Figure 1. FoxO1 signaling pathway involved in skeletal muscle differentiation.
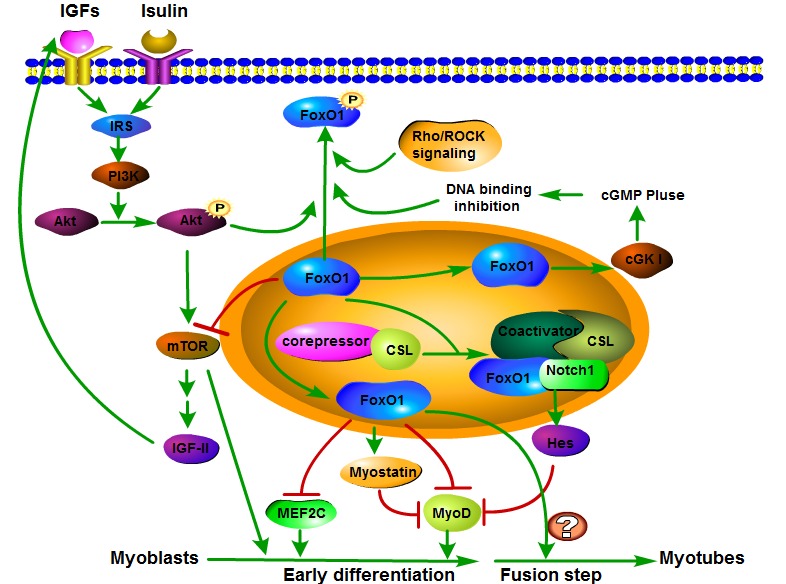
The FoxO1 upstream signals including IGFs, insulin and IRS regulate FoxO1 transcriptional activity through phosphorylating FoxO1 in a PI3K-Akt dependent manner. Phosphorylated FoxO1 will be excluded from nucleus and thus loses its capacity of binding to target regulatory elements. In addition, other signals, such as cGKI and Rho/ROCK signaling, directly mediate FoxO1 transcriptional activity by phosphorylation. Myostatin, MEF2C, MyoD and mTOR are downstream factors of FoxO1. FoxO1 negatively regulates myoblast early differentiation through promoting myostatin and inhibiting MEF2C, MyoD and mTOR. Then the decrease of MEF2C, MyoD and mTOR delays myoblast early differentiation. In addition, the relationship among FoxO1, mTOR, IGF-II and PI3K/Akt pathway presents a feedback loop that can preferably fine-tune the regulation of muscle differentiation. Moreover, FoxO1 can inhibit early step of myoblast differentiation through interacting with Notch signaling and promoting corepressor clearance and recruiting the coactivator of Csl, leading activation of Hes family, which is considered to be a myoblast differentiation repressor. Notably, although FoxO1 suppresses the early muscle differentiation process, FoxO1 is required for myoblast terminal differentiation fusion into myotubes. However, the molecular mechanism in which FoxO1 is required for myotube fusion has remained poorly understood.
