Summary
Electroencephalographic slow-wave activity (0.5–4 Hz) during non rapid-eye-movement (NREM) sleep is a marker for cortical reorganization, particularly within the prefrontal cortex. Greater slow-wave activity during sleep may promote greater waking prefrontal metabolic rate, and in turn, executive function. However, this process may be affected by age. Here we examined whether greater NREM slow-wave activity was associated with higher prefrontal metabolism during wakefulness and whether this relationship interacted with age. Fifty-two participants ages 25–61 were enrolled in studies that included polysomnography and a 18[F]-fluoro-deoxy-glucose positron emission tomography scan during wakefulness. Absolute and relative measures of NREM slow-wave activity were assessed. Semi-quantitative and relative measures of cerebral metabolism were collected to assess whole brain and regional metabolism, focusing on two regions of interest: the dorsolateral prefrontal cortex and the orbitofrontal cortex. Greater relative slow-wave activity was associated with greater dorsolateral prefrontal metabolism. Age and slow-wave activity significantly interacted in predicting semi-quantitative whole brain metabolism and outside regions of interest in the posterior cingulate, middle temporal gyrus, and the medial frontal gyrus, such that greater slow-wave activity was associated with lower metabolism in the younger participants and greater metabolism in the older participants. These results suggest that slow-wave activity is associated with cerebral metabolism during wakefulness across the adult lifespan within regions important for executive function.
INTRODUCTION
Sleep and executive function both exhibit significant age-related decline and are both subserved by the prefrontal cortex (PFC). NREM slow-wave activity (SWA) (< 5 Hz) in sleep electroencephalography (EEG) reflects synchronous neural activity generated by the prefrontal cortex (PFC) (Uhlhaas et al., 2010, Massimini et al., 2004) and significantly decreases from adolescence through middle adulthood and into older adulthood (Carrier et al., 2001, Landolt et al., 1996). Goal-driven, executive function also depends on an intact PFC (Stuss and Alexander, 2000) and deteriorates with aging (Verhaeghen and Cerella, 2002).
Due to this common underlying substrate between SWA and executive function, there is increasing interest in whether age-related changes in these domains influence one another (Anderson and Horne, 2003, Wilckens et al., 2012, Wilckens et al., in press, Mander et al., 2013, Muzur et al., 2002). For instance, neural synchrony underlying SWA contributes to the substantial decrease in PFC metabolism during NREM sleep, which may be one mechanism by which sleep restores executive function (Maquet et al., 1997). Further, neural synchrony underlying SWA may reinforce cortical connections within PFC networks (Wilckens et al., in press, Mander et al., 2013, Picchioni et al., 2013, Terry et al., 2004), potentially resulting in increased waking metabolism in the PFC and improved waking function. Thus, an age-related decrease in SWA may diminish the restorative value of sleep to the PFC and in turn, negatively impact executive abilities (Wilckens et al., 2012, Harrison et al., 2000). By the same model, older individuals who have high SWA despite advancing age may benefit from the restorative effects of SWA on PFC function. This view is supported by cross-sectional data showing that greater SWA is associated with better executive function behaviorally in older adults (Anderson and Horne, 2003, Wilckens et al., in press).
Alternatively, these cross-sectional data could be explained by an alternative hypothesis that a use-dependent increase in SWA may occur with greater daytime executive processing: Increased cognitive activity during the day may lead to an increase in SWA the following night (Tononi and Cirelli, 2006). Thus, with poorer executive function in older age, SWA may be diminished. It is also plausible that SWA and executive function may influence each other in a bidirectional manner.
Prior cross-sectional studies of age-related changes in sleep and executive function, however have not directly measured PFC function during wakefulness (Anderson and Horne, 2003, Wilckens et al., in press, Wilckens et al., 2014, Blackwell et al., 2006, Nebes et al., 2009). Thus, extant studies have yet to address whether PFC function is the underlying mechanism in the link between SWA, age, and executive function. Specifically, there are no studies, to our knowledge, that have examined how age and sleep interact in relation to waking PFC function.
Positron emission tomography (PET) studies have consistently found that SWA is associated with reduced regional metabolism in the PFC, specifically the orbitofrontal cortex (OFC) (Maquet et al., 1997, Braun et al., 1997, Picchioni et al., 2009), and less consistently the dorsolateral prefrontal cortex (DLPFC) (Braun et al., 1997). These studies raise the question of whether reduced PFC metabolism with greater SWA “restores” PFC function and in turn, has daytime functional significance (Muzur et al., 2002). If SWA-related decreases in PFC metabolism during NREM sleep have functional relevance for daytime PFC function, then we would expect to find a corresponding increase in PFC metabolism during wakefulness associated with NREM SWA within overlapping PFC regions.
The present FDG PET study aims to examine how age, SWA, and their interaction relate to PFC metabolism during wakefulness. Consistent with the view that SWA “restores” PFC function (Muzur et al., 2002), we hypothesized that greater SWA would be associated with greater wake PFC metabolism, independently of age. In line with the view that greater SWA may counteract effects of age on PFC function, we hypothesized that SWA would moderate the relationship between age and wake metabolism. We hypothesized that older participants with greater SWA would exhibit higher wake PFC metabolism compared to older participants with less SWA, reflecting similar metabolic rates between young adults and older adults with high SWA. We hypothesized that relationships among SWA and wake PFC metabolism would be evident within both the DLPFC and the OFC, given that these regions have been associated with SWA during NREM sleep (Maquet et al., 1997) and aging (Raz et al., 1997) independently in prior studies. While our primary hypotheses focused on the DLPFC and OFC, we also explored these relationships in the entire brain using a semi-quantitative measure of whole-brain metabolism and at the voxel-level to determine whether hypothesized relationships applied to the whole brain and outside a priori regions of interest (ROI).
METHODS and MATERIALS
Overview
Participants were adults enrolled as good sleeping control participants in three protocols within the Sleep and Chronobiology Lab at the University of Pittsburgh (MH024653, MH061566, W81XWH-08-1-0637). Each of these protocols included overnight in-lab polysomnography (PSG) sleep studies and 18[F]-fluoro-2-deoxy-d-glucose (FDG) PET scans during wakefulness. Participants were compensated for their time and provided informed consent as approved by the University of Pittsburgh Institutional Review Board.
Participants
Participants were all recruited within the community for each of the three protocols. Participants from all three protocols were excluded if they had a current major medical or psychiatric condition, history of substance abuse, or any sleep disorder. All participants included in the present analyses had PSQI scores < 10 and Apnea Hypopnea Index scores < 20. An apnea hypopnea index less than 20 limited the sample to individuals with no greater than mild to moderate obstructive sleep apnea which has been shown to have no significant effects on daytime function including attention and executive function (Quan et al., 2014, Quan et al., 2006). Two of the protocols assessed insomnia severity (MH024653 and W81XWH-08-1-0637) for which all participants had an insomnia severity index < 5. Participants in these two protocols were also excluded if they were taking any medications that affect sleep including antidepressants. Participants in protocol MH061566 were excluded if they were taking any antidepressant, anxiolytic, or antipsychotic medication. All participants in protocol W81XWH-08-1-0637 were veterans, but none had current post traumatic stress disorder (PTSD) as assessed with the Pittsburgh Sleep Quality Index Addendum (PSQI-A) for PTSD-related sleep disturbances (all scores = 0) and the Clinician Administered PTSD scale (Blake et al., 1995).
Fifty-two participants completed wake FDG PET scans with usable data. Reasons for failed scans included presence of more than eight 20-second epochs of Stage 1 sleep within the first 20 minutes of uptake, or one or more epochs of Stage 2, 3, 4 or REM sleep, based on Rechtschaffen and Kales (1968) criteria.
PET and MR study procedures
PET study procedures were originally described in Nofzinger et al. (1998). PET scans were conducted at the University of Pittsburgh PET Facility on a Siemens/CTI ECAT HR+ PET scanner. Intravenous catheters were placed in each arm with normal saline infused at the minimal rate to keep the vein open. One catheter was used to inject the radioligand, and the other was used to collect blood samples needed for the determination of blood glucose and plasma radioactivity concentrations, respectively. Participants were asked to lie supine with eyes closed, and wakefulness was continuously monitored with PSG. After 20 minutes of wakefulness, an intravenous bolus of FDG was injected via one of the indwelling catheters. Participants continued to lie supine during the 20-minute uptake period before being transported to the PET center for a 10–15 minute transmission scan and a 30-minute emission scan beginning at 60 minutes post injection, acquired as six × 300-second frames. Six venous samples were drawn for radioactivity at 45, 55, 65, 75, 85, and 90 min following injection, and plasma glucose was assayed from the first and last samples. These values were used for semi-quantitative whole-brain metabolism estimates (Hunter et al., 1996).
All participants received a structural MR imaging scan prior to the PET study to screen for pathology and to measure brain volume. The scan sequence was a T1 weighted structural scan acquired at the University of Pittsburgh Magnetic Resonance Research Center. All three protocols used the same PET scanner, whereas MR imaging was performed at 1.5T or 3.0T as a result of a scanner upgrade. MR imaging was performed at 1.5T in n=11 using a GE Signa 1.5 Tesla scanner (GE Medical Systems, Milwaukee, WI) or at 3.0 T in n=40 using a Siemens MAGNETOM Trio 3 Tesla Scanner (Siemens Medical Solutions USA, Malverne, PA). The 1.5T structural MR images were acquired at a coronal orientation using 3D SPGR (TR/TE = 5/25 ms; flip angle = 40°; FOV = 24×18cm, 124 slices, slice thickness = 1.5mm, in-plane resolution 0.9375 mm × 0.9375 mm). The 3.0T MR images were acquired at a sagittal orientation using a MPRAGE sequence (TR/TE=2300/2.98 ms, 256 slices, slice thickness 1.2mm, flip angle = 9, in-plane resolution 1 mm × 1 mm).
Normalized FDG PET images were created only for scans with motion less than 2 mm for any frame of the FDG PET scans’ 6 frames. Relative metabolism was assessed in Statistical Parametric Mapping (SPM) version 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) software package as follows: The six FDG PET frames were motion-corrected if necessary and averaged using established methods (Woods et al. 1993) and then co-registered to their corresponding structural AC-PC aligned MR image. The MR images were normalized to the ICBM 152 template (Montreal Neurological Institute) based on tissue priors applied as part of the unified segmentation technique (Ashburner and Friston, 2005) in SPM. The transformation matrix was applied to the FDG PET images which were then smoothed using a 10-mm FWHM Gaussian filter.
Regional cerebral metabolic rate of glucose uptake (rCMRglu) was calculated to examine regional metabolism at the ROI and voxel level. ROI brain masks were constructed based on Automated Anatomical Labeling (AAL) -defined regions in the WFU PickAtlas (Maldjian et al., 2003, Maldjian et al., 2004, Tzourio-Mazoyer et al., 2002). A semi-quantitative index of glucose uptake was used to measure whole brain metabolism (excluding cerebral spinal fluid (CSF) and non-brain tissue). Semi-quantitative whole brain metabolism was estimated using a modified simplified kinetic method based on blood venous samples (Hunter et al., 1996).
To account for known age differences in gray matter volume (Taki et al., 2011), and the fact that gray matter has the highest FDG uptake (Lin and Alavi, 2009), sensitivity analyses were performed including an estimate of whole brain normalized gray matter volume as a covariate derived from the structural MR image for ROI and voxel-wise analyses. Normalized gray matter volume was estimated as the ratio of gray matter to intracranial volume. Intracranial volume was estimated with the entire volume of the brain, CSF, and tissue inside of the subject’s skull. Each unstripped high-resolution image was run through the FSL (Functional MRI of the Brain Software Library) brain extraction tool to generate a binary brain mask. Manually stripped high-resolution images were then used to clean up the mask around the subject’s cerebellum. Finally, the number of voxels in the resulting mask was calculated. Gray matter volume was estimated using FAST (Smith, 2002), which segmented the subject’s high-resolution image into gray matter, white matter, and CSF. Volume estimates were available for all but one participant.
Semi-quantitative whole brain values were partial volume-corrected (Meltzer et al., 1990) for gray matter volume on a pixel-by pixel basis. As a result, FDG PET images with lower gray matter had a larger correction. Thus, age differences in gray matter between participants were corrected for with semi-quantitative whole brain analyses and therefore did not require sensitivity analyses with gray matter volume estimates as a covariate.
PSG data collection and processing
PSG was recorded during an uninterrupted night preceding the PET scan, as well as an initial apnea screen night that served as an adaptation night. EEG was recorded from F3, F4, C3, and C4 electrodes, referenced to A1–A2. Records were visually scored in 20-second epochs using Rechtschaffen and Kales (1968) criteria because the protocols were completed before American Academy of Sleep Medicine (AASM) scoring was adopted. Spectral analysis was performed on EEG channels C3 and C4 with a 512-point fast Fourier transform using epochs scored as NREM sleep (Vasko et al., 1997). Following decimation, the EEG signal was parsed into 0.5 Hz bins over 4-second epochs and weighted by a Hamming window. Four-second epochs identified as movement artifact by an automated algorithm (Brunner et al., 1996) and visual inspection were rejected. Analyses were confined to NREM sleep because this is where the most SWA is observed. Spectral density data from the C4 channel within the 0.5–4 Hz (delta) band were analyzed for the present study to measure SWA. C4 was chosen based on the rationale that sleep scoring was done based on C4. Absolute and relative delta power (SWA) were calculated across the uninterrupted night. We included both absolute and relative measures of SWA (aSWA, rSWA) based on the rationale that greater SWA in absolute terms was hypothesized to relate to cerebral metabolism, and it is uninfluenced by fluctuations of power in higher frequencies. Further, rSWA, which accounts for overall power, ensured that associations involving SWA were not driven by the effect of overall power on metabolism. Relative power was calculated by dividing power within the 0.5 to 4 Hz bandwidth by power within the 0.5 to 32 Hz bandwidth.
Statistical Analyses
A series of multiple regression analyses were performed to test bivariate associations involving age and SWA, as well as SWA × age interactions within ROIs, across the whole brain using the semi-quantitative method and at the voxel level. Separate regression analyses were performed for absolute and relative SWA. ROI analyses with rCMRglu focused on the DLPFC and the OFC because of our a priori hypotheses regarding SWA and regional brain metabolism. Both ROI and voxel-wise analyses were performed in SPM8. Multiple regression analyses testing associations with rCMRglu were performed on all voxels within the ROI mask. Significant clusters were identified within each ROI. ROI analyses used a voxel-level height threshold of p = 0.05 and a cluster-level threshold of p = 0.05 correcting for multiple comparisons at the cluster level via 1000 Monte Carlo simulations conducted using the AlphaSim program within Analysis of Functional Neuroimages (AFNI). Analyses of whole brain metabolism using the semi-quantitative method were performed in SPSS with a significance level of p = 0.05. Exploratory voxel-wise analyses were performed in SPM to identify whether relationships between SWA and cerebral metabolism existed outside of a priori ROIs. For voxel-wise analyses, a voxel-level height threshold of p < 0.05 and a family-wise error (FWE)-corrected cluster threshold of p < .05 were used to determine significance. A more conservative multiple correction approach (FWE rather than Alpha Sim) was used for voxel-wise analyses given the exploratory nature of the voxel-wise analyses. Significant clusters were identified within ROIs and across the brain at the voxel-level.
Sensitivity analyses for ROI and voxel-wise analyses included age and gray matter volume as covariates in separate regressions to determine whether bivariate associations remained significant after accounting for age and gray matter volume. Sensitivity analyses were only performed for significant associations.
Interactions were tested using moderation regression analyses. Moderation analyses allowed us to determine whether older participants with greater SWA had greater wake metabolism (based on a SWA × age interaction). Here we define a moderator (SWA) as an independent variable that interacts with another independent variable (age) in predicting a dependent variable (metabolism), whereby the moderator is characterized statistically in terms of interactions (Cohen et al., 2003). Given the cross-sectional nature of the present study, we emphasize that this analysis does not assess a causal moderating effect. To test interactions in ROIs and at the voxel-level, age and SWA variables were centered and the product of the centered variables was calculated outside of SPM. All three variables were then entered into the multiple regression model in SPM. The interaction term was identified as the variable of interest in the SPM contrast. For semi-quantitative whole brain metabolism analyses in SPSS, age and SWA were centered and entered simultaneously as the first step in a hierarchical regression model. The product of the centered variables was entered as the second step in the regression model. In the case of a significant interaction term, post-hoc bivariate regression analyses were performed separately in the young and older half of the sample using a median split to interpret the interaction.
RESULTS
Sample characteristics and predictor variables
Table 1 displays participant sample characteristics. Table 2 displays relationships among predictor variables.
Table 1.
Participant characteristics
| Entire sample | Range | Younger half | Older half | |
|---|---|---|---|---|
| n | 52 | 26 | 26 | |
| Age (years) | 39.04 (10.02) | 25–61 | 30.75 (4.02) | 47.32 (6.78) |
| n Female (%) | 27 (51.9%) | 10 (38.5%) | 17 (65.4%) | |
| n White/Caucasian (%) | 42 (80.8%) | 19 (73.1%) | 23 (88.5%) | |
| n Current medications reported | 1.5 (2.36) | 0–9 | 0.92 (2.33) | 2.08 (2.28) |
| PSQI | 2.4 (1.61) | 0–9 | 2.4 (1.47) | 2.35 (1.77) |
| AHI | 3.63 (3.97) | 0–19.53 | 2.61 (2.79) | 4.61 (4.69) |
| Total sleep time (min) | 402.04 (47.07) | 279–512.33 | 410.76 (55.77) | 393.33 (35.39) |
| WASO (min) | 33.03 (32.18) | 5–160.33 | 28.17 (33.02) | 37.90 (31.20) |
| aSWA (μV2 Hz) | 25.90 (13.05) | 1.5–56.51 | 29.42 (12.47) | 22.38 (12.88) |
| rSWA (μV2 Hz) | 0.81 (0.07) | 0.65–0.90 | 0.83 (0.06) | 0.78 (0.63) |
| Semi-quantitative whole brain metabolism (μmol−1100 mL−1 min−1) | 13.40 (1.77) | 10.05–17.42 | 13.83 (1.60) | 12.94 (1.86) |
| n = 47 | n = 24 | n = 23 | ||
| n Stage 1 epochs during uptake period | 1.03 (1.87) | 0–8 |
AHI, apnea–hypopnea index; aSWA, absolute slow wave activity; rSWA, relative slow wave activity; WASO, wake time after sleep onset; PSQI, Pittsburgh Sleep Quality Index. Unless specified otherwise, values represent means and standard deviations.
Table 2.
Correlation matrix of associations among predictors
| Age | Grey matter volume | aSWA | rSWA | |
|---|---|---|---|---|
| Age | – | −0.63*** | −0.22 | −0.47* |
| Grey matter/ICV | – | 0.19 | 0.32* | |
| aSWA | – | 0.59*** |
aSWA, absolute slow wave activity; rSWA, relative slow wave activity; ICV, intracranial volume.
P < 0.05;
P < 0.001.
Age and wake metabolism
Results for semi-quantitative whole brain metabolism are presented in Table 3. Results for age and rCMRglu in ROIs are presented in Table 4. Results for exploratory voxel-wise analyses are presented in Table 5.
Table 3.
Hierarchical regression table for predictors of semi-quantitative whole brain metabolism. Note that bivariate association P-values for R2Δ and b are equivalent expressions
| Predictor | R2D | F | P | B (SE) | b | t | P | |
|---|---|---|---|---|---|---|---|---|
| Bivariate associations | Age | 0.10 | 5.22 | 0.03 | −0.06 (0.02) | −0.32 | −2.28 | 0.03 |
| aSWA | 0.03 | 1.18 | 0.28 | 0.02 (0.02) | 0.16 | 1.08 | 0.28 | |
| rSWA | 0.10 | 4.84 | 0.03 | 8.20 (3.73) | 0.31 | 2.20 | 0.03 | |
| Moderation models | Age | 0.11 | 2.80 | 0.07 | −0.52 (0.03) | −0.30 | −2.08 | 0.04 |
| aSWA | 0.01 (0.02) | 0.10 | 0.66 | 0.51 | ||||
| Age 3 aSWA | 0.12 | 6.89 | 0.01 | 0.01 (0.002) | 0.36 | 2.63 | 0.01 | |
| Age | 0.14 | 3.42 | 0.04 | −0.38 (0.03) | −0.22 | −1.38 | 0.18 | |
| rSWA | 5.30 (4.3) | 0.20 | 1.25 | 0.22 | ||||
| Age 3 rSWA | 0.13 | 7.25 | 0.01 | 0.86 (0.32) | 0.36 | 2.69 | 0.01 |
aSWA, absolute slow wave activity; rSWA, relative slow wave activity; SE, standard error.
Significant relationships are highlighted in bold font.
Table 4.
Clusters within regions of interest (ROIs) showing age and slow wave activity (SWA) associations with regional cerebral metabolic rate of glucose uptake (rCMRglu)
| Predictor | ROI | Peak MNI coordinates (x,y,z) | Max cluster size | R2D | t | Z | df | P |
|---|---|---|---|---|---|---|---|---|
| Age | DLPFC | 30, 8, 60 | 849 | 0.17 | 3.67 | 3.43 | 1, 50 | 0.06 |
| OFC | −34, 18, −14 | 390 | 0.12 | 2.68 | 2.58 | 1, 50 | 0.23 | |
| aSWA | DLPFC | 32, 10, 58 | 37 | 0.07 | 1.93 | 1.89 | 1, 50 | 0.91 |
| OFC | – | – | – | – | – | – | – | |
| rSWA | DLPFC | −34, 14, 34 | 990 | 0.17 | 4.56 | 4.15 | 1, 50 | 0.03† |
| OFC | −38, 42, −8 | 152 | 0.10 | 2.58 | 2.49 | 1, 50 | 0.54 | |
| Age 9 aSWA | DLPFC | −32, 26, 26 | 439 | 0.03 | 3.83 | 3.56 | 1, 48 | 0.27 |
| OFC | −32, 32, −10 | 555 | 0.03 | 3.54 | 3.32 | 1, 48 | 0.11 | |
| Age 9 rSWA | DLPFC | −28, 50, 8 | 125 | 0.09 | 2.09 | 2.04 | 1, 48 | 0.74 |
| OFC | −34, 32, −8 | 185 | 0.11 | 2.78 | 2.66 | 1, 48 | 0.47 |
aSWA, absolute slow wave activity; rSWA, relative slow wave activity; DLPFC, dorsolateral prefrontal cortex; OFC, orbitofrontal cortex.
Significant relationships are highlighted in bold font.
significance after controlling for grey matter volume; –: no suprathreshold clusters. MNI coordinates and t-values represent peak-level statistics. Cluster size and P-values represent Alpha Sim-corrected cluster-level statistics.
Table 5.
Clusters showing bivariate associations of age and slow wave activity (SWA) with regional cerebral metabolic rate of glucose uptake (rCMRglu)
| Predictor | Hemisphere | Significant cluster regions | Peak MNI coordinates | Max cluster size | R2D | t | Z | df | P |
|---|---|---|---|---|---|---|---|---|---|
| Age (negative) | Right | Inferior frontal | 54, 16, 26 | 11 295 | 0.22 | −4.12 | −3.81 | 1, 50 | 0.009† |
| Right | Superior frontal | 30, 6, 62 | |||||||
| Left | Supplementary motor area | 0, 6, 54 | |||||||
| Age (positive) | Bilateral | Cerebellum | 8, −40, −24 | 27 031 | 0.32 | 4.30 | 3.95 | 1, 50 | <0.001 |
| Bilateral | Parietal white matter | −30, −32, 34 | |||||||
| aSWA | −6, −6, 74 | 3283 | 0.17 | 3.14 | 2.99 | 1, 50 | 0.59 | ||
| rSWA (positive) | Left | Inferior frontal | −34, 14, 32 | 10 598 | 0.20 | 4.81 | 4.34 | 1, 50 | 0.013† |
| Bilateral | Superior frontal | 8, 28, 52 | |||||||
| Bilateral | Precentral | −36, 0, 54 | |||||||
| Age 9 aSWA | Left | Middle temporal gyrus | −38, −64, 18 | 32 106 | 0.31 | 4.68 | 4.23 | 1, 48 | <0.001*,† |
| Bilateral | Posterior cingulate/BA 31 | 10, −28, 44 | |||||||
| Right | Medial frontal gyrus | 14, 52, −2 | |||||||
| Age 9 rSWA | −24, −54, 56 | 1026 | 0.13 | 2.66 | 2.56 | 1, 48 | 0.998 |
aSWA, absolute SWA; rSWA, relative SWA.
Significance after controlling for age.
significance after controlling for grey matter volume. MNI coordinates and t-values represent peak-level statistics. Cluster size and P-values represent family-wise error (few)-corrected cluster-level statistics.
Age and semi-quantitative whole brain metabolism were negatively correlated. There was a marginally significant negative correlation between age and wake metabolism in the DLPFC. The association between age and wake metabolism in the OFC did not reach significance.
Exploratory voxel-wise analyses revealed that age was negatively associated with rCMRglu in a frontal lobe cluster (Figure 1) that overlapped with the DLPFC ROI, primarily encompassing the right superior frontal gyrus (BA 6, 9, and 10), right superior middle and medial frontal gyri, right inferior frontal gyrus (orbital and frontal operculum), and left supplementary motor area. This relationship remained significant after controlling for gray matter volume, cluster size = 29492, t(1,48) = 4.79, z = 4.31, FWE p <0.001. There was also a positive correlation between age and rCMRglu in the cerebellum and parietal lobe white matter, but this relationship did not approach significance after accounting for gray matter volume (max cluster size = 429, t(1, 48) = 3.79, z = 3.53, FWE p = 1.0).
Figure 1.
Heat map of t values for the negative (left) and positive (right) association between age and rCMRglu at the voxel level. Crosshairs indicate peak voxel on a canonical single subject T1 image. Greater age was associated with lower metabolism in the superior frontal cortex, and higher metabolism in the cerebellum.
SWA and wake metabolism
Results for SWA and wake semi-quantitative whole brain metabolism are presented in Table 3 Results for SWA and wake rCMRglu in ROIs are presented in Table 4, and results from exploratory voxel-wise analyses are presented in Table 5.
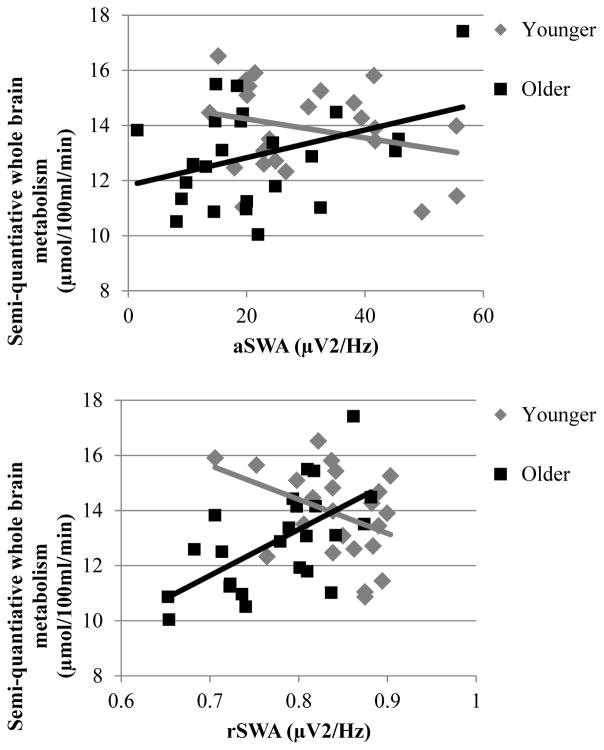
Bivariate relationships between SWA and semi-quantitative whole brain metabolism did not reach significance. However, the negative correlation between age and semi-quantitative whole brain metabolism was significantly moderated by SWA. In a follow-up analysis using a median split (median age = 36.95) to determine what drove this interaction, we found that, among the older half of the sample, greater SWA was associated with greater semi-quantitative whole brain metabolism, (aSWA: r = 0.37, p = 0.08; rSWA: r = 0.61, p = 0.002). This relationship was in the negative direction and did not reach significance in the younger half of the sample, (aSWA: r = −0.27, p = 0.21; rSWA: r = −0.39, p = 0.06). Figure 2 illustrates the SWA × age interaction with semi-quantitative whole brain metabolism, revealing that associations with age were diminished at high levels of SWA. Scatter plots of relationships among SWA, age, and semi-quantitative whole brain metabolism are presented as supplementary material.
Figure 2.
Semi-quantitative whole brain metabolism as a function of aSWA (upper panel) and rSWA (lower panel) in the younger and older half of participants. The older half of participants with higher SWA exhibited higher semi-quantitative whole brain metabolism.
Wake rCMRglu in the DLPFC was positively associated with rSWA (Figure 3), but was not associated with aSWA. Sensitivity analyses revealed that the significant relationship between rSWA and the DLPFC cluster remained significant after accounting for gray matter volume (max cluster size = 1128, t(1, 48) = 4.08, z = 3.76, Alpha Sim p = 0.01), but not age (max cluster size = 536, t(1, 49) = 3.84, z = 3.58, Alpha Sim p = 0.20). No significant associations were found between SWA and metabolism for the OFC. SWA was not a significant moderator in the relationship between age and rCMRglu in the DLPFC or OFC.
Figure 3.
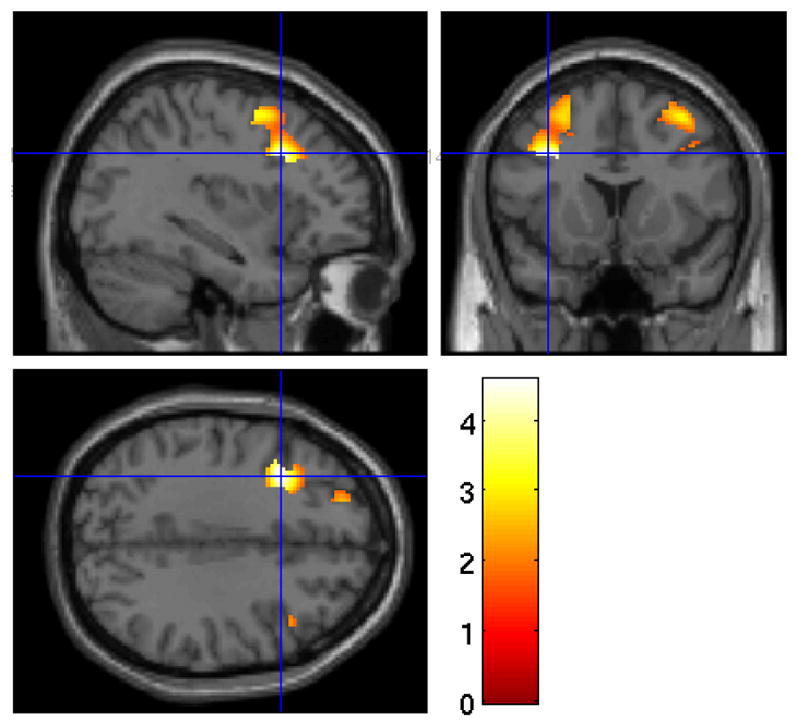
Heat map of t values for the association between rSWA and DLPFC rCMRglu. Crosshairs indicate peak voxel on a canonical single subject T1 image. Greater DLPFC metabolism was associated with greater rSWA.
Exploratory voxel-wise analyses revealed that greater rSWA was associated with greater rCMRglu in a cluster primarily encompassing the right superior frontal cortex overlapping with the DLPFC ROI (BA6 to BA8, including medial and lateral aspects of the superior frontal cortex), as well as the inferior frontal gyrus, and the precentral gyrus (Figure 4). This relationship with rSWA remained significant in this cluster after accounting for gray matter volume (cluster size = 14399, t(1,48) = 4.29, z = 3.93, FWE p = 0.002) and was marginally significant after controlling for age (cluster size = 6942, t(1,49) = 4.13, z = 3.80, FWE p = 0.08).
Figure 4.
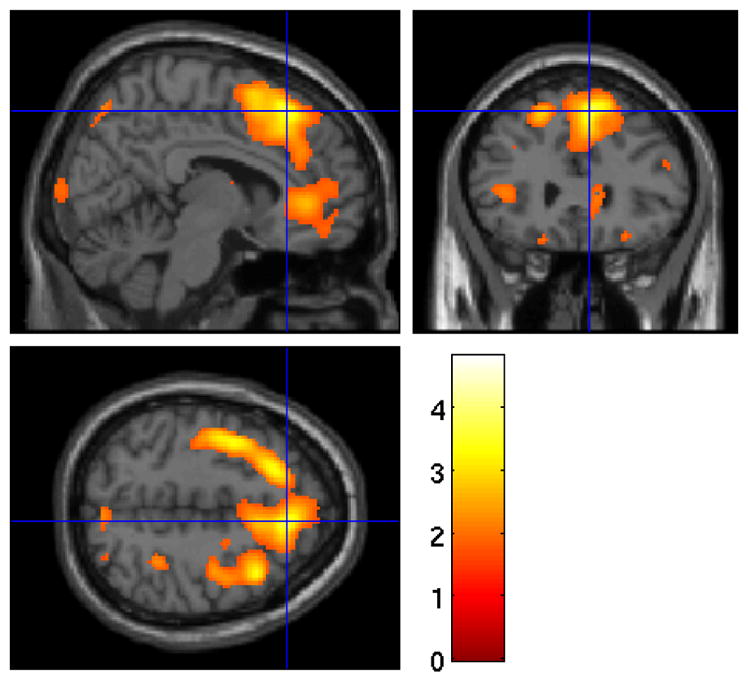
Heat map of t values for the association between rSWA and rCMRglu at the voxel level. Crosshairs indicate peak voxel on a canonical single subject T1 image. Greater metabolism in the right superior frontal gyrus was associated with greater rSWA.
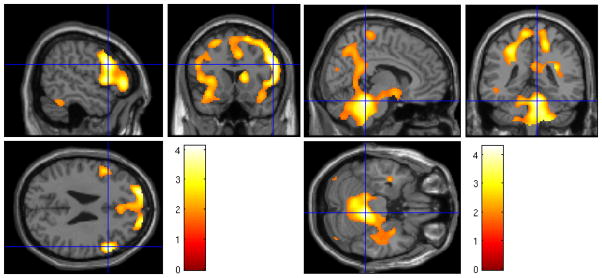
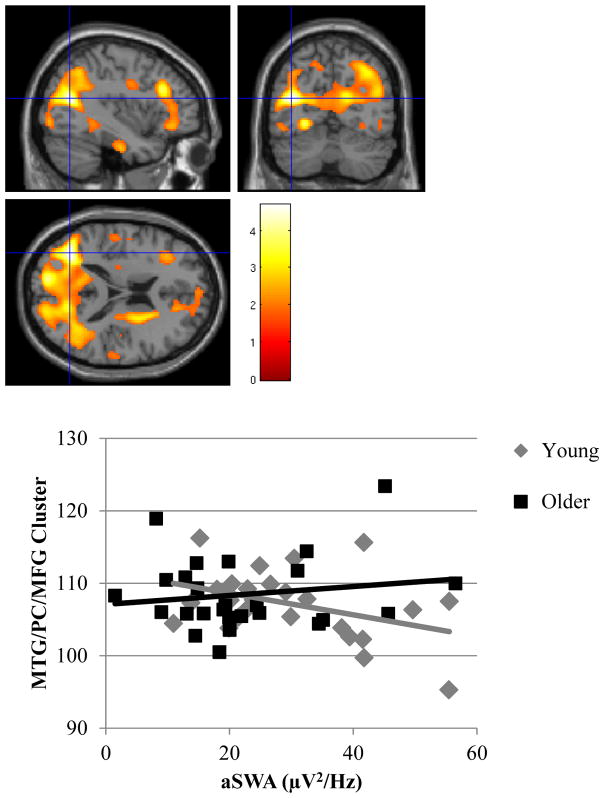
There was a significant aSWA × age interaction in a large cluster primarily encompassing the posterior cingulate cortex (BA 31), peaking within the middle temporal gyrus, and extending to the medial frontal gyrus (Figure 5). The interaction remained significant in this cluster after controlling for gray matter volume (cluster size = 48477, t(1,46) = 6.12, z = 5.21, FWE p < 0.001). In a follow-up analysis using a median split (median age = 37.58) to determine what drove this interaction, we found that among the younger half of the sample, greater SWA was associated with lower metabolism within this cluster (r = -0.40, p = 0.04). This relationship was in the positive direction and not significant for the older half of the sample (r = 0.16, p = 0.45) (Figure 5). Scatter plots of relationships among aSWA, age, and rCMRglu in this cluster are presented as supplementary material.
Figure 5.
Upper panel: Heat map of t values for the interaction between aSWA and age for rCMRglu at the voxel level in the middle temporal gyrus, posterior cingulate, and medial frontal gyrus. Crosshairs indicate peak voxel on a canonical single subject T1 image. Lower Panel: Extracted values for rCMRglu in the significant middle temporal gyrus/posterior cingulate/medial frontal (MTG/PC/MFG) cluster as a function of aSWA in the younger and older half of the sample. The younger half of participants with higher aSWA exhibited lower rCMRglu in this cluster.
Summary of Results
Greater age was associated with lower semi-quantitative whole brain metabolism and superior frontal rCMRglu. Greater SWA was associated greater rCMRglu in the DLPFC and superior frontal cortex. SWA moderated relationships between age and semi-quantitative whole brain metabolism and rCMRglu in posterior regions outside of a priori ROIs.
DISCUSSION
Sleep and brain function change with aging. The current findings demonstrate that SWA and age interact in their relationship with cerebral metabolism across young and middle adulthood. We tested whether greater age and SWA were associated with cerebral metabolism during wakefulness in dorsolateral and orbitofrontal regions of the PFC. We found that age and SWA were associated with wake metabolism in the DLPFC, but not the OFC. This finding partially confirms our hypotheses that age and SWA are associated with higher wake metabolism in the PFC. Given that OFC metabolism was not associated with greater SWA, and a decrease in OFC metabolism is commonly found during NREM sleep (Braun et al., 1997, Maquet et al., 1997), the present pattern of results does not necessarily suggest a “restorative” model whereby greater SWA during NREM sleep leads to greater PFC metabolism during wakefulness. Nonetheless, these results do not rule out the possibility for restorative value of SWA to the DLPFC.
The association between SWA and DLPFC wake metabolism remained significant independent of gray matter volume, but not age, in line with the high correlation between these factors. However, at the voxel-level, the relationship between SWA and rCMRglu in the superior frontal gyrus was relatively unaffected when controlling for age. These findings suggest a potential role of SWA in daytime frontal cortex function that is independent of age, which may have implications for dependent cognitive functions. An important next step is to determine whether the SWA-related reduction in PFC metabolism is related to or impacts daytime PFC functions, such as inhibitory control.
Interactions between SWA and Age
Age and SWA did not significantly interact in relation to DLPFC or OFC metabolism. However, we found an SWA × age interaction using the whole-brain semi-quantitative method: SWA moderated the association between age and metabolism such that older participants with greater SWA exhibited greater whole brain metabolism. The younger half of the sample exhibited a non-significant negative relationship with SWA. This interaction suggests that SWA may counteract effects of age on cerebral metabolism rate. This view is consistent with our previous findings (Wilckens et al., 2014) demonstrating that task-switching abilities were similar between young and older adults with high sleep continuity. Nonetheless, it should be emphasized that this interaction reflects statistical moderation and does not imply causation.
The semi-quantitative whole brain metabolism interaction should be interpreted in the context of the similar interaction found with exploratory voxel-wise analyses. A significant aSWA × age interaction was found in regions posterior to a priori ROIs. The cluster encompassed the posterior cingulate cortex and middle temporal gyrus and extended to the medial frontal gyrus- regions associated with the default mode network (Buckner et al., 2008). While this interaction was driven by a similar pattern to that found using semi-quantitative whole brain metabolism (a positive relationship with SWA in the older half and a negative relationship in the younger half), the relationship at the voxel-level was only significant in the younger half of the sample. Given that the FDG PET scan occurred at rest, it is possible that this reflects a significant association between greater SWA and lower metabolism within the default mode network, a network that is associated with executive function abilities (Hampson et al., 2006, Andrews-Hanna et al., 2007). This negative relationship could reflect that the greater reliance on posterior brain regions commonly found in older age (Davis et al., 2008) also occurs in younger adults with less SWA. Alternatively, greater SWA in young adults may arise from voluntary sleep restriction. Indeed, sleep deprivation has been shown to disrupt default mode network function (De Havas et al., 2012) and task-related function of the cingulate cortex in young adults (Tomasi et al., 2009). We cannot adjudicate between these and other speculations based on the present results and it is important to interpret this result cautiously, as it was not based on a priori hypotheses. This will be an important avenue of future investigation.
Limitations and Future Directions
The present study has several limitations. We assumed in our interpretation that greater NREM SWA had an effect on daytime wake metabolism. However, waking PFC function may also affect SWA production (Mander et al., 2013, Nicholas et al., 2002, Mander et al., 2015). Thus in the present study we cannot infer directionality. Future studies aimed at increasing SWA, especially in older individuals, may shed light on whether SWA does indeed have an impact on PFC function during wakefulness. Given that SWA tends to be stable night to night within an individual (Israel et al., 2012), our results may reflect chronic as opposed to acute relationships between SWA and cerebral metabolism. Nonetheless, SWA is known to change in the long term with age (Carrier et al., 2001), thus, changes in SWA within an individual could conceivably have an impact on PFC function in the long-term.
This study used data from three different protocols, contributing to heterogeneity of the sample. Nonetheless, these studies were similar in that participants were all good sleeping controls with similar exclusion criteria and were all collected in the same sleep and PET imaging laboratories. Additionally, the larger heterogeneous sample has the advantage of increasing the study’s generalizability.
Although there are benefits to examining wake cerebral metabolism during rest (the scan is unbiased by the task performed), our results may have been further elucidated had participants engaged in a cognitive task and had we examined EEG activity during the FDG PET scan. For instance, in the age × aSWA interaction, we do not know whether lower posterior cingulate metabolism with greater SWA reflects more efficient neural processing, a compensatory process, or greater default mode metabolism during rest. Future studies incorporating cognitive tasks and wake EEG during a wake FDG PET scan will further elucidate the behavioral and cognitive relevance of SWA to cerebral metabolism. Additionally, a focus on the PFC in relation to SWA and executive function may be too simplistic of a model (Alvarez and Emory, 2006). Rather, the role SWA in the broader executive control network (Shirer et al., 2012) and the default mode network should be an area of future investigation.
Finally, a substantial literature demonstrates effects of aging on medial temporal lobe structure and function (Raz et al., 2004), and a rapidly expanding literature suggests an important role of sleep in medial temporal lobe function and memory (Walker and Stickgold, 2006). This study was not focused on the medial temporal lobes and exploratory voxel-wise analyses did not show significant relationships with the medial temporal lobes. Nevertheless, whether greater SWA is associated with greater medial temporal lobe function during wakefulness is an important question for future research.
Conclusion
SWA is associated with daytime cerebral metabolism across young and middle adulthood. SWA is associated with higher DLPFC metabolism and interacts with age in its relationship with whole brain metabolism, particularly in posterior brain regions. Identifying whether age-related changes in sleep have a direct effect on PFC function may elucidate key targets for mitigating cognitive decline in older adulthood.
Supplementary Material
Acknowledgments
MH024653, HL082610 (Buysse); MH019986 (Reynolds); DA032557 (Hasler); MH061566 (Siegle); W81XWH-08-1-0637 (Germain)
Footnotes
Author contributions:
Study design: Buysse, Nofzinger, Franzen, Germain, Hall, Kupfer
Conducted the study: Buysse, Nofzinger, Germain, Siegle, Franzen, Price
Data processing and analysis: Wilckens, James, Aizenstein, Hasler, Rosario-Rivera, Price
Wrote and provided critical feedback on the paper: Wilckens, Aizenstein, Nofzinger, James, Hasler, Rosario-Rivera, Franzen, Germain, Hall, Kupfer, Price, Siegle, Buysse
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40:349–57. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Brunner D, Vasko R, Detka C, Monahan J, Reynolds C, III, Kupfer D. Muscle artifacts in the sleep EEG: Automated detection and effect on all-night EEG power spectra. J sleep res. 1996;5:155–64. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- Cohen JPC, West SG, SAL . Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3 Lawrence Erlbaum Associates, Publishers; Mahwah, NJ: 2003. [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior–anterior shift in aging. Cereb cortex. 2008;18:1201–09. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults--a model for healthy aging? Sleep. 2000;23:1067–73. [PubMed] [Google Scholar]
- Hunter GJ, Hamberg LM, Alpert NM, Choi NC, Fischman AJ. Simplified measurement of deoxyglucose utilization rate. J Nucl Med. 1996;37:950–54. [PubMed] [Google Scholar]
- Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012;35:1285. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbély AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- Lin EC, Alavi A. Normal Variants and Benign Findings. In: LIN EC, ALAVI A, editors. PET and PET/CT: A Clinical Guide. Thieme Medical Publishers; New York, NY: 2009. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–55. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–39. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mander BA, Marks SM, Vogel JW, et al. [beta]-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015 doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Degueldre C, Delfiore G, et al. Functional neuroanatomy of human slow wave sleep. J Neurosci. 1997;17:2807–12. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–70. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Jr, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J comput assist tomog. 1990;14:561–70. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–81. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–87. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. J sleep res. 2002;11:247–53. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Mintun MA, Price J, et al. A method for the assessment of the functional neuroanatomy of human sleep using FDG PET. Brain Res Protoc. 1998;2:191–98. doi: 10.1016/s1385-299x(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Picchioni D, Duyn JH, Horovitz SG. Sleep and the functional connectome. NeuroImage. 2013;80:387–96. doi: 10.1016/j.neuroimage.2013.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchioni D, Killgore WD, Balkin TJ, Braun AR. Positron emission tomography correlates of visually-scored electroencephalographic waveforms during non-Rapid Eye Movement sleep. Int J Neurosci. 2009;119:2074–99. doi: 10.1080/00207450903139770. [DOI] [PubMed] [Google Scholar]
- Quan SF, Budhiraja R, Batool-Anwar S, et al. Lack of impact of mild obstructive sleep apnea on sleepiness, mood and quality of life. Southwest J Pulm Crit Care. 2014;9:44. doi: 10.13175/swjpcc082-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea–hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue K, Head D, Kennedy K, Acker J. Differential aging of the medial temporal lobe a study of a five-year change. Neurology. 2004;62:433–38. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- Shirer W, Ryali S, Rykhlevskaia E, Menon V, Greicius M. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb cortex. 2012;22:158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum brain mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–98. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, et al. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLos one. 2011;6:e22734. doi: 10.1371/journal.pone.0022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry JR, Anderson C, Horne JA. Nonlinear analysis of EEG during NREM sleep reveals changes in functional connectivity due to natural aging. Human brain mapping. 2004;23:73–84. doi: 10.1002/hbm.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Telang F, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb cortex. 2009;19:233–40. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Vasko RC, Brunner DP, Monahan JP, et al. Power spectral analysis of EEG in a multiple-bedroom, multiple-polygraph sleep laboratory. Int J Med Inform. 1997;46:175–84. doi: 10.1016/s1386-5056(97)00064-6. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neurosci Biobehav Rev. 2002;26:849–57. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: the role of the PFC and sleep. Neural plast. 2012;2012 doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Nebes R, Hall M, Monk TH, Buysse DJ. Changes in Cognitive Performance are Associated with Changes in Sleep in Older Adults with Insomnia. Behav Sleep Med. doi: 10.1080/15402002.2014.1002034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Erickson KI, Wheeler ME. Sleep continuity and total sleep time are associated with task-switching and preparation in young and older adults. J sleep res. 2014;23:508–16. doi: 10.1111/jsr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.