Abstract
Background
The role of surgical therapy in gastric cancer patients with distant metastases remains controversial. This retrospective analysis was performed to identify whether gastric cancer patients with distant metastases might benefit from surgery.
Patients and methods
A total of 5185 patients from the SEER database who were initially diagnosed with histologically confirmed gastric cancer with distant metastases from 2004 to 2009 were included. Patients were divided into the following three groups: patients who underwent resection of both the primary tumor and distant metastatic tumors (‘PMTR’ group), patients who only underwent resection of the primary tumor (‘PTR’ group) and patients who did not undergo any surgery (‘No surgery’ group). We employed the Kaplan-Meier analysis, the log-rank test and multivariate Cox proportional hazards regression models to estimate the survival time of the different groups.
Results
A total of 5185 patients had a median survival time (MST) of 9.0 months. The improvement in survival of the ‘PMTR’ and ‘PTR’ groups was significantly different compared with that of the ‘No surgery’ group (MST, 12.0 vs 12.0 vs 9.0 months, respectively, P<0.001; 1-year survival rate, 49.6% vs 49.1% vs 30.1%, respectively, P<0.001; 3-year survival rate, 12.5% vs 15.1% vs 5.8%, respectively, P<0.001), whereas no significant difference was found between the ‘PMTR’ group and ‘PTR’ group (P=0.642). Multivariate Cox proportional analysis showed that surgery was an independent prognostic factor (‘PMTR’, hazard ratio (HR) =0.648, 95% confidence interval (CI) 0.574-0.733, P<0.001; ‘PTR’, HR=0.631, 95% CI 0.583-0.684, P<0.001).
Conclusions
This retrospective analysis demonstrated that combined PTR and metastasectomy or PTR alone were independent prognostic factors for survival improvement in gastric cancer patients with distant metastases. Because no statistically significant difference in survival was observed between the ‘PMTR’ group and ‘PTR’ group, PTR, which is a more minor surgery, might be more appropriate than PMTR in clinical practice for gastric cancer patients with distant metastases.
Keywords: gastric cancer with distant metastases, surgery, outcomes
INTRODUCTION
Gastric cancer is the fourth most common type of cancer worldwide and is the second cause of cancer-related death globally [1]. In 2014, in the United States, the numbers of new cases and deaths from gastric adenocarcinoma were estimated to be 22,220 and 10,990, respectively [2, 3]. The incidence of gastric cancer is the second highest in China and is > 20 per 100,000 in men [4]. Because of early tumor detection, curative surgical resection and appropriate adjuvant therapy, the survival of patients with early primary gastric cancer has improved. In a previous study in Japan, the resection rate in patients with early primary gastric cancers was 95.4% and the 5-year survival rate of patients who underwent resection was 70.7% [5]. However, because of the atypical early symptoms, limited popularity of routine gastroscopy examination and other factors, 35% of patients present with evidence of distant metastases at the time of diagnosis [6].As a result, the treatment of gastric cancer patients with distant metastases remains poor. Although systemic chemotherapy with or without new molecular targeting agents is currently the standard treatment modality, the median survival time (MST) of gastric cancer patients with distant metastases is < 12 months and the 5-year survival is < 10% without surgical treatment [7, 8].
Researchers have begun to seek and explore new and more effective treatment options for gastric cancer patients with distant metastases. With advancements in current research and the emergence of a large amount of evidence-based medical findings, some scholars have proposed active surgical treatment for gastric cancer patients with distant metastases [9, 10, 11]. However, because the outcomes of palliative resections in gastric cancer patients with distant metastases are extremely poor [12, 13], the benefits of surgery in these cases remain debatable. Thus, this retrospective analysis was performed to analyze the significance of surgery for gastric cancer with distant metastases and to investigate the prognostic factors associated with surgery to identify candidates with gastric cancer with distant metastases who are most likely to benefit from curative surgical treatment.
PATIENTS AND METHODS
Patient selection
The Surveillance, Epidemiology, and End Results Program (SEER) database was sponsored by the National Cancer Institute. In the SEER database, information on cancer cases from 18 population-based cancer registries, which represent approximately 27.8% of the population in the United States, was collected. The database of the SEER program includes information on patient demographics, primary tumor site, tumor histology, stage at initial diagnosis, surgery, radiotherapy, and survival.
Our retrospective study contained 5185 patients from the SEER database (SEER*Stat 8.2.1) who were initially diagnosed with histologically confirmed gastric cancer with distant metastases between 2004 and 2009. The histological type was restricted to adenocarcinoma. Exclusion criteria included patients less than 18 years of age, survival time less than 3 months after confirmed diagnosis, patients with previously diagnosed malignancies, metastasectomy without primary tumor resection or if resection of the primary tumor was unknown, and occult gastric cancer (no evidence of primary tumor). The remaining patients were divided into three groups as follows: patients who underwent resection of both the primary tumor and distant metastatic tumors (‘PMTR’ group), patients who underwent resection of the primary tumor alone (‘PTR’ group) and patients who did not undergo surgery (‘No surgery’ group).
Statistical analysis
Survival curves, the median survival time (MST), 1-year survival rate and 3-year survival rate were estimated with the Kaplan-Meier method, and the log-rank test was performed to evaluate survival in the different groups. Hazard ratios (HRs) along with 95% confidence intervals (CI) were calculated using the multivariate Cox proportional hazard regression model to determine the influences of other factors including surgery, age, race, gender, tumor site, grade, histological type, T-stage, N-stage, and radiation status, on survival. Statistical tests were two-sided and P < 0.05 was considered statistically significant. SPSS 12.0 (SPSS Chicago, IL, USA) software was used for the statistical analysis.
RESULTS
Patient characteristics
A total of 5185 eligible patients were included: 322 (6.2%) patients underwent resection of both primary and distant metastatic tumors (‘PMTR’ group), 885 (17.1%) patients underwent PTR alone (‘PTR’ group), and 3978 (76.7%) patients did not undergo any surgery (‘No surgery’ group). A total of 946 (18.24%) patients were over 75 years old and 1843 (35.54%) were male. Patient demographics and characteristics are summarized in Table 1.
Table 1. The Characteristics of patients with gastric cancer with distant metastases.
| Variance | N,% | PMTR,% | PTR,% | No surgery,% | Total |
|---|---|---|---|---|---|
| Total | 5185(100) | 322 (6.2) | 885 (17.1) | 3978(76.7) | |
| Age | |||||
| ≤75years old | 4239(81.76) | 268(6.3) | 718(16.9) | 3253(76.7) | |
| >75years old | 946(18.24) | 54(5.7) | 167(17.7) | 725(76.6) | |
| P value | 0.704 | ||||
| Race | |||||
| white | 3778(72.86) | 231(6.1) | 585(15.5) | 2962(78.4) | |
| black | 656(12.65) | 35(5.3) | 128(19.5) | 493(75.2) | |
| other | 739(14.25) | 55(7.4) | 169(22.9) | 515(69.7) | |
| unknown | 12(0.23) | 1(8.3) | 3(25.0) | 8(66.7) | |
| P value | <0.001 | ||||
| Gender | |||||
| male | 1843(35.54) | 155(8.4) | 320(17.4) | 1368(74.2) | |
| female | 3342(64.46) | 167(5.0) | 565(16.9) | 2610(78.1) | |
| P value | <0.001 | ||||
| Site of tumor | |||||
| body | 1809(34.90) | 168(9.3) | 472(26.1) | 1169(64.6) | |
| cardia | 2163(41.70) | 92(4.3) | 245(11.3) | 1826(84.4) | |
| fundus | 218(4.20) | 10(4.6) | 29(13.3) | 179(82.1) | |
| pylorus | 114(2.20) | 11(9.6) | 38(33.3) | 65(57.0) | |
| stomach | 881(17.00) | 41(4.7) | 101(11.5) | 739(83.9) | |
| P value | <0.001 | ||||
| Grade | |||||
| well | 80(1.54) | 2(2.5) | 11(13.8) | 67(83.8) | |
| moderate | 928(17.90) | 54(5.8) | 154(16.6) | 720(77.6) | |
| poor | 3034(58.51) | 239(7.9) | 625(20.6) | 2170(71.5) | |
| undifferentiated | 109(2.10) | 13(11.9) | 35(32.1) | 61(56.0) | |
| unknown | 1034(19.94) | 14(1.4) | 60(5.8) | 960(92.8) | |
| P value | <0.001 | ||||
| Histological type | |||||
| non-signet-ring cell | 3957(76.32) | 232(5.9) | 610(15.4) | 3115(78.7) | |
| signet-ring cell | 1228(23.68) | 90(7.3) | 275(22.4) | 863(70.3) | |
| P value | <0.001 | ||||
| T-stage* | |||||
| T1 | 782(15.1) | 10(1.3) | 39(5.0) | 733(93.7) | |
| T2 | 215(4.1) | 10(4.7) | 327(16.7) | 463(78.6) | |
| T3 | 676(13.0) | 89(13.2) | 274(43.2) | 279(43.6) | |
| T4 | 1768(34.1) | 198(11.2) | 223(27.5) | 796(60.7) | |
| Tx | 1744(33.6) | 15(0.9) | 22(1.3) | 1707(97.9) | |
| P value | <0.001 | ||||
| N-stage* | |||||
| N0 | 1449(27.95) | 50(3.5) | 137(9.5) | 1262(84.2) | |
| N1 | 1611(31.07) | 62(3.8) | 172(10.7) | 1377(85.5) | |
| N2 | 278(5.36) | 64(23.0) | 156(56.1) | 58(20.9) | |
| N3 | 540(10.41) | 139(25.7) | 385(71.3) | 16(3.0) | |
| Nx | 1307(25.21) | 7(0.5) | 35(2.7) | 1265(96.8) | |
| P value | <0.001 | ||||
| Radiation | |||||
| done | 1074(20.71) | 67(6.2) | 180(16.8) | 827(77.0) | |
| no radiation | 4034(77.80) | 246(6.1) | 692(17.2) | 3096(76.8) | |
| unknown | 77(1.49) | 9(11.7) | 13(16.9) | 55(71.4) | |
| P value | 0.383 |
PMTR: patients who underwent resection of both primary tumor and distant metastatic tumors; PTR: patients received primary tumor resection alone; No surgery: patients did not undergo any surgery.
* T-stage and N-stage according to the 7th edition of AJCC TNM staging.
Survival analyses
The results of the Kaplan-Meier analysis and log-rank test showed that a total of 5185 patients had a MST of 9.0 months, a 1-year survival rate of 34.6%, and a 3-year survival rate of 7.9%. Among them, the MST of the ‘PMTR’ group and the ‘PTR’ group was significantly longer compared with that of the No surgery group (12.0 vs 12.0 vs 9.0 months, P < 0.001), whereas no significant difference was observed between the ‘PMTR’ and the ‘PTR’ groups (P = 0.642). The 1-year survival rates were 49.6%, 49.1% and 30.1% for the ‘PMTR’, ‘PTR’ and ‘No surgery’ groups, respectively (P < 0.001) and the 3-year survival rates were 12.5%, 15.1% and 5.8%, respectively (P < 0.001). The survival curves are shown in Figure 1.
Figure 1. The survival curves of gastric cancer with distant metastases in different grups.
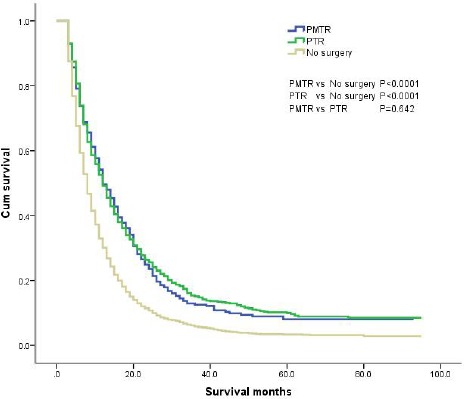
PMTR: patients who underwent resection of both primary tumor and distant metastatic tumors; PTR: patients received primary tumor resection alone; No surgery: patients did not undergo any surgery.
Outcomes of the different subgroups
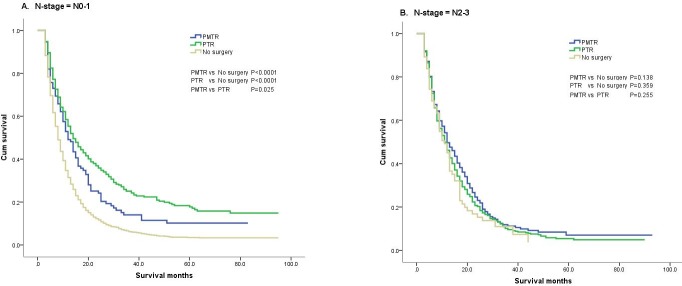
We compared the survival benefit of patients according to the subgroups, which accounted for age, race, gender, grade, tumor site, histological type, T-stage, N-stage and radiation status, by Kaplan-Meier analysis and log-rank test. Among the different subgroups, the survival benefits of patients in the ‘PMTR’ and ‘PTR’ groups were better than those seen in patients in the ‘No surgery’ group (Table 2). Specifically, in the N0-1 subgroup, the results showed that the survival improvement of patients in the ‘PMTR’ and ‘PTR’ groups was significantly higher compared with that of patients in the ‘No surgery’ group (MST, 12.0 vs 14.0 vs 8.0 months, P < 0.001; 1-year survival rate, 49.1% vs 55.4% vs 31.4%, P < 0.001; 3-year survival rate, 14.0% vs 25.1% vs 6.2%, P < 0.001). Moreover, patients with stage N0-1 cancer in the ‘PTR’ group had an increased survival benefit compared with those in the ‘PMTR’ group (P < 0.05). However, among those with stage N2-3 cancer, no statistically significant differences were found in the MST, the 1-year or the 3-year survival rate among the three groups (P > 0.05) (Figure 2).
Table 2. Survival rate(%) and MST(Months) of patients with gastric cancer with distant metastases.
| PMTR | PTR | No Surgery | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survival rate(%) | MST | Survival rate(%) | MST | Survival rate(%) | MST | |||||
| 1Y | 3Y | 1Y | 3Y | 1Y | 3Y | |||||
| Total | 49.6 | 12.5 | 12.0 | 49.1 | 15.1 | 12.0 | 30.1 | 5.8 | 9.0 | <0.001 |
| Age | ||||||||||
| ≤75y | 52.9 | 13.6 | 14.0 | 51.3 | 15.9 | 13.0 | 30.9 | 6.2 | 8.0 | <0.001 |
| >75y | 32.9 | 6.4 | 9.0 | 39.4 | 11.5 | 8.0 | 26.5 | 4.3 | 7.0 | <0.001 |
| Race | ||||||||||
| white | 51.9 | 11.4 | 14.0 | 47.2 | 15.4 | 12.0 | 30.2 | 5.4 | 8.0 | <0.001 |
| black | 38 | 10.4 | 11.0 | 54.1 | 14.2 | 13.0 | 26.1 | 5.2 | 7.0 | <0.001 |
| other | 46.2 | 16.4 | 12.0 | 51.6 | 14.1 | 13.0 | 32.5 | 8.6 | 8.0 | <0.001 |
| Gender | ||||||||||
| male | 55.9 | 13.8 | 15.0 | 51.1 | 16.5 | 13.0 | 30.7 | 5.6 | 8.0 | <0.001 |
| female | 42.9 | 11 | 11.0 | 45.5 | 12.5 | 11.0 | 28.8 | 6.3 | 8.0 | <0.001 |
| Site of tumor | ||||||||||
| body | 47.8 | 13.7 | 12.0 | 58.2 | 15.6 | 12.0 | 30.1 | 6.4 | 80.0 | <0.001 |
| cardia | 60.6 | 13.8 | 16.0 | 57.3 | 23.3 | 13.0 | 32.1 | 6.6 | 9.0 | <0.001 |
| fundus | 40 | 0 | 11.0 | 58.6 | 18.7 | 14.0 | 26.2 | 2.8 | 7.0 | 0.007 |
| pylorus | 43.6 | 10.9 | 12.0 | 41.7 | 19.3 | 8.0 | 24.6 | 1.5 | 6.0 | 0.004 |
| stomach | 36.6 | 9.8 | 10.0 | 44.1 | 19.3 | 12.0 | 26.4 | 4.2 | 7.0 | <0.001 |
| Grade | ||||||||||
| well | 50 | 0 | 10.0 | 45.5 | 0 | 11.0 | 35.5 | 3.1 | 9.0 | 0.899 |
| moderate | 64.2 | 28.5 | 17.0 | 66.8 | 21.7 | 17.0 | 35.1 | 6.7 | 9.0 | <0.001 |
| poor | 47.6 | 9.7 | 12.0 | 45.2 | 12.9 | 11.0 | 28.9 | 5.3 | 8.0 | <0.001 |
| undifferentiated | 25.2 | 0 | 11.0 | 48.6 | 15.7 | 12.0 | 35.4 | 10.3 | 9.0 | 0.145 |
| Histological type | ||||||||||
| non-signet-ring cell | 53.7 | 15.1 | 14.0 | 53 | 16.7 | 13.0 | 30.8 | 6.2 | 8.0 | <0.001 |
| signet-ring cell | 38.8 | 5.1 | 11.0 | 40.3 | 11.3 | 10.0 | 27.4 | 4.4 | 7.0 | <0.001 |
| T-stage* | ||||||||||
| T1-2 | 60 | 25 | 14.0 | 63.3 | 35.6 | 24.0 | 30.7 | 6.1 | 8.0 | <0.001 |
| T3-4 | 49.4 | 11.8 | 12.0 | 47.7 | 12.8 | 12.0 | 32 | 6 | 8.0 | <0.001 |
| N-stage* | ||||||||||
| N0-1 | 49.1 | 14 | 12.0 | 55.4 | 25.1 | 14.0 | 31.4 | 6.2 | 8.0 | <0.001 |
| N2-3 | 49.6 | 11.2 | 12.0 | 46.2 | 9.6 | 11.0 | 45 | 11 | 11.0 | 0.272 |
| Radiation | ||||||||||
| done | 63.9 | 24.5 | 17.0 | 58.3 | 20.5 | 16.0 | 31.1 | 5.6 | 8.0 | <0.001 |
| no radiation | 46.7 | 9.5 | 12.0 | 46.3 | 13.4 | 11.0 | 29.7 | 5.8 | 8.0 | <0.001 |
PMTR: patients who underwent resection of both primary tumor and distant metastatic tumors; PTR: patients received primary tumor resection alone; No surgery: patients did not undergo any surgery.
* T-stage and N-stage according to the 7th edition of AJCC TNM staging.
Figure 2. The survival curves of three groups in N-stage subgroups.
A. N0-1 subgroup; B: N2-3 subgroup.
The superiority in the ‘PMTR’ and ‘PTR’ groups was completely diminished compared with the ‘No surgery’ group only in the subgroups that contained patients with well differentiated and undifferentiated tumors. Considering the far smaller number of patients who underwent surgery in these two subgroups, the results should be interpreted with caution.
Multivariate analyses for survival
The multivariate Cox proportional hazard regression analysis showed that surgery was an independent prognostic factor (‘PMTR’, hazard ratio (HR) = 0.577, 95% confidence interval (CI) 0.491-0.677, P < 0.001; ‘PTR’, HR = 0.559, 95% CI 0.493-0.633, P < 0.001). We also analysed all the aforementioned factors in the subgroups and found that age, histological type, N-stage and radiation status were also independent prognostic factors (Table 3).
Table 3. Multivariate analysis (Cox Proportional Hazard Model) of overall survival for patients with gastric cancer with distant metastases.
| Variable (reference) | Wald | HR | 95.0% CI for HR | P |
|---|---|---|---|---|
| Surgery (no surgery) | ||||
| PMTR | 45.222 | 0.577 | 0.491-0.677 | <0.001 |
| PTR | 84.258 | 0.559 | 0.493-0.633 | <0.001 |
| Age (≤75 years old) | 27.819 | 1.351 | 1.208-1.511 | <0.001 |
| Race (white) | ||||
| black | 0.149 | 1.025 | 0.902-1.165 | 0.700 |
| other | 2.111 | 0.915 | 0.812-1.031 | 0.146 |
| Gender (male) | 0.638 | 1.038 | 0.948-1.137 | 0.424 |
| Site of tumor (body) | ||||
| cardia | 0.459 | 0.965 | 0.872-1.069 | 0.498 |
| fundus | 2.248 | 1.174 | 0.952-1.449 | 0.134 |
| pylorus | 0.155 | 1.056 | 0.807-1.382 | 0.693 |
| stomach | 0.078 | 1.021 | 0.882-1.182 | 0.779 |
| Grade (well) | ||||
| moderate | 0.869 | 0.865 | 0.638-1.173 | 0.351 |
| poor | 0.148 | 1.060 | 0.787-1.427 | 0.701 |
| undifferentiated | 0.014 | 0.978 | 0.672-1.423 | 0.906 |
| Histology type (non-signet-ring cell) | 13.467 | 1.214 | 1.095-1.347 | <0.001 |
| T-stage (T3-4)* | 3.439 | 0.908 | 0.820-1.005 | 0.064 |
| N-stage (N2-3)* | 6.010 | 0.858 | 0.760-0.970 | 0.014 |
| Radiation (no radiation) | 6.989 | 0.871 | 0.786-0.965 | 0.008 |
PMTR: patients who underwent resection of both primary tumor and distant metastatic tumors; PTR: patients received primary tumor resection alone; No surgery: patients did not undergo any surgery.
* T-stage and N-stage according to the 7th edition of AJCC TNM staging.
Prognostic factors associated with surgery
The multivariate Cox proportional hazard regression analysis showed that in the ‘PMTR’ group, age and radiation status were independent prognostic factors, while in the ‘PTR’ group, in addition to age and radiation status, T-stage and N-stage were also independent prognostic factors (Table 4).
Table 4. Multivariate analysis (Cox Proportional Hazard Model) of overall survival of subgroups in group ‘PMTR’, group ‘PTR’ and group ‘No surgery’ respectively.
| Variance | PMTR | PTR | No surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95.0% CI | P* | HR | 95.0% CI | P** | HR | 95.0% CI | |
| Age(≤75years old) | |||||||||
| >75years old | 0.004 | 1.675 | 1.182-2.374 | 0.005 | 1.336 | 1.090-1.637 | 0.001 | 1.286 | 1.108-1.492 |
| Race(white) | |||||||||
| black | 0.927 | 0.979 | 0.620-1.546 | 0.480 | 0.918 | 0.724-1.164 | 0.329 | 1.085 | 0.921-1.277 |
| other | 0.866 | 0.970 | 0.680-1.384 | 0.152 | 0.863 | 0.705-1.056 | 0.322 | 0.920 | 0.780-1.085 |
| Gender | |||||||||
| female | 0.166 | 1.212 | 0.923-1.591 | 0.226 | 1.107 | 1.939-1.305 | 0.819 | 0.986 | 0.872-1.115 |
| Site of tumor(body) | |||||||||
| cardia | 0.964 | 0.993 | 0.724-1.361 | 0.652 | 1.043 | 0.870-1.249 | 0.400 | 0.942 | 0.820-1.082 |
| fundus | 0.240 | 1.508 | 0.760-2.991 | 0.906 | 0.974 | 0.626-1.515 | 0.129 | 1.224 | 0.943-1.588 |
| pylorus | 0.380 | 1.374 | 0.676-2.793 | 0.819 | 0.952 | 0.623-1.454 | 0.698 | 1.084 | 0.722-1.628 |
| stomach | 0.796 | 1.060 | 0.682-1.648 | 0.741 | 0.955 | 0.725-1.257 | 0.694 | 1.039 | 0.857-1.261 |
| Grade(well) | |||||||||
| moderate | 0.464 | 0.466 | 0.060-3.596 | 0.033 | 0.505 | 0.270-0.948 | 0.955 | 1.010 | 0.707-1.443 |
| poor | 0.681 | 0.681 | 0.090-5.162 | 0.182 | 0.663 | 0.362-1.213 | 0.326 | 1.191 | 0.841-1.686 |
| undifferentiated | 1.244 | 1.244 | 0.149-10.379 | 0.107 | 0.564 | 0.281-1.132 | 0.960 | 0.987 | 0.599-1.626 |
|
Histological type (non-signet-ring cell) |
|||||||||
| signet-ring cell | 0.110 | 1.278 | 0.946-1.727 | 0.364 | 1.086 | 1.909-1.299 | 0.006 | 1.223 | 1.059-1.411 |
| T-stage*(T3-4) | |||||||||
| T1-2 | 0.282 | 0.732 | 0.415-1.292 | 0.001 | 0.587 | 0.425-0.810 | 0.710 | 0.979 | 0.875-1.095 |
| N-stage*(N2-3) | |||||||||
| N0-1 | 0.949 | 0.991 | 0.740-1.326 | 0.001 | 0.751 | 0.631-0.894 | 0.179 | 1.218 | 0.913-1.624 |
| Radiation(no radiation) | |||||||||
| done | 0.025 | 0.682 | 0.488-0.954 | 0.004 | 0.745 | 0.609-0.911 | 0.733 | 0.977 | 0.856-1.116 |
PMTR: patients who underwent resection of both primary tumor and distant metastatic tumors; PTR: patients received primary tumor resection alone; No surgery: patients did not undergo any surgery.
HR= hazard ratio, CI = confidence interval.
* T-stage and N-stage according to the 7th edition of AJCC TNM staging.
DISCUSSION
In recent years, surgical treatment for gastric cancer with distant metastases has remained controversial. Although systemic chemotherapy is the standard treatment strategy, the MST of these patients is only approximately 12 months, and long-term survival beyond 5 years is rare [14, 15]. Recently, it was reported that in selected cases, especially in patients with gastric cancer with liver metastases, aggressive surgical treatment may lead to unexpected results [16, 17, 18, 19, 20]. In a review based on 19 studies, Kerkar et al. reported that the 5-year survival rate of gastric cancer patients with liver metastases who underwent liver resection was 26.5% (range: 0-60%) [16]. Similarly, Kodera et al. analysed 17 studies that involved patients with gastric cancer with liver metastases and showed that the MST ranged from 9.0 to 38.8 months among patients who underwent surgical resection [17]. Recently, two meta-analyses also verified that hepatectomy might be associated with the significant improvement in overall survival [18, 19]. In an analysis of 39 studies that involved patients with gastric cancer with liver metastases who underwent hepatectomy, Markar et al. found that the median 1-year, 3-year, and 5-year survival rates were 68%, 31%, and 27%, respectively [18]. In another meta-analysis of 11 observational studies, Martella et al. reported a significantly higher survival rate in the patients who underwent the most aggressive surgery for liver metastases (HR = 0.54, 95% CI 0.46-0.95) compared with patients who underwent palliative treatment only [19]. Studies of gastric cancer patients with distant metastases other than those to the liver who underwent curative surgery are scarce. In a retrospective study initiated by HAN et al., gastric cancer patients with distant metastases, including metastases to the liver, para-aortic lymph nodes, peritoneum and ovary, were enrolled, and those who were good responders to induction chemotherapy underwent curative R0 resection [20]. Finally, the median survival of patients was as high as 22.9 months. In the meta-analysis, a survival benefit and clinical significance were also found for palliative gastrectomy for metastatic gastric cancer (HR = 0.62; 95%CI 0.49-0.78; P < 0.0001) [21]. All these studies demonstrated that surgery was a potential approach to improve the outcome of selected patients with metastatic gastric cancer.
Recently, the opposite result was reported in the REGATTA randomized controlled trial [22]. REGATTA was an open-label, randomized, phase 3 trial with 44 participating centres and hospitals in Japan, South Korea, and Singapore. Patients aged 20-75 years old with advanced gastric cancer confined to either the liver (H1), peritoneum (P1), or para-aortic lymph nodes (16a1/b2) and who had a single non-curable factor were enrolled and randomly assigned (1:1) in each country to chemotherapy alone or gastrectomy (D1 lymphadenectomy) followed by chemotherapy. The overall survival at 2 years as the primary endpoint for all randomly assigned patients was 31.7% (95% CI 21.7-42.2) for those assigned to chemotherapy alone compared with 25.1% (16.2-34.9) for those assigned to gastrectomy plus chemotherapy. The median overall survival was 16.6 months (95% CI 13.7-19.8) for patients assigned to chemotherapy alone and 14.3 months (11.8-16.3) for those assigned to gastrectomy plus chemotherapy (hazard ratio 1.09, 95% CI 0.78-1.52; one-sided P = 0.70). Based on the result of this trial, compared with chemotherapy alone, resection of the primary tumor plus chemotherapy did not improve survival. In contrast to the aforementioned retrospective study, the majority of the enrolled patients in this trial experienced peritoneal metastasis (75%). This undesirable composition of the enrolled patients may have led to the negative result in this trial.
However, not all patients with metastatic gastric cancer obtained a survival benefit from surgery, and these candidates for surgery shared some general characteristics. In the study by Samarasam et al., 107 of 151 patients underwent surgical resection and 44 underwent non-resectional surgery [23]. The MST of the patients who underwent surgical resection was 24.0 months, but the MST was 12.0 months for those who underwent non-resectional surgery. The patients were divided into four groups according to widespread tumor growth (T+), unresectable lymph node involvement (L+), liver metastasis (H+) and peritoneal metastasis (P+). All patients with one positive sign underwent resection, and the resultant MST was 24.3 months. Patients with two positive signs had a survival advantage in favour of surgical resection, in contrast to those who underwent non-resectional surgery (13.0 vs 8.0 months). When three signs (6.0 vs 12.0 months) and four signs (2.0 vs 2.6 months) were present, the survival advantage of patients who underwent surgical resection disappeared. Similarly, Hartgrink et al. found that among 156 patients who underwent palliative resection and 77 who did not undergo resection, the MST was greater in the resection group (8.1 vs 5.4 months; P < 0.001) [24]. A significant difference in survival benefit was found in patients with one metastatic site between the resection group and the non-resection group (MST, 10.5 vs 6.7 months; P = 0.034) while no significant survival advantage was observed in patients who underwent resection of two or more metastatic sites (5.7 vs 4.6 months, P = 0.084). In addition, they found that although patients aged over 70 years gained a 3-month improvement in survival after surgery, the morbidity and perioperative mortality rates in this older age group were much higher (50% and 20%, respectively).
The results of the current study were similar to those described above. Compared with patients who did not undergo surgery, those who underwent PMTR or PTR experienced significant improvements in survival. Furthermore, in the subgroup analyses, with the exception of the N2-N3 stage subgroup, the factors of age, race, gender, tumor site, grade, histological type, T-stage, and radiation status did not influence the surgery-associated benefits seen in these patients. This may imply that patients with N2-N3 stage cancer would not benefit from surgery. However, no significant difference was found in terms of a survival benefit between the patients who underwent PMTR and those who underwent PTR. Additionally, in clinical practice, PMTR may lead to more treatment complications and risks. This suggests that, in the same cases, PTR may represent a better option compared with PMTR.
Thus far, many investigators have agreed that age, histological type, N-stage and radiation status were independent prognostic factors [16, 17, 18, 19, 20, 21]. In our study, using a multivariate analysis, we arrived at the same conclusions and found that surgery was an additional independent prognostic factor. This implied that compared with those who did not undergo surgery, patients who underwent surgery might obtain a survival benefit and experience a significant decrease in the risk of death. Similarly, younger patients, those with non-signet-ring cell adenocarcinoma, those with early N-stage cancers and those who received radiation therapy might be more likely to experience a survival benefit compared with other patients. In addition, younger patients and those who received radiation were more likely to benefit from PMTR. However, in the ‘PTR’ group, younger patients, those with early T-stage tumors, those with early N-stage tumors, and those who received radiation therapy were more prone to undergo PTR.
Some limitations may have influenced the results of our study. First, our study had limitations that are inherent to the methodology of retrospective analyses, including selection bias and potential confounders. Thus, we integrated the data of the following patient groups to reduce bias as much as possible because of insufficient sample capacity: T1 and T2 were integrated into the T1-2 subgroup, T3 and T4 were integrated into the T3-4 subgroup, N0 and N1 were integrated into the N0-1 subgroup, and N2 and N3 were integrated into the N2-3 subgroup. Second, information such as the performance status of the patients, the site and number of metastases, whether patients underwent synchronous or metachronous surgery, chemotherapy status, and comorbidities were not included in the SEER database. Finally, the determination of the T-stage and N-stage of patients who underwent surgery depended on the postoperative pathologic results, while for those who did not undergo surgery, they were determined according to the imaging results.
In conclusion, we sought to evaluate whether gastric cancer patients with distant metastases would benefit from surgery. The results showed that surgical treatment was able to improve effective survival time except patients with N2-N3 stage cancer. From the results of this study, we considered that patients who were younger and those with early stage primary tumors might obtain a greater survival benefit from surgical treatment than others. Additionally, radiation therapy may strengthen the survival benefit that is gained from surgical treatment. Furthermore, the improvements in the survival of patients who underwent PMTR and PTR were not statistically significant. As a result of more treatment complications and risks that are associated with PMTR in clinical practice, PTR may represent a better option for gastric cancer patients with distant metastases. Our study was a retrospective analysis with limitations and our conclusions should be further validated by a more prospective randomized trial.
ACKNOWLEDGMENTS AND FUNDING
This study was supported by Key Projects in the National Science and Technology Pillar Program during the Twelfth Five-year Plan Period, No. 2014BAI09B07 and by the Natural Science Foundation of Zhejiang Province, No.LY13H160019
Footnotes
CONFLICTS OF INTEREST
All authors have declared no conflicting interests, including but not limited to, commercial, personal, political, intellectual, or religious interests related to the article.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Institution NC. SEER Stat Fact Sheets: Esophagus. In: National Cancer Institution, 2015: Suggested citation: National Cancer Institution. SEER Stat Fact Sheets: Esophagus [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, Ono H, Tanabe S, Kaminishi M. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013(16):1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu JL, Deng MG, Li W, Zou RH, Li BK, Zheng Y, Lao XM, Zhou K, Yuan YF. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol. 2013(39):694–700. doi: 10.1016/j.ejso.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Toshiki S, Yoshiaki M, Tomonori H, Noriaki F. Survival Benefit of Surgical Treatment for Liver Metastases from Gastric Cancer. J Gastrointest Surg. 2015(19):1043–1051. doi: 10.1007/s11605-015-2775-6. [DOI] [PubMed] [Google Scholar]
- 8.Yagi Y, Seshimo A, Kameoka S. Prognostic factors in stage IV gastric cancer: univariate and multivariate analyses. Gastric Cancer. 2000(3):71–80. doi: 10.1007/pl00011699. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki H, Ninomiva M, Yano S. Evaluation of multimodlity therapy for synchronous liver metastases of gastric cancer. Gan To Kagaku Ryoho. 2004;31:1924–1926. [PubMed] [Google Scholar]
- 10.Roh HR, Suh KS, Lee HJ. Outcome of liver resection for metastatic gastic cancer. Am Surg. 2005(71):95–99. [PubMed] [Google Scholar]
- 11.Hirai I, Kimora W, Fuse A. Surgical management for metastatic liver tumors. Hepatogastroenterology. 2006(53):757–763. [PubMed] [Google Scholar]
- 12.Baba H, Okuyama T, Hiroyuki O, Anai H, Korenaga D, Maehara Y, Akazawa K, Sugimachi K. Prognostic factors for non-curative gastric cancer: univariate and multivariate analysis. J Surg Oncol. 1992(51):104–108. doi: 10.1002/jso.2930510208. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Yan M, Chen J, Xiang M, Zhu ZG, Yin HR, Lin YZ. Survival benefit of non-curative gastrectomy for gastric cancer patients with synchronous distant metastasis. J Gastrointest Surg. 2010(14):282–88. doi: 10.1007/s11605-009-1095-0. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi I, Kakeji Y, Emi Y, Sakurai M, Yonemura Y, Kimura Y, Maehara Y. S-1 in the treatment of advanced and recurrent gastric cancer: current state and future prospects. Gastric Cancer. 2003(6):28–33. doi: 10.1007/s10120-003-0228-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK, Shin DB, Kim HT, Kim HJ, Kang WJ, Suh CI, Bang YJ. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993;71:3813–3818. doi: 10.1002/1097-0142(19930615)71:12<3813::aid-cncr2820711205>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Sid P, Clinton D, Itzhak A. Liver resections in metastatic gastric cancer. HPB. 2010(12):589–596. doi: 10.1111/j.1477-2574.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodera Y, Fujitani K, Fukushima N, Ito S, Muro K, Ohashi N, Yoshikawa T, Kobayashi D, Tanaka C, Fujiwara M. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. 2014(17):206–212. doi: 10.1007/s10120-013-0299-x. [DOI] [PubMed] [Google Scholar]
- 18.Markar SR, Mikhail S, Malietzis G, Athanasiou T, Mariette C, Sasako M, Hanna GB. Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg. 2016(263):1092–101. doi: 10.1097/SLA.0000000000001542. [DOI] [PubMed] [Google Scholar]
- 19.L1 Martella, Bertozzi S, Londero AP, Steffan A, De Paoli P, Bertola G. Surgery for Liver Metastases From Gastric Cancer: A Meta-Analysis of Observational Studies. Medicine. 2015(94):e1113. doi: 10.1097/MD.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han DS, Suh YS, Kong SH, Lee HJ, Im SA, Bang YJ, Kim WH, Yang HK. Outcomes of Surgery Aiming at Curative Resection in Good Responder to Induction Chemotherapy for Gastric Cancer With Distant Metastases. J Surg Oncol. 2013(107):511–516. doi: 10.1002/jso.23284. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, Zhou B, Xu H. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013(13):577. doi: 10.1186/1471-2407-13-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, J Park do, Yoshikawa T, Hahn S, Nakamura K, et al. REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016(17):309–18. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 23.Samarasam I, Chandran BS, Sitaram V, Perakath B, Nair A, Mathew G. Palliative gastrectomy in advanced gastric cancer: is it worthwhile? ANZ J Surg. 2006;76:60–63. doi: 10.1111/j.1445-2197.2006.03649.x. [DOI] [PubMed] [Google Scholar]
- 24.Hartgrink HH, Putter H, E Klein Kranenbarg, Bonenkamp JJ, van de Velde CJ. Dutch Gastric Cancer Group. Value of palliative resection in gastric cancer. Br J Surg. 2002(89):1438–1443. doi: 10.1046/j.1365-2168.2002.02220.x. [DOI] [PubMed] [Google Scholar]