose 5 nM trametinib with EGF treatment to ensure a clear combinatory effect (Fig 4E). In the partially responsive group (C45, CB3), ERK phosphorylation was suppressed more by combination treatment than trametinib alone, with (Fig 4E) or without (S2 Fig) EGF stimulation. In contrast, combination treatment did not suppress ERK phosphorylation in the resistant group (C75, C48).
Combination of cetuximab and trametinib was more effective in the partially responsive group than in the resistant group in vivo
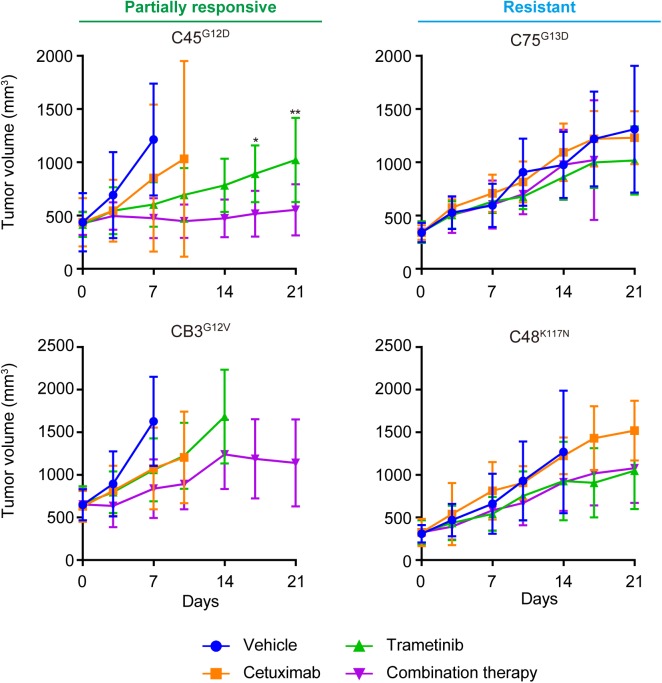
We examined the combined effect of cetuximab and trametinib in vivo (Fig 5) using xenograft models generated by subcutaneously injecting the CTOSs. Mono-therapy with either cetuximab or trametinib had significant effects in the partially responsive group (C45, CB3), and the combination of cetuximab and trametinib enhanced the effect; CB3 tumors stopped growing after 14 days with combination therapy. In contrast, the lines in the resistant group (C75, C48) exhibited a marginal effect with trametinib alone, and combination therapy seemingly had no effect.
Fig 5. Combination of cetuximab and trametinib was more effective in the partially responsive group in vivo.
Growth curves of subcutaneous tumors generated by four CTOS lines (the partially responsive group, C45 and CB3; the resistant group, C75 and C48). Blue, treated with vehicle; orange, cetuximab (20 mg/kg) alone; green, trametinib (0.3 mg/kg) alone; purple, combination of cetuximab (20 mg/kg) and trametinib (0.3 mg/kg). Mean±SD is shown. N = 6 in each treated group. *P < 0.05, **P < 0.01, mono therapy with trametinib versus combination; two-way ANOVA with Bonferroni post-test. The type of KRAS mutant is indicated in superscript to the left of the line name.
Discussion
We applied the CTOS method in this study of in vivo and ex vivo drug sensitivity assays. The in vivo results were consistent with previous reports of the cetuximab resistance in KRAS mutant CRCs. Here we revealed that KRAS mutant CRC tumors have a wide range of sensitivity to cetuximab and can be divided into two groups: partially responsive and resistant. Lines in the partially responsive group exhibit remarkable suppression of ERK phosphorylation after cetuximab treatment in the presence of EGF stimulation. Combination therapy with cetuximab and trametinib, a MEK inhibitor, was more effective than mono-treatment in the partially responsive group but not the resistant group.
Recently, the primary culture of cancer cells has progressed remarkably and is expected to be applied to the prediction of sensitivity for individual patients. A phenotypic assay can be the basis for studying mechanisms and developing biomarkers for a drug, especially when multiple factors are involved [26]. CTOSs can be prepared efficiently from patient tumors, as well as PDX or CTOS-derived xenografts. In addition, CTOSs can be proficiently cryopreserved as spheroids. We previously reported that the ex vivo growth assay for selected CTOSs correlates with the in vivo assay for various cancers and drugs[19, 22, 23, 27]. To the best of our knowledge, this is the first report to perform and compare in vivo and ex vivo cetuximab sensitivity assays side by side for a panel of CRC primary cultures. The results of the in vivo assays using CTOS-derived xenografts were compatible with previous reports using PDX models. Furthermore, the ex vivo signaling assay reflected the in vivo sensitivity, although the ex vivo growth did not completely reflect it.
The CTOS line C132 was sensitive to cetuximab in vivo but resistant ex vivo when the standard CTOS medium containing HER3 ligand was used. As the growth conditions in culture can never be exactly the same as the microenvironment in vivo, the culture conditions need to be adjusted to conform the results of the ex vivo growth assay to those of the in vivo assay or patient responses. Various factors, such as the selection of growth factors and cell matrices and co-culture with fibroblasts, should be optimized. On the other hand, signaling assays are more promising because they assess the ‘potential capacity’ of cancer cells without long-term culture. In this study, the suppression of ERK phosphorylation under high-dose EGF-stimulated conditions was the best predictor of cetuximab sensitivity in vivo, although the functional role of suppressing ERK phosphorylation is still not clear.
KRAS mutant CRCs are thought to be generally resistant to cetuximab, as the overall survival, progression-free survival, and response rate have not improved in clinical trials [5, 6]. All KRAS mutant xenograft tumors in this study grew after cetuximab treatment, which is consistent with findings from previous clinical and pre-clinical studies [5, 14–17]. However, by comparing the tumors to vehicle-treated controls, which is only allowed in experimental settings, we demonstrated that KRAS mutant CRCs can be divided into two groups. Tumor growth was significantly suppressed by cetuximab treatment in the partially responsive group but not the resistant group. In previous clinical reports, 10% of KRAS mutant CRC exhibited disease control [28], and mutations in codon 13 (G13D) have been suggested to be more sensitive to cetuximab than mutations in codon 12 [29, 30]. Thus, diverse sensitivities to cetuximab, even in KRAS mutants, have been suggested in clinical studies, although the characteristics and mechanisms are poorly understood. When the previous reports using PDX are examined carefully, they also indicate that KRAS mutants exhibit various levels of partial response to cetuximab [16, 17]. Thus, a partial response to cetuximab in KRAS mutant CRC was observed in both CTOS xenografts and PDX models, although the clinical relevance remains to be clarified. In this study, attributing the different sensitivities to the type of KRAS mutation is not likely, as all four of the KRAS mutations in codon 12 were in the relatively sensitive (partially responsive) group, and two of the G13D (C97 and C75) lines were in different sensitivity groups. Neither hotspot mutation in NRAS, BRAF, PIK3CA nor that of other genes which were reportedly related to cetuximab resistance [15, 31–33] was able to distinguish the two groups. Triad mutations of APC, KRAS, and genes in PI3K-AKT pathway were found only in the resistant group. Since the number of the resistant lines are too small, further study should be performed to ascertain whether the triad mutations can be a biomarker of the resistant group.
In this study, the CTOS method enabled ex vivo signaling assays of cancer cells in xenograft tumors. Because KRAS is a downstream molecule in EGFR signaling, KRAS mutant CRCs are thought to be resistant to cetuximab due to constitutive activation of downstream signaling [34–36]. In this study, increased ERK phosphorylation was suppressed by cetuximab in all of the CTOS lines in the partially responsive group, indicating that ERK phosphorylation is still dependent on upstream signaling in the majority of KRAS mutant lines. The results are a sharp contrast to most previous reports using established CRC cell lines, in which ERK phosphorylation is mostly resistant to cetuximab in KRAS mutants [36–39]. The discrepancy may be attributed to the different culture conditions, or the partially responsive group may be rare in the established cell lines. On the other hand, the two lines in the resistant group did not respond to cetuximab treatment in regards to both growth and ERK phosphorylation. Particularly in C48 CTOSs, ERK phosphorylation increased with cetuximab treatment. The activation of compensatory pathways or relief of a negative feedback loop [40] may be involved.
Encouraged by the partial response to cetuximab in the partially responsive group, we investigated combination therapy with a MEK inhibitor. MEK is a downstream molecule of EGFR and RAS signaling, and MEK inhibitor is expected to enhance the effect of cetuximab [36, 38]. In this study, ERK phosphorylation and the growth of CTOSs were suppressed in two KRAS mutants in the partially responsive group after combination treatment. In contrast, the growth of CTOSs in two KRAS mutants in the resistant group were not affected by combination treatment. As for ERK phosphorylation, cetuximab had no additional effect on MEK inhibition (Fig 4E). The activation of pathway molecules between EGFR and ERK may be independent of EGFR activation. Alternatively, pathways other than MEK-ERK can be involved in cetuximab resistance, as MEK inhibition had a minor effect on growth (Fig 4B and 4C).
Previous studies offer some examples in which the combination of cetuximab and a MEK inhibitor is effective, even in resistant-type cell lines [36, 38]. The discrepancy may be due to the difference between CTOSs and established cell lines, or a difference in the MEK inhibitors used [37]. Resistant CTOS lines need to be accumulated to confirm the characteristics and investigate the mechanism.
Taken together, our data support CTOSs of the same origin as a model shuttling system between in vivo and ex vivo assays, especially for assessing the diversity of drug sensitivity. In addition, this approach may be applicable to existing PDX models, although further studies are required to clarify the similarity and difference between CTOS-derived xenograft and PDX model. Furthermore, ex vivo assays using CTOS technology may be useful for screening drugs and combination treatments or selecting patients for the therapies.
Supporting information
Pearson’s correlation coefficients, R, and p-values are shown. In vivo growth reduction was the average rate of growth reduction day 11 after the first treatment with 60 mg/kg cetuximab in vivo. Ex vivo growth reduction was the average rate of growth reduction day 7 after treatment with 100 nM cetuximab ex vivo. The reduced intensity of ERK/AKT phosphorylation, which was adjusted by β-actin, was detected by Western blotting with (EGF+) or without (EGF-) stimulation ex vivo.
(TIF)
Western blotting of lysates from the reconstituted spheroids treated with or without 100 nM cetuximab, 1 nM trametinib, or a combination of 100 nM cetuximab and 1 nM trametinib for 2 h without EGF stimulation. The type of KRAS mutant is indicated in superscript to the left of the line name.
(TIF)
ATP content relative to that of non-treated spheroids is shown. Effective results (<0.50) are indicated in pink.
(PDF)
Acknowledgments
The authors thank A. Mizukoshi, N. Kanto, and T. Yasuda for technical assistance and M. Izutsu for secretarial assistance. The SCADS Inhibitor Kit was kindly provided by the Screening Committee of Anticancer Drugs supported by a Grant-in-Aid for Scientific Research on Innovative Areas, Scientific Support Programs for Cancer Research, from The Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by grants-in-aid from P-DIRECT (15cm0106155h0002), and P-CREATE (16cm0106203h0001), Japan Agency for Medical Research and Development (MI, HO, and HE) and the Takeda Science Foundation (MI). Charles River Laboratory Japans provided support in the form of salaries for YA, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, Group EGW. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii1–9. [DOI] [PubMed] [Google Scholar]
- 2.https://www.nccn.org/patients/guidelines/colon/index.html.
- 3.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. 10.1016/S1470-2045(15)70127-0 [DOI] [PubMed] [Google Scholar]
- 4.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67(6):2643–8. 10.1158/0008-5472.CAN-06-4158 [DOI] [PubMed] [Google Scholar]
- 5.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65. 10.1056/NEJMoa0804385 [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17. 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
- 7.Peeters M, Oliner KS, Parker A, Siena S, Van Cutsem E, Huang J, et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res. 2013;19(7):1902–12. 10.1158/1078-0432.CCR-12-1913 [DOI] [PubMed] [Google Scholar]
- 8.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34. 10.1056/NEJMoa1305275 [DOI] [PubMed] [Google Scholar]
- 9.Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26(1):13–21. 10.1093/annonc/mdu378 [DOI] [PubMed] [Google Scholar]
- 10.Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69(8):3364–73. PubMed Central PMCID: PMCPMC2821899. 10.1158/0008-5472.CAN-08-4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillet JP, Calcagno AM, Varma S, Marino M, Green LJ, Vora MI, et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A. 2011;108(46):18708–13. PubMed Central PMCID: PMCPMC3219108. 10.1073/pnas.1111840108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–50. PubMed Central PMCID: PMCPMC3928688. 10.1038/nrclinonc.2012.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. PubMed Central PMCID: PMCPMC4167608. 10.1158/2159-8290.CD-14-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes M, Vrignaud P, Vacher S, Richon S, Lievre A, Cacheux W, et al. Evaluating patient-derived colorectal cancer xenografts as preclinical models by comparison with patient clinical data. Cancer Res. 2015;75(8):1560–6. 10.1158/0008-5472.CAN-14-1590 [DOI] [PubMed] [Google Scholar]
- 15.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1(6):508–23. 10.1158/2159-8290.CD-11-0109 [DOI] [PubMed] [Google Scholar]
- 16.Krumbach R, Schuler J, Hofmann M, Giesemann T, Fiebig HH, Beckers T. Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur J Cancer. 2011;47(8):1231–43. 10.1016/j.ejca.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 17.Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goere D, Mariani P, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18(19):5314–28. 10.1158/1078-0432.CCR-12-0372 [DOI] [PubMed] [Google Scholar]
- 18.Kondo J, Endo H, Okuyama H, Ishikawa O, Iishi H, Tsujii M, et al. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A. 2011;108(15):6235–40. PubMed Central PMCID: PMCPMC3076886. 10.1073/pnas.1015938108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endo H, Okami J, Okuyama H, Kumagai T, Uchida J, Kondo J, et al. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J Thorac Oncol. 2013;8(2):131–9. Epub 2013/01/19. 10.1097/JTO.0b013e3182779ccf [DOI] [PubMed] [Google Scholar]
- 20.Okuyama H, Yoshida T, Endo H, Nakayama M, Nonomura N, Nishimura K, et al. Involvement of heregulin/HER3 in the primary culture of human urothelial cancer. J Urol. 2013;190(1):302–10. Epub 2013/01/15. 10.1016/j.juro.2012.12.106 [DOI] [PubMed] [Google Scholar]
- 21.Yamaki T, Suenaga Y, Iuchi T, Alagu J, Takatori A, Itami M, et al. Temozolomide suppresses MYC via activation of TAp63 to inhibit progression of human glioblastoma. Sci Rep. 2013;3:1160 Epub 2013/01/31. PubMed Central PMCID: PMC3557454. 10.1038/srep01160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima A, Endo H, Okuyama H, Kiyohara Y, Kimura T, Kamiura S, et al. Radiation sensitivity assay with a panel of patient-derived spheroids of small cell carcinoma of the cervix. Int J Cancer. 2015;136(12):2949–60. 10.1002/ijc.29349 [DOI] [PubMed] [Google Scholar]
- 23.Kiyohara Y, Yoshino K, Kubota S, Okuyama H, Endo H, Ueda Y, et al. Drug screening and grouping by sensitivity with a panel of primary cultured cancer spheroids derived from endometrial cancer. Cancer Sci. 2016;107(4):452–60. PubMed Central PMCID: PMCPMC4832863. 10.1111/cas.12898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23(9):1803–10. 10.1200/JCO.2005.08.037 [DOI] [PubMed] [Google Scholar]
- 25.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3(99):99ra86 PubMed Central PMCID: PMCPMC3268675. 10.1126/scitranslmed.3002442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–45. 10.1016/j.cell.2015.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshii Y, Furukawa T, Waki A, Okuyama H, Inoue M, Itoh M, et al. High-throughput screening with nanoimprinting 3D culture for efficient drug development by mimicking the tumor environment. Biomaterials. 2015;51:278–89. 10.1016/j.biomaterials.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 28.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25(22):3230–7. 10.1200/JCO.2006.10.5437 [DOI] [PubMed] [Google Scholar]
- 29.De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304(16):1812–20. 10.1001/jama.2010.1535 [DOI] [PubMed] [Google Scholar]
- 30.Tejpar S, Celik I, Schlichting M, Sartorius U, Bokemeyer C, Van Cutsem E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012;30(29):3570–7. 10.1200/JCO.2012.42.2592 [DOI] [PubMed] [Google Scholar]
- 31.Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3(6):658–73. PubMed Central PMCID: PMCPMC4078408. 10.1158/2159-8290.CD-12-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanella ER, Galimi F, Sassi F, Migliardi G, Cottino F, Leto SM, et al. IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med. 2015;7(272):272ra12 10.1126/scitranslmed.3010445 [DOI] [PubMed] [Google Scholar]
- 33.Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature. 2015;526(7572):263–7. PubMed Central PMCID: PMCPMC4878148. 10.1038/nature14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6(9):519–27. 10.1038/nrclinonc.2009.111 [DOI] [PubMed] [Google Scholar]
- 35.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7(9):493–507. PubMed Central PMCID: PMCPMC2929287. 10.1038/nrclinonc.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misale S, Arena S, Lamba S, Siravegna G, Lallo A, Hobor S, et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6(224):224ra26 10.1126/scitranslmed.3007947 [DOI] [PubMed] [Google Scholar]
- 37.Yoon J, Koo KH, Choi KY. MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Cancer Res. 2011;71(2):445–53. 10.1158/0008-5472.CAN-10-3058 [DOI] [PubMed] [Google Scholar]
- 38.Troiani T, Napolitano S, Vitagliano D, Morgillo F, Capasso A, Sforza V, et al. Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition. Clin Cancer Res. 2014;20(14):3775–86. 10.1158/1078-0432.CCR-13-2181 [DOI] [PubMed] [Google Scholar]
- 39.Napolitano S, Martini G, Rinaldi B, Martinelli E, Donniacuo M, Berrino L, et al. Primary and Acquired Resistance of Colorectal Cancer to Anti-EGFR Monoclonal Antibody Can Be Overcome by Combined Treatment of Regorafenib with Cetuximab. Clin Cancer Res. 2015;21(13):2975–83. 10.1158/1078-0432.CCR-15-0020 [DOI] [PubMed] [Google Scholar]
- 40.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68(20):8322–32. 10.1158/0008-5472.CAN-07-6720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pearson’s correlation coefficients, R, and p-values are shown. In vivo growth reduction was the average rate of growth reduction day 11 after the first treatment with 60 mg/kg cetuximab in vivo. Ex vivo growth reduction was the average rate of growth reduction day 7 after treatment with 100 nM cetuximab ex vivo. The reduced intensity of ERK/AKT phosphorylation, which was adjusted by β-actin, was detected by Western blotting with (EGF+) or without (EGF-) stimulation ex vivo.
(TIF)
Western blotting of lysates from the reconstituted spheroids treated with or without 100 nM cetuximab, 1 nM trametinib, or a combination of 100 nM cetuximab and 1 nM trametinib for 2 h without EGF stimulation. The type of KRAS mutant is indicated in superscript to the left of the line name.
(TIF)
ATP content relative to that of non-treated spheroids is shown. Effective results (<0.50) are indicated in pink.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.