Abstract
Terrestrial vertebrate frugivores constitute one of the major guilds in tropical forests. Previous studies show that the meso-scale distribution of this group is only weakly explained by variables such as altitude and tree basal area in lowland Amazon forests. For the first time we test whether seasonally limiting resources (water and fallen fruit) affect the dry season distribution in 25 species of terrestrial vertebrates. To examine the effects of the spatial availability of fruit and water on terrestrial vertebrates we used a standardized, regularly spaced arrangement of camera-traps within 25km2 of lowland Amazon forest. Generalized linear models (GLMs) were then used to examine the influence of four variables (altitude, distance to large rivers, distance to nearest water, and presence vs absence of fruits) on the number of photos on five functional groups (all frugivores, small, medium, large and very large frugivores) and on seven of the most abundant frugivore species (Cuniculus paca, Dasyprocta leporina, Mazama americana, Mazama nemorivaga, Myoprocta acouchy, Pecari tajacu and Psophia crepitans). A total of 279 independent photos of 25 species were obtained from 900 camera-trap days. For most species and three functional groups, the variation in the number of photos per camera was significantly but weakly explained by the GLMs (deviance explained ranging from 6.2 to 48.8%). Generally, we found that the presence of water availability was more important than the presence of fallen fruit for the groups and species studied. Medium frugivores, large-bodied frugivores, and two of the more abundant species (C. paca and P. crepitans) were recorded more frequently closer to water bodies; while none of the functional groups nor the most abundant species showed any significant relationship with the presence of fallen fruit. Two functional groups and two of the seven most common frugivore species assessed in the GLMs showed significant results with species-specific responses to altitude. Our findings provide a more detailed understanding of how frugivorous vertebrates cope with periods of water and fruit scarcity in lowland Amazon forests.
Introduction
Environmental features such as water availability and food resources may influence the spatial distribution of wildlife to varying degrees depending on species-specific factors. For example, in subtropical semi-arid regions many species of mammals showed a positive association between occupancy patterns and permanent water sources [1–5]. Similarly, a preference for areas close to water bodies has also been documented in tropical forests for some species of terrestrial birds [6, 7].
In tropical forests, frugivorous species represent the majority of the mammalian and avian biomass [8] and depend on both unripe and ripe fruits as well as some species of flowers as key diet resources [9], with the degree of dependency related with the specificity of their diet [6, 10–12]. However, the availability of resources (e.g. food and water) is not constant in tropical forests and has been found to be extremely variable in both space and time [13–15]. Even continuous tracts of tropical forests can show distinct vertebrate species distributions [16], a phenomena that could be related with habitat suitability and resource availability. Although fruit production varies in space and time, a period of fruit scarcity exists everywhere [17]. Some studies use precipitation as indicator of food availability for frugivores, with the dry season a period of limited food and water availability for Amazon vertebrate frugivores [14, 15, 18, 19].
Seasonal shortages are sufficient to cause several effects in a local frugivore community [14]. A species’ response to fruit and water availability may be influenced by its life-history traits (e.g., dietary guild, body mass, home range size) and competitive pressure [2–4, 20, 21]. Species traits are also used to explain variations on species’ abundances in responses to environmental variables and anthropogenic disturbances [5, 21–27].
Studies on the relationships between life-history traits and water and fruit dependency are still scarce for neotropical terrestrial vertebrates. Although numerous studies have monitored vertebrates in lowland Amazonia [7, 24, 25, 28], the relative importance of different resources (e.g. water and fruit) have not been quantified for terrestrial vertebrates. As Amazonia holds both, the highest diversity of terrestrial and aquatic frugivorous vertebrates [11, 19] and the widest spectrum of morphological fruit types anywhere on Earth [29] it is surprising that more studies on this topic are not found.
The differences in temperature and precipitation regimes predicted by climate change models in the coming decades and their possible influence on resource availability (e.g., fruits and water) [1] may influence population parameters of frugivore species. There is a need to understand the ecological factors affecting the distribution of different species and obtain basic information of the species-specific responses to cope with water and fruit scarcity. This information may inform models for understanding or predicting the potential impact of anthropogenic and climate change on the species in the future, including the potential resilience of frugivores to disturbances [2].
In this study we used camera-traps to survey mid-sized and large- bodied vertebrates with a standardized sampling regime that has been utilized in other tropical studies to survey terrestrial vertebrates within a 25 km2 area [7]. Here we focused on evaluating the spatial effects of (1) fruit availability, (2) water availability and (3) altitude on vertebrate frugivores during the peak of the dry season, a period of reduced resources in eastern Amazonia. We included altitude as a proxy for biological variables as it affects soil, water availability, climate and other abiotic and biotic variables [30–34] and has also been shown to be important for the vertebrate groups in the study area [7]. Additionally, we examined the importance of species life-history traits in the responses to these environmental variables. We predict that both the presence of fallen fruit and water availability would affect functional groups and species of terrestrial frugivores. To test these predictions we evaluated whether water and fruit availability act with altitude to explain abundance patterns in terrestrial vertebrates during the dry season in a continuous forest site.
Materials and methods
Ethics statement
Data collection used non-invasive, remotely activated camera traps and did not involve direct contact or interaction with animals, thus no ethical approval was required. Fieldwork was conducted under research permit number IBAMA/SISBIO 47859–1 to DN and FM, issued by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio).
Study area
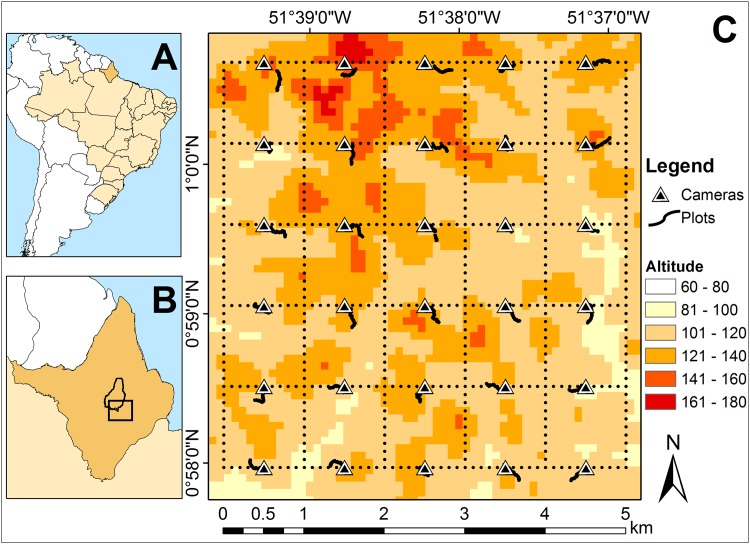
This study was conducted in Amapá National Forest (Floresta Nacional Amapá –hereafter ANF), a sustainable-use protected area of approximately 412,000 ha, centered in the state of Amapá in north-eastern Brazilian Amazonia (0°55’29”N, 51°35’45”W, Fig 1) [35]. The ANF consists of continuous tropical rainforest vegetation, predominantly never-flooded closed canopy ‘‘terra firme” forest [35].
Fig 1. Location of the study region in the Amapá National Forest (ANF), Amapá State, eastern Brazilian Amazon.
(A) Amapá State in Brazil; (B) ANF (polygon) in Amapá State; (C) Altitude (m) across the grid system (dotted lines), non-linear plots placed along topographic contours and linear plots along the trails (solid back lines) where the study was conducted. Camera traps were placed at 30 regularly spaced sample points (triangles).
ANF currently experiences low levels of anthropogenic perturbation (there has been no mechanized logging within the boundaries of ANF) and is part of a large (> 4 million hectares) connected group of protected areas [35] that maintain both continuous undisturbed forests and the complete regional community of medium-sized and large-bodied vertebrates [7].
The regional climate is classified by Köppen-Geiger as Am (Equatorial monsoon) [36], with an annual rainfall greater than 2000 mm [37]. The driest months are September to November (total monthly rainfall < 150 mm) and the wettest months from February to April (total monthly rainfall > 300 mm) [37] (S1 Fig).
Sampling design
Data were collected between October and December 2015, months that historically represent the peak of the dry season in the study area (monthly mean ± SD = 81 mm ± 74 mm, range = 13 mm– 268 mm, for 2013 to 2015) [37] (S1 Fig). Our study months were particularly dry compared with previous years, with a total rainfall of 124 mm (monthly range = 13 mm– 89 mm) between October-December [37] (S1 Fig). Our research was conducted within a 25 km2 RAPELD grid (RAP surveys in the Long-term Ecological Research Sites whose Brazilian acronym is PELD, hence RAPELD) of the Brazilian Program for Biodiversity Research (PPBio) [38, 39] (Fig 1). The RAPELD grid is a standardized arrangement of trails and permanent plots [39]. The current study used the 30 permanent regularly spaced sample points and plots that are distributed at 1 km intervals along the east-west trails in the RAPELD grid [7, 38, 39] (Fig 1). This sample size and arrangement has been shown to be generally robust and representative for quantifying meso-scale spatial patterns in lowland Amazon biodiversity [40].
Vertebrate sampling
We used camera traps equipped with infrared triggers (Bushnell Trophy Cam, 8MP, Overland Park, KS, USA) to sample terrestrial vertebrates in the RAPELD grid. Due to financial constraints we did not have sufficient cameras to survey all 30 points simultaneously. Thus, cameras were placed at 20 points for 30 consecutive days then immediately transferred to the remaining 10 points. This division of study areas into blocks, with cameras not operating simultaneously within an area due to logistical constrains, is a common practice in studies with camera traps [7, 41–44]. As the sampling was conducted over a relatively short period of time, we assume that this division does not introduce any systematic bias. All cameras were unbaited, installed at 30–40 cm above the ground and facing the trails to ensure the capture of a wide spectrum of vertebrates (from small to large-bodied species). Cameras were deployed for 30 days, functioning continuously (24 hours a day). We configured cameras to film for 40 seconds post-activation, with intervals of 15 seconds between videos, with date-time stamp enabled.
In order to estimate the relative abundance of vertebrates, we considered as independent, videos with over a 30 min interval, when the same species was recorded during the same day on the same camera [7, 45, 46]. This minimum 30-min interval reduces the temporal dependence between camera trap detections [45] and has been widely used in studies with camera traps [47–49]. Vertebrates were identified using field guides of mammals and birds [50–52]. All identifications were double-checked by two researchers with more than 10 years experience (FM and DN).
Species traits
We conducted a literature survey to obtain two morphological and ecological traits (trophic guild and body mass) for the 25 vertebrates studied (Table 1). All 25 vertebrates were classified into frugivores and non-frugivores based on previously published data (Table 1). We grouped mammals and birds into small species (< 1 kg), medium species (1–5 kg), large species (5–15 kg) and very large species (>15 kg) according to the average body size of adult individuals [26, 53] (Table 1). Finally, we defined five functional groups as follows (Table 1): (i) All frugivores (all frugivores from all body sizes), (ii) Small frugivores (frugivores < 1 kg), (iii) Medium frugivores (frugivores with 1–5 kg), (iv) Large frugivores (frugivores with 5–15 kg) and (v) Very large frugivores (frugivores > 15 kg).
Table 1. Trophic guild, body mass, functional group, number of occupied sites, relative abundances and number of independent videos (Detections) for all 25 species examined.
| Class/Order/Family | Species | Trophic Guild* | Body Mass (kg) | Functional group | Occupied Sites | RA**(Detections) |
|---|---|---|---|---|---|---|
| Birds | ||||||
| Gruiformes | ||||||
| Psophiidae | Psophia crepitans | Fr/In [54, 55] | 1.50 [24] | Medium frugivore | 21 | 0.61 (55) |
| Cracidae | Crax alector | Fr/Sp [24] | 3.40 [24] | Medium frugivore | 4 | 0.07 (6) |
| Tinamiformes | ||||||
| Tinamidae | Crypturellus erythropus | In/Fr [54] | 0.42 [54] | Small frugivore | 4 | 0.07 (6) |
| Tinamidae | Tinamus major | Sp/Fr/In [54] | 1.20 [24] | Medium frugivore | 4 | 0.04 (4) |
| Mammals | ||||||
| Didelphimorphia | ||||||
| Didelphis marsupialis | In/Fr/Vp [28] | 1.05 [28] | Medium frugivore | 3 | 0.03 (3) | |
| Artiodactyla | ||||||
| Cervidae | Mazama americana | Fr/Fo [28] | 30.0 [28] | Very large frugivore | 9 | 0.36 (32) |
| Mazama nemorivaga | Fr/Fo [28] | 18.0 [28] | Very large frugivore | 9 | 0.18 (16) | |
| Tayassuidae | Pecari tajacu | Sp/Fr/Vp [28] | 25.0 [28] | Very large frugivore | 14 | 0.49 (44) |
| Perissodactyla | ||||||
| Tapiridae | Tapirus terrestris | Fr/Fo [28] | 150.0 [28] | Very large frugivore | 4 | 0.07 (6) |
| Carnivora | ||||||
| Canidae | Speothos venaticus | Vp [28] | 6.32 [56] | - | 1 | 0.01 (1) |
| Felidae | Leopardus pardalis | Vp [28] | 11.90 [24] | - | 2 | 0.09 (8) |
| Leopardus wiedii | Vp [28] | 3.25 [24] | - | 1 | 0.01 (1) | |
| Puma concolor | Vp [28] | 51.60 [24] | - | 2 | 0.02 (2) | |
| Panthera onca | Vp [28] | 80.00 [24] | - | 1 | 0.01 (1) | |
| Mustelidae | Eira barbara | Fr/In/Vp [28] | 4.80 [28] | Medium frugivore | 2 | 0.02 (2) |
| Procyonidae | Nasua nasua | In/Vp/Fr [28, 57] | 3.10 [28] | Medium frugivore | 1 | 0.01 (1) |
| Procyon cancrivorus | In/Vp/Fp/Fr [28, 57] | 6.93 [56] | Large frugivore | 1 | 0.01 (1) | |
| Cingulata | ||||||
| Dasypodidae | Dasypus kappleri | In [28] | 9.50 [24] | - | 2 | 0.06 (5) |
| Dasypus novemcinctus | In [28] | 5.50 [28] | - | 4 | 0.06 (5) | |
| Priodontes maximus | In [28] | 38.00 [24] | - | 1 | 0.01 (1) | |
| Pilosa | ||||||
| Myrmecophagidae | Myrmecophaga tridactyla | In [28] | 22.33 [24] | - | 3 | 0.03 (3) |
| Rodentia | ||||||
| Cuniculidae | Cuniculus paca | Sp/Fr [28] | 8.00 [28] | Large frugivore | 6 | 0.31 (28) |
| Dasyproctidae | Myoprocta acouchy | Sp/Fr [54] | 0.95 [24] | Small frugivore | 9 | 0.41 (37) |
| Dasyprocta leporina | Sp/Fr [54] | 3.50 [24] | Medium frugivore | 7 | 0.11 (10) | |
| Sciuridae | Sciurus aestuans | Sp/Fr [28, 52] | 0.19 [24] | Small frugivore | 1 | 0.01 (1) |
*Trophic guild: (Fr) frugivore, (Fo) folivore, (Sp) seed predator, (Fu) fungus, (In) Invertebrate predator, (Vp) terrestrial vertebrate predator, (Fp) fish predator.
**Average relative abundance (number of independent videos of each species divided by 900 camera-trap days and multiplied by 10 camera-trap days, rounded to two decimal points).
Environmental variables
We used four environmental variables to explain the dry season distribution of the vertebrate groups and species (S1 Table). Fallen fruit availability was sampled in three plots (250 x 2 m) at each of the 30 permanent sampling points where camera traps were located: (1) the RAPELD permanent plot and (2) another two trail plots located 250 m before and after the location of the camera along the east-west trails. All fallen fruits within the plots were identified in situ using field guides [58, 59]. Identification of all fruits found in the field was also confirmed with the aid of a trained technician from the Amapá State Scientific Research and Technology Institute (Instituto de Pesquisas Científicas e Tecnológicas do Estado de Amapá—IEPA) and the Emilio Goeldi Museum of Pará State—MPEG. Only known animal-consumed fruit species were considered [14, 18, 60] (S2 Table). Finally, fruit availability was expressed as the presence or absence of fruits for each of the 30 sample points.
To evaluate the spatial distribution of water availability we used two variables at different scales: (1) distance from camera traps to the nearest large river and (2) distance from camera traps to the nearest stream with water. The distance from the camera traps to the nearest large river was estimated by using shapefiles of the Araguari and Falsino rivers (available at http://hidroweb.ana.gov.br/HidroWeb.asp?TocItem=4100), and measured as a straight line (Euclidian) distance with ArcGIS version 10.2 [61]. Distance from the camera traps to the nearest stream with water was estimated in situ, using a handheld Global Positioning System (GPS) while walking along the east-west trails in the RAPELD grid during the camera trapping survey period. The distance from each camera station to the nearest stream with water was calculated as a straight line (Euclidian) distance using ArcGIS version 10.2 [61].
To estimate the altitude of the terrain at the camera trap locations, we used a digital elevation model (DEM) produced by the Shuttle Radar Topographic Mission (SRTM) [62], with a 3 arc-second spatial resolution (approximately 90 m at the Equator), downloaded from http://www.cgiar-csi.org/data/srtm-90m-digital-elevation-database-v4-1. Altitude values for each camera trap were then obtained by overlaying camera trap coordinates with the SRTM DEM.
Data analysis
The relative abundance of each species was expressed as the number of independent videos of each species divided by the sampling effort (900 camera trap-days) and multiplied by 10 trap-days (rounded to two decimal points), with which we were able to make comparisons with other studies of Amazon forest vertebrates [7, 46].
To assess whether the sampling effort was sufficient to record the majority of species, we constructed and compared cumulative species curves with function specaccum of the Vegan package [63]. To predict the total number of species that could potentially be detected in the study area, we used the First order jackknife estimator to extrapolate the species richness (i.e., estimate the number of undetected species) based on the frequency of recorded species (function specpool, package Vegan) [63].
We used Spearman correlation values to evaluate independence between environmental variables. Variables with weak correlations (rs < 0.50) were retained for use in subsequent analyzes.
To test for differences in the ecological relationships of different functional groups and species we used Generalized Linear Models (GLMs, error distribution family = Tweedie) [64]. GLMs were preferred to alternatives such as occupancy models because the number of videos (i.e., potential of recaptures) and naïve occupancy (proportion of cameras with records) was low for most species. These low sample sizes meant that it was only possible to obtain reliable occupancy estimates for two of the seven most commonly recorded species (S1 File).
The GLMs were run separately for each species and for the five functional groups of vertebrates (Table 1). For the GLM analysis we selected only groups/species with at least one video in five or more different cameras within the study area. In addition to the additive effect of the four variables we also included the interaction between distance to nearest water and presence of fruits. To improve numerical stability of the GLMs and interpretation of the interaction term, the continuous variables were standardized (centered and scaled by the standard deviation). All analyses were performed with the R language and environment for statistical computing [65].
Results
Sampling effort and species richness
Following a sampling effort of 900 trap-days (30 days for each camera-trap), we obtained 423 videos of which 279 were considered independent of 25 vertebrate species (Table 1). We obtained an overall capture rate of 0.31 videos per trap-day (279 independent videos /900 trap-days). This total included four bird and 21 mammal species, representing 10 orders: Birds—Tinamiformes, Galliformes, Gruiformes; Mammals—Artiodactyla, Perissodactyla, Carnivora, Cingulata, Pilosa, Didelphimorphia and Rodentia (Table 1). From the 25 vertebrate species we detected a total of 16 frugivores (Table 1, S3 Table), including four bird and 12 mammal species (four rodents, four ungulates, three carnivores and one marsupial).
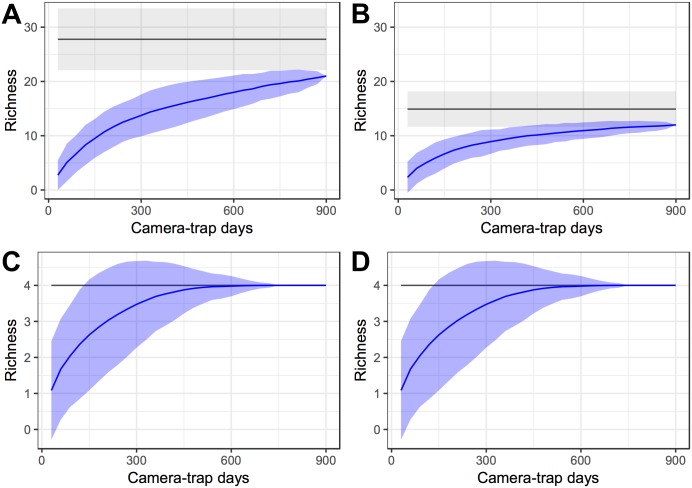
The species accumulation curves reached an asymptote for all birds, frugivorous mammals and frugivorous birds (Fig 2). Although the accumulation curve did not reach an asymptote for all mammals (Fig 2A), we obtained 75.63% of the expected mammal species. For all birds we obtained 100% of the species pool. This suggests that sampling effort was sufficient for frugivorous vertebrates.
Fig 2. Cumulative curves for mammal and bird species sampled with camera traps in the dry season in the Amapá National Forest.
Detection of species recorded in 900 camera-trap days randomized 1000 times and results used to derive mean (blue line) 95% confidence intervals of the mean (blue polygon). First order jackknife estimates of extrapolated species richness and 95% confidence intervals are showed with black line and light gray shaded area, respectively. (A) Cumulative curve for all mammal species; (B) Cumulative curve for frugivorous mammals; (C) Cumulative curve for all bird species; (D) Cumulative curve for frugivorous birds.
Functional groups
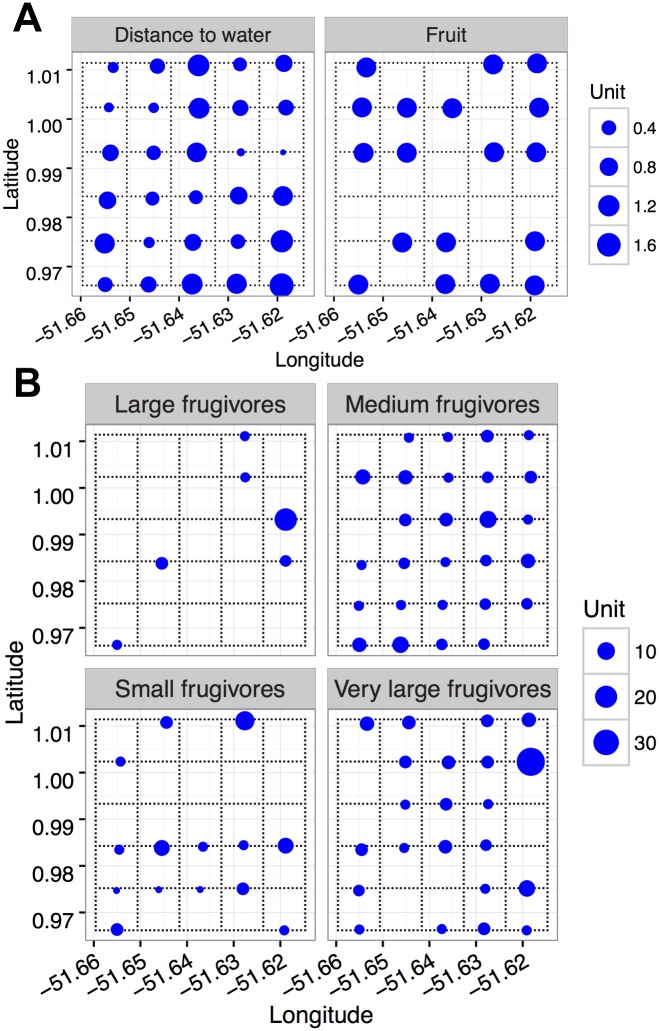
As none of the four environmental variables were strongly correlated (Spearman r ranging between 0.00 and 0.26) we retained all in subsequent analyses. The explanatory power of the GLMs was low for almost all groups (Table 2), with a maximum deviance explained of 49% (for large frugivores) and a minimum of 10% (for small frugivorous). Two groups (all frugivores and small frugivores) were not significantly influenced by any of the environmental variables measured. The groups containing medium (1–5 kg) and large frugivores (5–15 kg) were negatively influenced by distance to nearest water. The groups of large and very large frugivores were significantly influenced by altitude. However, while large frugivores were negatively influenced by altitude, the very large frugivores were positively influenced by this variable. None of the groups showed a statistically significant association with the presence of fruits and only a marginally significant (P < 0.10) interaction between distance to the nearest water and presence of fruits was found for medium frugivores (Fig 3, Table 2).
Table 2. Parameter (Slope) estimates of explanatory variables from the GLMs on the abundance of groups of vertebrates in the eastern Brazilian Amazon.
| All frugivoresa | Small frugivoresb | Medium frugivoresc | Large frugivoresd | Very large frugivorese | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slope (SE)f | t value | Slope (SE)f | t value | Slope (SE)f | t value | Slope (SE)f | t value | Slope (SE)f | t value | |
| Intercept | 1.828 (0.751) | 2.43* | 0.871 (1.540) | 0.57 | -0.020 (0.624) | -0.03 | -2.740 (2.039) | -1.34 | 1.390 (1.125) | 1.24 |
| Altitude | 0.059 (0.166) | 0.35 | -0.097 (0.382) | -0.25 | -0.233 (0.144) | -1.61 | -1.145 (0.488) | -2.35* | 0.665 (0.265) | 2.51* |
| Distance to large rivers | 0.119 (0.186) | 0.64 | 0.255 (0.405) | 0.63 | -0.125 (0.132) | -0.95 | -0.052 (0.378) | -0.14 | 0.243 (0.316) | 0.77 |
| Distance to nearest water | -0.301 (0.208) | -1.45 | -0.486 (0.528) | -0.92 | -0.481 (0.192) | -2.51* | -2.459 (0.906) | -2.72* | 0.400 (0.294) | 1.36 |
| Fruit (presence vs absence) | 0.321 (0.754) | 0.43 | -0.906 (1.530) | -0.59 | 0.727 (0.611) | 1.19 | -0.311 (1.756) | -0.18 | -0.248 (1.171) | -0.21 |
| Distance to nearest water X Fruit (presence vs absence) | 0.209 (1.071) | 0.20 | -0.428 (2.343) | -0.18 | 1.649 (0.843) | 1.96† | 3.058 (3.189) | 0.96 | -1.420 (1.593) | -0.89 |
| Model deviance explained (%)g | 13.90 | 9.90 | 29.50 | 48.80 | 29.20 | |||||
| Model AICh | 198.77 | 105.90 | 125.65* | 71.88*** | 149.14† | |||||
Significance values:
†p<0.10,
*p <0.05,
***p<0.001.
a Includes relative abundances of all frugivores recorded in the study area;
b Includes relative abundances of only small frugivores (< 1 kg);
c Includes relative abundances of only medium frugivores (1–5 kg);
d Includes relative abundances of only large frugivores (5–15 kg);
e Includes relative abundances of only very large frugivores (> 15 kg);
f Slope for variables and Standard Error (SE);
g Percentage of Deviance Explained for each model (%);
h Akaike Information Criterion value for each model (AIC);
Fig 3. (A) Distance to the nearest water (km) and presence/absence of fruits and (B) number of photos of functional groups of frugivores per sampling point on a 25 km2 grid, during the dry season in the Amapá National Forest, Brazil.
Species
Psophia crepitans was the most frequently recorded species with 55 records (0.61 records/10 trap-days), followed by Pecari tajacu with 44 records (0.49 records/10 trap-days), Myoprocta acouchy with 37 records (0.41 records/10 trap-days) and Mazama americana with 32 records (0.36 records/10 trap-days) (Table 1). Of the seven most common species assessed in the GLMs, three showed statistically significant results (Table 3). The percentage of variation explained by the models ranged from a minimum of 6% for Mazama nemorivaga to a maximum of 48% for Cuniculus paca. Of these seven species, the rodent C. paca, the ungulate M. americana and the rodent D. leporina were the species where the model provided the highest percentage of explanation for their relative abundances, ranging from 36 to 48% (Table 3).
Table 3. Parameter (Slope) estimates of explanatory variables from the GLMs on the abundance of most common species of vertebrates in the eastern Brazilian Amazon.
| Cuniculus paca | Dasyprocta leporina | Mazama americana | Mazama nemorivaga | Myoprocta acouchy | Pecari tajacu | Psophia crepitans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope (SE)a | t value | Slope (SE)a | t value | Slope (SE)a | t value | Slope (SE)a | t value | Slope (SE)a | t value | Slope (SE)a | t value | Slope (SE)a | t value | |
| Intercept | -2.709 (2.031) | -1.33 | -1.745 (1.551) | -1.13 | -1.612 (1.418) | -1.14 | -2.098 (1.910) | -1.10 | 0.244 (1.633) | 0.15 | 1.915 (1.458) | 1.31 | -0.566 (0.892) | -0.64 |
| Altitude | -1.134 (0.487) | -2.33* | -0.164 (0.420) | -0.39 | 1.657 (0.380) | 4.37*** | 0.707 (0.431) | 1.64 | -0.017 (0.394) | -0.04 | 0.286 (0.300) | 0.95 | -0.133 (0.194) | -0.69 |
| Distance to large rivers | -0.053 (0.378) | -0.14 | 0.255 (0.343) | 0.74 | 0.171 (0.470) | 0.36 | -0.066 (0.492) | -0.14 | 0.242 (0.426) | 0.57 | 0.141 (0.379) | 0.37 | -0.345 (0.181) | -1.90† |
| Distance to nearest water | -2.432 (0.900) | -2.70* | 0.320 (0.575) | 0.56 | 0.220 (0.454) | 0.49 | 0.188 (0.446) | 0.42 | -0.434 (0.558) | -0.78 | 0.251 (0.312) | 0.80 | -0.924 (0.336) | -2.75* |
| Fruit (presence vs absence) | -0.331 (1.753) | -0.19 | -0.461 (1.795) | -0.26 | 0.551 (1.404) | 0.39 | 1.449 (1.932) | 0.75 | -0.335 (1.614) | -0.21 | -1.343 (1.500) | -0.90 | 0.566 (0.811) | 0.70 |
| Distance to nearest water X Fruit (presence vs absence) | 3.013 (3.173) | 0.95 | 1.324 (1.979) | 0.67 | 0.309 (1.891) | 0.16 | 1.032 (2.302) | 0.45 | 0.185 (2.412) | 0.08 | -3.555 (2.518) | -1.41 | 1.904 (1.297) | 1.47 |
| DE (%)b | 48.10 | 36.00 | 45.60 | 6.20 | 21.10 | 16.30 | 36.20 | |||||||
| Model AICc | 71.76*** | 58.92† | 82.05*** | 91.51 | 88.31 | 106.88 | 108.86** | |||||||
Significance values:
†p<0.10,
*p <0.05,
**p<0.01,
***p<0.001.
a Slope for variables and Standard Error (SE);
b Percentage of Deviance Explained for each model (DE(%));
c Akaike Information Criterion value for each model (AIC);
Cuniculus paca and P. crepitans were the only species with two marginally or significant variables in the GLMs. M. americana was significantly associated with one variable in the model, while D. leporina, M. nemorivaga, P. tajacu and M. acouchy were not associated significantly with any of the environmental variables (Table 3).
The variable altitude had a positive influence on the relative abundance of one ungulate (M. americana) and negative influence for C. paca. The distance to large river had a marginal negative influence only for the bird P. crepitans. Finally, distance to nearest water had a negative effect on C. paca and P. crepitans.
Discussion
Our study showed that water availability is more important than the presence of fallen fruit during an event of resource scarcity in a lowland Amazon forest. Thus, our findings support the prediction that water availability would affect functional groups and species of terrestrial frugivores. In contrast we found no support for the prediction that fallen fruit would also affect meso-scale patterns in terrestrial frugivores. Distance to nearest water was an important variable that explained meso-scale variation in two functional groups (medium and large frugivores) and two terrestrial frugivore species. These observations allow an improved understanding of species-specific responses to water scarcity, and also provide baseline information for monitoring tropical frugivore responses to future environmental changes. However, we must remain cautious in our conclusions as we only surveyed a single dry season and our results should be interpreted carefully.
Sampling effort and species richness
The difference between the observed and extrapolated species richness values obtained indicates that our sampling effort was sufficient to record the full spectrum of frugivorous birds and mammals in the study area. Indeed, the 25 species recorded in our study is similar to the composition and number of species recorded for other Amazonian regions [66–68]. A previous camera-trap survey in the same study area reported a total richness of 25 species with a sampling effort of 1800 trap-days (900 trap-days each for the dry and rainy seasons), with 21 vertebrate species recorded during the 2013 dry season [7]. Based on these findings we consider the results from the present study to be representative of the terrestrial vertebrate species in the study area.
For mammals, the species compositions of the two studies during the dry season were similar, with a difference that Tamandua tetradactyla was detected only in 2013 [7] and Priodontes maximus was detected only in 2015. We also detected four species that were not recorded in a previous study [7] during the dry season but were registered in the rainy season: Sciurus aestuans, Procyon cancrivorus, Nasua nasua and Speothos venaticus. Such differences are consistent with the findings from previous studies that show spatial and temporal variations in the species recorded using camera-trap surveys in tropical forests [22, 66, 69, 70].
Four mid-sized and large-bodied terrestrial bird species were recorded in our study. Due to their body sizes and habit of foraging on the ground [6, 55], these birds are likely to be recorded by camera trap studies [7, 24]. The same bird species were previously recorded during the 2013 dry season in the study area, with similar relative abundances to our study [7]. Although our extrapolated bird richness values suggest that we registered all the bird species that could possibly be recorded with camera traps, the frequency with which they were registered was generally low. This result may suggest that camera traps might not be ideal for monitoring this group of birds, and that complementary techniques maybe necessary. Indeed, a combination of indirect and direct techniques have been proven to be more efficient than cameras traps only [71].
Camera trapping studies are often conducted during the dry season in tropical forests. For example, dry season surveys form the basis of the camera-trap protocols implemented globally by the Tropical Ecology Assessment and Monitoring Network [70, 72]. This preference for dry season surveys is mainly due to the logistical constraints associated with rainy season surveys (e.g. restricted access and cameras malfunctioning). A previous study in our study area [7] found fewer records of some frugivore species during the dry compared with the rainy season. In our study, several vertebrates were represented by only a few photographs, showing the difficulty of detecting some frugivorous during the dry season (e.g., Tapirus terrestris, Crax alector, Crypturellus erythropus and Tinamus major).
Differences between functional groups
The completeness of our survey enabled us to examine if different resources (fruit and water) explained the relative abundance of a broad spectrum of terrestrial frugivores. For example, our functional body sizes ranged over three orders of magnitude (<1 to > 100 kg). All our functional groups include species that are ubiquitous across tropical and sub-tropical regions of South America [52, 53]. As such we can expect a wide range of physiological and behavioral adaptations and acclimations to seasonal changes in resources both within and between groups.
Our results agree with previous studies that found other factors to be more important than fruit availability to explain variation in the abundance of terrestrial frugivores [1, 2, 4, 73]. Medium frugivores were influenced only by distance to the nearest water. Large frugivores were most strongly influenced by distance to nearest water and altitude, while very large frugivores were only influenced by altitude. Medium and large frugivores were associated negatively with distance to nearest water showing a significant increase in the number of records in areas closer to water bodies within the study area. This finding supports previous studies that show water availability may play a stronger role in driving the behavior of large bodied terrestrial mammals than food searching during the dry season [1–4].
The group of medium to large-bodied vertebrates is one of the most affected by subsistence hunting [18, 26] and it has been shown that in semi-arid regions these vertebrates can be more easily hunted closer to water during the dry season [74]. Typically hunter behavior does not seem to show great variation in the neotropics and regional differences are more related to the use of certain species (notably primates) by native Americans and European descendants [75]. Although the rivers are the main means of transport of habitants in the Amazon region [26], we did not find any negative effect on the relative abundance of these frugivorous groups close to the large rivers, which supports the idea that there is currently little anthropogenic impact within the ANF [7].
Altitude was the best predictor of the relative abundances of the large and very large-bodied frugivores. However, while large frugivores were negatively associated with altitude, very large frugivores were positively associated with the same variable. A similar pattern was observed in some large-bodied frugivores that remained in the highlands during the rainy season but moved to lowlands in the dry season, potentially responding to the renewed supply of resources following the rainy season [11, 76]. SRTM altitude varies from sea level to 1216 m across the >5 million km2 of Brazilian Amazonia. However, the mean altitude is 159.5 m, and there is low meso-scale variation (SD values < = 40) in 90% of the area [40]. Nevertheless, even with this low variation, altitude is a key determinant of Amazonian biodiversity [31–34], affecting soil, water availability, climate and other biotic and abiotic variables [30].
We found only weak associations between the sampled community of terrestrial frugivores and the meso-scale availability of fallen fruits. A lack of association of the interaction between distance to the nearest water and presence of fruits was also confirmed for all groups, with the exception of medium frugivores that were weakly associated with this interaction. The simplest and most likely explanation for this lack of association is the generalist nature of the frugivore species. All species consume fruits but all may also consume a variety of alternative foods [6, 10, 52, 53, 77, 78]. As such, our findings support the idea that terrestrial fruit-frugivore relationships tend to be less strong and less affected by fruit availability compared with canopy fruit-frugivore relationships [9, 17, 18, 79].
Studies from other Amazon terra firme forests can help to understand our observations of the groups of small to large frugivores in the ANF. Previous studies suggest that species dietary diversity and ability to adapt to a changing resource base are important traits in determining vertebrate responses to relative food reduction within terra firme forests [25]. It has also been shown that fruit–frugivore networks are also highly diffuse in this biome [11]. The interaction between medium to large-bodied frugivores and fruit resources suggests generalization in terra firme forests, compared with greater specialization in varzea forests for this group [11]. One driver of generalization in fruit–frugivore relationships could be dietary complementarity [79]. Diets of the studied groups are rarely entirely frugivorous, with fruit consumed in varying ratios depending on species-specific interactions with habitat, season, fruit availability and the availability of alternative food sources [17, 18, 78, 80]. Thus, for small frugivorous mammals, there is evidence that leaf and fiber consumption increases during periods of fruit scarcity [78]. It has also been suggested that small-bodied species are less likely to be affected by habitat changes because they may be able to diverge through microhabitat specialization [25], supporting our results in this body-sized group. Large and very-large frugivores are also not strictly dependent on fruits, for example, deer and tapir form part of the browser-grazer community and have the ability to digest leaves [81, 82]. Previous studies show that within the very-large frugivores, digestive physiology is more important than body size for resource portioning of diet [77], with all species consuming a mix of fruit, leaves and fiber that varies with habitat and season [77, 81, 82]. Thus, a weak relationship between fruit availability and these functional groups is not surprising.
Differences between species
Our findings support those from previous studies, which found a greater number of detections of Cuniculus paca in low-lying areas near permanent water sources [7]. On the other hand, Mazama americana showed a greater number of detections within the study area uplands. These observations support the importance of altitude as a driver and modulator of species meso-scale distribution patterns [40]. Similarly, a previous study [7] found the same association in relation to streams for both Mazama americana and Pecari tajacu within the ANF. These results contrast to those from other studies in more arid regions, which found that the dry season distributions of some ungulates were strongly influenced by water availability [1–4]. Keuroghlian, Eaton [83] reported seasonal movements of some ungulates apparently driven by availability of key fruits in a tropical forest. However, dry season fruit availability was not a significant variable for ungulates in our study.
Only one bird species (P. crepitans) had sufficient records for analysis. Fruit and seeds are known to form the bulk of the diet in P. crepitans, C. alector and T. major, however, it is known that P. crepitans also eats invertebrates in relatively large quantities [6]. We found that during the dry season, the presence of fruit had no significant effect on the relative abundance of P. crepitans. Chatterjee and Basu [73] suggest that other factors such as insect abundance may be important for frugivore bird groups that also rely on insects as a secondary dietary component. For these authors, a combination of fruit availability and insect availability should explain the variation in frugivore bird density in space and time, rather than fruit availability alone. Although our observations might support this conclusion, C. alector and T. major had few records (videos in < 5 different camera traps) and the relationship with fruit availability could not be evaluated. However, our study supports findings that P. crepitans has a preference for areas close to water availability and close to large rivers within Amazonian forests [6, 7].
Although camera traps are efficient for rapid inventories during the dry season [84], we must remain cautious in our conclusions. Capture frequencies with camera traps can give an idea of the relative abundance of different species, but may be affected by a variety of factors such as species-specific behavior (e.g. use or avoidance of trails, partly arboreal versus exclusively terrestrial, or habitat specialist versus generalist) [66, 69]. For this reason we limit our conclusions to differences in spatial encounter rates and do not attempt to imply population parameters (e.g. density).
Conclusions
Our models could only partially explain dry season abundance patterns in the recorded species and groups. The lack of association between frugivores and fruit availability could suggest that, at the meso-scale level (25 km2), other factors may have more decisive roles during a period of resource scarcity. We found that, at this scale, the distribution of frugivore species and functional groups can be partly explained by variables such as water availability and altitude. However, a substantial survey effort is necessary to ensure a representative sample of terrestrial frugivores and to better understand the processes driving the spatial distribution of these vertebrate groups.
Supporting information
(DOC)
Weather station data available from the Brazilian National Water Agency (station ID: 8052000). Monthly totals are presented from three years (2013, 2014 and 2015). Boxplots show means and 95% confidence limits estimated via nonparametric bootstrap. The blue line and shaded areas are the mean value and 95% confidence intervals from a GAM model illustrating the trend in rainfall.
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
The Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) and the Federal University of Amapá (UNIFAP) provided logistical support. We thank IBAMA for authorization to conduct research in ANF (IBAMA/SISBIO permit 47859–1). We thank the Long-term Biodiversity Monitoring Program (PPBIO) for providing the grid system used during field activities. We are grateful to Cremilson and Cledinaldo Alves Marques for their invaluable assistance during fieldwork. We thank to Patrick Cantuária and Tonny Medeiros of the Instituto de Pesquisas Científicas e Tecnológicas do Estado de Amapá (IEPA) and to Antônio Magalhães of the Emilio Goeldi Museum of Pará State for helping with the botanical identifications. We are grateful to Yuri Breno da Silva, Victor Rodríguez-Chuma and Alexander Arevalo for their help during data collection and identification. We are grateful to Danilo Russo and two anonymous referees for comments on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Brazilian National Counsel of Technological and Scientific Development (“Conselho Nacional de Desenvolvimento Científico e Tecnológico”—CNPq) [grant number MCTI/CNPQ/Universal 14/2014: 446926/2014-0]. OSLP received a MSc scholarship from the Federal Agency for Support and Evaluation of Graduate Education, Ministry of Education (“Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”—CAPES). FM receives a productivity scholarship from CNPq (Process 301562/2015-6). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.O'Farrill G, Gauthier SK, Rayfield B, Bodin O, Calme S, Sengupta R, et al. The potential connectivity of waterhole networks and the effectiveness of a protected area under various drought scenarios. Plos One. 2014;9(5):e95049 10.1371/journal.pone.0095049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyna-Hurtado R, Chapman CA, Calme S, Pedersen EJ. Searching in heterogeneous and limiting environments: foraging strategies of white-lipped peccaries (Tayassu pecari). Journal of Mammalogy. 2012;93(1):124–33. [Google Scholar]
- 3.Reyna-Hurtado R, O’Farrill G, Chávez C, Serio-Silva JC, Castillo-Vela G. Large terrestrial mammals In: Islebe GA, Calmé S, León-Cortés JL, Schmook B, editors. Biodiversity and Conservation of the Yucatán Peninsula. Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 4.Reyna-Hurtado R, Rojas-Flores E, Tanner GW. Home range and habitat preferences of white-lipped peccaries (Tayassu pecari) in Calakmul, Campeche, Mexico. Journal of Mammalogy. 2009;90(5):1199–209. [Google Scholar]
- 5.Rich LN, Miller DAW, Robinson HS, McNutt JW, Kelly MJ. Using camera trapping and hierarchical occupancy modelling to evaluate the spatial ecology of an African mammal community. Journal of Applied Ecology. 2016;53(4):1225–35. [Google Scholar]
- 6.Erard C, Thery M, Sabatier D. Régime alimentaire de Tinamus major (Tinamidae), Crax alector (Cracidae) et Psophia crepitans (Psophiidae), en forêt guyanaise. Gibier Faune Sauvage. 1991;8:183–210. [Google Scholar]
- 7.Michalski LJ, Norris D, de Oliveira TG, Michalski F. Ecological relationships of meso-scale distribution in 25 neotropical vertebrate species. PLoS One. 2015;10(5):e0126114 10.1371/journal.pone.0126114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terborgh JW, Fitzpatrick JW, Emmons LH. Annotated checklist of the bird and mammal species of Cocha Cashu Biological Station, Manu National Park, Peru. Fieldiana Zoology, New Series. 1984;21:1–29. [Google Scholar]
- 9.Fleming TH, Kress WJ. A brief history of fruits and frugivores. Acta Oecologica-International Journal of Ecology. 2011;37(6):521–30. [Google Scholar]
- 10.Gayot M, Henry O, Dubost G, Sabatier D. Comparative diet of the two forest cervids of the genus Mazama in French Guiana. Journal of Tropical Ecology. 2004;20:31–43. [Google Scholar]
- 11.Hawes JE, Peres CA. Ecological correlates of trophic status and frugivory in neotropical primates. Oikos. 2014;123(3):365–77. [Google Scholar]
- 12.Kitamura S, Yumoto T, Poonswad P, Chuailua P, Plongmai K, Maruhashi T, et al. Interactions between fleshy fruits and frugivores in a tropical seasonal forest in Thailand. Oecologia. 2002;133(4):559–72. [DOI] [PubMed] [Google Scholar]
- 13.Myneni RB, Yang W, Nemani RR, Huete AR, Dickinson RE, Knyazikhin Y, et al. Large seasonal swings in leaf area of Amazon rainforests. Proceedings of the National Academy of Sciences. 2007;104(12):4820–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smythe N. Relationships between fruiting seasons and seed dispersal methods in a neotropical forest. The American Naturalist. 1970;104(935):25–35. [Google Scholar]
- 15.van Schaik CP, Terborgh JW, Wright SJ. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of ecology and Systematics. 1993:353–77. [Google Scholar]
- 16.Pontes ARM, Paula MD, Magnusson WE. Low primate diversity and abundance in Northern Amazonia and its implications for conservation. Biotropica. 2012;44(6):834–9. [Google Scholar]
- 17.Terborgh J. Community aspects of frugivory in tropical forests In: Estrada A, Fleming TH, editors. Frugivores and seed dispersal. Princeton: Princeton University; 1986. p. 371–84. [Google Scholar]
- 18.Smythe N. Competition and resource partitioning in the guild of neotropical terrestrial frugivorous mammals. Annual Review of Ecology and Systematics. 1986;17:169–88. [Google Scholar]
- 19.Hanya G, Aiba S. Fruit fall in tropical and temperate forests: implications for frugivore diversity. Ecological Research. 2010;25(6):1081–90. [Google Scholar]
- 20.Adler GH, Endries M, Piotter S. Spacing patterns within populations of a tropical forest rodent, Proechimys semispinosus, on five Panamanian islands. Journal of Zoology. 1997;241:43–53. [Google Scholar]
- 21.Aliaga-Rossel E, Kays RW, Fragoso JMV. Home-range use by the Central American agouti (Dasyprocta punctata) on Barro Colorado Island, Panama. Journal of Tropical Ecology. 2008;24:367–74. [Google Scholar]
- 22.Anile S, Devillard S. Study design and body mass influence RAIs from camera trap studies: evidence from the Felidae. Animal Conservation. 2016;19(1):35–45. [Google Scholar]
- 23.Benchimol M, Peres CA. Predicting primate local extinctions within "real-world" forest fragments: A Pan-neotropical analysis. American Journal of Primatology. 2014;76(3):289–302. 10.1002/ajp.22233 [DOI] [PubMed] [Google Scholar]
- 24.Benchimol M, Peres CA. Predicting local extinctions of Amazonian vertebrates in forest islands created by a mega dam. Biological Conservation. 2015;187:61–72. [Google Scholar]
- 25.Bicknell J, Peres CA. Vertebrate population responses to reduced-impact logging in a neotropical forest. Forest Ecology and Management. 2010;259(12):2267–75. [Google Scholar]
- 26.Peres CA. Effects of subsistence hunting on vertebrate community structure in Amazonian forests. Conservation Biology. 2000;14(1):240–53. [Google Scholar]
- 27.Peres CA, Emilio T, Schietti J, Desmouliere SJ, Levi T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(4):892–7. 10.1073/pnas.1516525113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peres CA. Nonvolant mammal community structure in different Amazonian forest types In: Eisenberg JF, Redford KH, editors. Mammals of the Neotropics. 3 Chigago: University of Chicago Press; 1999. p. 564–81. [Google Scholar]
- 29.Gentry AH. A Field Guide to the Families and Genera of Woody Plants of Northwest South America (Colombia, Ecuador, Peru), with supplementary notes on herbaceous taxa. International C, editor. Chicago: University of Chicago Press; 1996. [Google Scholar]
- 30.Korner C. The use of 'altitude' in ecological research. Trends in Ecology & Evolution. 2007;22(11):569–74. [DOI] [PubMed] [Google Scholar]
- 31.Costa FRC, Magnusson WE. The need for large-scale, integrated studies of biodiversity—the experience of the Program for Biodiversity Research in Brazilian Amazonia. Natureza & Conservacao. 2010;8(1):3–12. [Google Scholar]
- 32.Hoorn C, Wesselingh FP, ter Steege H, Bermudez MA, Mora A, Sevink J, et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010;330(6006):927–31. 10.1126/science.1194585 [DOI] [PubMed] [Google Scholar]
- 33.Laurance SGW, Laurance WF, Andrade A, Fearnside PM, Harms KE, Vicentini A, et al. Influence of soils and topography on Amazonian tree diversity: a landscape-scale study. Journal of Vegetation Science. 2010;21(1):96–106. [Google Scholar]
- 34.Tuomisto H, Ruokolainen K, Kalliola R, Linna A, Danjoy W, Rodriguez Z. Dissecting Amazonian biodiversity. Science. 1995;269(5220):63–6. 10.1126/science.269.5220.63 [DOI] [PubMed] [Google Scholar]
- 35.ICMBio. Plano de Manejo da Floresta Nacional do Amapá: Instituto Chico Mendes de Conservação da Biodiversidade; 2014 [03 August 2016]. http://www.icmbio.gov.br/portal/unidadesdeconservacao/biomas-brasileiros/amazonia/unidades-de-conservacao-amazonia/1956-flona-do-amapa.
- 36.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Koppen-Geiger climate classification updated. Meteorologische Zeitschrift. 2006;15(3):259–63. [Google Scholar]
- 37.ANA. Sistema de Monitoramento Hidrológico (Hydrological Monitoring System). Agência Nacional de Águas[[nl]]National Water Agency, http://www.hidroweb.ana.gov.br 2016 [20.07.2016].
- 38.Magnusson WE, Lima AP, Luizão R, Luizão F, Costa FRC, Castilho CV, et al. RAPELD: a modification of the Gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotropica. 2005;5(2):19–24. [Google Scholar]
- 39.Magnusson W, Braga-Neto R, Pezzini F, Baccaro F, Bergallo H, Penha J, et al. Biodiversidade e monitoramento ambiental integrado (Biodiversity and integrated environmental monitoring). Santo André, SP: Áttema Editorial; 2013. [Google Scholar]
- 40.Norris D, Fortin MJ, Magnusson WE. Towards monitoring biodiversity in amazonian forests: How regular samples capture meso-scale altitudinal variation in 25 km2 plots. Plos One. 2014;9(8):e106150 10.1371/journal.pone.0106150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrote G, de Ayala RP, Pereira P, Robles F, Guzman N, García FJ, et al. Estimation of the Iberian lynx (Lynx pardinus) population in the Doñana area, SW Spain, using capture–recapture analysis of camera-trapping data. European Journal of Wildlife Research. 2011;57(2):355–62. [Google Scholar]
- 42.Royle JA, Karanth KU, Gopalaswamy AM, Kumar NS. Bayesian inference in camera trapping studies for a class of spatial capture–recapture models. Ecology. 2009;90:3233–44. [DOI] [PubMed] [Google Scholar]
- 43.Rocha DG, Sollmann R, Ramalho EE, Ilha R, Tan CKW. Ocelot (Leopardus pardalis) Density in Central Amazonia. Plos One. 2016;11(5):e0154624 10.1371/journal.pone.0154624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilting A, Mohamed A, Ambu LN, Lagan P, Mannan S, Hofer H, et al. Density of the Vulnerable Sunda clouded leopard Neofelis diardi in two commercial forest reserves in Sabah, Malaysian Borneo. Oryx. 2012;46(3):423–6. [Google Scholar]
- 45.Norris D, Michalski F, Peres CA. Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. Journal of Mammalogy. 2010;91(3):551–60. [Google Scholar]
- 46.Michalski F, Peres CA. Disturbance-mediated mammal persistence and abundance-area relationships in Amazonian forest fragments. Conservation Biology. 2007;21(6):1626–40. 10.1111/j.1523-1739.2007.00797.x [DOI] [PubMed] [Google Scholar]
- 47.Benchimol M, Peres CA. Widespread Forest Vertebrate Extinctions Induced by a Mega Hydroelectric Dam in Lowland Amazonia. Plos One. 2015;10(7):e0129818 10.1371/journal.pone.0129818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Brien TG, Kinnaird MF, Wibisono HT. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation. 2003;6:131–9. [Google Scholar]
- 49.Oliveira TG, Michalski F, Botelho ALM, Michalski LJ, Calouro AM, Desbiez ALJ. How rare is rare? Quantifying and assessing the rarity of the bush dog Speothos venaticus across the Amazon and other biomes. Oryx. 2016:Available from: 10.1017/S0030605316000624. [DOI] [Google Scholar]
- 50.Sick H. Ornitologia Brasileira. Rio de Janeiro: Nova Fronteira; 1997. 902 p. [Google Scholar]
- 51.Reis NR, Peracchi AL, Pedro WA, Lima IP. Mamíferos do Brasil. Londrina: Editora da Universidade Estadual de Londrina; 2006. 437 p. [Google Scholar]
- 52.Emmons LH, Feer F. Neotropical rainforest mammals: A field guide. Chicago: The University of Chicago Press; 1997. 307 p. [Google Scholar]
- 53.Eisenberg JF, Redford KH. Mammals of the Neotropics—The Central Neotropics: Ecuador, Peru, Bolivia, Brazil. Chicago: The University of Chicago Press; 1999. 609 p. [Google Scholar]
- 54.Peres CA, Palacios E. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal-mediated seed dispersal. Biotropica. 2007;39(3):304–15. [Google Scholar]
- 55.Erard C, Thery M, Sabatier D. Fruit characters in the diet of syntopic large frugivorous forest bird species in French Gluiana. Revue D Ecologie-La Terre Et La Vie. 2007;62(4):323–50. [Google Scholar]
- 56.Jones KE, Bielby J, Cardillo M, Fritz SA, O’Dell J, Orme CDL, et al. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology. 2009:2648. [Google Scholar]
- 57.Oliveira TG, Pereira JA. Intraguild predation and interspecific killing as structuring forces of carnivoran communities in South America. Journal of Mammalian Evolution. 2014;21(4):427–36. [Google Scholar]
- 58.Barroso GM. Frutos e sementes: morfologia aplicada à sistemática de dicotiledôneas: Ed. UFV, Universidade Federal de Viçosa; 1999. [Google Scholar]
- 59.Cornejo F, Janovec J. Seeds of amazonian plants: Princeton University Press; 2010. [Google Scholar]
- 60.Peres CA. Identifying keystone plant resources in tropical forests: the case of gums from Parkia pods. Journal of Tropical Ecology. 2000;16(2):287–317. [Google Scholar]
- 61.ESRI. ArcGIS Desktop: Release 10.2 Redlands, CA: Enviromental Systems Research Institute (ESRI); 2015. [Google Scholar]
- 62.Rabus B, Eineder M, Roth A, Bamler R. The shuttle radar topography mission—a new class of digital elevation models acquired by spaceborne radar. ISPRS Journal of Photogrammetry and Remote Sensing. 2003;57(4):241–62. [Google Scholar]
- 63.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. R package version 2.4–0. https://CRAN.R-project.org/package=vegan. 2016.
- 64.Dunn PK. Tweedie: Tweedie exponential family models. R package version 2.2.1. https://cran.r-project.org/web/packages/tweedie. 2014.
- 65.R Development Core Team. R 3.1.1: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing; 2015. [Google Scholar]
- 66.Tobler MW, Carrillo-Percastegui SE, Pitman RL, Mares R, Powell G. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Animal Conservation. 2008;11(3):169–78. [Google Scholar]
- 67.Negrões N, Revilla E, Fonseca C, Soares AMVM, Jácomo ATA, Silveira L. Private forest reserves can aid in preserving the community of medium and large-sized vertebrates in the Amazon arc of deforestation. Biodiversity and Conservation. 2011;20:505–18. [Google Scholar]
- 68.Pickles RSA, McCann NP, Holland AP. Mammalian and avian diversity of the Rewa Head, Rupununi, Southern Guyana. Biota Neotropica. 2011;11:237–51. [Google Scholar]
- 69.Harmsen BJ, Foster RJ, Silver S, Ostro L, Doncaster CP. Differential use of trails by forest mammals and the implications for camera-trap studies: A case study from Belize. Biotropica. 2010;42(1):126–33. [Google Scholar]
- 70.Ahumada JA, Silva CEF, Gajapersad K, Hallam C, Hurtado J, Martin E, et al. Community structure and diversity of tropical forest mammals: data from a global camera trap network. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:2703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silveira L, Jacomo ATA, Diniz JAF. Camera trap, line transect census and track surveys: a comparative evaluation. Biological Conservation. 2003;114(3):351–5. [Google Scholar]
- 72.TEAM Network. Terrestrial Vertebrate Protocol Implementation Manual, v. 3.1. Arlington, VA, USA: Tropical Ecology, Assessment and Monitoring Network, Center for Applied Biodiversity Science, Conservation International; 2011. http://www.teamnetwork.org/files/protocols/terrestrial-vertebrate/TEAMTerrestrialVertebrate-PT-EN-3.1.pdf. [Google Scholar]
- 73.Chatterjee S, Basu P. Differential effect of fruit availability on avian frugivore guilds in a moist deciduous forest of India. Proceedings of the Zoological Society. 2015;68:147–54. [Google Scholar]
- 74.Reyna-Hurtado R, Naranjo E, Chapman CA, Tanner GW. Hunting and the conservation of a social ungulate: the white-lipped peccary Tayassu pecari in Calakmul, Mexico. Oryx. 2010;44(1):89–96. [Google Scholar]
- 75.Silvius KM, Bodmer RE, Fragoso JMV. People in nature: Wildlife conservation in South and Central America. New York: Columbia University Press; 2004. 464 p. [Google Scholar]
- 76.Carrillo E, Saenz JC, Fuller TK. Movements and activities of white-lipped peccaries in corcovado National Park, Costa Rica. Biological Conservation. 2002;108(3):317–24. [Google Scholar]
- 77.Bodmer RE. Influence of digestive morphology on resource partitioning in Amazonian ungulates. Oecologia. 1991;85(3):361–5. [DOI] [PubMed] [Google Scholar]
- 78.Dubost G, Henry O. Comparison of diets of the acouchy, agouti and paca, the three largest terrestrial rodents of French Guianan forests. Journal of Tropical Ecology. 2006;22:641–51. [Google Scholar]
- 79.Schleuning M, Bluethgen N, Floerchinger M, Braun J, Schaefer HM, Boehning-Gaese K. Specialization and interaction strength in a tropical plant-frugivore network differ among forest strata. Ecology. 2011;92(1):26–36. [DOI] [PubMed] [Google Scholar]
- 80.Bodmer RE. Fruit patch size and frugivory in the lowland tapir (Tapirus terrestris). Journal of Zoology. 1990;222:121–8. [Google Scholar]
- 81.Bodmer RE. Ungulate frugivores and the browser-grazer continuum. Oikos. 1990;57(3):319–25. [Google Scholar]
- 82.Bodmer RE, Ward D. Frugivory in large mammalian herbivores In: Danell K, Bergström R, Duncan P, Pastor J, editors. Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge: Cambridge University Press; 2006. p. 232–60. [Google Scholar]
- 83.Keuroghlian A, Eaton DP, Longland WS. Area use by white-lipped and collared peccaries (Tayassu pecari and Tayassu tajacu) in a tropical forest fragment. Biological Conservation. 2004;120(3):411–25. [Google Scholar]
- 84.Jiménez CF, Quintana H, Pacheco V, Melton D, Torrealva J, Tello G. Camera trap survey of medium and large mammals in a montane rainforest of northern Peru. Revista Peruana de Biología. 2010;17(2):191–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Weather station data available from the Brazilian National Water Agency (station ID: 8052000). Monthly totals are presented from three years (2013, 2014 and 2015). Boxplots show means and 95% confidence limits estimated via nonparametric bootstrap. The blue line and shaded areas are the mean value and 95% confidence intervals from a GAM model illustrating the trend in rainfall.
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.