Abstract
This report emerges from a workshop convened by the National Eye Institute (NEI) as part of the “Audacious Goals Initiative” (AGI). The workshop addressed the replacement of retinal ganglion cells (RGCs) from exogenous and endogenous sources, and sought to identify the gaps in our knowledge and barriers to progress in devising cellular replacement therapies for diseases where RGCs die. Here, we briefly review relevant literature regarding common diseases associated with RGC death, the genesis of RGCs in vivo, strategies for generating transplantable RGCs in vitro, and potential endogenous cellular sources to regenerate these cells. These topics provided the clinical and scientific context for the discussion among the workshop participants and are relevant to efforts that may lead to therapeutic approaches for replacing RGCs. This report also summarizes the content of the workshop discussion, which focused on: (1) cell sources for RGC replacement and regeneration, (2) optimizing integration, survival, and synaptogenesis of new RGCs, and (3) approaches for assessing the outcomes of RGC replacement therapies. We conclude this report with a summary of recommendations, based on the workshop discussions, which may guide vision scientists seeking to develop therapies for replacing RGCs in humans.
Keywords: retinal ganglion cell biology, Muller glia, cell-based therapies, endogenous replacement strategies
Introduction
In developed countries, losing vision is among the most feared ailments, and across all countries blindness creates enormous economic and social burdens1 (and references cited therein). In developing countries, the majority of visual disability is due to uncorrected refractory errors and cataracts.2–4 However, in developed countries, where the barriers to accessing modern medicine are low, visual disability results more commonly from diseases that result in the death of retinal neurons, principally photoreceptors and retinal ganglion cells. In humans, the death of these cells results in irreversible blindness. This bleak outcome is the motivation for developing multipronged approaches for treating blinding diseases.5 One approach is to transplant cells that are capable of replacing the depleted neurons. Another is to induce cells endogenous to the retina to regenerate supplementary neurons, a capacity already possessed by some cold-blooded vertebrates. Implicit in either approach are the requirements that new neurons are correctly specified, survive, and make functional synaptic connections that restore useful vision. The NEI, as part of the AGI, has convened a series of workshops focused on regenerating neurons and their synaptic connections within the human retina and visual system. The first three workshops addressed, respectively, regenerating the opic nerve,6 regenerating and integrating photoreceptors,7 and reconnecting neurons in the visual system.8 This is a report from the fourth workshop addressing the replacement of retinal ganglion cells (RGCs) from exogenous and endogenous sources.
Diseases Associated with Loss of Retinal Ganglion Cells
Retinal ganglion cells integrate synaptic signals in the retina and transmit the resulting visual information to the brain. This visual information is carried by long axons that course over the surface of the retina, exit the eye through the optic disc (optic nerve head), and form the optic nerve. During their intraretinal course, the axons of RGCs are unmyelinated. Once axons exit the globe, they become myelinated, and the optic nerve then closely resembles other white matter tracts of the central nervous system.
Diseases that result in the death of RGCs are broadly categorized as optic neuropathies.9,10 The most common optic neuropathy is glaucoma, the leading cause of irreversible blindness worldwide.11 A recent population-based survey estimated that in the United States, glaucoma affects 2.3 million individuals 60 years of age and older.12 Glaucoma results in a progressive and selective loss of RGCs axons and death of the cell bodies.13,14 Interestingly, there can be a prolonged asymptomatic phase of glaucoma, and substantial ganglion cell death can occur in the absence of clinically detectable visual field defects.15 Treatment for glaucoma can decelerate but not overcome the progressive death of RGCs. Consistent with the progressive nature of this disease, the end stage of damage can result in loss of all RGCs.
Ischemic optic neuropathy is similar to a stroke within the optic nerve which injures axons, leading to the death of RGCs.16–18 As seen in strokes of other axonal tracts in the central nervous system, and unlike glaucoma, ischemic optic neuropathy has a rapid onset and variable degrees of injury, which can range from normal acuity with some visual field defects to profound defects in both acuity and visual fields. There are different forms of ischemic optic neuropathy, based on the pathogenic mechanism. Ischemic optic neuropathy due to underlying giant cell arteritis generally leads to more severe vision loss, compared with nonarteritic anterior ischemic optic neuropathy, and after occurring in one eye, can become bilateral within days.
The hereditary optic neuropathies are genetic disorders that result in simultaneous or sequential bilateral vision loss due to the death of RGCs.19–22 This disease may appear in children or adults and may be isolated to the optic nerve, such as in Leber hereditary optic neuropathy and dominant optic atrophy, or may be present in the optic nerve as a component of a more broadly distributed neurodegenerative process.19 In contrast to glaucoma, inherited optic neuropathies first present as central scotomas, indicating preferential death of RGCs that transmit high acuity, macular, and foveal vision.
Toxins, nutritional deficiency, or trauma can also create optic neuropathies that lead to the death of RGCs.23–25 Toxic optic neuropathies can result from drugs such as ethambutol or linezolid, methanol, or certain heavy metals. Vitamin B12 deficiency can cause an optic neuropathy in vegans or those who do not absorb dietary B12. Finally, it is well established that trauma to the mammalian optic nerve, such as from direct or indirect trauma, results in rapid death of RGCs.26
Genesis of Retinal Ganglion Cells In Vivo
The targeted replacement of RGCs by transplanting cells or regenerating cells from endogenous sources will necessarily rely upon a fundamental understanding of the mechanisms that control the embryonic genesis of RGCs. Retinal histogenesis from undifferentiated progenitors relies on the coordinated interplay of intrinsic and extrinsic cellular signaling, which establishes patterns of cell proliferation, initiates the onset of cell cycle withdrawal, specifies neuronal identities, and governs cellular differentiation.27,28 The retina has long served as a model for delineating intrinsic cellular mechanisms, whereby multipotent progenitors produce discrete cell types. This work, in part, has led to the concept of hierarchical gene-regulatory networks. In its simplest form, a gene-regulatory network in the retina is the combination of transcription factors, and the target genes they regulate, which sequentially determine cell fates, neuronal differentiation, and the assembly of synaptic circuits.29 The most well-studied gene regulatory networks in the retina are those that produce rod and cone photoreceptors30 and RGCs.31–33
Initially, all retinal progenitors require the homeodomain transcription factor, Pax6, both to adopt retinal identities and to sustain proliferation. The first evidence of RGC specification is the expression of the bHLH transcription factor, Atoh7 (originally named Ath5), in progenitors as they exit the cell cycle.34 Atoh7 functions as a RGC competence factor, and the loss of Atoh7 results in retinas with largely normal architecture, but the nearly complete absence of ganglion cells.35–37 Subsequent to identifying Atoh7 in the gene-regulatory network for RGCs, efforts were begun to identify both its upstream regulators and downstream targets.32,38 A current model of this network places Atoh7 downstream of Pax6 and Hes132 and upstream of the transcription factors, Pou4f2 and Isl1, which together are sufficient to specify RGC fates, but also function to regulate RGC differentiation.39 This gene regulatory network is sufficient to broadly specify RGCs, but does not account for the genesis of the known ganglion cell subtypes. There may be as many as 30 subtypes of RGCs in mouse, each with distinct morphology, synaptic inputs, functions, and central targets.40,41 A significant challenge that remains is to define RGC subtypes in humans, identify the gene networks that determine each, and establish their unique subtype-specific functional properties.
Genesis of Retinal Ganglion Cells In Vitro
Knowledge gained from decades investigating retinal development in vivo has laid the foundation for using pluripotent stem cells to model retinal development in vitro.42 As a result, we can now generate human retinal neurons in almost unlimited quantities.43–46 Fundamental insights into human retinal development were gained by the discovery that in vitro pluripotent stem cells, when first differentiated into forebrain progenitors, will spontaneously self-organize into three-dimensional (3D) embryonic retinas. In the presence of basement membrane components, forebrain progenitors develop optic vesicle-like structures that invaginate to form bilayered optic cups that produce retinal neurons, including RGCs, in a histogenic sequence characteristic of the vertebrate retina in vivo.42,47,48 RGCs generated from these 3D retinas can grow long axons.49,50 When forebrain aggregates are grown in the absence of basement membrane components, they form 3D optic vesicle-like spheres that fail to undergo further morphogenesis, but nonetheless, differentiate into layered retinas, complete with RGCs and synaptic neuropil.51–53
Despite the striking recapitulation of retinal development in vitro, limitations of human 3D retinas are their slow development, which hews closely to the human developmental timeframe, and the genesis of retinal neurons in proportions that are present in vivo (where RGCs are less than 1% of the total). These features limit the utility of using retinal organoids to generate retinal neurons that may be used for transplantation. To overcome these limitations, approaches have been developed to differentiate human pluripotent stem cells directly into RGCs54,55 or convert cells isolated from the human retina into multipotent progenitor states and then into neurons, including RGCs. For example, human RPE contains a small population of self-renewing stem cells that can be differentiated into multiple cell lineages, including neuronal lineages.56,57 Müller glia isolated from the human retina can dedifferentiate in vitro into retinal stem cells, which can be propagated and redifferentiated into RGCs.58,59
Regeneration of Retinal Neurons In Vivo
Unlike mammals, a small number of vertebrates have the capacity to spontaneously regenerate the retina. Two endogenous cellular sources can give rise to regenerated retina, the retinal pigmented epithelium (RPE) and Müller glia.60 The RPE originates from the same neuroectodermal lineage as the retina, but early in development the RPE adopts a nonneuronal function, which, from that point forward, supports aspects of retinal neurogenesis and, later, photoreceptor physiology. The ability of the RPE to regenerate retina is limited to some amphibians and the embryonic chick.61–63 In these models, after excision or degeneration of the retina, a subset of cells in the RPE detach from Bruch's membrane, expel their pigment granules and proliferate to create a retinal epithelium overlying the original, albeit repaired RPE. This epithelium then, following the embryonic histogenic pattern, differentiates into a fully functional retina, including a new optic nerve that innervates central targets. The signaling pathways that underlie the transdifferentiation of RPE into retinal progenitors are yet to be thoroughly described,64,65 though the initial signaling events are thought to be the loss of cell–cell and cell–basement membrane contacts, re-expression of transcription factors that specify a retinal identity and the activation of a fibroblast growth factor (FGF) signaling pathway.63 Interestingly, transdifferentiation of the RPE in the chick is transient and limited to early embryonic stages.
Müller glia are the only retinal glial cell that are derived from the retinal epithelium. Further, transcriptome analysis shows that mature Müller glia express numerous genes in common with mitotic, late-stage retinal progenitors.66 Therefore, it is perhaps not surprising that in some vertebrates Müller glia retain the ability to enter the cell cycle and, thereby, can function as an intrinsic retinal stem cell. The post-hatch chick and teleost fish have emerged as the most informative models for investigating the stem cell properties of Müller glia (reviewed in Refs. 67–71). In zebrafish, Müller glia sustain the persistent, growth-associated genesis of rod photoreceptors that is characteristic of teleost fish.60 In addition, Müller glia in zebrafish respond to the presence of dying neurons by partially dedifferentiating, undergoing a single asymmetric cell division,72 and spawning rapidly dividing progenitors that can regenerate a single subtype of retinal neuron following its ablation73 or regenerate an entire retina following extensive cell death.74 Presently, there is an intense research effort focused on deciphering the intercellular signaling pathways by which Müller glia become neurogenic when in the presence of dying cells. A key node in these signaling pathways is the proneural transcription factor Ascl1a. Within Müller glia, multiple pathways appear to converge on the regulation of this gene, and if the injury-induced upregulation of ascl1A is blocked, Müller glia fail to enter the cell cycle and neuronal regeneration is forestalled.75 In a recent study using medaka fish, a conditional gene expression paradigm was used to further investigate regenerative mechanisms in Müller glia. This study showed that in an uninjured retina, the conditional expression of atoh7 was sufficient to force quiescent Müller glia into the cell cycle.76 Müller glia expressing atoh7 gave rise to mitotic retinal progenitors that then differentiated into mature retinal neurons. Importantly, the supernumerary neurons generated by the forced expression of atoh7 were largely RGCs, a result that links atoh7 both to the genesis and regeneration of RGCs.
In mammals, Müller glia are normally mitotically quiescent. In response to cell death, Müller glia become reactive and gliotic, but only rarely enter the cell cycle.77 Whether or not Müller glia have a native ability to effect neuronal regeneration is controversial.70 However, in mice, if neuronal death is coupled with the intraocular injection of growth factors, Müller glia will dedifferentiate, enter the cell cycle, and support modest neuronal regeneration.78
Further, if retinal cell death is combined with the conditional expression of ascl1, Müller glia initiate a regenerative response resembling that described for zebrafish.79 Importantly, in the absence of cell death, the forced expression of ascl1 does not alter the Müller glia phenotype or induce entry into the cell cycle. Though this neuronal regeneration does not approach that observed in fish, it confirms that in mammals Müller glia in vivo can be induced to respond to cell death by adopting a neurogenic phenotype and regenerate retinal neurons.
Finally, recent studies show that in mammals, retinal neurons can be regenerated from an endogenous cellular source when cell death is coupled with cell fusion–mediated reprogramming. Cell–cell fusion, the merging of plasma membranes to integrate intercellular components, is a tightly regulated process that occurs naturally during development and in a variety of pathologies.80 Fusion in vivo between stem cells and adult somatic cells can reprogram somatic cells into multipotent progenitors.81,82 In a manner dependent on Wnt signaling, hematopoietic stem cells transplanted into lesioned retinas of adult mice will spontaneously fuse with host retinal neurons and Müller glia.83,84 When coupled with injuries that kill inner retinal neurons, the cell hybrids become mitotic, revert to a neuronal lineage, and differentiate into amacrine cells and RGCs.83 When coupled with the selective death of photoreceptors, hematopoietic stem cells fuse exclusively with Müller glia, which become neurogenic and selectively regenerate photoreceptors.84
While our knowledge of the mechanisms governing the development and regeneration of retinal neurons has advanced, many fundamental questions remain. Importantly, we still do not fully understand why there are profound differences in regenerative capacity across species, why this process is so limited in warm-blooded vertebrates, including humans, and how regeneration could be enhanced and optimized to effectively treat human disease. Therefore, the goal of this AGI workshop was to build upon our knowledge of retinal development and regeneration to delineate opportunities and barriers and to begin to map a path toward RGC replacement in human disease.
Discussion: Gaps in Scientific Knowledge and Barriers to Progress
Cell Sources for RGC Regeneration and Replacement
Exogenous Sources
While the focus of the workshop was on endogenous sources for RGC replacement, the group first discussed recent advances made in using exogenous sources to generate RGCs. Either human embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs) were identified as a viable source for donor cells because they can be derived in unlimited numbers, and can be directed to generate retinal progenitor cells that under defined conditions differentiate into RGCs or other retinal cell types. Recent studies were discussed demonstrating that RGC-like cells derived from human iPSCs have morphological, phenotypic, and functional characteristics expected of RGCs (reviewed in Refs. 85–87). The panel identified some advantages to these approaches, including modeling aspects of human retinal development, as well as enabling the use of genetic tools and in vitro manipulations to promote RGC differentiation. For example, making use of reporter lines to identify cells that have undergone differentiation could help refine and optimize differentiation conditions.55
Ultimately, exogenous cells may also provide a source of storable tissue for transplant studies, although a number of challenges were highlighted. For example, it is still difficult to produce RGCs in numbers and over a time course that is scalable. In addition, the effect of freezing and storing cells prior to transplantation needs to be assessed. Moreover, because ESCs and iPSCs will differentiate into a heterogeneous variety of cell types, strategies need to be developed to either purify cells that will make RGCs, or more efficiently direct these cells to generate RGCs, for example by expressing specific transcription factors. Furthermore, it is currently unknown what stage of RGC development is best for transplantation, but photoreceptor replacement studies suggest that transplanted cells shouldn't be too primitive, but also shouldn't be too mature (reviewed in Ref. 88). It was also emphasized that the more general challenge of immune rejection will need to be addressed, unless cells are derived from that host, obtained from super donors with certain human leukocyte antigen (HLA) types, or engineered to evade immune detection.
One challenge to transplanting exogenous cells is to understand the role of the recipient environment, (e.g., damaged versus intact retina). It is still unknown whether signals in the recipient environment are sufficient to direct exogenous cells to differentiate as RGCs. For example, if RGCs are the primary cell type that is damaged or injured, will this help promote replacement of the cell that is normally there but now absent by disease, or preferentially generate cells in the area of the pathology? In zebrafish there seems to be propensity to replace missing cells, including some recognition of which cells are missing, with those being the ones regenerated in greater amounts (reviewed in Ref. 71). Whether the host environment will play a role in human transplant studies remains to be seen.
Endogenous Sources
The primary focus of the workshop was on utilizing endogenous sources for RGC replacement, building upon advances in retinal regeneration achieved in model systems, as highlighted above. There are multiple cell populations with the potential to contribute to retinal cell regeneration, including Müller glia, RPE cells, and stem cells within the ciliary epithelium. In considering which cell population to target for endogenous regeneration, the group emphasized the importance of understanding the mobility of cells and the cues that direct them to move through the retina. In particular, it will be important to define the cues that indicate loss of RGCs and trigger regeneration and control cell migration to the ganglion cell layer. Müller glia are among the most promising target for reprogramming due to their ability to contribute to retinal regeneration in species such as fish and post-hatch chick (see above). Furthermore, Müller glia–derived progenitors can move through the layers and potentially populate the RGC layer to become RGCs. Age may be a critical variable and may reflect epigenetic changes in Müller glia as the retina matures because, for mammals, Müller glia in younger retinas are more amenable to being redirected toward neural fates.79,89 In addition, much remains to be learned about how to more selectively direct Müller glia to regenerate RGCs.
While RPE cells in some contexts contribute to retinal regeneration, the potential for endogenous RPE cells to selectively replace RGCs was felt to be somewhat limited. In particular, it may be difficult to induce RPE cells in an endogenous intact retina to migrate through the retinal layers to the RGC layer. However, it was mentioned that in proliferative vitreoretinopathy, RPE cells can migrate through the retina and contribute to the formation of epiretinal membranes, which illustrates how RPE cells can respond and acquire different properties that could potentially be harnessed.57,90,91 It was noted that when transcription factors have been expressed in human RPE to push them into the neural lineage, they take on quite a different morphology and growth characteristics,92 suggesting they could be reprogrammed, although this has yet to be shown in an in vivo system selectively depleted of RGCs. The possibility of targeting ciliary epithelial stem cells was also addressed, although these cells would have to move from the margins of the eye to the ganglion cell layer. It is possible to direct these cells to differentiate into neurons. However, the cells retain pigment granules, so their application to RGC replacement may be limited.93
Other less conventional cell sources for RGC replacement were also discussed. For example, astrocytes can be redirected toward neuronal fates,94,95 and if in proximity to RGCs, they are theoretically targetable. One challenge is that astrocytes play important roles in the optic nerve, so redirecting them to alternate fates could be detrimental. However, it might be possible to use them as a source for replacing RGCs, while also decreasing potentially negative effects of depleting astrocytes. An innovative approach suggested targeting displaced amacrine cells, which already express Isl1 and Sox2, so they could potentially be redirected to an RGC fate. A recent study showed that Lgr5-positive amacrine cells have progenitor-like properties and generate new neurons in adult mice, so they may be amenable to RGC-directed reprogramming.96 This intriguing possibility was considered worth pursuing.
While retina regeneration studies have advanced in recent years, there is still a paucity of specific knowledge regarding the regeneration of RGCs, so the group discussed how the production of functional RGCs could be optimized. Overall, one clear priority is to thoroughly define the transcription factors and signaling pathways that promote efficient reprogramming of endogenous cells and RGC differentiation, particularly in humans. It was suggested that these efforts should consider the role of progenitor competence and timing, because during normal development RGCs are born during a restricted developmental window.28 One question that was raised is whether all RGC types need to be generated. If the goal is to help a nonseeing person to see, what characteristics of RGCs are necessary to process and transmit information? Current approaches address gene expression, morphology, and connectivity to mimic normal RGCs. But it is important to define what are the most important characteristics to mimic. It was suggested that it may be unnecessary to generate an exact RGC, but rather a neuron that has the essential functionality (i.e., a generic projection neuron). The essential functional characteristics are that it must project through the optic nerve, arrive at the correct targets, make appropriate afferent connections, and perform basic processing of visual information. It was recommended that fully characterizing human RGCs and defining requisite criteria should be a priority, including detailing functional properties and the array of molecular markers that are characteristic of RGCs.
Optimizing Integration, Survival, and Synaptogenesis of New RGCs
Targeting and Delivery
The panel considered the best strategies for targeting endogenous cells and introducing genes or factors into the host retina for reprogramming. Gene therapy approaches in the eye have focused on viral vectors, particularly adeno-associated virus (AAV), for gene delivery.97 The group discussed efforts to optimize targeting of ocular tissues, and specific retinal types, by screening large libraries of AAV with novel changes in the capsid. Because there are species differences in the specificity and targeting of these vectors, these screens are being performed in rodents, canines, and primates. To optimize viral targeting of human cells, it was suggested that vectors could be screened on the human 3D eyecup in vitro. AAV variants have already been identified that specifically target Müller glia in the rodent retina. It may ultimately be possible to target each major subclass of RGC, as well as targeting other cell types such as RPE. One important consideration raised for reprogramming of cells is the timing of delivery of factors, because the timing and order of expression of new genes is likely to be critical for Müller glia reprogramming.98 It was pointed out that while much focus has been on AAV, because it has a simple genome, it is limited because it only packages 4.7 kilobases, so alternate vectors were discussed.99 Adenovirus was identified as better in that regard, but this virus puts major histocompatibility complex (MHC) on the outside of the cell, which can trigger a significant immune response, thereby limiting its application, particularly for individuals with systemic diseases.
Integration
For transplantation of cells from exogenous sources, an important consideration is how to enhance the delivery and integration of transplanted cells. It was suggested that it may help to temporarily make the inner limiting membrane more porous and better able to receive the cells delivered to the eye, which could increase the likelihood of functional integration of RGCs. The role of the host environment is also critical. If there is ongoing disease, will the transplanted cells die for the same reason as the original RGCs? Will it be possible to engineer cells that may be more resistant to the same lesion? It was pointed out that changes to the extracellular matrix, which is significant in degeneration, need to be considered when transplanting cells into the damaged retina. During the initial testing phase, it was suggested that investigators choose a disease model in which there is not ongoing injury or damage, or where the causal risk factor can be mitigated. A chronic disease like glaucoma might be risky to choose for proof-of-concept studies. In many cases, we do not fully understand the pathophysiology of several optic neuropathies, which poses challenges for restoring RGCs in a potentially hostile environment.
The panel pointed out that much remains to be learned about the long-term survival and stability of regenerated or transplanted RGCs, and that this may need to be optimized. To achieve this goal, it will be necessary to identify, visualize, and follow new cells over time. This may be challenging in humans, because it is not clear whether it will be safe to transplant cells expressing standard exogenous reporters (such as green fluorescent protein). It may be necessary to develop a technology to mark and visualize the cells in humans in a safe way, or introduce technology to eliminate the cells if something goes wrong. It was noted that even without visible reporters, advances in adaptive optics and other in vivo imaging technologies may enable visualization of new cells, but not necessarily whether they have made connections. For transplantation of cells from exogenous sources, the panel emphasized the importance of monitoring the integration of transplanted cells. What are the criteria to prove that the cell that is being imaged and believed to be integrated is really the cell that was transplanted? While fluorescent reporters are valuable, it is critical to control for cell fusion, and for transfer of material (e.g., protein or RNA) between transplanted cells and recipient host cells.100,101 These studies must also distinguish RGC replacement from rescue of remaining RGCs or other indirect effects. For example, transplanted cells may provide neurotrophic support and improve the function of remaining RGCs, which may be a desirable outcome, particularly if RGCs are partially damaged but still able to recover. However, it is important to understand mechanisms and optimize beneficial effects, perhaps by targeting the early stage of disease when there is only initial damage to RGCs.
Connectivity
The group next discussed another important consideration for successful regeneration or replacement of RGCs, optimizing synaptogenesis, and connectivity. The focus was on assays that reveal RGC features that are functionally relevant, such as synaptic integration into the inner plexiform layer (IPL), the electrophysiologic response to light, as well as axon growth and guidance to the correct targets in the brain. Because previous workshops tackled the complex challenges of regenerating the optic nerve and targeting the brain, this discussion focused on connectivity within the retina. To assess successful integration within the retina at a structural level, it was proposed that one could visualize and count the number of synapses and dendrites and assess the morphology of neurons formed. New fluorescent reporter approaches and imaging techniques should help in this visualization at nanoscale level. High-resolution in vivo imaging tools exist, but making them more available to all investigators would speed assessment and discovery. The group recommended that to make the best use of these imaging tools, common standards need to be developed to allow comparison of results. The panel noted that differences in RGC subtypes could be an important consideration, because survival, synaptogenesis, and connectivity could vary among subtypes. It may be important to consider whether regenerated RGCs recreate subtype-specific synaptic circuits within the IPL. Utilizing or generating reporters for each RGC subclass using CRISPR technology could investigate this. It was emphasized that if ex vivo screens are utilized to test and optimize parameters for integration, they need to be performed in a system where there is an IPL present to allow for appropriate connectivity. To optimize integration into human retina, high throughput screens could be performed using explants of human or monkey retina, or 3D stem cell–derived eyecups before testing in vivo in animal models. However, it will be first important to establish the best in vitro test to predict success in vivo.
Approaches for Assessing the Outcomes of RGC Replacement Therapies
Assessing RGC Function
The final discussion focused on assessing whether successful regeneration or replacement of functional RGCs has been achieved. The panel emphasized the importance of standardized functional assays to monitor RGC responses and connectivity at the cellular level in vivo. Several strategies were discussed for probing new RGCs to determine whether they have integrated and connected. The goal is to visualize the cells that have been transplanted or regenerated, stimulate the cells, and know that they are the ones that are carrying the signal. In model systems, this could involve optogenetic approaches to noninvasively stimulate RGCs, and then perform electrophysiological recordings in the brain to assess whether stimulated cells have actually connected. It was pointed out that in the spinal cord transplant field, researchers are using chemical or optogenetic methods to reversibly silence transplanted cells to study their functional contributions, a strategy that could be applied to RGCs. In addition, it may be possible to determine whether new RGCs have formed dendritic connections in the IPL by stimulating photoreceptors and monitoring the responses of the new RGCs. To assess whether the cells are there and still alive, an innovative strategy could determine if mitochondria are flowing within RGC axons, because these organelles can be visualized. In general, the panel recommended the development of standardized criteria for successful integration to allow studies to be compared across labs.
Assessing Visual Function
The panel then grappled with the important question of determining whether treatments lead to improved visual outcomes. It is first important to decide if the goal is ambulatory vision or high-acuity vision. If ambulatory vision is the goal, then it was suggested that it might suffice to replace just a few subtypes of RGCs. One interesting proposal was to utilize intrinsically photosensitive RGCs to obviate the whole problem of connectivity on the afferent side. A related question is how many RGCs are needed to restore useful vision, and how should they be distributed across the retina. It was proposed that perhaps as few as 10,000 cells may be sufficient for useful vision, although it depends on the quality of vision that is desired. If the quality of vision that many of us enjoy is needed, then it will require many more cells. In sensory substitution experiments, a 256 × 256 grid of sensory input to the tongue is sufficient for a person to obtain enough information about the visual world to ambulate,102 so it may require fewer cells than usually considered necessary for ambulatory vision. It is also likely that the cortical fill-in phenomenon will permit scene recognition even in a limited visual field. It is known that patients with end-stage glaucoma can have a tiny amount of visual field and yet have useful vision.
The group also considered the challenge of detecting improvement of visual function after treatment. If the goal is to assess recovery of vision, it will be important to establish sensitive and quantitative functional criteria. Another consideration is selecting appropriate diseases, or stages of disease, to target for therapy in proof-of-concept studies. If relatively few RGCs are needed for light perception, than it may be difficult to detect improvement unless subjects (experimental animals or patients) start with no light perception and no evidence of RGC function.
The stage of disease has other potential implications for success of treatment, because retinal circuits undergo remodeling after extended periods of neuronal loss.103 This can impact target structures in the lateral geniculate nucleus and visual cortex. If the goal is to have new RGCs recreate stereotypical synaptic circuits, then either acute injury or early stage disease should be targeted for therapy to ensure that there has not been time for upstream and/or downstream remodeling of synaptic circuits. This will help ensure that the supporting structures are still present and there is no progressive damage. At the same time, the group recognized that studies should not focus on early disease because naturally occurring improvement could still take place, which could confound studies of efficacy. We summarize these challenges in Box 1.
A final point is that animal models do not always adequately represent humans, and that many aspects of disease are specific to humans. If, based on animal models, it appears that a therapy will be safe, then it will be necessary to empirically test it in a wide range of human diseases and at different stages of severity. In other words, clinical research must be included as part of the experimental pathway toward developing therapies.
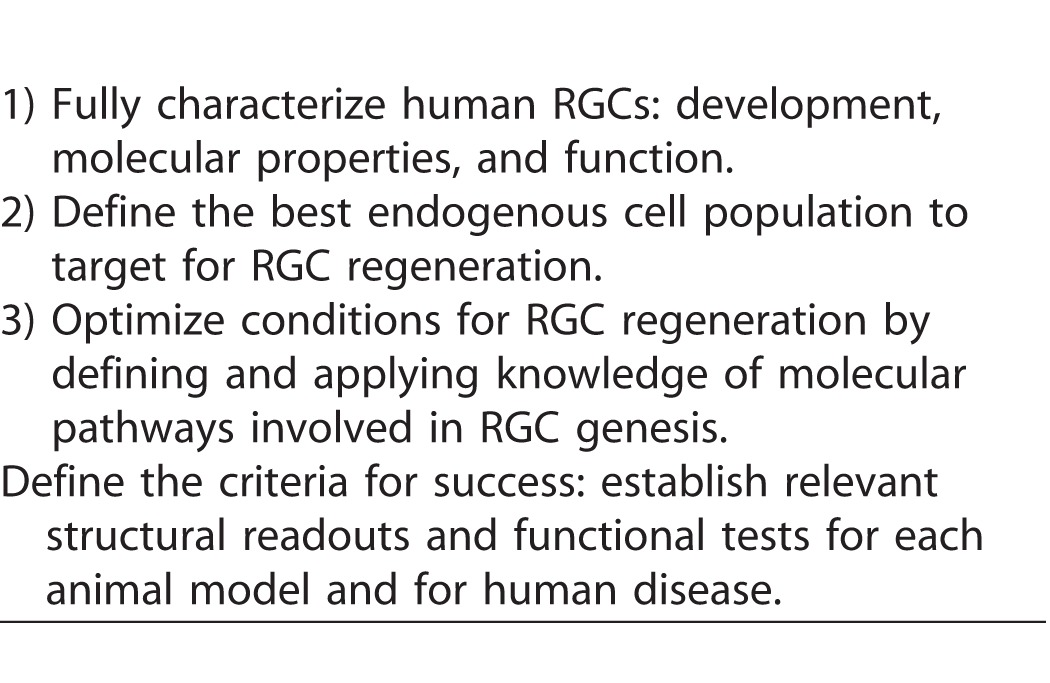
The panel discussion ended by articulating key recommendations (Box 2) to accelerate progress toward functional RGC regeneration. There was strong consensus that while these are early days for RGC replacement and regeneration, key intermediate objectives could be achieved within a reasonable time frame. These intermediate objectives (Box 2) could set the course for a path forward, and would help accelerate discoveries to ultimately achieve RGC regeneration and restoration of vision in human disease.
Table 1.
Summary of Gaps in Knowledge and Barriers to Progress

Table 2.
Key Recommendations to Accelerate Progress Toward Functional RGC Regeneration

Table 3.
Intermediate Objectives
Acknowledgments
The authors thank Lynne Peeples for providing detailed notes from the workshop discussion.
Appendix A
Adah Almutairi, PhD
Associate Professor
Principal Investigator
Laboratory of Bioresponsive Materials
Co-Director
Center of Excellence in Nanomedicine & Engineering
University of California, San Diego
aalmutairi@ucsd.edu
Mark S. Blumenkranz, MD
Director
Byers Eye Institute
H.J. Smead Professor
Department of Ophthalmology
Stanford University
mark.blumenkranz@stanford.edu
Alain Chedotal, PhD
Director of Research
Institut de la Vision
Université Pierre et Marie Curie
alain.chedotal@inserm.fr
Maria Pia Cosma, ScD
ICREA Senior Investigator, Group Leader
Reprogramming and Regeneration Group Leader
Gene Regulation, Stem Cells and Cancer Program
Center for Genomic Regulation
pia.cosma@crg.eu
Andy Fischer, PhD
Professor
Department of Neuroscience
The Ohio State University College of Medicine
andrew.fischer@osumc.edu
John Flannnery, PhD
Professor
Vision Science, Neuroscience Division, Molecular and Cell Biology
Helen Wills Neuroscience Institute
University of California, Berkeley
flannery@berkeley.edu
Tom Glaser, PhD
Professor
Department of Cell Biology and Human Anatomy
School of Medicine
University of California, Davis
tmglaser@ucdavis.edu
Jeffrey Goldberg, MD, PhD
Professor and Chair
Department of Ophthalmology
Stanford University
jlgoldbe@stanford.edu
Peter Hitchcock, PhD (Co-Chair)
Professor and Associate Dean
Professor, Ophthalmology and Visual Sciences
Professor, Cell and Developmental Biology
Kellogg Eye Center
University of Michigan
peterh@umich.edu
David Hyde, PhD
Professor of Biology
Center for Stem Cells and Regenerative Research
University of Notre Dame
dhyde@nd.edu
Paul Kaufman, MD
Ernst H. Bárány Professor of Ocular Pharmacology
Departments of Ophthalmology and Visual Sciences and Animal Health and Biomedical Sciences
Institute on Aging
University of Wisconsin-Madison
paul.kaufman@wisc.edu
Leonard A. Levin, MD, PhD
Professor and Chair of Ophthalmology
McGill Academic Eye Centre
McGill University
Physician-in-Chief of Ophthalmology
McGill University Health Centre
leonard.levin@mcgill.ca
Astrid Limb, PhD
Professor of Retinal Stem Cell Biology and Therapeutics
Ocular Biology
Institute of Ophthalmology
Fellow
Royal College of Pathologists
g.limb@ucl.ac.uk
Jason Meyer, PhD
Assistant Professor
Stark Neurosciences Research Institute
University of Indiana – Purdue University, Indianapolis
meyerjas@iupui.edu
Xiuqian Mu, PhD
Associate Professor
Department of Ophthalmology
Jacobs School of Medicine and Biomedical Sciences
University at Buffalo, State University of New York
xmu@buffalo.edu
Yehoash Raphael, PhD
Professor
Department of Otolaryngology - Head and Neck Surgery
Kresge Hearing Research Institute
University of Michigan Medical School
yoash@umich.edu
Pamela Raymond, PhD
Stephen S. Easter Collegiate Professor
Department of Molecular, Cellular, and Developmental Biology
College of Literature, Science, and the Arts
University of Michigan
praymond@umich.edu
Douglas Rhee, MD
Professor and Chair
Department of Ophthalmology and Visual Sciences
University Hospitals Eye Institute
douglas.rhee@uhhospitals.org
Joel Schuman, MD
Professor and Chairman of Ophthalmology
New York University Langone Medical Center
joel.schuman@nyumc.org
Paul Sieving, MD, PhD
Director
National Eye Institute
National Institutes of Health
pas@nei.nih.gov
Sally Temple, PhD
Scientific Director
Neural Stem Cell Institute
Regenerative Research Foundation
sallytemple@neuralsci.org
Monica Vetter, PhD (Co-Chair)
Professor and Chair
Department of Neurobiology and Anatomy
University of Utah
monica.vetter@neuro.utah.edu
Valerie Wallace, PhD
Senior Scientist and Division Head
Krembil Research Institute
University Health Network
Department of Ophthalmology and Vision Science
Department of Laboratory Medicine and Pathobiology
University of Toronto
vwallace@uhnresearch.ca
Don Zack, MD, PhD
Co-Director
Johns Hopkins Center for Stem Cells and Ocular Regenerative Medicine
Professor of Ophthalmology
Johns Hopkins University
donzack@gmail.com
References
- 1. Scott AW,, Bressler NM,, Folkes S,, Wittenborn JS,, Jorkasky J. Public attitudes about eye and vision health. JAMA Ophthalmol. 2016; 134: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 2. Dawson CR,, Schwab IR. Epidemiology of cataract - a major cause of preventable blindness. Bull World Health Organ. 1981; 59: 493–501. [PMC free article] [PubMed] [Google Scholar]
- 3. Congdon NG,, Friedman DS,, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003; 290: 2057–2060. [DOI] [PubMed] [Google Scholar]
- 4. Resnikoff S,, Pascolini D,, Mariotti SP,, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008; 86: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dowling JE. Restoring vision to the blind: introduction. Trans Vis Sci Tech. 2014; 3 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldberg JL,, Guido W; and the AGI Workshop Participants. Report on the National Eye Institute audacious goals initiative: regenerating the optic nerve. Invest Ophthalmol Vis Sci. 2016; 57: 1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gamm DM,, Wong R; and the AGI Workshop Panelists. Report on the National Eye Institute Audacious goals initiative: Photoreceptor Regeneration and Integration Workshop. Trans Vis Sci Tech. 2015; 4 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crair MC,, Mason CA. Reconnecting eye to brain. J Neurosci. 2016; 36: 10707–10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghaffarieh A,, Levin LA. Optic nerve disease and axon pathophysiology. Int Rev Neurobiol. 2012; 105: 1–17. [DOI] [PubMed] [Google Scholar]
- 10. Almasieh M,, Wilson AM,, Morquette B,, Cueva Vargas JL,, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181. [DOI] [PubMed] [Google Scholar]
- 11. Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004; 82: 887–888. [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta P,, Zhao D,, Guallar E,, Ko F,, Boland MV,, Friedman DS. Prevalence of Glaucoma in the United States: The 2005-2008 National Health and Nutrition Examination Survey. Invest Ophthalmol Vis Sci. 2016; 57: 2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quigley HA. Glaucoma. Lancet. 2011; 377: 1367–1377. [DOI] [PubMed] [Google Scholar]
- 14. Nickells RW. The cell and molecular biology of glaucoma: mechanisms of retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2012; 53: 2476–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999; 18: 39–57. [DOI] [PubMed] [Google Scholar]
- 16. Zhang C,, Guo Y,, Slater BJ,, Miller NR,, Bernstein SL. Axonal degeneration, regeneration and ganglion cell death in a rodent model of anterior ischemic optic neuropathy (rAION). Exp Eye Res. 2010; 91: 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonul S,, Koktekir BE,, Bakbak B,, Gedik S. Comparison of the ganglion cell complex and retinal nerve fibre layer measurements using Fourier domain optical coherence tomography to detect ganglion cell loss in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2013; 97: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 18. Biousse V,, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015; 372: 2428–2436. [DOI] [PubMed] [Google Scholar]
- 19. Yu-Wai-Man P,, Griffiths PG,, Chinnery PF. Mitochondrial optic neuropathies - disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011; 30: 81–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newman NJ. Treatment of hereditary optic neuropathies. Nat Rev Neurol. 2012; 8: 545–556. [DOI] [PubMed] [Google Scholar]
- 21. Meyerson C,, Van Stavern G,, McClelland C. Leber hereditary optic neuropathy: current perspectives. Clin Ophthalmol. 2015; 9: 1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez Sanchez MIG,, Crowston JG,, Mackey DA,, Trounce IA. Emerging mitochondrial therapeutic targets in optic neuropathies. Pharmacol Ther. 2016; 165: 132–152. [DOI] [PubMed] [Google Scholar]
- 23. Grzybowski A,, Zülsdorff M,, Wilhelm H,, Tonagel F. Toxic optic neuropathies: an updated review. Acta Ophthalmol. 2015; 93: 402–410. [DOI] [PubMed] [Google Scholar]
- 24. Kumaran AM,, Sundar G,, Chye LT. Traumatic optic neuropathy: a review. Craniomaxillofac Trauma Reconstr. 2015; 8: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singman EL,, Daphalapurkar N,, White H,, et al. Indirect traumatic optic neuropathy. Mil Med Res. 2016; 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berkelaar M,, Clarke DB,, Wang YC,, Bray GM,, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994; 14: 4368–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agathocleous M,, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009; 25: 45–69. [DOI] [PubMed] [Google Scholar]
- 28. Bassett EA,, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012; 35: 1–9. [DOI] [PubMed] [Google Scholar]
- 29. Gregory-Evans CY,, Wallace VA,, Gregory-Evans K. Gene networks: dissecting pathways in retinal development and disease. Prog Retin Eye Res. 2013; 33: 40–66. [DOI] [PubMed] [Google Scholar]
- 30. Yang H-J,, Ratnapriya R,, Cogliati T,, Kim J-W,, Swaroop A. Vision from next generation sequencing: multi-dimensional genome-wide analysis for producing gene regulatory networks underlying retinal development, aging and disease. Prog Retin Eye Res. 2015; 46: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mu X,, Klein WH. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin Cell Dev Biol. 2004; 15: 115–123. [DOI] [PubMed] [Google Scholar]
- 32. Souren M,, Martinez-Morales J-R,, Makri P,, Wittbrodt B,, Wittbrodt J. A global survey identifies novel upstream components of the Ath5 neurogenic network. Genome Biol. 2009; 10: R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kiyama T,, Mao C-A,, Cho J-H,, et al. Overlapping spatiotemporal patterns of regulatory gene expression are required for neuronal progenitors to specify retinal ganglion cell fate. Vision Res. 2011; 51: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown NL,, Kanekar S,, Vetter ML,, Tucker PK,, Gemza DL,, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998; 125: 4821–4833. [DOI] [PubMed] [Google Scholar]
- 35. Brown NL,, Patel S,, Brzezinski J,, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001; 128: 2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kay JN,, Finger-Baier KC,, Roeser T,, Staub W,, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron. 2001; 30: 725–736. [DOI] [PubMed] [Google Scholar]
- 37. Wang SW,, Kim BS,, Ding K,, et al. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001; 15: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mu X,, Fu X,, Sun H,, Beremand PD,, Thomas TL,, Klein WH. A gene network downstream of transcription factor Math5 regulates retinal progenitor cell competence and ganglion cell fate. Dev Biol. 2005; 280: 467–481. [DOI] [PubMed] [Google Scholar]
- 39. Wu F,, Kaczynski TJ,, Sethuramanujam S,, et al. Two transcription factors, Pou4f2 and Isl1, are sufficient to specify the retinal ganglion cell fate. Proc Natl Acad Sci U S A. 2015; 112: E1559–E1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanes JR,, Masland RH. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci. 2015; 38: 221–246. [DOI] [PubMed] [Google Scholar]
- 41. Baden T,, Berens P,, Franke K, Román Rosón M, Bethge M, Euler T. The functional diversity of retinal ganglion cells in the mouse. Nature. 2016; 529: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sasai Y,, Eiraku M,, Suga H. In vitro organogenesis in three dimensions: self-organising stem cells. Development. 2012; 139: 4111–4121. [DOI] [PubMed] [Google Scholar]
- 43. Wright LS,, Phillips MJ,, Pinilla I,, Hei D,, Gamm DM. Induced pluripotent stem cells as custom therapeutics for retinal repair: progress and rationale. Exp Eye Res. 2014; 123: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki IK,, Vanderhaeghen P. Is this a brain which I see before me? Modeling human neural development with pluripotent stem cells. Development. 2015; 142: 3138–3150. [DOI] [PubMed] [Google Scholar]
- 45. Chamling X,, Sluch VM,, Zack DJ. The potential of human stem cells for the study and treatment of glaucoma. Invest Ophthalmol Vis Sci. 2016; 57: ORSFi1- . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sinha D,, Phillips J,, Joseph Phillips M,, Gamm DM. Mimicking retinal development and disease with human pluripotent stem cells. Invest Ophthalmol Vis Sci. 2016; 57: ORSFf1- . [DOI] [PubMed] [Google Scholar]
- 47. Eiraku M,, Takata N,, Ishibashi H,, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011; 472: 51–56. [DOI] [PubMed] [Google Scholar]
- 48. Nakano T,, Ando S,, Takata N,, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012; 10: 771–785. [DOI] [PubMed] [Google Scholar]
- 49. Tanaka T,, Yokoi T,, Tamalu F,, Watanabe S-I,, Nishina S,, Azuma N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci Rep. 2015; 5: 8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maekawa Y,, Onishi A,, Matsushita K,, et al. Optimized culture system to induce neurite outgrowth from retinal ganglion cells in three-dimensional retinal aggregates differentiated from mouse and human embryonic stem cells. Curr Eye Res. 2016; 41: 558–568. [DOI] [PubMed] [Google Scholar]
- 51. Meyer JS,, Howden SE,, Wallace KA,, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011; 29: 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Phillips MJ,, Wallace KA,, Dickerson SJ,, et al. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012; 53: 2007–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohlemacher SK,, Sridhar A,, Xiao Y,, et al. Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells. 2016; 34: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boucherie C,, Mukherjee S,, Henckaerts E,, Thrasher AJ,, Sowden JC,, Ali RR. Brief report: self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells. 2013; 31: 408–414. [DOI] [PubMed] [Google Scholar]
- 55. Sluch VM,, Davis C-HO,, Ranganathan V,, et al. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci Rep. 2015; 5: 16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salero E,, Blenkinsop TA,, Corneo B,, et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012; 10: 88–95. [DOI] [PubMed] [Google Scholar]
- 57. Saini JS,, Temple S,, Stern JH. Human retinal pigment epithelium stem cell (RPESC). Adv Exp Med Biol. 2016; 854: 557–562. [DOI] [PubMed] [Google Scholar]
- 58. Lawrence JM,, Singhal S,, Bhatia B,, et al. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007; 25: 2033–2043. [DOI] [PubMed] [Google Scholar]
- 59. Singhal S,, Bhatia B,, Jayaram H,, et al. Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012; 1: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hitchcock P,, Ochocinska M,, Sieh A,, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004; 23: 183–194. [DOI] [PubMed] [Google Scholar]
- 61. Chiba C. The retinal pigment epithelium: an important player of retinal disorders and regeneration. Exp Eye Res. 2014; 123: 107–114. [DOI] [PubMed] [Google Scholar]
- 62. Fuhrmann S,, Zou C,, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res. 2014; 123: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hidalgo M,, Locker M,, Chesneau A,, Perron M. Stem cells and regeneration in the xenopus retina. : Pébay A, Regenerative Biology of the Eye. Stem Cell Biology and Regenerative Medicine. New York, NY: Springer New York; 2014: 83–99. [Google Scholar]
- 64. Yoshii C,, Ueda Y,, Okamoto M,, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev Biol. 2007; 303: 45–56. [DOI] [PubMed] [Google Scholar]
- 65. Luz-Madrigal A,, Grajales-Esquivel E,, McCorkle A,, et al. Reprogramming of the chick retinal pigmented epithelium after retinal injury. BMC Biol. 2014; 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blackshaw S,, Harpavat S,, Trimarchi J,, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004; 2: E247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gallina D,, Todd L,, Fischer AJ. A comparative analysis of Müller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014; 123: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gorsuch RA,, Hyde DR. Regulation of Müller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res. 2014; 123: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lenkowski JR,, Raymond PA. Müller glia: stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014; 40: 94–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hamon A,, Roger JE,, Yang X-J,, Perron M. Müller glial cell-dependent regeneration of the neural retina: an overview across vertebrate model systems. Dev Dyn. 2016; 245: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wan J,, Goldman D. Retina regeneration in zebrafish. Curr Opin Genet Dev. 2016; 40: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nagashima M,, Barthel LK,, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013; 140: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yoshimatsu T,, Orazi FDDR,, Gamlin CR,, et al. Presynaptic partner selection during retinal circuit reassembly varies with timing of neuronal regeneration in vivo. Nat Commun. 2016; 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sherpa T,, Lankford T,, McGinn TE,, et al. Retinal regeneration is facilitated by the presence of surviving neurons. Devel Neurobio. 2014; 74: 851–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ramachandran R,, Zhao X-F,, Goldman D. Ascl1a/Dkk/{beta}-catenin signaling pathway is necessary and glycogen synthase kinase-3{beta} inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011; 108: 15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lust K,, Sinn R,, Pérez Saturnino A,, Centanin L,, Wittbrodt J. De novo neurogenesis by targeted expression of atoh7 to Müller glia cells. Development. 2016; 143: 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dyer MA,, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000; 3: 873–880. [DOI] [PubMed] [Google Scholar]
- 78. Karl MO,, Hayes S,, Nelson BR,, Tan K,, Buckingham B,, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008; 105: 19508–19513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ueki Y,, Wilken MS,, Cox KE,, et al. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A. 2015; 112: 13717–13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Willkomm L,, Bloch W. State of the art in cell-cell fusion. Methods Mol Biol. 2015; 1313: 1–19. [DOI] [PubMed] [Google Scholar]
- 81. Lluis F,, Cosma MP. Cell-fusion-mediated somatic-cell reprogramming: a mechanism for tissue regeneration. J Cell Physiol. 2010; 223: 6–13. [DOI] [PubMed] [Google Scholar]
- 82. Sanges D,, Lluis F,, Cosma MP. Cell-fusion-mediated reprogramming: pluripotency or transdifferentiation? Implications for regenerative medicine. Adv Exp Med Biol. 2011; 713: 137–159. [DOI] [PubMed] [Google Scholar]
- 83. Sanges D,, Romo N,, Simonte G,, et al. Wnt/β-catenin signaling triggers neuron reprogramming and regeneration in the mouse retina. Cell Rep. 2013; 4: 271–286. [DOI] [PubMed] [Google Scholar]
- 84. Sanges D,, Simonte G,, Di Vicino U,, et al. Reprogramming Müller glia via in vivo cell fusion regenerates murine photoreceptors. J Clin Invest. 2016; 126: 3104–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gill KP,, Hewitt AW,, Davidson KC,, Pébay A,, Wong RCB. Methods of retinal ganglion cell differentiation from pluripotent stem cells. Trans Vis Sci Tech. 2014; 3 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sluch VM,, Zack DJ. Stem cells, retinal ganglion cells and glaucoma. Dev Ophthalmol. 2014; 53: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu N,, Doorenbos M,, Chen DF. Induced pluripotent stem cells: development in the ophthalmologic field. Stem Cells Int. 2016; 2016: 2361763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jayakody SA,, Gonzalez-Cordero A,, Ali RR,, Pearson RA. Cellular strategies for retinal repair by photoreceptor replacement. Prog Retin Eye Res. 2015; 46: 31–66. [DOI] [PubMed] [Google Scholar]
- 89. Löffler K,, Schäfer P,, Völkner M,, Holdt T,, Karl MO. Age-dependent Müller glia neurogenic competence in the mouse retina. Glia. 2015; 63: 1809–1824. [DOI] [PubMed] [Google Scholar]
- 90. Casco-Robles MM,, Islam MR,, Inami W,, et al. Turning the fate of reprogramming cells from retinal disorder to regeneration by Pax6 in newts. Sci Rep. 2016; 6: 33761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tamiya S,, Kaplan HJ. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp Eye Res. 2016; 142: 26–31. [DOI] [PubMed] [Google Scholar]
- 92. Li X,, Ma W,, Zhuo Y,, Yan R-T,, Wang S-Z. Using neurogenin to reprogram chick RPE to produce photoreceptor-like neurons. Invest Ophthalmol Vis Sci. 2010; 51: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cicero SA,, Johnson D,, Reyntjens S,, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A. 2009; 106: 6685–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Berninger B,, Costa MR,, Koch U,, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007; 27: 8654–8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Heinrich C,, Blum R,, Gascón S,, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010; 8: e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen M,, Tian S,, Glasgow NG,, et al. Lgr5 +amacrine cells possess regenerative potential in the retina of adult mice. Aging Cell. 2015; 14: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Day TP,, Byrne LC,, Schaffer DV,, Flannery JG. Advances in AAV vector development for gene therapy in the retina. Adv Exp Med Biol. 2014; 801: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014; 15: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Trapani I,, Puppo A,, Auricchio A. Vector platforms for gene therapy of inherited retinopathies. Prog Retin Eye Res. 2014; 43: 108–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pearson RA,, Gonzalez-Cordero A,, West EL,, et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun. 2016; 7: 13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Santos-Ferreira T,, Llonch S,, Borsch O,, Postel K,, Haas J,, Ader M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat Commun. 2016; 7: 13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Stronks HC,, Mitchell EB,, Nau AC,, Barnes N. Visual task performance in the blind with the BrainPort V100 Vision Aid. Expert Rev Med Devices. 2016; 13: 919–931. [DOI] [PubMed] [Google Scholar]
- 103. Strettoi E. A survey of retinal remodeling. Front Cell Neurosci. 2015; 9: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]


