Abstract
The amygdala is an integrator of affective processing, and a key component of a network regulating social behavior. While decades of lesion studies in non-human primates have shown alterations in social interactions after amygdala damage, acute manipulations of the amygdala in primates have been under-explored. We recently reported (Wellman, Forcelli, Aguilar, & Malkova, 2016) that acute pharmacological inhibition of the basolateral complex of the amygdala (BLA) or the central nucleus of the amygdala increased affiliative social interactions in experimental dyads of macaques; this was achieved through microninjection of a GABA-A receptor agonist. Prior studies in rodents have shown similar effects achieved by blocking NMDA receptors or AMPA receptors within the BLA. Here, we sought to determine the role of these receptor systems in the primate BLA in the context of social behavior. In familiar dyads, we microinjected the NMDA receptor antagonist AP7 or the AMPA receptor antagonist NBQX and observed behaviors and social interactions in the immediate post-injection period. In striking contrast with our prior report using GABA agonists, and in contrast with prior reports in rodents using glutamate antagonists, we found that neither NMDA or AMPA blockade increase social interaction. Both treatments, however, were associated with decreases in locomotion and manipulation and increases in passive behavior. These data suggest that local blockade of glutamatergic neurotransmission in BLA is not the functional equivalent of local activation of GABAergic signaling, and raise interesting questions regarding the functional microcircuitry of the non-human primate amygdala in the context of social behavior.
Keywords: social, macaque, primate, pharmacological inactivation, glutamate
INTRODUCTION
The amygdala is widely recognized as a critical hub in the brain networks mediating social behavior. In the eighty years following the foundational studies of Kluver and Bucy (Klüver & Bucy, 1937), lesion studies in non-human primates have revealed profound deficits in social behavior, including decreased social dominance, alterations in anxiety, and reduced affiliative interactions (Kalin, Shelton, & Davidson, 2004; A. Kling & Brothers, 1992; Machado & Bachevalier, 2006; Malkova, Mishkin, Suomi, & Bachevalier, 2010; Martine Meunier & Bachevalier, 2002; M. Meunier, Bachevalier, Murray, Málková, & Mishkin, 1999; Rosvold, Mirsky, & Pribram, 1954). These effects have been reported both after damage to the amygdala in adult animals, as well as after damage in the neonatal period (Bachevalier, Málková, & Mishkin, 2001; Eliza Bliss-Moreau, Bauman, & Amaral, 2011; Eliza Bliss-Moreau, Moadab, Bauman, & Amaral, 2013; Eliza Bliss-Moreau, Toscano, Bauman, Mason, & Amaral, 2010; E. Bliss-Moreau, Toscano, Bauman, Mason, & Amaral, 2011; Malkova et al., 2010; Moadab, Bliss-Moreau, & Amaral, 2015; Raper, Stephens, Sanchez, Bachevalier, & Wallen, 2014; Raper, Wilson, Sanchez, Machado, & Bachevalier, 2013).
While the majority of lesion studies found reduced social interaction after amygdala damage, one study following neurotoxic lesions of the amygdala resulted in increased social behavior (Emery et al., 2001; Machado et al., 2008). Moreover, we recently reported that transient pharmacological inhibition of the basolateral complex of the amygdala (BLA; i.e., the lateral, basal, and accessory basal nuclei) by microinjection of the GABA-A receptor agonist, muscimol, induced a profound increase in social interactions in familiar dyads of macaques (Forcelli et al., 2016; Wellman et al., 2016). We found a similar profile when central nucleus of the amygdala (CeA) was injected with muscimol (Wellman et al., 2016). In one of these studies, which was the first to separately examine the BLA and CeA, we also found that disinhibition of the BLA, but not CeA resulted in reduced social interactions (Wellman et al., 2016).
The use of transient pharmacological manipulations has the potential benefit of avoiding compensatory reorganization after damage, but, more importantly in the context of the present study, allows for the separate probing of discrete receptor systems on a within-subject basis. For example, in a rat social interaction test, microinjection of NMDA or AMPA receptor antagonists into BLA significantly increase social behavior (Sajdyk & Shekhar, 1997). While the microinjection approach has long been standard in rodent models, their use has remained limited in the primate. Following up on our recent study, and based on prior findings in rodents, here, we sought to examine the contribution of glutamatergic neurotransmission within the BLA to social behavior in primates. We employed highly familiar experimental dyads (to avoid any confounds associated with social-novelty induced anxiety), and microinjected, in separate sessions, the NMDA receptor antagonist AP7 or the AMPA receptor antagonist NBQX into the BLA. Following microinjection, we assessed general behavior and dyadic social interactions. We consider these results in the context of our recent findings (Forcelli et al., 2016; Wellman et al., 2016).
MATERIALS AND METHODS
Animals
Four pigtail macaques (Macaca nemestrina) were used in this study, 2 female (SA, JN) and 2 male (CH, ZK). They were born and raised as infants in the Infant Primate Research Laboratory at the University of Washington Regional Primate Research Facility, in a way similar to that described previously (Novak & Sackett, 1997). At ~6 months of age, the animals were transferred to Georgetown University where all experimental procedures were conducted. Here, all monkeys were pair-housed within two joined individual cages (size: 61 × 74 × 76 cm each). These animals were part of a larger colony and were raised in groups of three to four monkeys of the same age and thus were also routinely paired with other animals not included in this study. The combinations of pairs within and across these 3–4 monkey groups rotated over days and weeks to identify compatible pairs. Because of this housing design, all animals in this study were highly familiar with each other. For the experiments in this study, stable dyads were formed and used for all social measures for a given infused subject.
Monkeys were housed in a temperature (24C) and humidity controlled room with a standard 12-hr light/dark cycle. When not performing concurrent cognitive testing, animals were given full feed (Primate Lab Diet, #5049, Purina Mills Inc. International, Brentwood, MD) supplemented with fresh fruit. Water was also available ad libitum in the home cage. Care and housing of the monkeys met or exceeded the standards as stated in the Guide for Care and Use of Laboratory Animals (National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), & National Academies Press (U.S.), 2011), ILAR recommendations and AAALAC accreditation standards. The study was conducted under a protocol approved by the Georgetown University Institutional Animal Care and Use Committee (Protocol No. 2016 - 1115. Neural Substrates of Social and Emotional Behavior).
The present experiments began after the animals were extensively socialized and behaviorally trained (including chair-training), at approximately two years of age. In addition to the experimental procedures described here, all subjects were trained on various cognitive tasks administered at the Wisconsin General Testing Apparatus; the tasks included visual object discrimination, visual delayed non-matching to sample, crossmodal auditory-visual matching task, and reinforcer devaluation. As part of those experiments, some animals received drug infusions in BLA (animals JN, and ZK); (Wellman, Gale, & Malkova, 2005)). Additionally, three of the four subjects in this study also received microinjections in BLA or CeA for another study of social behavior (JN, ZA, CH; (Wellman et al., 2016)); SA was experimentally naive at the time of this study.
Surgical Implantation of Cranial Infusion Platform and Localization of Infusion sites
Monkeys were implanted with stereotaxically positioned chronic infusion platforms as we have described extensively elsewhere (DesJardin et al., 2013; Dybdal et al., 2013; Forcelli et al., 2014, 2016; Holmes et al., 2012; Malkova et al., 2015; Wellman et al., 2016, 2005; West, DesJardin, Gale, & Malkova, 2011). This platform enabled us to target the amygdala based on the coordinates assessed by structural MRI scans. Briefly, each monkey received a T1-weighted magnetic resonance imaging (MRI) scan to calculate pre-operative coordinates for the platform implantation. This scan occurred between two weeks and two months prior to surgery. The infusion platform was implanted under anesthesia and aseptic conditions followed by a postoperative regimen of analgesics and antibiotics determined in consultation with the facility veterinarian.
Postoperatively, each monkey received at least one T1-weighted scan with tungsten microelectrodes (FHC, Bowdoinham, ME) placed dorsal to the infusion sites calculated based on the pre-operative MRI. Post-operative scans occured a minimum of two weeks after surgery. The position of these electrodes, which were visible on the scan, were then used to adjust the final infusion coordinates as needed. Electrodes were typically placed 5 to 10 mm dorsal to the structure of interest. Our platform allows for 2 mm resolution in the anteroposterior and mediolateral planes, and sub-mm resolution in the dorsoventral plane.
Drug Solutions and Intracerebral Infusions
AP7, an NMDA receptor-specific antagonist (2-amino-7-phosphonoheptanoic acid; RBI) and NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide; Sigma), an AMPA receptor-specific antagonist, were used for microinfusions. NBQX (as the disodium salt) was dissolved in a small volume of dilute NaOH, neutralized with dilute HCl, and adjusted to a final concentration of 20 mM by the addition of sterile water. AP7 was dissolved in sterile saline at a final concentration of 20 mM. Drug solutions were filtered (20 µm pore size) before storing as 1 ml frozen aliquots (20°C). 1 µl volumes of drug were infused for final doses of 20 nmol per site. This is the same dose of NBQX we had previously found to be effective at disrupting performance on the delayed non match to sample task after microinjection into perirhinal cortex (Malkova et al., 2015). The dose of AP7 is 40% of that employed in our previous study (Malkova et al., 2015).
Drug infusions were performed aseptically, while the monkey was seated in a standard primate chair (Crist Instruments, Inc) with minimal restraint. Infusions were performed using procedures we have previously described (Malkova et al., 2015). We bilaterally targeted the BLA via removable cannulae that were inserted acutely for each infusion. Cannulae were connected via sterile tubing, to a Hamilton syringe. The syringe was driven by an infusion pump calibrated to deliver 0.2 µl per minute; the cannulae were allowed to remain in place for a minimum of 1 min prior to drug injection to prevent reflux up the cannula track. The entire infusion procedure lasted 10–15 min, after which behavioral observations were initiated.
Each animal received 3 injections with AP7 and 3 injections with NBQX. Each infusion was preceded (by 24 h) by a matched baseline session (a total of 6 baseline sessions). Each subject also received 1 injection with saline (0.9% sodium chloride, 1 µl) as a control.
Behavioral Assessment
As described above, animals were observed during baseline and drug infused sessions (a total of twelve sessions per animal). For this purpose, the experimental monkey was placed into an observation cage (61 × 74 × 76 cm) with one partner and the pair was videotaped for 15 minutes with no experimenters present in the room; the pair was then returned to their home cages. 24 hours later, the experimental monkey was drug-infused, and immediately after the infusion, the two monkeys were again placed in the observation cage and were videotaped.
The videotapes were analyzed using the software program The Observer (Noldus Information Technology, Wageningen, Netherlands) according to an established ethogram (Wellman et al., 2016) modified from those previously used: (Bachevalier et al., 2001; Malkova et al., 2010). A list of operational definitions for the behavioral categories is provided in Table 1. Analyses of two observers (LLW and PAF) were used for statistical analysis, however, additional observers were trained to achieve a high level of inter-observer correlation (r=0.9 or better) and analyzed a subset of videotapes.
Table 1.
| General (Non-Social) | |
| Behavior | Description |
| Locomotion | Walks, runs, climbs or jumps |
| Manipulation | Handles, chews, licks, moves, or smells objects or cage parts |
| Passive | Inactive, stays in one location |
| Self-Directed | Engages in self-directed behaviors, i.e. self-grooms, hugs head, self-grabs and bites, presses face with hands, self-holds, closes fists, self-clutches, sexually self-stimulates, prone, or head on chest |
| Motor Stereotypies | Repeatedly paces, somersaults, circles, swings |
| Vocalization | Emits calls |
| Social | |
| Behavior | Description |
| Approach | Initiates social contact; moves body or head towards the conspecific |
| Mounting | Mounts the conspecific |
| Aggression | Makes threatening gestures (i.e., mouth threat, head or body lunge, cage shake) towards or hits, grabs or bites the conspecific |
| Isolation | Sits alone |
| Contact | Touches or holds the conspecific, not covered by any other behavior |
| Play | In contact with the conspecific, includes chasing, wrestling, and “rough and tumble” behaviors |
| Grooming | Subject grooms the conspecific |
| Solicitation of Grooming |
Subject presents for grooming |
| Withdrawal | Moves away from the conspecific when approached |
| Total Contact | Includes contact, mounting, grooming, and play |
Histology
Animals were perfused and brains processed for localization of infusion sites, as we have previously described (Dybdal et al., 2013; Forcelli et al., 2014; Wellman et al., 2005). One animal (SA) was unavailable for histological processing, however, histological analysis was performed on animals JA, CH, and ZA. Representative photomicrographs are presented in Figure 1.
Figure 1.

We found a close correspondence between histologically-verified infusion sites and those determined by MRI (see A and D for subject ZA and B and E for subject JA). Electrode tips were located within the basal, accessory basal, ventrobasal, and lateral subnuclei of the amygdala. (A) Infusion site for ZA (symbols match those used in Figs 2 and 3), (B) Infusion sites for JA, (C) Infusion sites for ZA. (D and E) MRI showing electrodes tips placed dorsal to the intended infusion site. Arrows indicate cannula tip (A–C) or electrode tip (D-E). Dotted line in E indicates the intended extension from the original electrode artifact. (F) Schematic showing the intended infusion region (shaded) encompassing the basal nuclei and the lateral nucleus. Symbols indicate positions of infusions.
Statistical Analysis
Data were analyzed using SPSS (IBM, Armonk, NY) and GraphPad Prism (GraphPad Software, Inc, La Jolla, CA).
Passive behavior, locomotion, manipulation, and total contact were transformed (square root of the value + 1) to normality; these behaviors were analyzed using parametric methods. Approach, play, grooming and soliciting grooming occurred rarely, were severely non-normal, and were thus analyzed using nonparametric methods.
At the onset of these studies, we were uncertain if behavioral responses would be impacted by repeated drug infusion; for this reason, we included baselines as control sessions prior to each drug infusion. We performed saline infusions for each subject (2 for SA, 1 each for JA, CH, and ZA), and compared these sessions statistically to the mean of the sessions for each subject [paired t-test for passive, manipulation, locomotion and total contact; Wilcoxon test for approach, play, grooming, and soliciting grooming]. In no case did saline differ from baseline (Ps = 0.09–0.99). Thus, for statistical analysis, we collapsed across baselines and saline sessions, providing a more stable estimate of behavior in the absence of experimental manipulations. Consistent with our recent report (Wellman et al., 2016), we chose not to collect additional saline injections, as this would have doubled the number of brain penetrations and increased the risk of brain damage. To determine if repeated infusions altered responses, we performed one-way ANOVA (for passive, manipulation, locomotion and total contact) for each experimental treatment (Baseline, AP7 and NBQX), with session as a repeated measure. There was no main effect of session for any behavior or condition (Fs = 0.04–2.1, Ps = 0.21–0.90). Friedman’s test (for approach, play, grooming and soliciting grooming) likewise failed to show an effect of session (Ps =0.36–0.91); the exception to this was the NBQX condition for play, an effect that was entirely driven by an an outlier (see below).
Following the above analysis, we next examined the data for extreme values using the conventional Tukey criteria of values falling > 3× the interquartile range. We excluded three sessions: two from JA (2 AP7 sessions) and one CH (one NBQX session), as these sessions produced extreme values in two or more behavioral categories.
After outlier removal, passive behavior, manipulation, locomotion and total contact were entered into a linear mixed model. Treatment and treatment-by-category interactions were specified as fixed effects (repeated within monkey), with monkey as a random factor. We examined simple effects of treatment within each behavioral category. Pairwise comparisons within each behavioral category with a significant simple effect of treatment were performed (Baseline vs. AP7; Baseline vs. NBQX) and corrected for multiple comparisons using the step-down method of Holms. P values < 0.05 (two-tailed) were considered to be statistically significant.
For approach, play, grooming and soliciting grooming a single estimate for each behavior within each treatment for each monkey was calculated as the mean of the values after outlier removal (as above). These means were analyzed by Freidman's test with Dunn's post-hoc test. P values < 0.05 (two-tailed) were considered to be statistically significant.
RESULTS
Histological Verification of Infusion Sites
As shown in Fig 1, our intended sites closely matched the sites confirmed histologically (Fig 1A–C) and via MRI (Fig 1D–E). For ZA (Fig 1A) cannulae were localized to the accessory basal nucleus and to the lateral nucleus. For JA (Fig 1B) cannulae were located at the border of the accessory basal and basal nuclei, and at the border of the basal and lateral nuclei. For CH (Fig 1C), injections were localized to the border of the basal and lateral nuclei. For SA, histology was unavailable, but the infusion site was aimed at the border of the lateral and basal nuclei based on MRI scans. Sub-nuclei are labeled according to the conventions of (Chareyron, Banta Lavenex, Amaral, & Lavenex, 2011). This close co-registration between infusion sites on MRI and histological reconstruction (compare Fig 1A to Fig 1D and Fig 1B to Fig 1E) is consistent with our prior reports. Further consistent with our prior studies, even with repeated microinjections, damage was minimal.
Effects of glutamatergic blockade
Mixed model analysis of the behaviors presented in Fig 2A–C and Fig 3A, revealed a significant effect of treatment (F2,23=10.8, P<0.0005) and behavior by treatment interaction (F9,141.4=25.4, P<1×10−25). Pairwise comparisons revealed that both the AP7 and NBQX treated conditions differed from baseline (Ps <0.0005 and <0.05, Holm corrected, respectively). Simple effects of treatment for each behavior are described below.
Figure 2. NMDA or AMPA receptor antagonists in BLA increase passive and decrease active behaviors.
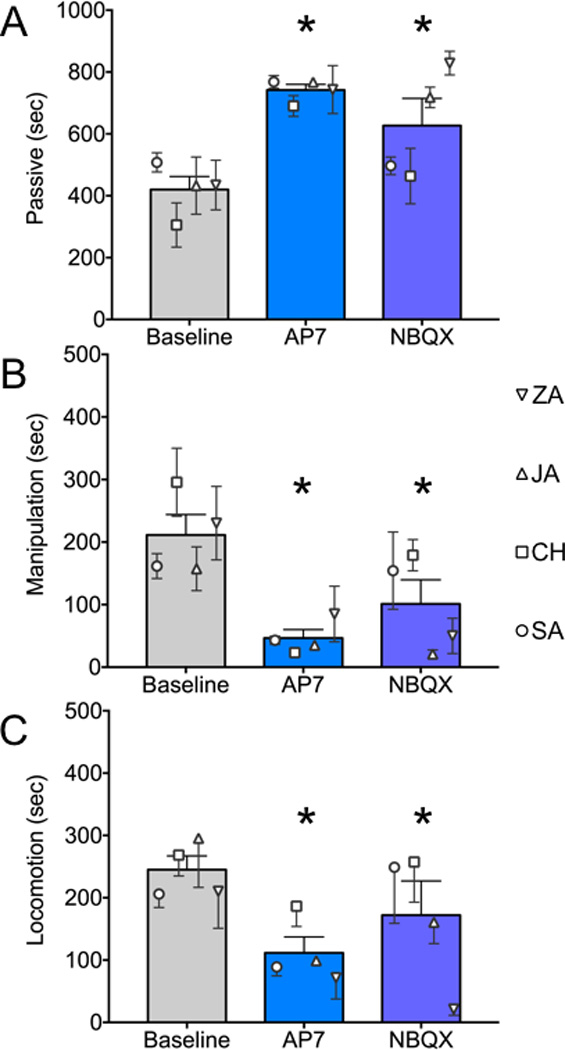
(A) Passive behavior, as defined in Table 1, under baseline, AP7 infused or NBQX infused conditions. (B) Manipulation (as defined in Table 1). (C) Locomotion (as defined in Table 1). Symbols indicate the mean (+SEM) value for each animal. Bars show group means + SEM. * = significantly different than baseline, P<0.05, Sidak-corrected.
Figure 3. Effect of NMDA or AMPA blockade in BLA on social behaviors.

(A) Total contact (as defined in Table 1); while the main effect of treatment for this behavior approached, but did not reach statistical significance, comparison between AP7 and Baseline sessions revealed a decrease in contact after AP7 infusion (P<0.05) (B) Play behavior (C) Approach, (D) Grooming and (E) Soliciting Grooming. Symbols follow the conventions in Figs 1 and 2. Symbols indicate the mean (+SEM) value for each animal. Bars show group means + SEM. * = significantly different than baseline, P<0.05, Sidak-corrected.
Passive behavior (Fig 2A) differed significantly across treatments (F2,173.9=10.7, P<0.00005). Under baseline conditions, the experimental subjects were passive for a mean of 420 sec, equating to ~47% of the observation period. Passive behavior was increased by microinjection of either AP7 or NBQX into the BLA (Ps <0.0001 and <0.005, Holm corrected, respectively). After AP7 injection, a mean of 82% of the session [742 sec], was spent passive, whereas with NBQX microinjection a mean of 70% of the session [627 sec] was spent passive.
As with passive behavior, manipulation and locomotion likewise differed significantly across treatments (F2,159.9=12.5, P<0.00001 and F2,159.9=4.8, P<0.01, respectively). Unlike time spent passive, the duration of time spent engaged in manipulation and locomotion decreased after microinjection of either AP7 (Ps < 0.00005 and < 0.01, Holms corrected, respectively) or NBQX (Ps < 0.005 and 0.05, Holms corrected, respectively) into BLA. For manipulation, this equated to approximately 24% of the observation period under baseline conditions, and only 5% and 11% under AP7 and NBQX-infused, conditions respectively. Similarly, locomotion accounted for 27% of the observation period under baseline conditions and only 12 and 19% respectively after AP7 or NBQX infusion.
In addition to these general behavioral categories, we also assessed social behaviors. While withdrawal, aggression, and mounting were present in our ethogram, they were absent in all conditions in this study and thus are not presented here. As a general measure of social behavior, we assessed time spent in contact with the conspecific, which accounted for 19% of the observation period. The effect of treatment on total contact (Fig 3A) approached, but did not reach, the level of statistical significance (F2,173.6=2.7, P=0.07). Comparisons between AP7 and baseline (P<0.05) indicated a decrease in social contact (all 4 animals) after AP7 treatment, whereas no such pattern was present after NBQX treatment (P=0.15). The only social behavior to differ as a function of treatment was play (Fig 3B), which was significantly decreased by NBQX, but not AP7 treatment (Friedman’s test, X2=6.5, df=2, P<0.05; Dunn’s post-test, P<0.05). Even though AP7 treatment did not reach the level of statistical significance, it is worth noting that the duration of play was numerically decreased in all four animals. Approach, grooming and solicitation of grooming did not differ as a function of treatment (Friedman’s test, X2=2; X2=2.3; X2=3.9 respectively; all Ps >0.2).
DISCUSSION
Here we report that blockade of glutamatergic neurotransmission (either AMPA or NMDA-receptor mediated) within the basolateral complex of the amygdala resulted in a suppression of active behavior without a resultant increase in social behavior. In fact, we found a borderline-significant decrease in social contact. These data stand in stark contrast to our prior findings (Forcelli et al., 2016; Wellman et al., 2016), wherein we reported that transient inhibition of the BLA by microinjection of muscimol resulted in a striking pro-social effect. These data also differ from prior studies in rats, which likewise demonstrated a pro-social effect of NMDA or AMPA receptor blockade (Sajdyk & Shekhar, 1997).
Our finding of increased passive behavior following glutamate receptor blockade (present study) is consistent with our recent reports following GABA agonism in BLA (Forcelli et al., 2016; Wellman et al., 2016). In both of these studies, locomotion was decreased concurrent with an increase in passive behavior. These findings are in part consistent with prior reports in rodents. For example, we have previously found a decrease in response vigor in rats after microinjection of muscimol in the BLA (West et al., 2012) during an operant reinforcer devaluation task. By contrast, microinjection of NMDA receptor antagonists in the BLA of mice (Masneuf et al., 2014) has been reported to suppress both anxiety-like behavior and to increase locomotor activity. The degree to which locomotor increases in the mice were due to a decrease in anxiety, rather than a general pro-locomotor effect is an open question. Consistent with this finding, glucocorticoids (which are anxiogenic in rodents) trigger increases in excitatory postsynaptic currents in neurons of the amygdala. This effect is mediated by glucocorticoid receptors expressed on glutamatergic, but not GABAergic neurons (Hartmann et al., 2016). However, in the present experiments, our animals were not tested in an environment likely to promote anxiety, rather, they were tested in their home cage with a highly familiar conspecific. This suggests that modulation of anxiety is unlikely to have contributed to the changes in behavior we observed. The possibility remains that had we tested animals in isolation, or with a novel, rather than familiar conspecific, the outcomes would be different.
Our present findings may also relate to the report by Kling et al in cebus monkeys with lesions to the temporal pole (A. S. Kling, Tachiki, & Lloyd, 1993). These animals displayed classic Klüver-Bucy syndrome phenotypes, especially early in the post-operative period. Behavioral alterations included lethargy, tameness, and decreased social status. Interestingly, these animals displayed decreased glutamate in the amygdala (which was left intact during surgery) as assessed by microdialysis (A. S. Kling et al., 1993). In particular, the decrease in active behaviors we found with glutamate antagonists in BLA may relate to the tameness and lethargy seen in prior studies.
With respect to social behavior, in two recent studies, we found that muscimol microinjection into BLA resulted in an increase in total social contact, this increase averaged 2-fold in one study (Wellman et al., 2016) examining dyads of pigtail macaques (as were used in the present study) and 3-fold in the other (Forcelli et al., 2016) examining dyads of rhesus macaques. In the former study, we also found a 1.7-fold increase in passive behaviors. Here, we found a 2-fold increase in passive behaviors after microinjection of AP7 and a 1.6-fold increase in passive behaviors after microinjection of NBQX. The finding that passive behavior was increased to a similar degree following glutamate antagonist treatments in this study, as compared to GABA agonist treatment in our prior study, provides an important positive control in light of our failure to detect increases in social behavior after glutamate antagonist infusion. Similarly, when we compare our doses of AP7 and NBQX to those used in the rat, (100 pmol and 50 pmol of AP7 and NBQX, respectively) (Sajdyk & Shekhar, 1997), we employed a 200× and 40× greater dose; this is consistent with the 32–39% greater volume of the basolateral complex in the monkey as compared to the rat (Chareyron et al., 2011). Thus, we think it unlikely that ineffective coverage or drug concentration can explain the differences between the present study and prior reports.
How then, can we interpret our findings in light of the pro-social effect reported after intra-BLA NMDA or AMPA blockade in rodents? The critical difference may again, rest with anxiety. The social interaction task employed by Shekhar and colleagues (Sajdyk & Shekhar, 1997) is commonly used as a measure of anxiety-like behavior in rodents (File & Seth, 2003); in this task, animals are briefly exposed to a novel conspecific. By contrast, our animals were highly familiar with one another, and thus, anxiety associated with social novelty is unlikely to have played a role. The degree to which glutamate antagonists would increase social behavior in experimentally unfamiliar dyads remains an open question; if the pro-social effect is secondary to anxiolysis, one might expect an increase in social behavior. Arguing against this, however, we have reported that GABA agonists in BLA increase social behavior in familiar dyads (whereas systemic diazepam does not), suggesting that at least in our experimental setup anxiety is not a critical contributor (Wellman et al., 2016).
Rodent data also shed light on our finding that both NMDA- and AMPA-receptor blockade produced equivalent effects. The classic mode of NMDA receptor function requires membrane depolarization to displace the blockade of the receptor pore by Mg2+; a likely source of the needed membrane depolarization is AMPA-mediated signaling. However, in rodent BLA, under normal physiological conditions (i.e., resting membrane potential, physiological concentrations of Mg2+ ions), NMDA-mediated currents are still detectible in BLA principal cells, even in the presence of AMPA antagonist (Rainnie, Asprodini, & Shinnick-Gallagher, 1991). These data suggest that at rest, NMDA-receptor mediated neurotransmission may occur, even in the absence of AMPA-mediated events. These data raise the possibility that the effects of AMPA antagonist infusion in the present study were due to per se blockade of AMPA transmission, and not due to reduced NMDA receptor mediated transmission following AMPA blockade.
Principal cells within the basolateral nucleus in mice display powerful, bicuculline-sensitive tonic GABA currents (Marowsky, Rudolph, Fritschy, & Arand, 2012), with a lesser degree of tonic current seen in neurons of the lateral nucleus. In our recent paper, when we blocked GABA-mediated synaptic transmission in the BLA, thus disinhibiting the structure, we found a decrease in social behavior (Wellman et al., 2016). This is consistent with the high degree of preferential co-localization between GABAergic terminals and putative excitatory neurons (with sparser input to GABAergic neurons) within the BLA (McDonald & Augustine, 1993). Bicuculline application within the amygdala would thus suppress both tonic and phasic GABAergic input to principal cells, with a net increase in excitability of these neurons.
By contrast, when we applied muscimol to the amygdala, we found increased social behavior. Microinjection of muscimol will indiscriminately act on both projection neurons and interneurons expressing GABA receptors. While action of muscimol on GABAergic interneurons would suppress their firing, and reduce GABA release, suppression of these neurons, may, however, have a negligible total effect, as direct activation of post-synaptic GABA receptors on principal cells (e.g., glutamatergic neurons) would have the net effect of suppressing amygdala outputs. With respect to glutamatergic blockade, electrophysiological experiments conducted in slices of the rat amygdala have demonstrated that bath application of AP-V or CNQX both significantly attenuated (and in some cases abolished) inhibitory post-synaptic currents recorded from principal cells in the BLA (Rainnie et al., 1991). Thus, suppression of excitatory neurotransmission may have a secondary consequence of decreasing GABAergic signaling within the BLA in the present study; this could have an intermediate effect on output neurons, as decreased glutamatergic drive onto these neurons may have been compensated for by decreased tonic and phasic GABAergic input. Consistent with this interpretation, we found a 49% decrease in social contact in the present study when AP7 was infused into BLA; this matches the magnitude of suppression in social contact seen in our prior study after infusion of bicuculline into the BLA (49%, (Wellman et al., 2016)).
The precise intra-amygdala and amygdalofugal circuit that is impacted by focal application of AP7 or NBQX in our studies remains unknown, however, there are several plausible candidates. (1) The basal and lateral nuclei send input to the CeA (Aggleton, 1985; Fudge & Tucker, 2009; Pitkanen & Amaral, 1998), which we have previously demonstrated to play a role in the control of social behavior (Wellman et al., 2016). The CeA in turn has projections to the bed nucleus of the stria terminalis (Oler et al., 2016) and brainstem regions regulating arousal (Jongen-Rêlo & Amaral, 1998), which may be positioned to modulate approach. (2) Recent studies in rodents have identified projections from the BLA to prelimbic/infralimbic cortex (the rodent homolog of the anterior cingulate) and projections from BLA to ventral hippocampus as modulators of social interaction. Using optogenetic methodologies, when these projections were silenced, reduced social interaction was observed in the resident-intruder test. Moreover, inhibition of these pathways facilitated social interaction (A. C. Felix-Ortiz, Burgos-Robles, Bhagat, Leppla, & Tye, 2016; Ada C. Felix-Ortiz & Tye, 2014). Similar pathways have been described from the amygdala to hippocampus (Saunders, Rosene, & Van Hoesen, 1988) and from the amygdala to the anterior cingulate (Amaral & Price, 1984; Porrino, Crane, & Goldman-Rakic, 1981). The degree to which selective modulation of these projections would alter social behavior in the primate remains unexplored. (3) The amygdala, and the BLA, in particular, provide an important source of input to the nucleus accumbens (Cho, Ernst, & Fudge, 2013; deCampo & Fudge, 2013). This projection may play a role both in social reward (Báez-Mendoza & Schultz, 2013), as well as modulation of motor activity. Perhaps suppression of activity within the BLA biases ventral striatal circuitry away from action initiation by reducing excitatory input. The importance of amygdalostriatal projections has been well-documented in rodent studies (Stuber et al., 2011; Zorrilla & Koob, 2013) and remains to be examined in the primate. While each of these pathways may play a role in the control of social behavior, it is likely that each contributes to a different aspect of the behaviors we have described here and previously. For example, we have previously found that inactivation of the amygdala suppresses cowering in response to activation of the superior colliculus, but not other aspects of colliculus-evoked defensive responses (Forcelli et al., 2016). Similarly, in rodent studies, we have found that amygdala interactions with ventral pallidum influence prepulse inhibition, while separate outputs from the amygdala modulate overall startle reflexes (Forcelli, West, Murnen, & Malkova, 2012). Moreover, recent optogenetic studies have identified discrete outputs from the mouse BLA coding for valence of conditioned stimuli (Beyeler et al., 2016; Namburi et al., 2015).
With the recent application of next-generation methods for neuronal modulation and circuit analysis (e.g., chemogenetics) in non-human primates (Aguilar, Elorette, Huizenga, Forcelli, & Malkova, 2015; Eldridge et al., 2016), dissecting the separate contributions of these pathways to social behavior, motivation and approach in primates is now within reach. At the present time, we are left with the conclusion that inhibition of glutamatergic neurotransmission within the BLA is not the functional equivalent of activation of GABAergic signaling within this structure. This pattern, which diverges from our previous reports of pharmacological manipulation within the primate hippocampus (Forcelli et al., 2014), underscores the importance of considering microcircuitry within the amygdala in the control of these behavioral processes. While the drug microinjection approach has been informative, our manipulations target all neurons in a given structure, perhaps obscuring effects of local microcircuits. Next-generation, viral mediated methods may enable cell-type specific targeting (e.g., glutamatergic vs. GABAergic neurons) within the primate amygdala, perhaps providing the microcircuit-level analysis needed to decipher the relative contributions of individual cell populations to these complex behaviors.
Acknowledgments
Funding: The study was supported in part by R01 MH084069 (LM), R01 MH082364 (LM), K02 HD042269 (LM), Cure Autism Now (now Autism speaks; LM), National Alliance for Autism Research (NAAR; now Autism speaks; LM), F32 MH067414 (LLW), and KL2 TR001432 (PAF). The authors wish to thank Carrie Silver, Caitlyn Clark, Bryn Gaertner, and Pete Maniatis for technical support.
ABBREVIATIONS
- AP7
2-amino-7-phosphonoheptanoic acid
- NBQX
2, 3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide
- BLA
basolateral complex of the amygdala
- CeA
central nucleus of the amygdala
References
- Aggleton JP. A description of intra-amygdaloid connections in old world monkeys. Experimental Brain Research. 1985;57(2):390–399. doi: 10.1007/BF00236545. [DOI] [PubMed] [Google Scholar]
- Aguilar B, Elorette C, Huizenga M, Forcelli P, Malkova L. Presented at the Society for Neuroscience. Chicago: 2015. Chemogenetic control of motor behavior in the non-human primate: DREADD-mediated silencing of the substantia nigra pars reticulata. [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of Comparative Neurology. 1984;230(4):465–496. doi: 10.1002/cne.902300402. https://doi.org/10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Málková L, Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]
- Báez-Mendoza R, Schultz W. The role of the striatum in social behavior. Frontiers in Neuroscience. 2013;7 doi: 10.3389/fnins.2013.00233. https://doi.org/10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Tye KM. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron. 2016;90(2):348–361. doi: 10.1016/j.neuron.2016.03.004. https://doi.org/10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behavioral Neuroscience. 2011;125(6):848–858. doi: 10.1037/a0025757. https://doi.org/10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Bauman MD, Amaral DG. The impact of early amygdala damage on juvenile rhesus macaque social behavior. Journal of Cognitive Neuroscience. 2013;25(12):2124–2140. doi: 10.1162/jocn_a_00483. https://doi.org/10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Developmental Psychobiology. 2010;52(5):487–503. doi: 10.1002/dev.20451. https://doi.org/10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience. 2011;178:123–132. doi: 10.1016/j.neuroscience.2010.12.038. https://doi.org/10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Banta Lavenex P, Amaral DG, Lavenex P. Stereological analysis of the rat and monkey amygdala. The Journal of Comparative Neurology. 2011;519(16):3218–3239. doi: 10.1002/cne.22677. https://doi.org/10.1002/cne.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Ernst M, Fudge JL. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(35):14017–14030. doi: 10.1523/JNEUROSCI.0170-13.2013. https://doi.org/10.1523/JNEUROSCI.0170-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCampo DM, Fudge JL. Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: comparison with ventral striatal afferents. The Journal of Comparative Neurology. 2013;521(14):3191–3216. doi: 10.1002/cne.23340. https://doi.org/10.1002/cne.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesJardin JT, Holmes AL, Forcelli PA, Cole CE, Gale JT, Wellman LL, Malkova L. Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(1):150–155. doi: 10.1523/JNEUROSCI.2924-12.2013. https://doi.org/10.1523/JNEUROSCI.2924-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdal D, Forcelli PA, Dubach M, Oppedisano M, Holmes A, Malkova L, Gale K. Topography of dyskinesias and torticollis evoked by inhibition of substantia nigra pars reticulata. Movement Disorders: Official Journal of the Movement Disorder Society. 2013;28(4):460–468. doi: 10.1002/mds.25215. https://doi.org/10.1002/mds.25215. [DOI] [PubMed] [Google Scholar]
- Eldridge MAG, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ, Richmond BJ. Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nature Neuroscience. 2016;19(1):37–39. doi: 10.1038/nn.4192. https://doi.org/10.1038/nn.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. https://doi.org/10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(2):586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. https://doi.org/10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, DesJardin JT, West EA, Holmes AL, Elorette C, Wellman LL, Malkova L. Amygdala selectively modulates defensive responses evoked from the superior colliculus in non-human primates. Social Cognitive and Affective Neuroscience. 2016 doi: 10.1093/scan/nsw111. https://doi.org/10.1093/scan/nsw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Palchik G, Leath T, Desjardin JT, Gale K, Malkova L. Memory loss in a nonnavigational spatial task after hippocampal inactivation in monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1320562111. https://doi.org/10.1073/pnas.1320562111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, West EA, Murnen AT, Malkova L. Ventral pallidum mediates amygdala-evoked deficits in prepulse inhibition. Behavioral Neuroscience. 2012;126(2):290–300. doi: 10.1037/a0026898. https://doi.org/10.1037/a0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Tucker T. Amygdala projections to central amygdaloid nucleus subdivisions and transition zones in the primate. Neuroscience. 2009;159(2):819–841. doi: 10.1016/j.neuroscience.2009.01.013. https://doi.org/10.1016/j.neuroscience.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Dedic N, Pöhlmann ML, Häusl A, Karst H, Engelhardt C, Schmidt MV. Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Molecular Psychiatry. 2016 doi: 10.1038/mp.2016.87. https://doi.org/10.1038/mp.2016.87. [DOI] [PubMed] [Google Scholar]
- Holmes AL, Forcelli PA, DesJardin JT, Decker AL, Teferra M, West EA, Gale K. Superior colliculus mediates cervical dystonia evoked by inhibition of the substantia nigra pars reticulata. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(38):13326–13332. doi: 10.1523/JNEUROSCI.2295-12.2012. https://doi.org/10.1523/JNEUROSCI.2295-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen-Rêlo AL, Amaral DG. Evidence for a GABAergic projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: a combined retrograde tracing and in situ hybridization study. The European Journal of Neuroscience. 1998;10(9):2924–2933. doi: 10.1111/j.1460-9568.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. https://doi.org/10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A, Brothers L. The Amygdala and Social Behavior. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. 1st. New York, NY: Wiley-Liss; 1992. pp. 353–377. [Google Scholar]
- Kling AS, Tachiki K, Lloyd R. Neurochemical correlates of the Klüver-Bucy syndrome by in vivo microdialysis in monkey. Behavioural Brain Research. 1993;56(2):161–170. doi: 10.1016/0166-4328(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy P. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in Rhesus monkeys. American Journal of Physiology. 1937;119:352–353. [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120(4):761–786. doi: 10.1037/0735-7044.120.4.761. https://doi.org/10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): consistent pattern of behavior across different social contexts. Behavioral Neuroscience. 2008;122(2):251–266. doi: 10.1037/0735-7044.122.2.251. https://doi.org/10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Forcelli PA, Wellman LL, Dybdal D, Dubach MF, Gale K. Blockade of glutamatergic transmission in perirhinal cortex impairs object recognition memory in macaques. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2015;35(12):5043–5050. doi: 10.1523/JNEUROSCI.4307-14.2015. https://doi.org/10.1523/JNEUROSCI.4307-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Suomi SJ, Bachevalier J. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta) Behavioral Neuroscience. 2010;124(6):742–760. doi: 10.1037/a0021622. https://doi.org/10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy J-M, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for α3 subunit-containing GABAA receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(25):8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012. https://doi.org/10.1523/JNEUROSCI.4404-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masneuf S, Lowery-Gionta E, Colacicco G, Pleil KE, Li C, Crowley N, Kash T. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology. 2014;85:190–197. doi: 10.1016/j.neuropharm.2014.04.015. https://doi.org/10.1016/j.neuropharm.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52(2):281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J. Comparison of emotional responses in monkeys with rhinal cortex or amygdala lesions. Emotion (Washington, D.C.) 2002;2(2):147–161. doi: 10.1037/1528-3542.2.2.147. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. The European Journal of Neuroscience. 1999;11(12):4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Moadab G, Bliss-Moreau E, Amaral DG. Adult social behavior with familiar partners following neonatal amygdala or hippocampus damage. Behavioral Neuroscience. 2015;129(3):339–350. doi: 10.1037/bne0000062. https://doi.org/10.1037/bne0000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Tye KM. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520(7549):675–678. doi: 10.1038/nature14366. https://doi.org/10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.) Guide for the care and use of laboratory animals. 8th. Washington, D.C: National Academies Press; 2011. Institute for Laboratory Animal Research (U.S.), & National Academies Press (U.S.) [Google Scholar]
- Novak MF, Sackett GP. Pair-rearing infant monkeys (Macaca nemestrina) using a “rotating-peer” strategy. American Journal of Primatology. 1997;41(2):141–149. doi: 10.1002/(SICI)1098-2345(1997)41:2<141::AID-AJP6>3.0.CO;2-X. https://doi.org/10.1002/(SICI)1098-2345(1997)41:2<141::AID-AJP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Oler JA, Tromp DPM, Fox AS, Kovner R, Davidson RJ, Alexander AL, Fudge JL. Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non-human primate: neuronal tract tracing and developmental neuroimaging studies. Brain Structure & Function. 2016 doi: 10.1007/s00429-016-1198-9. https://doi.org/10.1007/s00429-016-1198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: Projections originating in the lateral nucleus. The Journal of Comparative Neurology. 1998;398(3):431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. https://doi.org/10.1002/(SICI)1096-9861(19980831)398:3<431::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. The Journal of Comparative Neurology. 1981;198(1):121–136. doi: 10.1002/cne.901980111. https://doi.org/10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. Journal of Neurophysiology. 1991;66(3):986–998. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- Raper J, Stephens SBZ, Sanchez M, Bachevalier J, Wallen K. Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Developmental Psychobiology. 2014;56(8):1711–1722. doi: 10.1002/dev.21234. https://doi.org/10.1002/dev.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wilson M, Sanchez M, Machado CJ, Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinology. 2013;38(7):1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. https://doi.org/10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. Journal of Comparative and Physiological Psychology. 1954;47(3):173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Research. 1997;764(1–2):262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. The Journal of Comparative Neurology. 1988;271(2):185–207. doi: 10.1002/cne.902710203. https://doi.org/10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. https://doi.org/10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Forcelli PA, Aguilar BL, Malkova L. Bidirectional Control of Social Behavior by Activity within Basolateral and Central Amygdala of Primates. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2016;36(33):8746–8756. doi: 10.1523/JNEUROSCI.0333-16.2016. https://doi.org/10.1523/JNEUROSCI.0333-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25(18):4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. https://doi.org/10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, DesJardin JT, Gale K, Malkova L. Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(42):15128–15135. doi: 10.1523/JNEUROSCI.3295-11.2011. https://doi.org/10.1523/JNEUROSCI.3295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Forcelli PA, Murnen AT, McCue DL, Gale K, Malkova L. Transient inactivation of basolateral amygdala during selective satiation disrupts reinforcer devaluation in rats. Behavioral Neuroscience. 2012;126(4):563–574. doi: 10.1037/a0029080. https://doi.org/10.1037/a0029080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Amygdalostriatal projections in the neurocircuitry for motivation: a neuroanatomical thread through the career of Ann Kelley. Neuroscience & Biobehavioral Reviews. 2013;37(9):1932–1945. doi: 10.1016/j.neubiorev.2012.11.019. https://doi.org/10.1016/j.neubiorev.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


