SUMMARY
NG2-expressing glia (NG2 glia) are a uniformly distributed and mitotically active pool of cells in the central nervous system (CNS). In addition to serving as progenitors of myelinating oligodendrocytes, NG2 glia might also fulfill physiological roles in CNS homeostasis, although the mechanistic nature of such roles remains unclear. Here, we report that ablation of NG2 glia in the prefrontal cortex (PFC) of the adult brain causes deficits in excitatory glutamatergic neurotransmission and astrocytic extracellular glutamate uptake and induces depressive-like behaviors in mice. We show in parallel that chronic social stress causes NG2 glia density to decrease in areas critical to Major Depressive Disorder (MDD) pathophysiology at the time of symptom emergence in stress-susceptible mice. Finally, we demonstrate that loss of NG2 glial secretion of fibroblast growth factor 2 (FGF2) suffices to induce the same behavioral deficits. Our findings outline a pathway and role for NG2 glia in CNS homeostasis and mood disorders.
INTRODUCTION
NG2 glia are precursors for myelinating oligodendrocytes (OLs) and constitute a lineage distinct from astrocytes and microglia (Nishiyama et al., 1999). In addition, however, many lines of evidence now suggest that NG2 glia might play regulatory roles in maintaining CNS health in the adult brain, including that they are ubiquitously distributed throughout the CNS, even in regions of sparse myelination (Dawson et al., 2003), and that this distribution is tightly self-regulated (Birey and Aguirre, 2015; Hughes et al., 2013). NG2 glia also represent the most actively cycling cell population in the adult CNS (Nishiyama et al., 2009) and swiftly respond to insults, such as loss of spinal cord motor neurons in an amyotrophic lateral sclerosis (ALS) mouse model and stab-wound injuries by increasing proliferation rates and migrating to the site of insult (Hampton et al., 2004; Kang et al., 2010). Moreover, NG2 glia respond to the alterations in neurotransmission that follow deprivation of sensory experience in the developing brain (Hill et al., 2014; Mangin et al., 2012). Together, these studies point to potential roles for NG2 glia in adult brain physiology and possibly in non-myelin-related pathologies. The precise nature of such functionality has remained elusive.
The incomplete understanding of MDD pathophysiology and associated failures in developing effective therapeutics are largely attributed to the lack of robustly-defined underlying mechanisms (Sullivan et al., 2000; Tennant, 2002), which are likely to involve an interplay of multiple genetic and environmental factors encompassing many cell types (Nestler and Hyman, 2010; Petrik et al., 2012). Governing views on MDD etiology propose that in response to environmental stimuli such as stress, the normally optimized activities of specific brain circuits are altered as a result of maladaptive molecular and cellular changes (Mayberg, 2003; Ressler and Mayberg, 2007). Many studies have directly linked stress signals to neuronal malfunction, but there have been only a few investigations into the role of glia in this process. Glial cell loss, along with glutamate-related deficits, has been correlated with MDD pathophysiology. Reduced astrocyte (GFAP+) cell numbers have been shown both in animal models of depression and in postmortem analysis of MDD patients (Banasr and Duman, 2008; Ongür et al., 1998). Nonetheless, so far, there have been no studies that have defined the precise mechanistic roles of discrete glial subtypes and their molecular, cellular, and physiological correlates, either in rodent models or in postmortem patient samples of MDD. (Schroeter et al., 2010).
Here, we report a role for NG2 glia in the maintenance of CNS physiology. We show that pharmacologically induced loss of NG2 glia in the prefrontal cortex (PFC) is followed by astrocytic and neuronal glutamate abnormalities that have previously been reported in MDD and its rodent models (Banasr et al., 2010; Yuen et al., 2012) and, in this setting, lead to the acquisition of depression-like behaviors. We also show that chronic social stress decreases NG2 glial proliferation, and cell number. Finally, we show that the ensuing decrease in production of NG2 glia-derived factors (i.e., FGF2) during chronic stress may underlie the glutamate abnormalities and resulting maladaptive behavioral phenotype.
RESULTS
Validation of the NG2CRE/iDTR Mouse Line for Exploring Consequences of NG2 Glial Ablation
To directly explore potential roles for NG2 glia in adult CNS physiology, we developed a transgenic line in which NG2 glia can be transiently depleted (“NG2CRE/iDTR” mice; “iDTR”; Birey and Aguirre, 2015). In this mouse line, the diphtheria toxin (DT) receptor (DTR; Rosa26-stop-DTR) is under the control of the NG2 promoter (Buch et al., 2005), rendering NG2 glia susceptible to death when DT is administered. In the iDTR mice with a ROSA26-stop-YFP reporter cassette, we did not detect any YFP+ neurons (NeuN+ cells) or astrocytes (GFAP+ or GS+ cells) in the prefrontal cortex (PFC); rather, virtually all of the YFP+ cells were NG2 glia (YFP+ NG2+ or YFP+ Olig2+ cells), with small numbers of mature OLs (YFP+CC1+ cells, which are generated from NG2 glia), and a small percentage of NG2+ pericytes (SMA+) (see Figure S1A available online). YFP+ expression in the striatum, which was previously reported to have high NG2 glial-derived astrocyte output in development (Zhu et al., 2011), similarly showed no coexpression with astrocyte-lineage markers (Figure S1B). To rule out the presence of DTR-expressing astrocytes, we prepared primary cortical cultures from PFC obtained from the iDTR mice (Figure S1C). The cells were treated once daily with DT (50 ng/ml) for 7 days, following which GFAP expression levels and GFAP+ cell numbers were analyzed. No differences were discernable between control (NG2CRE only: “CTRL”) and iDTR cells, confirming that the astrocytes in the adult iDTR brain do not express DTR and are therefore not susceptible to DT (Figures S1D and S1E).
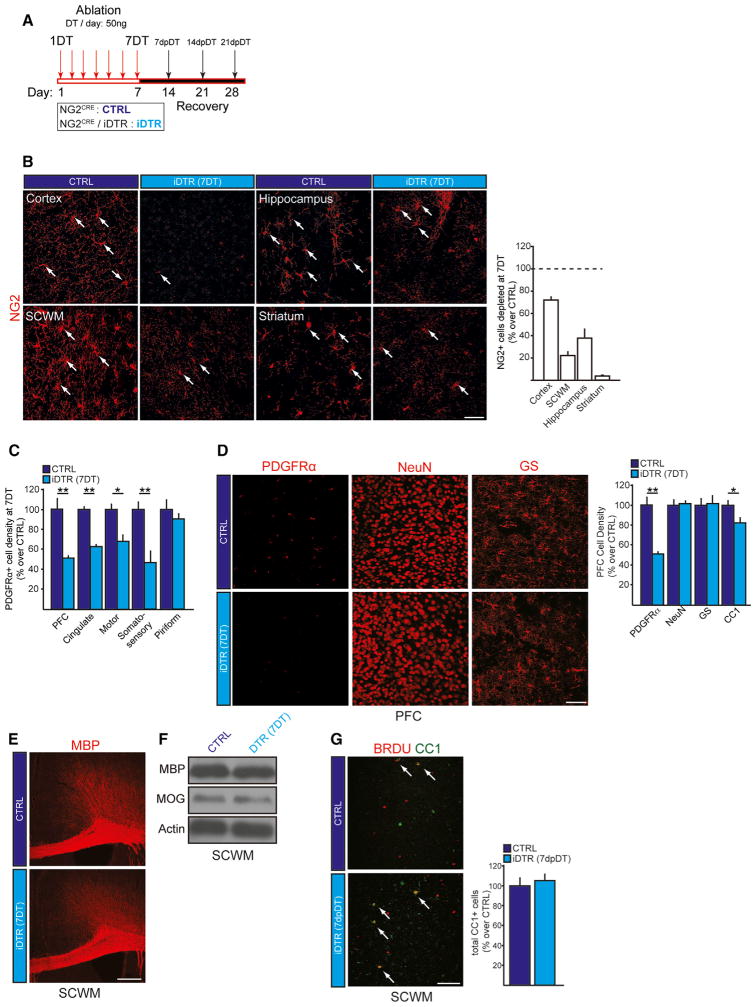
For the ablation experiments, we adapted a previously established DT administration paradigm (Buch et al., 2005): DT (50 ng) was injected intraperitoneally (i.p.) once daily into the adult mice (postnatal day 60; P60) for 7 days (each injection day termed “1–7DT”; Figure 1A). Using this approach, we achieved a reduction in NG2 glial density of 60%–75% in the cerebral cortex and 25%–40% in the hippocampus and subcortical white matter (SCWM) at 7DT (Figure 1B). Most of the cortical regions were equally affected (Figure 1C). As expected, the DT administration did not affect astrocytic or neuronal densities (Figure 1D). We detected a small reduction of mature OL numbers (CC1+ cells); however, this reduction did not lead to demyelination at 7DT (Figures 1E and F). Furthermore, lineage tracing analysis using a long-term 5-bromo-2′-deoxyuridine (BrdU) administration protocol (Aguirre and Gallo, 2007) demonstrated that the number of mature OLs in the PFC (BrdU+ CC1+ cells) returned to normal levels at 5–7 days post DT (dpDT) (Figure 1G). In summary, these findings validated NG2CRE/iDTR as a useful tool to specifically and robustly ablate NG2 glia in the adult brain without affecting other neural cell types.
Figure 1. NG2CRE/iDTR, a Model of NG2 Glial Cell Ablation.
(A) Paradigm used for NG2 glial cell ablation in the iDTR adult mouse brain using systemic Diphtheria Toxin (DT) administration. DT (50 ng/ul) was injected once daily for 7 days; days post DT (dpDT).
(B) Representative images and quantification of depletion efficiencies in different brain regions including cortex, hippocampus, subcortical white matter and striatum. Arrows, NG2+ glial cells.
(C) NG2 glial cell numbers in different cortical regions of control and iDTR mice at 7DT.
(D) NG2 glia (PDGFRα+), neuron (NeuN+), astrocyte (glutamine synthase; GS+), and mature oligodendrocyte (CC1+) numbers in the PFC of control and iDTR at 7DT.
(E) Representative confocal images of MBP immunostaining from control and iDTR mice at 7DT.
(F) Protein extracts from the PFC were used to analyze total expression levels of MBP and MOG at 7DT.
(G) The number of mature oligodendrocytes is rapidly replenished during the DT paradigms as assessed by the newly formed mature oligodendrocytes (BrdU+CC1+ cells) at 5 days after 7DT (5dpDT). Error bars, mean ± SEM (*p < 0.05, **p < 0.01; t-test) n = 5–7 per group. Scale bars, 40 μm.
See also Figures S1–S3.
NG2 Glia Ablation Does Not Affect Brain Vasculature or Induce Major Inflammation
NG2 is also expressed in pericytes in the adult CNS (Ozerdem et al., 2001), which are essential for the maintenance of vasculature cytoarchitecture and the blood-brain barrier (BBB) (Daneman et al., 2010). A small subset of the YFP+ cells were found to express the pericyte marker SMA (YFP+ SMA+ cells) in the cortices of NG2CRE/iDTR/YFP mice (Figure S2A). DT administration might therefore kill these cells along with the NG2 glia, adversely affecting brain vasculature and/or the BBB. However, the characterization with anti-SMA, anti-laminin, and anti-PE-CAM antibodies to assess the vascular cellular composition demonstrated that there were no apparent differences between the CTRL and the iDTR mice at 7DT or any other timepoint (Figures S2A and S2B). We next performed tail-vein injections of FITC-conjugated dextran (40 kDa) at 7DT to quantitatively assess the cortical vascular network (McCaslin et al., 2011), and found that there were no differences in the vasculature area coverage, total vasculature length per area and number of branchpoints in the PFC of iDTR mice at 7DT (Figures S2C and S2D). In addition, NG2 immunostaining of dextran-injected brains indicated that while NG2 glia (NG2+ glia FITC-Dextranneg cells; arrows) were absent in the PFC at 7DT, while NG2+ pericytes enwrapped around the vasculature (NG2+ FITC-Dextran+ cells; arrowheads) were preserved at 7DT (Figure S2E). Finally, we used the dye Evans blue to examine BBB integrity. No traces of the dye were detected in the brain parenchyma of either the CTRL or iDTR mice at 7DT (Figure S2F).
We anticipated that we would observe microglial activation in response to the significant NG2 glial cell loss, since one of the roles microglia undertake is to clear cell debris. Microglial (Iba1+ cells) morphological changes that were detected in the PFC of iDTR mice at 7DT returned to resting levels at 7dpDT (Figure S3A). The transcript levels at 7DT of several proinflammatory cytokines were not significantly elevated (Figure S2B). To selectively assess microglial cytokine levels following NG2 glial ablation, we isolated RNA from microglia (CD11b+ cells) isolated by FACS from the PFCs of CTRL and iDTR mice at 7DT (Figure S3C). Similar CD11b+ cell numbers and cytokine expression levels between CTRL and iDTR confirmed the absence of microglial activation on a molecular level in the iDTR mice at 7DT (Figures S3D and S3E). Taken together, we conclude that NG2 glial ablation does not disturb the brain vasculature or cause significant microglial activation and pro-inflammatory response, validating our iDTR system as a tool for exploring roles for NG2 glia in the adult CNS.
NG2 Glia Ablation Adversely Affects Glutamatergic Signaling in Excitatory Pyramidal Neurons
Morphological and physiological interactions between NG2 glia, and astrocytes and neurons, such as extension of glutamatergic synapses onto NG2 glia in the hippocampus (Bergles et al., 2000), are well described (reviewed in Wigley et al., 2007). Although it is now evident that neurons and astrocytes can elicit NG2 glia migration, proliferation, and lineage progression (Hill et al., 2014; Mangin et al., 2012; Ziskin et al., 2007), it has remained unknown whether NG2 glia, in turn, affect astrocytic and neuronal functioning.
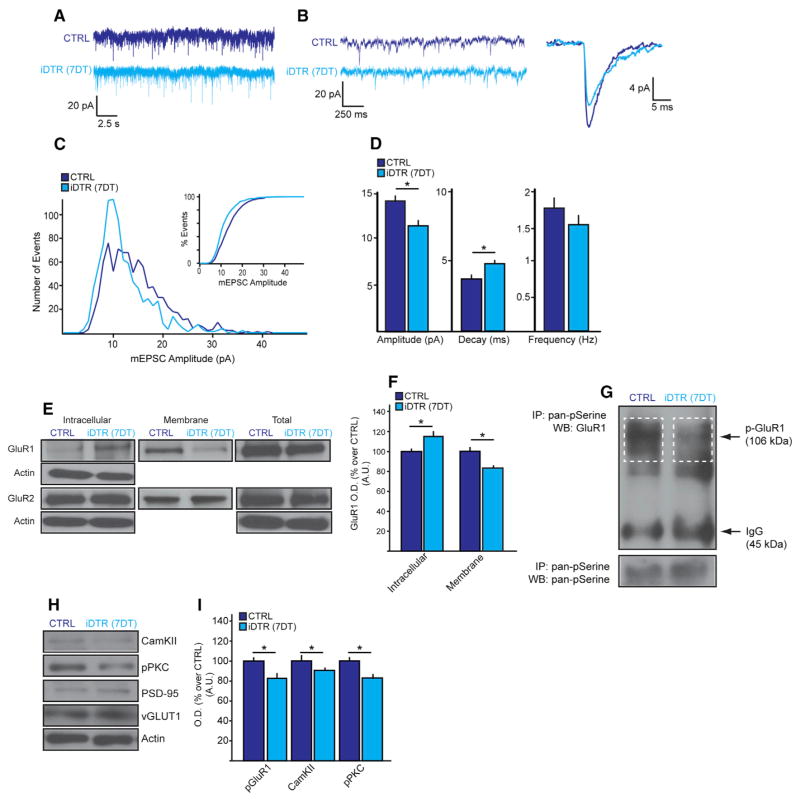
To explore the influence of NG2 glia on excitatory neurotransmission, we assessed the postsynaptic glutatmate receptor function by recording miniature excitatory postsynaptic currents (mEPSC) from pyramidal excitatory neurons in the PFC slices, from pyramidal excitatory glutamatergic following NG2 glial ablation. At 7DT, the mEPSC amplitude was decreased and the decay increased, whereas the frequency was unchanged (Figures 2A–2D). No such changes were observed in the dorsal striatum and the frequency and decay were increased in the somatosensory cortex, suggesting regionally heterogeneous responses of glutamatergic neurotransmission to NG2 glial loss (Figure S4). Consistent with the altered mEPSC dynamics in the PFC, levels of membrane-bound glutamate receptor 1 (GluR1), but not GluR2, were decreased, although the total levels of both subtypes were unchanged (Figures 2E and 2F). GluR1 pan-phosphorylation, which is known to modulate the receptor’s synaptic presence (Lee et al., 2003), was also decreased (Figure 2G), along with the levels of phosphorylated PKC and CaMKII, which are upstream regulators of GluR1 membrane translocation and phosphorylation (Brooks and Tavalin, 2011) (Figures 2H and 2I). The levels of vesicular glutamate transporter VGLUT1 and postsynaptic density marker PSD-95 were unchanged, suggesting the lack of a systemic insult on the excitatory synapse (Figures 2H and 2I). Our analysis showed that NG2 glia loss in the PFC adversely affects postsynaptic, phosphorylation-dependent glutamate receptor trafficking and the associated glutamatergic neurotransmission.
Figure 2. NG2 Glia Ablation Causes Deficits in the Glutamatergic Synaptic Transmission in the PFC.
(A and B) Sample recordings (25 s) of miniature EPSCs (mEPSC) taken from PFC pyramidal neurons (n = 23–5 per group). (A) Sample recordings at higher magnification lasting 2.5 s and (B) individual mEPSC lasting 250 ms.
(C) Histogram of mEPSC amplitude. Insert, a percent distribution of mEPSC amplitudes.
(D) Average amplitude (left), decay (middle), and frequency (right) mEPSCs.
(E and F) Membrane translocation of glutamate receptor 1 (GluR1) and GluR2.
(G–I) GluR1 serine phosphorylation (G) and total protein expression levels of CaMKII, pPKC, vGLUT1, and PSD95 (H) quantified in (I). Error bars, mean ± SEM (*p < 0.05; t-test); n = 3–4 per group unless indicated otherwise.
See also Figure S4.
NG2 Glia Ablation Suppresses Astrocytic Glutamate Uptake
One of the crucial astrocytic dysfunctions that adversely affect excitatory signaling in neurons is sub-optimal clearance of excess glutamate from the synaptic cleft due to decreased expression of the glutamate transporters GLT-1 and GLAST, which can ultimately result in excitotoxicity and maladaptive neural activity (Gómez-Galán et al., 2013; Hashimoto et al., 2007; Miguel-Hidalgo et al., 2010).
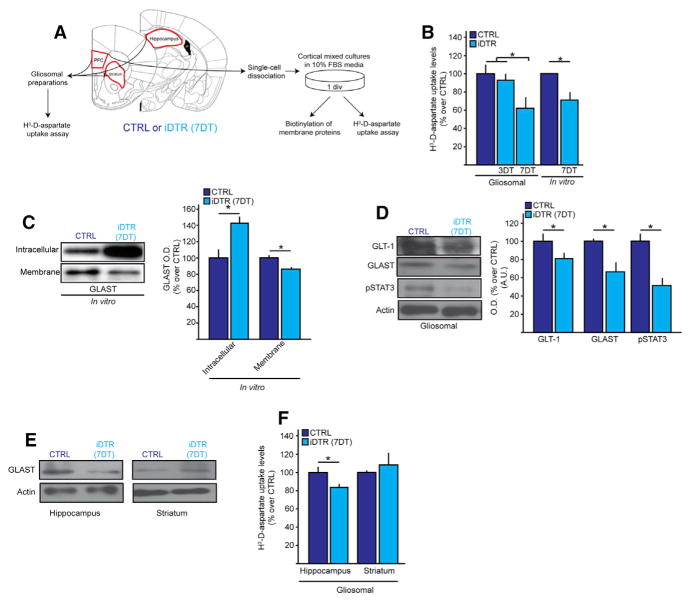
To test whether aberrant glutamatergic neurotransmission in the PFC following NG2 glia loss is partly mediated by astrocytic dysfuction, we analyzed if NG2 glia modulate astrocytic glutamate uptake. For this, we measured levels of uptake of the non-metabolizable glutamate analog [3H]-D-aspartate, using both gliosomal preparations from fresh PFC extracts and primary cortical cultures harvested from the PFCs of CTRL and iDTR mice at 7DT (Figure 3A). Both methods revealed a 30%–40% decrease in [3H]-D-aspartate uptake levels in the iDTR mice (Figure 3B). In accordance, intracellular accumulation and decreased membrane expression of GLAST were observed in the cortical mixed-culture preparations obtained from the PFC of iDTR mice at 7DT (Figure 3C). Total GLAST and GLT-1 protein levels were also decreased along with expression of pSTAT3, which regulates glutamate transporter expression (Raymond et al., 2011) (Figure 3D). GLAST levels and [3H]-D-aspartate uptake were similarly affected in the hippocampus of iDTR mice at 7DT, but not in the striatum (Figures 3E and 3F), mirroring the regions with highest ablation efficiencies. These results point to glutamate uptake as a major output of CNS homeostasis that is compromised both in stress pathology and after NG2 glial loss. Taken together, these findings underscore roles of NG2 glia in CNS physiology, loss of which disturbs glutamate-associated astrocytic and neuronal functions.
Figure 3. NG2 Glia Ablation Leads to Impaired Glutamate Transporter Expression and Glutamate Uptake Deficit in the PFC.
(A) Schematics of experimental protocol used for 3H-D-aspartate uptake assays.
(B) 3H-D-aspartate uptake assayed in CTRL and iDTR PFC gliosomes and in primary cortical astrocyte cultures at 3DT and 7DT; n = 8–10 per group.
(C) Intracellular and cell membrane-bound levels of GLAST from PFC CTRL and iDTR of in primary cortical astrocyte cultures at 7DT.
(D) Protein expression levels of glutamate transporter GLAST, GLT-1 and pSTAT3 in the PFCs of CTRL and iDTR mice at 7DT.
(E) 3H-D-aspartate uptake assayed in CTRL and iDTR hippocampal and striatal gliosomes at 7DT.
(F) Protein levels of GLAST in the hippocampus and striatum at 7DT. Error bars, mean ± SEM (*p < 0.05). n = 4–5 per group unless indicated otherwise.
NG2 Glia Loss Predisposes to a Depressive-like State in Mice
Given the extensive loss of function in glutamatergic signaling and extracellular glutamate clearance in the neurons and astrocytes following NG2 glial loss, we next investigated whether these deficits translate into behavioral aberrations. Indeed, such glutamate-related astrocytic and neuronal functions have consistently been implicated in both chronic stress models and MDD pathophysiology (Almeida et al., 2010; Bechtholt-Gompf et al., 2010; Hashimoto et al., 2007; Miguel-Hidalgo et al., 2010; Caudal et al., 2010); e.g., chronic stress induces a persistent potentiation of glutamate receptor membrane trafficking and glutamatergic transmission in the rat PFC (Yuen et al., 2012). Yet, little is known about whether glia plays any roles in the manifestation of such maladaptive outcomes.
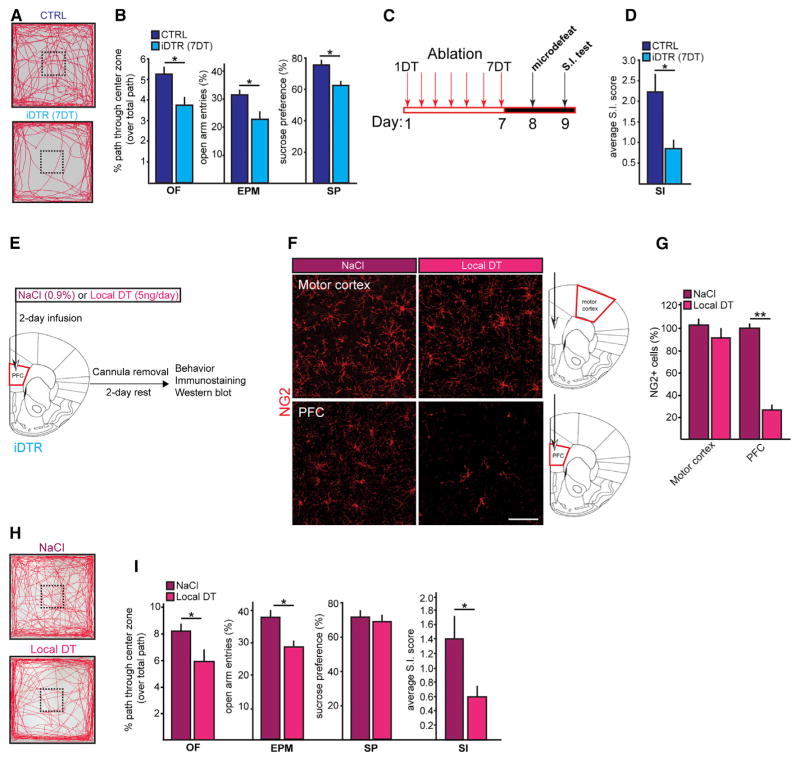
To this end, we employed behavioral tasks to examine whether ablation of NG2 glia drives depressive-like behaviors in mice, namely anxiety-like (measured by open field [OF] and elevated plus maze tests [EPM]), anhedonia-like (measured by sucrose preference test [SP]), and social avoidance behaviors (measured by social interaction test [SI]). We observed decreases in the center entries and open-arm entries over total path traveled in the OF (Figures 4A and 4B) and EPM, respectively, by the iDTR mice at 7DT (Figure 4B). Anhedonia-like behavior was also observed in the iDTR mice at 7DT as indicated by the decrease in the sucrose water preference over regular water (Figure 4B). Social avoidance was examined using a sub-threshold “microdefeat” paradigm (Figure 4C), a 1-day defeat protocol, which reveals susceptibility-promoting interventions, since it, by itself, is not sufficient to induce susceptibility in wild-type mice (Christoffel et al., 2011; Golden et al., 2013). Following the microdefeat paradigm, the average social interaction score (SI score) for the iDTR group was reduced by about 60% (Figure 4D). These results indicate that the induced loss of NG2 glia in the cerebral cortex and hippocampus suffices to predispose to a battery of depressive-like behavioral symptoms.
Figure 4. NG2 Glia Ablation Causes Depressive-like Behaviors in Mice.
(A) Open field activity.
(B–D) Behavioral analyses at 7DT (n = 12–14 per group; t test; OF, open field test; EPM, elevated plus maze; SP, sucrose preference test; SI, social interaction test). (B) Center entry measures normalized to total distance traveled assessed OF. Percent open arm entry frequencies over all entries assessed by EPM. A total of 1% sucrose water preference assessed by SF. (C) Schematic for subthreshold microdefeat protocol. (D) Average SI scores following microdefeat.
(E–G) Paradigm for local NG2 glia ablation in the PFC. Representative images (F) and cell number quantification (G) of NG2 glia, mature oligodendrocytes (GSTPi+ cells), astrocyte (GS+cells), and microglia(Iba+cells)inthe PFC and motor cortexafter NaCl orDT infusionvia cannula intothe PFCof iDTRmice (n = 4 per group;t test).
(H) Protein expression levels of astrocytes (GFAP), function-associated molecular markers for astrocytes (GLAST, GLT-1), mature oligodendrocytes (CNPase; myelin MBP), pericytes (PDGFRβ) and microglial cell activation (iNOS) after DT infusion in the PFC using cannula implanted in this brain region.
(I) Open field activity after NaCl or DT infusion.
(J) Behavioral analyses performed after acute focal NG2 ablation (n = 11–16 per group; t test). Error bars, mean ± SEM (*p < 0.05, **p < 0.01). Scale bar, 40 μm.
See also Figure S5.
Focal Ablation of NG2 Glia in the PFC Induces Depressive-like Behavioral Deficits
Although there exists regional restrictions to the NG2 glia ablation by systemic (i.p.) DT administration (Figure 1B), the cellular loss is widespread throughout some areas in the CNS and thus does not identify the brain regions critical for eliciting the emergence of depressive-like behavioral deficits. To investigate whether restricting NG2 glial loss to a pathologically relevant brain region would suffice to create the behavioral deficits, we focally infused DT (5 ng/day for 2 days) using cannulas implanted directly into the PFC to spatially restrict NG2 glial ablation to this region of the brain (Figure 4E). Using this approach, we were able to achieve NG2 glial loss locally in the PFC without affecting the surrounding regions such as the adjacent motor cortex (Figures 4F and 4G). Furthermore, there were no major changes in other cell types in number and function, including astrocytes, microglia, mature OLs, and pericytes (Figures 4F–4H). PFC-focal ablation of NG2 glia recapitulated anxiety- and social avoidance-like behaviors, but not anhedonic behavior (Figures 4I and 4J), raising the possibility that the loss of NG2 glia in other areas (i.e., hippocampus; Snyder et al., 2011) might be responsible for the anhedonic behavior. Together, our data demonstrate that PFC-specific loss of NG2 glia suffices to trigger behavioral abnormalities largely comparable to those observed in the systemically ablated iDTR mice.
Ablating NG2 Glia Using an Independent Approach Similarly Disturbs Glutamate Signaling and Uptake, and Induces Depressive-like Behavior
To further address the possibility of an off-target transgenic animal-specific effect through use of the iDTR mice, we used an independent, tamoxifen-inducible CRE promoter, PDGFRαCRETM, to drive DTR expression in the NG2 glia (PDGFRαCRETM/iDTR/YFP mice). iDTR/YFP expression in PDGFRαCRETM mice was induced by daily i.p. injections of tamoxifen from P8 to P12. DT (100 ng; Figure S5A) was then administered as before approximately 7 weeks later. The great majority of PDGFRα+ cells coexpressed YFP, suggesting a good recombination efficiency in the adult PFC (Figure S5B). DT administration reduced the NG2 glial density in the PFC by 50% at 7DT (Figures S5B and S5C). We then asked whether the functional consequences of NG2 glia ablation observed in the iDTR mice are recapitulated using this mouse line. We thus examined astrocytic glutamate handling and found that [3H]-D-aspartate uptake (Figure S6D), and molecular signaling elements involved in the regulation of transport were decreased (Figure S5E), and observed anxiety- and anhedonia-like deficits as well as social avoidance (Figures S6F and S6G). Taken together, these findings confirm our earlier systemic and focal ablation outcomes, ruling out the possibility of transgenic line-specific bias as an explanation for the observed changes in glutamate uptake and molecular mechanisms driving behavioral deficits after NG2 glia ablation.
Restoration of NG2 glia Density Correlates with the Rescue of the Molecular, Cellular, and Behavioral Abnormalities
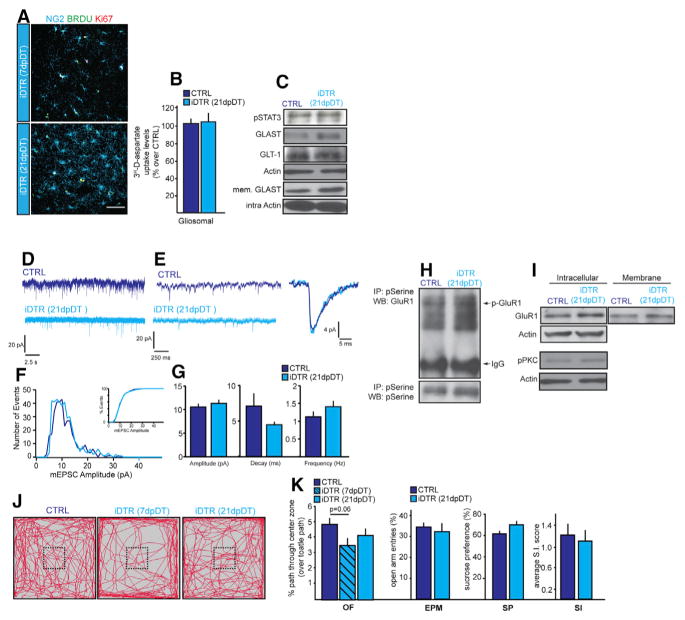
We next examined whether NG2 glia density recovers after the ablation event. To assess this, we marked newly generated NG2 glia using BrdU administered in drinking water after completion of the DT administration at 7DT. NG2 glia started to repopulate the PFC at 7 dpDT Figure 5A), and their numbers became comparable to those in CTRL mice by 21dpDT. The source of the newly generated NG2 glia in the PFC was proliferative local resident NG2 glia that had escaped depletion, as many of the NG2+BrdU+ glia in the regions surrounding the areas of depletion were Ki67+ at 7dpDT and 21dpDT (6 ± 3.3 × 106 um3 in the iDTR mice in comparison to 1 ± 1.5 cells × 106 um3 in the CTRL mice; Figure 5A).
Figure 5. Repopulation of NG2 Glia Density in the PFC after Their Depletion Is Associated with the Recovery of Molecular, Physiological, and Behavioral Stress-Related Deficiencies.
(A) Images and cell quantification of newly generated NG2 glia at 7dpDT and 21dpDT. BrdU (1 mg/mL) was giving in the drinking water after the last DT injection.
(B) 3H-D-aspartate uptake in the PFC of control and iDTR at 21dpDT.
(C) Protein expression levels of pSTAT3, GLAST, and GLT-1 in the PFC of control and iDTR at 21dpDT.
(D) Sample recordings (25 s) of miniature EPSCs from pyramidal neurons in the PFC. (CTRL, n = 10; iDTR, n = 15; t test).
(E) Sample recordings at higher magnification lasting 2 s and individual mEPSC.
(F) Histogram of mEPSC amplitude. Inset, percent distribution of mEPSC amplitudes.
(G) Average amplitude, decay, and frequency.
(H and I) Serine phosphorylation (H) and levels of intracellular and membrane-bound GluR1 (I) in the PFC at 21dpDT.
(J and K) Behavior analysis at 7dpDT and 21dpDT (n = 16–18 per group; two-way repeated-measures ANOVA (for OF analysis) and t test). (J) Open field activity and percent center entry frequencies over total distance traveled at 7dpDT and 21dpDT. (K) Percent open arm entry frequencies over all entries, 1% sucrose consumption, and average SI scores following microdefeat for behavioral analyses. Error bars, mean ± SEM (*p < 0.05). n = 4–5 per group unless indicated otherwise. Scale bar, 40 μm.
In parallel to the NG2 glia repopulation, virtually all of the molecular, cellular and behavioral measures similarly recovered to CTRL levels by 21dpDT (Figure 5), including [3H]-D-aspartate uptake (Figure 5B), glutamate transporter expression (Figure 5C), somatosensory cortex and PFC mEPSCs, (Figures 5D–5G and S4), membrane localization and phosphorylation status of GluR1 and pPKC (Figures 5H and 5I), and behavior (Figures 5J and 5K). Taken together, NG2 glial repopulation rescues the molecular, cellular, and behavioral ablation-specific CNS deficiencies.
Reduced NG2 Glia Density following Chronic Social Stress in Mice
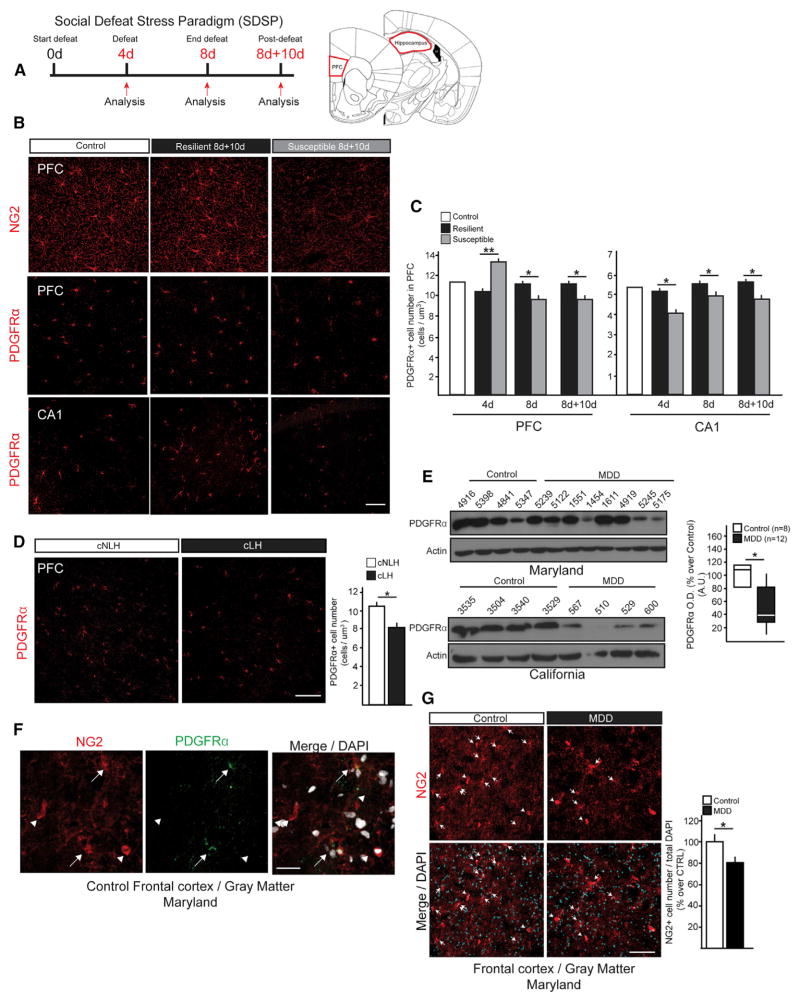
The prominent behavioral disturbances and MDD-related glutamatergic deficits in neurons and astrocytes (Almeida et al., 2010; Bechtholt-Gompf et al., 2010; Caudal et al., 2010; Yuen et al., 2012) observed following NG2 glial ablation led us to question whether NG2 glia density is reduced during the emergence of depressive-like behaviors. Chronic stress is a primary predictor of various cognitive and emotional disturbances in neuropsychiatric disorders, such as MDD and post traumatic stress disorder (PTSD). Based on this idea, we utilized a stress-based rodent model of MDD, the Social Defeat Stress Paradigm (SDSP) (Golden et al., 2011), to study the relationship between chronic social stress and NG2 glial density. In the SDSP model, adult mice are subjected to repeated bouts of physical aggression (“defeats”) from larger “bully” mice. About 60% of the mice that are subjected to social stress display social avoidance and other associated behavioral deficits (and are termed “susceptible”), whereas the nonresponding mice, which are termed “resilient,” do not, modeling the heterogeneity in individual responses to stress in the human population (Krishnan and Nestler, 2011).
Using the SDSP model, we examined NG2 glial densities in two of the regions known to be intimately involved in MDD pathophysiology, the prefrontal cortex (PFC) and the hippocampus (Campbell et al., 2004; Cotter et al., 2002). Cell density analyses were performed at 4 days of defeat (4d), 8 days of defeat (8d), and 8 days of defeat plus 10 days of recovery (8d+10d) (Figure 6A). Quantification of PDGFRα+ cells (as a marker for NG2 glia) indicated decreased NG2 glial density in the CA1 region of the hippocampus in susceptible mice compared to control and resilient animals at 4d, 8d, and 8d+10d (Figure 6B). The same analysis in the PFC, however, showed a significant increase in density at the early defeat time-point (4d) in the susceptible mice, but then decreased density at 8d and 8d+10 (Figure 6C). In contrast, NG2 glia density was not altered in the somatosensory cortex, dorsal striatum and other regions of hippocampus (data not shown), areas that are not directly implicated in the early pathogenesis of MDD. Together, these data suggest a very rapid and region-specific response to stress, as well as a differential response to acute, instead of chronic, stress in the PFC.
Figure 6. Reduced NG2 Glia Density in Animal Models of Depression and MDD Subjects.
(A) Experimental protocol for social defeat stress paradigm (SDSP) and schematic of anatomical brain regions investigated: prefrontal cortex (PFC), and Prelimbic (PrL) and infralimbic (IL) regions of the CA1 region of hippocampus.
(B–F) Representative images (B) and cell quantification (C) of PDGFRα+ cells in CA1 and PFC at 4d, 8d, and 8d+10d of susceptible animals (SUS) compared to resilient (RES) mice (n = 5–6 per group; t test). Representative images (D) and cell quantification (E) of proliferation dynamics of NG2 glial cells (NG2+ PCNA+ cells) in the PFC of control, RES, and SUS mice at 4d, 8d, and 8d+10d. (n = 4 per group). (F) Quantification of PDGFRα+ pGSK3β + cells of control, RES, and SUS mice in the PFC at 8d (n = 4 per group).
(G) Representative images and cell number of PDGFRα+ cells in PFCs of adult congenitally nonlearned helpless (cNLH) and learned helpless (cLH) rats (n = 4 per group; t test).
(H) Protein levels of PDGFRα from PFCs of the control and MDD subjects. Boxplot of PDGFRα expression levels in MDD subjects and age- and sex-matched controls (CTRL n = 8, MDD n = 12; paired t test).
(I) Characterization of human NG2 glial cells in the frontal cortex gray matter characterized using PDGFRα immunostaining. Arrows, NG2 glia; Arrowheads, pericytes.
(J) Representative images and cell number (percent over total DAPI+ nuclei) of NG2 glia in the PFCs of control, and MDD subjects. Arrows, NG2 glia (*p < 0.05, **p < 0.01). Error bars, mean ± SEM. Scale bars, 40 μm; Scale bars in (F), 20 μm.
Given the highly active proliferation profiles of NG2 glia in the normal adult CNS (Hughes et al., 2013; Psachoulia et al., 2009), we next asked whether the decreased density of NG2 glia could be attributed to reduced proliferative capacity after chronic stress. The fluctuations observed in the numbers of proliferating NG2 glia (PCNA+NG2+ cells) in the PFC mirrored the dynamic changes in density observed throughout the defined defeat time points (Figures 6D and 6E), suggesting that the shortage of NG2 glia in susceptible animals could be a consequence of decreased rates of proliferation. We next sought to pinpoint the upstream stress-responsive signaling elements that might mediate NG2 glia proliferation. A candidate pathway involves GSK3β, as it has been previously implicated both in the pathophysiology of MDD (Jope and Roh, 2006) and in the regulation of NG2 glial proliferation in a mouse model of hydrocephalus (Carter et al., 2012). Reduced numbers of NG2 glia co-expressing pGSK3β (pGSK3β+ PDGFRα+ cells) in the PFC of susceptible animals at 8d (Figure 6F) indicated that reductions in GSK3β phosphorylation may underlie the suppressed NG2 glial proliferation following chronic stress.
To inquire whether genetic propensity to depression is also correlated with NG2 glial density, we used a congenital rodent model of depression, congenitally learned helplessness (cLH). cLH rats were selectively bred for the phenotype of learned helplessness, which is quantifiable as significantly reduced lever pressing to escape electrical shocks (Henn and Vollmayr, 2005). A reduction in NG2 glia density was observed in the PFC of helpless (cLH), but not in non-helpless (cNLH) rats (Figure 6G), indicating that NG2 glia cell loss also correlates with endogenously presented depressive-like behavior in rodents, even in the absence of an acute or chronic stress stimulus.
NG2 Glia Density Is Reduced in the Frontal Cortices of MDD Patients
To investigate whether our findings in rodent models of stress might be clinically relevant, we quantified NG2 glial density in human subjects with MDD. We obtained post-mortem frontal cortex samples of MDD subjects from two independent brain tissue banks (control; n = 8, MDD; n = 12). PDGFRα protein levels in the frontal cortices of MDD subjects were significantly reduced compared to those found in age-matched controls (Figure 6H).
We then examined NG2 glia in situ in the frontal cortices of MDD subjects. We focused on cells with typical stellate morphology of NG2 glia—i.e., having multiple thin, branched processes that colocalized with PDGFRα, in order to exclude NG2+ pericytes from our analysis (Ozerdem et al., 2001) (Figure 6I). We detected reduced cell numbers of NG2 glia in the frontal cortices of MDD subjects as compared to age matched control subjects, supporting our western blot analyses (Figure 6J). Taken together, our data indicate that NG2 glial density is reduced as a consequence of chronic social stress in mice and in the post-mortem tissue of MDD subjects, suggesting that loss of NG2 glia is a clinically relevant phenomenon in stress-related disorders. Further confirmation of NG2 glial cell loss and how it interacts with certain variables (e.g., antidepressant use, age, sex) in MDD awaits future studies with larger cohorts of MDD patients.
NG2 Glia-Secreted Factors Modulate Key Astrocytic and Neuronal Functions Compromised by NG2 Glia Loss in the PFC
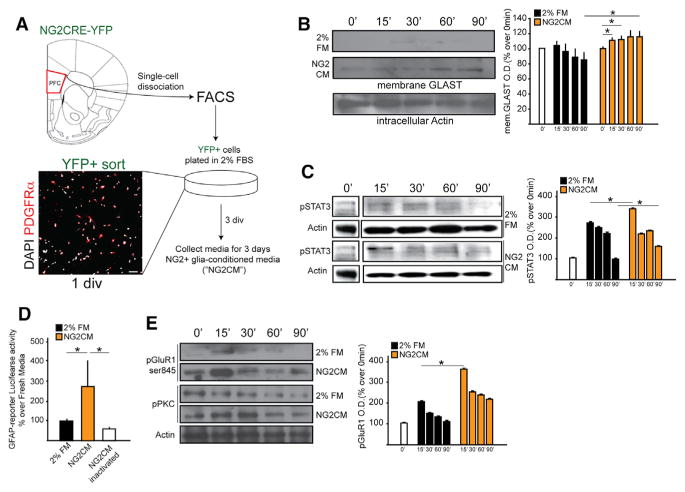
Glia secrete a plethora of factors including growth factors, neurotrophins, and cytokines that help regulate neuronal transmission. For example, astrocyte-secreted ATP has been proposed to be an anxiolytic, as genetically blocking its release in mice causes depressive-like behaviors (Cao et al., 2013); similarly, glial-released TNFα has causally linked to hippocampus-mediated long-term memory (Han et al., 2013). To investigate whether NG2 glia similarly modulate neuronal and astrocytic functions in a paracrine fashion, we generated media enriched in NG2 glia-secreted factors (NG2 glial-conditioned media; “NG2CM”) by FACS-isolating YFP+ cells from the PFC of NG2CRE/YFP mice and culturing them for 3 days in growth factor-free media containing 2% FBS. PDGFRα+ cells represented about 85%–88% of the FACS-isolated YFP+ cells (Figure 7A). Primary astrocytes and neurons were cultured in 2% FBS media or the NG2CM. NG2CM promoted GLAST membrane translocation (Figure 7B) and phosphorylation of STAT3 (Figure 7C) in astrocytes in a time-dependent manner. Similarly, NG2CM increased luciferase activity in astrocytes transfected with a GFAP-luciferase reporter construct containing STAT3 binding sites upstream of the GFAP-promoter (Asano et al., 2009; Figure 7D), and it stimulated GluR1 and PKC phosphorylation in primary neurons (Figure 7E). These results indicate NG2 glia-secreted factors support critical astrocytic and neuronal functions and hence that deficiency of these factors could mechanistically underlie the astrocytic and neuronal deficits observed following NG2 glial ablation.
Figure 7. NG2 Glia-Secreted Factors Mediate Astrocytic and Neuronal Functions.
(A) Schematics of experimental paradigm and representative image of post-FACS cells indicating sort purity (85%–90% of all DAPI cells were PDGFRα+).
(B) GLAST cellular localization at different time points of stimulation with 2% fresh medium (FM) or NG2 conditioned media (NG2-CM) in primary cortical astrocytes.
(C) Time course of phosphorylation of STAT3 (pSTAT3) in response to 2%FM and NG2CM in primary cortical astrocytes.
(D) GFAP-Luciferase reporter assay activity in primary cortical astrocytes in response to 2% FM or NG2CM.
(E) Time course of PKC and pGluR1 phosphorylation in response to 2% FM and NG2CM in primary hippocampal neurons. Error bars, mean ± SEM n = 4–5 per group (*p < 0.05; two-way ANOVA).
NG2 Glial Secretion of FGF2 in the PFC Is Downregulated following Chronic Social Stress
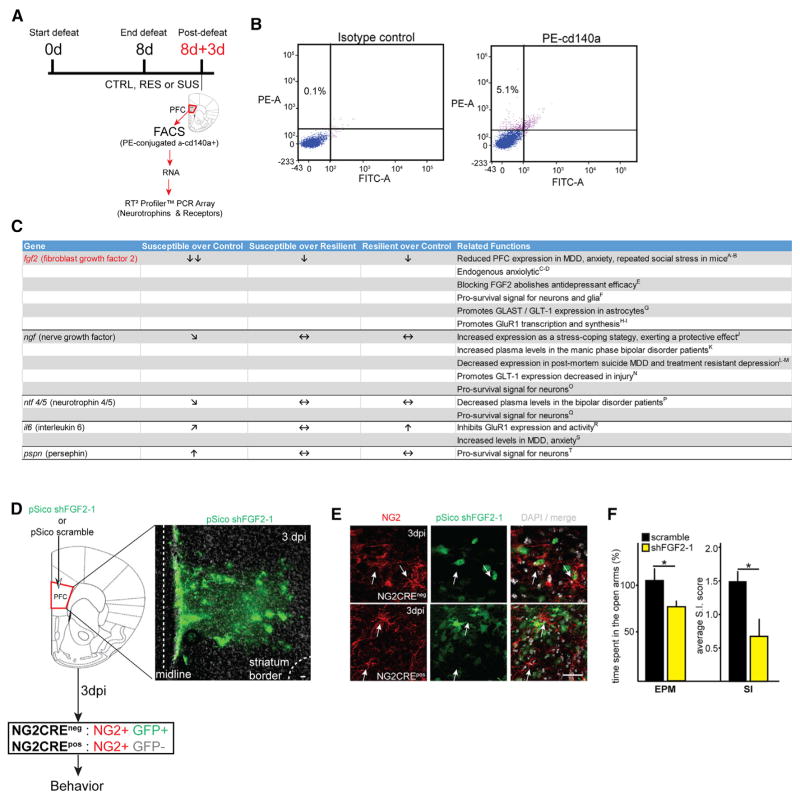
Next, in order to identify NG2 glia-secreted factors that might play a role in the development of susceptibility to depressive-like behavior, we isolated NG2 glia at 8d+3d from the PFC of susceptible, resilient, and control mice by FACS and quantified mRNA expression levels of an array of growth factors, neurotrophins, and cytokines using a gene-based array (The Mouse Neurotrophin and Receptors RT2 Profiler PCR Array; QIAGEN) (Figures 8A, 8B, and S6). Some of the top hits included secreted factors that have been previously associated with MDD (Figure 8C). Among these, FGF2 was the only factor that was significantly reduced in the NG2 glia isolated from susceptible compared to both control (fold change = −16) and resilient (fold change = −8) mice (Figures 8C and S6; Table S2). FGF2 has previously been strongly correlated in the pathophysiology of MDD and is downregulated in chronic stress models (Elsayed et al., 2012; Perez et al., 2009). Further in this context, FGF2 has been observed to regulate several neuronal and astrocytic functions that were also dysregulated in our system after NG2 glia depletion, namely GluR1- and GLAST-related glutamatergic functions (Figure 8C; see in-figure references). Our analysis here suggested that FGF2 secreted by NG2 glia could be a critical factor that modulates CNS homeostasis and is perturbed during chronic stress.
Figure 8. The Loss of NG2 Glia-Secreted FGF2 Participates in the Emergence of Depressive-like Behaviors.
(A) Schematic representation of NG2 glia isolation protocol by FAC-sorting to obtain total RNA and perform PCR array from control, RES, and SUS mice.
(B) Representative plots of FACS gates used to isolate NG2 glial cells (PDGFRα+ cells; PE+) for PCR pathway array analysis.
(C) Table showing the growth factors and neurotrophins with the highest differential expression among the groups and fold-changes along with reported functions in this context and relevant references. Double downward arrows, significant downregulation, >10-fold change; downward arrow, significant downregulation, <10-fold change; upward arrow, significant upregulation, <10-fold change; sideways downward arrow, downregulation trend; sideways upward arrow, upregulation trend; horizontal arrows, no change (n = 5 per group; see Supplemental Information for references and statistical analysis).
(D) Lentiviral shRNA strategy to knock down mouse FGF2 in NG2 glial cells in the PFC of adult mice. Representative confocal images showing infected cells (GFP+ cells) in the PFC at 3 days postinfection (3 dpi).
(E) At 3 dpi in the PFC of NG2CREneg mice (top panel, arrows), PDGFRα+ cells infected with shFGF2-1 were GFP+, while in the PFC of NG2CREpos mice PDGFRα+ were GFPneg (bottom panel, arrows).
(F) Representative behavioral measures assessed at 3 dpi of mice injected with scramble shRNA or shFGF2-1. Plots shows percentage of time spent in the open arms over total time assessed by EPM (n = 22/group) and average SI score assessed by SI (n = 11/group). dpi, days postinjection. Error bars, mean ± SEM (*p < 0.05; t test). Scale bars, 40 μm.
See also Figures S6 and S7.
NG2 Glia-Specific Knockdown of FGF2 Suffices to Induce Depressive-like Behaviors
Finally, we sought to determine whether the mechanism underlying the loss of NG2 glia predisposing to anxiety-like behavior in mice is mediated by FGF2. To test this hypothesis, we knocked down FGF2 specifically in the NG2 glial cells in the PFC and performed behavior analysis. To accomplish the FGF2 knockdown, we used NG2CRE mice and a CRE-dependent flox/flox shRNA lentiviral system (Ventura et al., 2004) to stably express shRNA directed to the mouse FGF2 after CRE recombination in NG2 glial cells. Using this approach, EGFP is initially expressed in all infected cells, but then turns off after the NG2 promoter-mediated recombination that simultaneously turns on the expression of shRNA against mouse FGF2 (Figure S7; see Experimental Procedures).
We cloned four different shFGF2 sequences into the pSico plasmid (shFGF2-1 - shFGF2-4; see Experimental Procedures for the shFGF2 sequences) in addition to a control scramble construct. To validate and optimize the shFGF2 viral constructs, we utilized NG2 glial cell-enriched cultures derived from the brain cortices of adult NG2CREpos mice and NG2CREneg mice (Figure S7a). Cell cultures were processed at several days of infection (dpi) and processed for either FACS or immunocytochemistry analysis to detect FGF2 mRNA and protein levels, respectively (Figure S7A). Note that at 32 hr in vitro (hiv), PDGFRα+ cells are EGFP+, while at 3DIV they were PDGFRα+ EGFPdim and at 5DIV virtually all the PDGFRα+ cells were EGFPneg (Figure S7B). Hence, we FAC-sorted PDGFRα+EGFP+, PDGFRα+EGFPneg, and PDGFRαnegEGFP+ cell populations to determine the efficiency of FGF2 downregulation in NG2 glial cells (Figure S7C). The shFGF2-1 construct achieved the highest efficiency of FGF2 downregulation in NG2 glia (Figures S7D–S7F; see also Experimental Procedures) as compared with shFGF2-2 and scramble constructs. Therefore we used the shFGF2-1 construct in all our functional and behavioral experiments. FGF2 downregulation in NG2 glia was further confirmed post-FACS by analyzing FGF2 fluorescence levels in PDGFRα+ EGFPneg cells from shFGF2-1 versus scramble construct (Figure S7F). This analysis thus validated our approach for NG2-glia-specific knockdown of FGF2.
We next knocked down FGF2 expression in NG2 glia in the PFC of NG2CRE mice and assessed behavioral tasks associated with depressive-like behavior (see also Supplemental Experimental Procedures). NG2CREneg (wild-type) mouse was then used to establish the conditions to target NG2 glial cells in the PFC using this lentiviral system (Figure 8D). In the PFC of these animals, a large percentage of the PDGFRα+ cells are targeted with the pSico lentiviral system (Figure 8E, top panel; arrows). Characterization of EGFP+ cells at this time points showed that a large percentage of these cells co-expressed PDGFRα (53 ± 6 PDGFα+ EGFP+ cells/EGFP+ cells; Figure 8E, top panel arrows). We next confirmed that shFGF2-1 resulted in transfection of NG2 glia in the PFC of NG2CREpos animals, as we did not detect any PDGFRα+ cells within the EGFP+ cells at this time points (Figure 8E, bottom panel arrows). Under those conditions, we also detected EGFP+ cells coexpressing the neuronal and astrocytic markers NeuN and GFAP, respectively (data not shown). Critically, at 3 dpi, mice infected with the shFGF2-1-mice displayed anxiety-like (EPM) and social avoidance behaviors (SI) as compared with scramble (Figure 8F), indicating that FGF2 loss restricted to NG2 glia in the PFC suffices to induce depressive-like behaviors in adult mice.
Our working model (Figure S8) proposes that the NG2 glia play key roles in maintaining CNS homeostasis by mediating astrocytic glutamate uptake and neuronal glutamatergic signaling, at least partially through secreted factors, one of which is FGF2. We conclude that chronic stress triggers loss of NG2 glia, which results in the loss of homeostatic secretion of critical NG2-glia-derived signaling cues, such as FGF2, which in turn creates the CNS cellular and molecular dysfunction previously shown to underlie MDD and other stress disorders.
DISCUSSION
In this study, we have defined a stringent functional link between NG2 glia and depressive-like behaviors, identifying FGF2 produced by the NG2 glia as a putative modulator of the NG2 glia-mediated CNS homeostasis. We employed a mouse model of chronic social stress to show that NG2 glia loss is a hallmark of stress-susceptible mice and propose that, through decreased FGF2 secretion, this loss creates region-specific deficits in neuronal and astrocytic glutamatergic signaling ultimately leading to MDD-like behavioral deficits.
NG2CRE/iDTR as a Suitable Model to Study NG2 Glia Ablation
In the developing forebrain, NG2 glia arise from the ventral germinal zones of the medial and lateral ganglionic eminences (Hill and Nishiyama, 2014). Lineage tracing studies which have temporally followed these early NG2 glia (Zhu et al., 2008a; Zhu et al., 2008b) found, in addition to the expected myelinating oligodendrocytes, forebrain and spinal cord astrocytes to be among their progeny. We generated the iDTR-YFP line to characterize CRE-expressing cells in the adult PFC. Our analysis showed that ~94% of the YFP+ cells were NG2 glia and 5% were oligodendrocytes, with no apparent YFP expression in astrocytes. Since NG2 glia represent ~5% of all glial cells (Dawson et al., 2003), the YFP+ oligodendrocytes could represent at most 1/20th of that amount, or 0.25% of the total number of glial cells. Since oligodendrocytes represent >50% of the glial cells, at most, 0.5%, or 1 out of every 200 oligodendrocytes, has a recombined iDTR/YFP cassette, which could even represent oligodendrocytes generated in adulthood from mature NG2 glia, as opposed to ones arising from developmental recombination events. Regardless, the very low frequency of YFP+ oligodendrocytes and the lack of demyelination after NG2 glia ablation in the PFC eliminates the concern that this model results in significant oligodendrocyte ablation in addition to NG2 glia ablation upon injection of DT. The additional evidence for the absence of off-target effects on astrocytes were shown by the lack of DT-driven cell death of primary astrocytes harvested from the iDTR mice. Of note, this is in line with the observations that a major source of astrocytes in the postnatal cortex in mice arises from the local protoplasmic astrocytes (Ge et al., 2012).
NG2 expression has been observed also in pericytes of the adult CNS (Ozerdem et al., 2001), which can render them susceptible to DT via CRE expression. Yet, we saw no alterations either in NG2+ pericytes or associated vasculature following NG2 glial cell ablation. A possible explanation for the lack of DTR activation in off-target cell types might ensue from the kinetics of CRE recombination, which requires a significant level and duration of CRE expression; below this threshold, very little recombination occurs (Mao et al., 2001) (reviewed in Richardson et al., 2011). Therefore, adult NG2 glia, which express high levels of NG2, as opposed to the low levels of expression seen during development, appear to be the only cells that undergo CRE-mediated excision and are thus susceptible to ablation via injection of DT.
We also excluded the minor microglial activation observed following NG2 glia ablation as a confounding factor, as it did not lead to an inflammatory response. Similar large-scale cell ablation studies have reported that microglia are largely unchanged in their phenotype after Ara-C-induced ablation of NG2 glia in the hypothalamus and DT-induced ablation of DCX+ immature neurons in the hippocampus (Robins et al., 2013; Vukovic et al., 2013). This is consistent with the findings that the pharmacological ablation of microglia is also insufficient either to prompt an inflammatory response by the surviving microglia progenitor population or to adversely affect brain vasculature (Elmore et al., 2014; Parkhurst et al., 2013).
NG2 Glia Dynamics in Stress and Stress-Related Disorders
Our data suggest that NG2 glia are a key cellular determinant of symptom emergence in stress-related disorders. Under chronic social stress, NG2 glial dynamics in the PFC went from transiently increased cell numbers during the early stages of stress to a robustly reduced cell density in areas that play a critical role in depression. This phenomenon was not a general response to stress, since the NG2 glial density changed neither in the PFC of resilient animals nor in areas involved in the pathophysiology of MDD, i.e., the somatosensory cortex and striatum of susceptible animals. NG2 glia have repeatedly been demonstrated to be the first cell type to respond to any type of insult to the adult CNS (Hampton et al., 2004; Hughes et al., 2013; Kang et al., 2010), and our data here show that stress is no exception: NG2 glia were rapidly perturbed in our rodent model of depression. The rapid influence of stress upon NG2 glia also stands in contrast to astrocytic perturbations observed in similar animal models of chronic stress-induced depression, in which a reduction in astrocyte density was observed only after a 35 day stress protocol (Banasr et al., 2010).
The maintenance of NG2 glial density in the PFC via control of proliferation rates is under tight autoregulation (Birey and Aguirre, 2015; Hughes et al., 2013), although the physiological reason for this has remained unclear. This dependence on replacement renders NG2 glia a unique cellular target for stress signals, putting the CNS functions they homeostatically support at risk when proliferation is suppressed, as we show here. Although identification of the upstream mechanisms that decrease the proliferation rates of NG2 glia is yet to be determined, a number of candidate pathways hold promise. It has been recently shown that lithium, a mood stabilizer, enhances the proliferation of NG2 glia by stimulating the phosphorylation of GSK3β (Carter et al., 2012), a pathway heavily implicated in MDD. In this context, 4 weeks of lithium treatment was shown to increase GSK3β serine-9 phosphorylation (Jope and Roh, 2006). The reduced number of pGSK3β+ PDGFRα+ cells suggests that downregulation of GSK3β phosphorylation in a subset of NG2 glia might underlie their reduced proliferation after chronic stress. Another mechanism that could account for NG2 glia loss is cell death, through increased levels of glucocorticoids due to over-activation of the HPA axis. Recently, it has been established that NG2 glia express glucocorticoid receptors (GRs) (Matsusue et al., 2014) and that chronic glucocorticoid administration in rats reduces their proliferation (Alonso, 2000). The hippocampus and PFC have high GR expression, consistent with the primary sites of NG2 glia loss in the susceptible mice after SDSP.
NG2 Glia-Secreted Factors Mediate Astrocytic and Neuronal Functions Lost after Ablation and Implicated in MDD
The emergence of depressive-like behaviors following NG2 glia ablation identifies a homeostatic loss-of-function in the adult CNS for them. In line with this idea, we sought to identify the systems affected by the lost NG2 glia modulatory functions that ultimately contribute to maladaptive behaviors. Our data suggest a direct role for NG2 glia in regulating essential glutamate-modulated systems, including astrocytic glutamate clearance and neuronal excitatory glutamatergic signaling. Whether deficiencies in optimal glutamate clearance directly cause the observed glutamatergic neurotransmission deficits awaits future studies. Nonetheless, the data above imply that NG2 glia participates in the regulation of optimized glutamate signaling and glutamate clearance. Given the nature of their immediate response to chronic stress, the loss of NG2 glia might be one of the first regulatory element to affect glutamate system in this setting.
The Loss of NG2 Glia-Secreted FGF2 Suffices to Drive Depressive-like Behaviors
We identified the growth factor FGF2 as a plausible factor through which NG2 glia mediate CNS homeostasis and through which their deficiency leads to anxiety-like behavior. Recent evidence points to FGF2 as a key factor in the pathophysiology of mood disorders, as it is one of the more intensively characterized growth factors in this context: FGF2 has been found at low levels in MDD and can rescue depressive-like behaviors in chronic stress models (Elsayed et al., 2012; Perez et al., 2009). Our PCR array data showed low levels of FGF2 mRNA levels in NG2 glia from susceptible animals, suggesting that a deficit of production of this factor might predispose to the development of depressive-like behavior by defective modulation of astrocytic and neuronal functions. Indeed, it has previously been shown that FGF2 stimulation can increase GLAST levels, glutamate uptake and phosphorylation of GluR (Figure 8C, see in-figure references). Strikingly, NG2 glia-specific knockdown of FGF2 in the PFC was sufficient to induce depressive-like behavior in mice, demonstrating that even a functional perturbation in a small population of NG2 glia is able to robustly influence properties of astrocyte and neurons systematically, leading to aberrations at the behavioral level. Although it is most likely that FGF2 works in concert with other factors to mediate the functions listed above, the loss of NG2 glia-derived FGF2 remains as a factor that plays a crucial role in the emergence of depressive phenotypes in the NG2 glia ablation and social defeat models.
Our findings reveal that NG2 glia play essential roles in maintaining normal adult brain function and that dysfunction of these roles and the ultimate loss of NG2 glia are implicated in the pathophysiology of MDD and related disorders. Further advancing our understanding of the cellular and molecular targets regulated by NG2 glia in stress disorders will provide novel therapeutic targets for antidepressant drug development.
EXPERIMENTAL PROCEDURES
Animals
Transgenic mouse lines were backcrossed to generate iDTR line. In the iDTR mouse line, the gene encoding DTR (simian Hbegf, heparin-binding epidermal growth factor-like growth factor) is under the control of the constitutive Rosa26 locus promoter, and its expression is blocked by an upstream loxP-flanked STOP sequence (see Elmore et al., 2014). The DTR is expressed after Cre recombinase removes the STOP cassette, rendering only NG2-expressing cells susceptible to DT. Wild-type littermates that are injected with DT were used as control animals for the experiments with systemic DT administration. No adverse side effects of DT were observed when administered to the controls.
Diphtheria Toxin Administration
Mice were analyzed at 2, 3, and 7 days after the first injection (acute depletion phase) and 2 days and 1, 2, and 3 weeks after 7DT administration (Figure 2A). These time points were chosen to include the onset of NG2 glia death and acute depletion (3–7 days) and recovery (3 weeks). For local DT infusions into the PFC, the following coordinates were used for cannula (Azlet) implantation: anterioposterior, +1.5 mm; mediolateral from bregma, 0.5 mm; and dorsoventral from dura, −1.4 mm.
Social Defeat Stress Paradigm
CD1 mice at 4–5 months of age were selected for aggressive behavior based on a 3 day screening period for aggression behavior prior to SDSP (see Golden et al., 2011 for full protocol procedures). Following 10 min of physical interaction, the victim C57BL/6J mice were removed and placed on the opposite side of the aggressor’s home cage behind a protective partition for the remainder of the 24 hr period. The victims are introduced a novel set of aggressors each day.
Electrophysiology
Glutamatergic synaptic transmission was investigated in the acute and recovery periods after DT administration (Maffei et al., 2004). For the acute period, recordings were performed at 7DT and 21dpDT in age-matched (P40) control and ablated animals. Coronal slices containing PFC, primary PFC, somatosensory cortex (S1), and striatum were prepared as previously described. Visualized patch clamp recordings were obtained from layer 2/3 pyramidal neurons in S1. AMPA mEPSCs were pharmacologically isolated and recorded in voltage clamps (at −70 mV). Neurons with series resistances below 15 MΩ and exhibiting less than 10% change throughout the recording were used for analysis. Cumulative and ranked distributions of mEPSC amplitudes were obtained from 50 events for each neuron.
pSico EGFP Lentiviral Flox/Flox Knockdown of Mouse shFGF2 in NG2 Glial Cells of the Adult PFC
NG2 glial cells were targeted for knockdown of mouse FGF2 using a CRE/Lox pSico lentiviral system(Ventura et al., 2004). Lentiviral (LV) stocks were produced and purified according to a previously published protocol (Taylor et al., 2006). The final titer was 125 μg/ml p24 and 1.6 × 109 infectious units (IU)/ml for the LV scramble control lentivirus, and 980 μg/ml p24 and 8 × 108 IU/ml for the LV shFGF2-1 construct. The vector stocks were diluted in Hank’s balanced salt solution (HBSS) (Invitrogen, Carlsbad, CA). Primary cortical mouse cultures as above were prepared to test mouse shFGF2 knockdown in NG2 glial cells. In order to determine the efficiency of lentiviral particles targeting NG2 glial cells, cultures were transduced in vitro with LV scramble or LV shFGF2-1 to -4, using 250,000 IU in 200 μl DMEM/F12 media. Cultures were processed for FAC sorting and immunocytochemistry at several days after infection (as indicated in Figure S7). For FAC sorting of PDGFRα+ cells after infection, cells were collected and incubated with isotype control or cd140a-PE antibodies and then sorted populations were processed for RT-PCR analysis and immunocytochemistry as above and previously described(Aguirre et al., 2010). The shRNA sequence for mouse FGF2 were cloned in the pSico vector (Addgene, catalog number 11578) and processed for LV lentiviral production. The sequences used for shRNA for mouse FGF2 are as follows: shFGF1-1 (GCCATACTGTTTCTTCCAATGTCTGCTAA); shFGF1-2 (CGTCAAACTACAACTCCAAGCAGAAGAGA); shFGF1-3 (GGCTCTACTGCAAGAACGGCGGCTTCTTC); and shFGF1-4 (TTGAACGACTGGAATCTAATAACTACAAT).
For targeting NG2 glia in the PFC, adult NG2CRE+ and NG2CREneg mice (P60–P90) were stereotaxically injected with scramble of LV shRNA GFP construct. Briefly, adult mouse males were anesthetized as previously described (Aguirre and Gallo, 2007) and placed in a stereotaxic frame. Burr holes were drilled to permit bilateral stereotaxic injection of 2 μl of LV shFGF2 or LV scramble (diluted in HBSS concentration mentioned above) at a rate of 0.2 μl/min into the PFC (stereotaxic coordinates from anterioposterior, +1.5 mm; mediolateral from bregma, 0.5 mm; and dorsoventral-below the surface of the dura, −1.4 mm). At 3 days after LV infection, mice were processed for immunohistochemistry and behavioral analysis.
Supplementary Material
Highlights.
NG2 glia loss affects the glutaminergic systems in the PFC
NG2 glia loss in the PFC induces behavioral deficits via loss of secreted FGF2
NG2 glia density is reduced in subjects with MDD and in susceptible mice after SDSP
Restoration of NG2 glia density rescues cellular and behavioral aberrations
Acknowledgments
We are grateful to the NICDH Brain, Tissue Bank for Developmental Disorders and Human Brain and Spinal Fluid Resource Center, West Los Angeles Health-care Center for the human brain tissue samples used in this study. This work was supported by NIHMH RO1 RMH099384A (A.A.) and R01 MH090264 (S.J.R.).
Footnotes
Supplemental Information includes eight figures, two tables, and Supplemental Experimental Procedures and can be found with this article at http://dx.doi.org/10.1016/j.neuron.2015.10.046.
AUTHOR CONTRIBUTIONS
F.B. performed all the experiments. M.K. and A.M. performed the electrophysiological experiments with A.A. and F.B.’s input. S.J.R., M.A.F., D.J.C., and J.K.R. assisted with the animal model of depression and behavior studies. M.C., F.I., and A.A. contributed to FACS and shRNA experiments, and I.H. performed the behavioral analysis for the shRNA experiments. A.A. and F.B. designed all the experiments. A.A. supervised the project. F.B., M.A.F., and A.A. wrote the manuscript.
References
- Aguirre A, Gallo V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007;3:209–220. doi: 10.1017/S1740925X08000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RF, Thomazi AP, Godinho GF, Saute JA, Wofchuk ST, Souza DO, Ganzella M. Effects of depressive-like behavior of rats on brain glutamate uptake. Neurochem Res. 2010;35:1164–1171. doi: 10.1007/s11064-010-0169-4. [DOI] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Asano H, Aonuma M, Sanosaka T, Kohyama J, Namihira M, Nakashima K. Astrocyte differentiation of neural precursor cells is enhanced by retinoic acid through a change in epigenetic modification. Stem Cells. 2009;27:2744–2752. doi: 10.1002/stem.176. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtholt-Gompf AJ, Walther HV, Adams MA, Carlezon WA, Jr, Ongür D, Cohen BM. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology. 2010;35:2049–2059. doi: 10.1038/npp.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Birey F, Aguirre A. Age-dependent Netrin-1 signaling regulates NG2+ glial cell spatial homeostasis in normal adult gray matter. J Neurosci. 2015;35:6946–6951. doi: 10.1523/JNEUROSCI.0356-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks IM, Tavalin SJ. Ca2+/calmodulin-dependent protein kinase II inhibitors disrupt AKAP79-dependent PKC signaling to GluA1 AMPA receptors. J Biol Chem. 2011;286:6697–6706. doi: 10.1074/jbc.M110.183558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19:773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- Carter CS, Vogel TW, Zhang Q, Seo S, Swiderski RE, Moninger TO, Cassell MD, Thedens DR, Keppler-Noreuil KM, Nopoulos P, et al. Abnormal development of NG2+PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med. 2012;18:1797–1804. doi: 10.1038/nm.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudal D, Godsil BP, Mailliet F, Bergerot D, Jay TM. Acute stress induces contrasting changes in AMPA receptor subunit phosphorylation within the prefrontal cortex, amygdala and hippocampus. PLoS ONE. 2010;5:e15282. doi: 10.1371/journal.pone.0015282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol Psychiatry. 2012;72:258–265. doi: 10.1016/j.biopsych.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484:376–380. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Galán M, De Bundel D, Van Eeckhaut A, Smolders I, Lindskog M. Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol Psychiatry. 2013;18:582–594. doi: 10.1038/mp.2012.10. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Rhodes KE, Zhao C, Franklin RJ, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Hill RA, Nishiyama A. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia. 2014;62:1195–1210. doi: 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci. 2014;17:1518–1527. doi: 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Li P, Scafidi J, Gallo V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat Neurosci. 2012;15:1192–1194. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- Matsusue Y, Horii-Hayashi N, Kirita T, Nishi M. Distribution of corticosteroid receptors in mature oligodendrocytes and oligodendrocyte progenitors of the adult mouse brain. J Histochem Cytochem. 2014;62:211–226. doi: 10.1369/0022155413517700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab. 2011;31:795–806. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dynamics. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Li P, Mangin JM, Huntsman M, Gallo V. Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J Neurosci. 2011;31:17864–17871. doi: 10.1523/JNEUROSCI.3179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Villemain A, Liu X, Djogo T, Kryzskaya D, Storch KF, Kokoeva MV. Extensive regenerative plasticity among adult NG2-glia populations is exclusively based on self-renewal. Glia. 2013;61:1735–1747. doi: 10.1002/glia.22554. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Sacher J, Steiner J, Blasig IE, Mueller K. Mood disorders are glial disorders: evidence from in vivo studies. Cardiovasc Psychiatry Neurol. 2010 doi: 10.1155/2010/780645. Published online May 27, 2010. http://dx.doi.org/10.1155/2010/780645. [DOI] [PMC free article] [PubMed]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant C. Life events, stress and depression: a review of recent findings. Aust N Z J Psychiatry. 2002;36:173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Borlikova GG, Ruitenberg MJ, Robinson GJ, Sullivan RK, Walker TL, Bartlett PF. Immature doublecortin-positive hippocampal neurons are important for learning but not for remembering. J Neurosci. 2013;33:6603–6613. doi: 10.1523/JNEUROSCI.3064-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat. 2007;210:661–670. doi: 10.1111/j.1469-7580.2007.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008a;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008b;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.