Abstract
Autosomal dominant mutations in Fibroblast Growth Factor Receptor 3 (FGFR3) cause Achondroplasia (Ach), the most common form of dwarfism in humans, and related chondrodysplasia syndromes that include Hypochondroplasia (Hch), Severe Achondroplasia with Developmental Delay and Acanthosis Nigricans (SADDAN), and Thanatophoric dysplasia (TD). FGFR3 is expressed in chondrocytes and mature osteoblasts where it functions to regulate bone growth. Analysis of the mutations in FGFR3 revealed increased signaling through a combination of mechanisms that include stabilization of the receptor, enhanced dimerization, and enhanced tyrosine kinase activity. Paradoxically, increased FGFR3 signaling profoundly suppresses proliferation and maturation of growth plate chondrocytes resulting in decreased growth plate size, reduced trabecular bone volume, and resulting decreased bone elongation. In this review we discuss the molecular mechanisms that regulate growth plate chondrocytes, the pathogenesis of Ach, and therapeutic approaches that are being evaluated to improve endochondral bone growth in people with Ach and related conditions.
Keywords: Achondroplasia, Hypochondroplasia, Thanatophoric dysplasia, Fibroblast Growth Factor Receptor, FGF, FGFR3, chondrogenesis, growth plate, endochondral ossification, therapy, skeletal dysplasia
Introduction
Achondroplasia (Ach) is the most common form of dwarfism in humans. It occurs with a frequency of 1 in 15–25,000 and 80% of cases are sporadic. Ach is an autosomal dominant genetic disease that has 100% penetrance. The short stature in Ach mainly results from shortening of the limbs with proximal segments affected disproportionally, a phenotype referred as rhizomelia. The head is large with frontal bossing and the midface is hypoplastic resulting from cartilage growth defects at the skull base. Narrowing of the foramen magnum and spinal stenosis are relatively common and often require neurosurgical corrections. The size of the trunk is relatively normal but is often deformed by excessive lumbar lordosis (Horton et al., 2007; Baujat et al., 2008).
Genetic linkage studies placed the Ach gene on the short arm of chromosome 4 and mutation analysis identified an arginine to glycine substitution at residue 380 (p.Gly380Arg) in Fibroblast Growth Factor Receptor 3 (FGFR3) in almost all Ach patients in Caucasian, African, and Asian populations (Rousseau et al., 1994; Shiang et al., 1994). Expression of FGFR3 in growth plate chondrocytes suggested a direct causal relationship between mutation in FGFR3 and growth plate function. Comparison of wild type and mutant FGFR3 showed that the mutant receptors had increased signaling that could be further enhanced in the presence of Fibroblast Growth Factor (FGF) ligands (Naski et al., 1996; Legeai-Mallet et al., 1998). This increased signaling may be due in part to increased protein stability resulting from decreased lysosomal degradation of the mutant receptor (Cho et al., 2004).
FGFs are signaling molecules that function during embryonic and postnatal development. In the adult, FGFs have roles in homeostasis and tissue repair (Ornitz and Itoh, 2015; Li et al., 2016). Eighteen FGF ligands have the capacity to activate four FGFR tyrosine kinase molecules. Alternative mRNA splicing of immunoglobulin-like domain III of FGFRs 1–3 produce b and c splice variants. In many tissues, b splice variants are expressed in epithelial cell types and c splice variants are expressed in mesenchymal derived cells (Belov and Mohammadi, 2013; Ornitz and Itoh, 2015; Li et al., 2016). These FGFR splice variants and cofactor molecules, which include heparan sulfate proteoglycans and Klotho-family proteins, also determine the strength and specificity of ligand binding and receptor activation (Ornitz, 2000; Polanska et al., 2009; Itoh et al., 2015). Binding to heparan sulfate also serves to limit FGF diffusion through tissue (Sun et al., 2016).
The identification of activating mutations in FGFR3 as the etiology of Ach and the related milder form of dwarfism, Hch, the severe and rare dwarfism, SADDAN, and the severe lethal chondrodysplasia, TD, immediately suggested that inhibitor therapies could be developed to lessen the severity of these diseases. Research over the past two decades has identified some of the mechanisms used by FGFR3 to regulate chondrocyte proliferation and differentiation in the growth plate. Also identified are signaling molecules and pathways that interact with FGFR3 that could be exploited to counteract the effects of hyperactivated FGFR3. Here, we review local signaling pathways acting on the growth plate, the mechanisms used by FGFR3 and interacting signaling pathways to regulate chondrogenesis, and the current efforts to develop therapies to treat patients with Ach and Hch, and potentially other forms of short-limbed dwarfism.
Growth plate structure and function
Longitudinal bone growth is driven by the proliferation and differentiation of chondrocytes in the growth plate, a structure located between the metaphysis and epiphysis of long bones. The definitive growth plate consists of three principal layers of cells that temporally and spatially follow a highly regulated developmental program (Figure 1) (Caplan and Pechak, 1987; Hall and Miyake, 1992; Hunziker, 1994; Olsen et al., 2000; Wagner and Karsenty, 2001; Karsenty and Wagner, 2002; Ornitz and Marie, 2002; Ornitz and Marie, 2015). Reserve (or resting) zone chondrocytes serve as a renewing population of progenitors that gives rise to proliferating chondrocytes. Proliferating chondrocytes form clonal columns of cells that differentiate into prehypertrophic and then hypertrophic chondrocytes. At the distal end of the growth plate, the extracellular matrix produced by hypertrophic chondrocytes begins to mineralize and the hypertrophic chondrocytes either die or further differentiate into osteoblasts that populate the primary spongiosa (Yang et al., 2014a; Yang et al., 2014b; Yeung Tsang et al., 2014; Zhou et al., 2014; Park et al., 2015). In this manner the growth plate functions as a template for trabecular (primary spongiosa or spongy) bone.
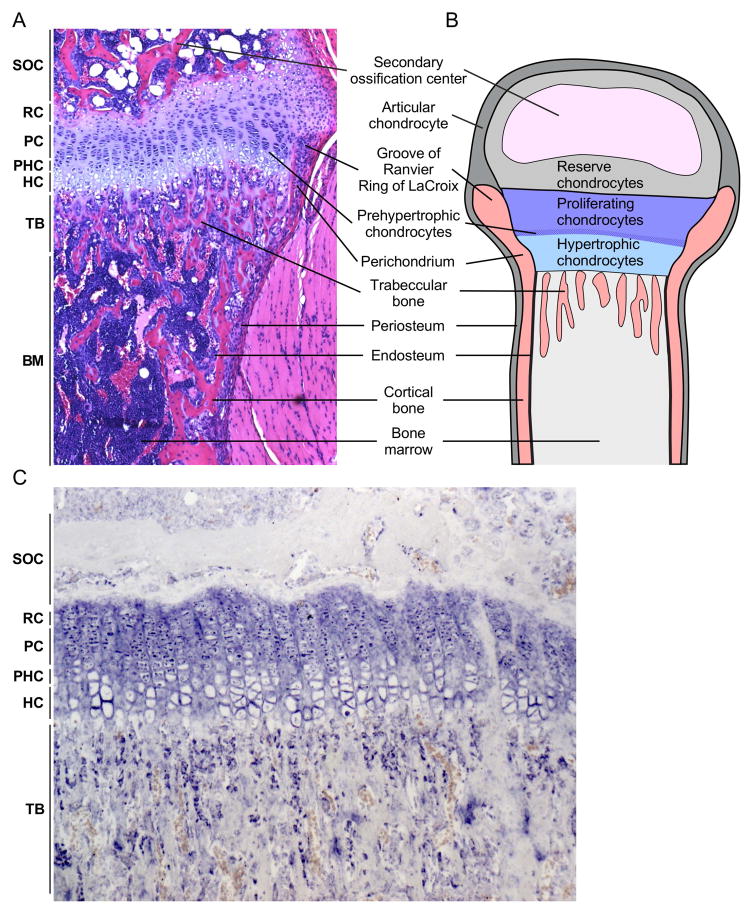
Figure 1. Histological organization of the postnatal growth plate.
A. Histological section of the mouse proximal tibia showing growth plate chondrocytes at different stages of differentiation (resting, proliferating, prehypertrophic, and hypertrophic), perichondrium, and trabecular and cortical bone. B. Schematic of the postnatal growth plate showing progression of chondrocyte development and juxtaposition to trabecular and cortical bone, the groove of Ranvier and ring of LaCroix, and the secondary ossification center. C. Fgfr3 expression (in situ hybridization) in proliferating and prehypertrophic chondrocytes and trabecular osteoblasts in a 21-day-old mouse tibia (image courtesy of K. Karuppaiah). SOC, secondary ossification center; RC, Reserve chondrocyte zone; PC, Proliferating chondrocyte zone; PHC, Prehypertrophic chondrocyte zone; HC, Hypertrophic chondrocyte zone; TB, trabecular bone.
The growth plate is surrounded by the perichondrium, a structure contiguous with the periosteum. The inner layer of the perichondrium is populated by densely packed cells in the groove of Ranvier and surrounding perichondrial ring of LaCroix (Ranvier, 1873; Ranvier, 1889; Shapiro et al., 1977). This structure is important for regulating longitudinal bone growth and serves as a source of progenitor cells that populate the periosteum and cortical bone (Robinson et al., 1999; Fenichel et al., 2006; Karlsson et al., 2009). The perichondrium thus serves as a template for the formation of cortical bone.
Chondrocyte hypertrophy accounts for approximately 60% of longitudinal bone growth (Hunziker et al., 1987; Hunziker and Schenk, 1989; Hunziker, 1994; Wilsman et al., 1996; Noonan et al., 1998). The rate of longitudinal bone growth is determined by chondrocyte proliferation, the rate of hypertrophic differentiation, the change in height of hypertrophic chondrocytes, and the amount of extracellular matrix produced by hypertrophic chondrocytes (Breur et al., 1991; Wilsman et al., 1996). Although the force driving bone elongation requires chondrocyte proliferation and hypertrophy, longitudinal bone growth also requires elongation of the perichondrium/periosteum, which must be synchronized with growth plate chondrogenesis.
Overview of signaling pathways regulating the growth plate
Proliferation and differentiation of chondrocytes in the growth plate is regulated by locally acting secreted growth factors, by endocrine factors, and by mechanical forces. Locally acting signals include Parathyroid hormone-like peptide (PTHLH or PTHRP), Indian hedgehog (IHH), Bone morphogenetic proteins (BMPs), Transforming growth factor β (TGFβ), Wingless-type MMTV integration site family members (WNTs), Notch, C-natriuretic peptide (CNP encoded by Nccp), Insulin-like growth factor 1 (IGF-1), Epidermal growth factor (EGF), Transforming growth factor α (TGFα), Vascular endothelial growth factor A (VEGFA), and FGFs. The functions of these pathways in skeletal growth and development have been extensively reviewed (reviewed in Long and Ornitz, 2013; Lui et al., 2014; Kozhemyakina et al., 2015; Rosello-Diez and Joyner, 2015; Maes, 2016). Endocrine factors include growth hormone (GH), Thyroid hormone (T3), Parathyroid hormone (PTH), FGF23, and sex steroids (reviewed in Perry et al., 2008; Rosello-Diez and Joyner, 2015; Maes, 2016; Yakar and Isaksson, 2016)]. Mechanical forces include those generated by hydrostatic forces, muscle contraction, and gravity. Hydrostatic compression of growth plate chondrocytes directly increases IHH signaling and chondrocyte proliferation (Shao et al., 2012). Chondrocyte proliferation and hypertrophy are also modulated by static and dynamic loading (Villemure and Stokes, 2009). For example, in the absence of muscle forces, proliferation decreased in embryonic chick growth plate (Germiller and Goldstein, 1997) and in mice lacking skeletal muscle, formation of the primary ossification center was delayed (Nowlan et al., 2010).
Focusing on local signals, IHH, PTHLH, BMP2, Wnt, CNP and FGFR3 are central factors for growth plate regulation (Figure 2). IHH and PTHLH form a negative feedback loop that controls chondrocyte proliferation and differentiation. IHH is made by prehypertrophic and early hypertrophic chondrocytes. During postnatal bone growth, after formation of the secondary ossification center, IHH signals to its receptor, PTCH1, in reserve zone chondrocytes to regulate expression of PTHLH (Chau et al., 2011). PTHLH, in turn, signals to its receptor, PTH1R (PTH type 1 receptor) in prehypertrophic chondrocytes and inhibits IHH expression and chondrocyte hypertrophy. BMP2 and BMP4 are expressed in prehypertrophic and hypertrophic chondrocytes and signal to BMPR1a (Bone Morphogenetic Protein Receptor Type 1A) in proximal proliferating chondrocytes and prehypertrophic chondrocytes to regulate chondrocyte proliferation (Feng et al., 2003; Nilsson et al., 2007; Shu et al., 2011). Inhibition of Wnt signaling by inactivating the Wintless (Wls) gene in chondrocytes or osteoblasts results in reduced chondrocyte hypertrophy and a smaller skeleton (Lu et al., 2013). CNP is expressed in proliferating and prehypertrophic chondrocytes and signals to natriuretic peptide receptor 2 (NPR2 or NPR-B) in proliferating and prehypertrophic chondrocytes (Chusho et al., 2001; Potter et al., 2006). Like BMP, IHH, PTHLH, and CNP promote chondrocyte proliferation (Karp et al., 2000; Chusho et al., 2001; Long et al., 2001; Hirai et al., 2011).
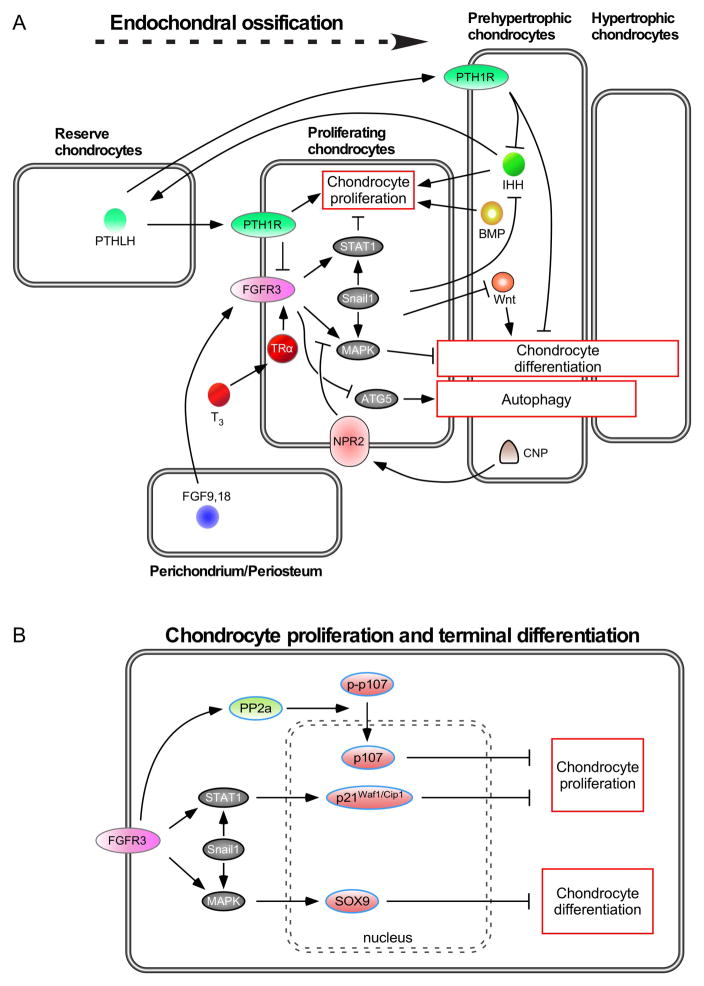
Figure 2. Signaling pathways in the postnatal growth plate.
A. During endochondral bone development, FGF9 and FGF18, derived from the perichondrium and surrounding tissue, signal to FGFR3 in chondrocytes. The balance of chondrocyte proliferation and differentiation is controlled by crosstalk of several signaling pathways. Expression of FGFR3 is enhanced by thyroid hormone (T3) and suppressed by PTHLH. FGFR3 signaling results in increased expression of Snail1, which is required for activation of STAT1 and MAPK signaling. Signaling from PTHLH, IHH and BMPs antagonizes the suppression of chondrocyte proliferation by FGFR3. Both FGFR3 and PTHLH function to suppress chondrocyte differentiation and antagonize the action of Wnt signaling, which promotes differentiation. FGFR3 negatively regulates the autophagy protein, ATG5. B. Activation of downstream signals, PP2a and STAT1, regulate p107, p21Waf1/Cip1 activation, respectively, which function to suppress chondrocyte proliferation. Activation of the MAPKs, ERK1 and ERK2, regulate Sox9 expression, which functions to suppress chondrocyte terminal differentiation and endochondral ossification.
Fgfr3 is expressed in proliferating and prehypertrophic chondrocytes during embryonic and postnatal development (Figure 1C) (Peters et al., 1993; Delezoide et al., 1998; Monsonego-Ornan et al., 2000; Pandit et al., 2002; Barnard et al., 2005; Karuppaiah et al., 2016). During establishment of the growth plate before formation of the secondary ossification center, FGFR3 signaling promotes chondrocyte proliferation (Iwata et al., 2000; Iwata et al., 2001; Havens et al., 2008). However, during postnatal skeletal growth, FGFR3 signaling inhibits chondrocyte proliferation and differentiation. The inhibition of chondrogenesis by FGFR3 underlies the etiology of Ach and related disorders in which activating mutations in FGFR3 suppress chondrogenesis during pre-pubertal skeletal growth (Colvin et al., 1996; Deng et al., 1996; Naski et al., 1996; Naski et al., 1998; Chen et al., 1999; Li et al., 1999; Pannier et al., 2010).
FGFR3 signaling in the growth plate
Mice expressing the FGFR3(p.Gly374Arg) activating mutation, which corresponds to the human FGFR3(p.Gly380Arg) mutation, develop an Ach-like phenotype with reduced chondrocyte proliferation and reduced hypertrophic differentiation and matrix production (Naski et al., 1998; Wang et al., 1999). The intracellular signaling mechanisms that mediate these phenotypes have revealed a complex network of signals that integrate FGFR3 signaling with several other signaling pathways.
FGFR signaling activates at least four downstream intracellular signaling pathways including, MAPK, PI3K/AKT, PLCγ, and STATs (reviewed in Ornitz and Itoh, 2015; Brewer et al., 2016). In the growth plate, FGFR3 activates STAT1 and the ERK1/2 and p38 branches of the MAPK pathway (Figure 2) (Su et al., 1997; Chen et al., 1999; Li et al., 1999; Chen et al., 2001; Legeai-Mallet et al., 2004; Raucci et al., 2004; de Frutos et al., 2007; Parafioriti et al., 2009). Activation and overexpression of STAT1 is a strong candidate for regulation (suppression) of chondrocyte proliferation downstream of FGFR3, as inactivation of the Stat1 gene rescued the chondrocyte proliferation defect in FGFR3(p.Gly374Arg) mice. However, these mice still developed an Ach-like phenotype, demonstrating that STAT1 is not sufficient to mediate the overall growth inhibitory effects of activated FGFR3 (Murakami et al., 2004). In contrast, expression of an activated MEK1 allele in chondrocytes of mice that lack a functional Stat1 gene resulted in an Ach-like phenotype with a prominently reduced hypertrophic chondrocyte zone, but no decrease in chondrocyte proliferation. This is consistent with chondrocyte hypertrophy contributing to bone elongation to a greater extent than chondrocyte proliferation (Murakami et al., 2004). The separation between regulation of proliferation and differentiation was further supported by the observation that CNP signaling enhances bone growth by increasing hypertrophic differentiation and matrix production through inhibition of MAPK signaling (Yasoda et al., 2004).
SNAIL1 is a transcription factor that has been shown to regulate chondrocyte differentiation through repression of Collagen II and Aggrecan transcription (Seki et al., 2003). Several studies have demonstrated that Snail1 functions downstream of FGFR3 and is essential for FGFR3 regulation of both chondrocyte proliferation and differentiation (de Frutos et al., 2007; Karuppaiah et al., 2016). Forced activation of SNAIL1 in mice suppressed chondrocyte proliferation and hypertrophy at late embryonic stages, a phenotype that resembled Ach (de Frutos et al., 2007). Further analysis revealed significantly reduced chondrocyte proliferation and a correlation between Snail1 expression and nuclear localization of STAT1. In addition to regulating STAT1, SNAIL1 activation also increases phosphorylated Erk1/2 and may enhance its nuclear localization (de Frutos et al., 2007; Smith et al., 2014). This function of SNAIL1 may be reinforced by a feed forward mechanism whereby activation of ERK2 phosphorylates and stabilizes SNAIL1 and increases its nuclear localization (Zhang et al., 2013). Downstream of SNAIL1, STAT1 and ERK1/2 activation results in suppression of chondrocyte proliferation and differentiation, respectively. Suppression of proliferation is mediated by activation of p107 (and p130) and expression of the cell cycle inhibitor, p21Waf1/Cip1 (Figure 2B) (Cobrinik et al., 1996; Su et al., 1997; Aikawa et al., 2001; Laplantine et al., 2002; Dailey et al., 2003; Legeai-Mallet et al., 2004; Kolupaeva et al., 2008; Kolupaeva et al., 2013). Chondrocyte differentiation is mediated in part by ERK1/2 (MAPK) regulation of Sox9, which must be suppressed to allow terminal hypertrophic differentiation and endochondral ossification (Hattori et al., 2010; Ikegami et al., 2011; Kim et al., 2011; Shung et al., 2012; Zhou et al., 2015b).
FGFR3 signaling also affects surrounding bone, directly and through the regulation of other growth factor signaling pathways in chondrocytes. For example, inactivation of FGFR3 globally or in chondrocytes results in increased expression of Ihh, Bmps 2, 4, 7, Tgfβ1, and Wnt4, and decreased expression of Noggin, resulting in increased bone mass (Naski et al., 1998; Zhou et al., 2015a; Wen et al., 2016), while activation of FGFR3 in chondrocytes results in decreased Ihh, BMP4, and Pthlh and leads to decreased bone mass (Figure 2A) (Naski et al., 1998; Chen et al., 2001; Su et al., 2010; Mugniery et al., 2012; Qi et al., 2014). Direct effects of FGFR3 on osteoblasts are supported by conditional knockouts of Fgfr3 in osteoblasts (OC-Cre), which result in impaired bone formation and remodeling (Xie et al., 2014). The function of osteoblasts is coupled to osteoclasts during bone formation and resorption, and recently it was demonstrated that Fgfr3 inactivation in osteoclasts (LysM-Cre) impaired bone resorption (Su et al., 2016).
Regulation of Fgfr3 expression
FGFR3 signaling is controlled in part by regulating the level of Fgfr3 mRNA and protein expression. Activating mutations in FGFR3 lead to increased FGFR3 protein expression, possibly through reduced receptor internalization and degradation (Cho et al., 2004; Legeai-Mallet et al., 2004; Qi et al., 2014). Paracrine and endocrine signals also regulate Fgfr3 expression in growth plate chondrocytes. These signals include FGF, Thyroid hormone (T3), and PTHLH. Overexpression of FGF9 in the perichondrium/periosteum activates a feed forward pathway that increases Fgfr3 expression and suppresses chondrocyte proliferation (Karuppaiah et al., 2016).
Mice lacking Thyroid Receptor α (TRαo/o), which is expressed in skeletal tissues, have skeletal hypothyroidism (reduced hypertrophic chondrocyte differentiation, delayed ossification, disorganized growth plate structure) (Gauthier et al., 2001). Mice with a mutant thyroid hormone receptor β (TRβpv/pv), which is expressed in the pituitary gland, have increased expression of TSH and develop thyrotoxicosis (elevated levels of T3 and T4) (O’Shea et al., 2003). These mice have reduced linear growth, advanced endochondral ossification, and craniosynostosis. These phenotypes can be explained in part through regulation of Fgfr3 in chondrocytes (Bassett and Williams, 2016). TRαo/o mice have reduced levels of Fgfr3 expression in growth plate chondrocytes, while hyperthyroid TRβpv/pv mice showed increased levels of Fgfr3 in growth plate chondrocytes (Barnard et al., 2005). This signaling could be direct (Figure 2), as analysis of the Fgfr3 promoter identified a putative thyroid hormone response element (McEwen et al., 1999). Additionally, treatment of cultured chondrocytes with T3 induced the expression of Fgfr3 (Barnard et al., 2005).
PTHLH signaling may directly regulate Fgfr3 by controlling a transcriptional regulatory element, which can be repressed by PTH through binding to a cAMP response element in the Fgfr3 promoter (McEwen et al., 1999). Treatment of primary chondrocytes with PTH(1-34) suppressed expression of Fgfr3 (Zhang et al., 2016) as did injection of PTH(1-34) in vivo (Karuppaiah et al., 2016). Although not investigated in chondrocytes, Fgfr3 expression was induced by hypoxia in a transcriptional and HIF1α-dependent manner in bladder cancer cells (Blick et al., 2013). Similar regulation could occur in the relatively hypoxic growth plate. Additionally, BMP2 induced expression of Fgfr3 through chromatin remodeling and SP1 sites in the Fgfr3 promoter (McEwen and Ornitz, 1998; Sun et al., 2009).
FGF ligands that regulate endochondral bone growth
Several FGFs are expressed in the growth plate and in the surrounding perichondrium and periosteum. During development, Fgf2, Fgf9, and Fgf18 are expressed in the perichondrium/periosteum and presumptive joint space and have been shown to regulate bone growth in vivo (Gonzalez et al., 1996; Liu et al., 2002; Ohbayashi et al., 2002; Hung et al., 2007; Reinhold and Naski, 2007). Fgf1, Fgf2, Fgf17 and Fgf19 are present in growth plate chondrocytes (Logan et al., 1991; Krejci et al., 2007), but of these, only Fgf2 has been shown to regulate bone growth in vivo. Mice congenitally lacking Fgf2 (Fgf2−/− mice) show normal growth plate morphology and function but have decreased bone mass, primarily seen in trabecular bone (Montero et al., 2000).
Mice that congenitally lack Fgf9 (Fgf9−/− mice) have decreased growth of long bones that affects the proximal skeletal elements to a greater extent than the distal elements (rhizomelia) (Hung et al., 2007). Mice that lack Fgf18 (Fgf18−/−) show a more uniform decrease in skeletal growth (Liu et al., 2007). For both of these ligands, chondrocyte proliferation is decreased, which is consistent with observed phenotypes in Fgfr3−/− mice during embryonic stages of bone growth, where FGFR3 signaling functions to promote chondrocyte proliferation (Iwata et al., 2000; Iwata et al., 2001; Hung et al., 2007; Liu et al., 2007). Mice that lack both Fgf9 and Fgf18 have a severe defect in bone growth that affects all skeletal elements (Hung et al., 2016). At late stages of development, Fgf9−/− and Fgf18−/− mice show an increase in the size of the hypertrophic chondrocyte zone. This phenotype closely matches that of Fgfr3−/− mice, which is consistent with FGFR3 functioning to suppress chondrocyte proliferation and differentiation at late stages of development and in the postnatal growth plate (Liu et al., 2002; Ohbayashi et al., 2002; Hung et al., 2007).
Autophagy in the growth plate
Macroautophagy is a lysosomal-dependant degradation process that maintains cellular homeostasis in response to cellular stress. The initiation of autophagosome formation requires the interactions of a subset of at least 18 autophagy related genes (Atg) (Feng et al., 2014). During growth plate development, autophagy regulates the maturation and the hypertrophy of chondrocytes (Shapiro et al., 2014). Autophagy is protective in articular cartilage and mice lacking Atg5 in chondrocytes develop age-related osteoarthritis (Bouderlique et al., 2016).
Genome-wide association studies have identified potential links between autophagy and human stature (Pan et al., 2010). Targeted genetic ablations of autophagy-related genes, Atg5 or Atg7, in chondrocytes results in mild growth retardation with reduced chondrocyte proliferation (Vuppalapati et al., 2015) and impairment of the secretion of collagen type 2, a major component of the cartilage extracellular matrix (Cinque et al., 2015).
A role for autophagy in FGF-regulation of chondrogenesis has recently been identified by several groups. Mice haploinsufficient or null for Fgf18 exhibited a low level of autophagy in chondrocytes resulting in decreased levels of Col2 in the growth plate (Cinque et al., 2015). Interestingly, this phenotype was attributed to signaling through FGFR4 rather than FGFR3. In contrast, Wang et al. showed that mice lacking Fgfr3 in growth plate chondrocytes had increased autophagy and mice expressing a constitutively active FGFR3 had reduced autophagy (Wang et al., 2015).
Diseases caused by mutations in FGFR3
Achondroplasia
The diagnosis of Ach is usually made at birth and 80% of cases of Ach arise as sporadic mutations in FGFR3. Ach is the most frequent form of dwarfism, characterized by short long bones, disproportional shortening of the proximal skeletal segments (rhizomelia), impaired elbow extension, tibial bowing, exaggerated lumbar lordosis, shortening of the vertebral pedicles and narrowing of the lumbar interpedicular distance, shortening of the femoral head, macrocephaly, midface hypoplasia, frontal bossing, hearing loss and a reduced size of the foramen magnum (Figure 3) (Horton et al., 2007; Baujat et al., 2008). Ach can also include partial premature fusion of the coronal and sagittal sutures, suggesting a role for FGFR3 in membranous ossification (Twigg et al., 2009; Di Rocco et al., 2014). Ach is a progressive disease and the severity of the phenotype is correlated with age. For example, with age, there is progressive disorganization of the skeletal growth plate (Legeai-Mallet et al., 2004). Bone age (a assessment of skeletal maturation based on comparisons of radiographs of the wrist, hand, and fingers with standardized radiographs) is delayed in the newborn Ach patient; however, during adolescence bone maturation accelerates and the bone age approaches the chronological age (Pannier et al., 2010). Ach patients have a significant kyphosis that leads to a progressive deformity. With age, Ach patients develop an excessive lumbar lordosis. A major complication, narrowing of the spinal canal due to degenerative changes of the spinal canal, can lead to nerve root compression and often requires surgical decompression (Baujat et al., 2008).
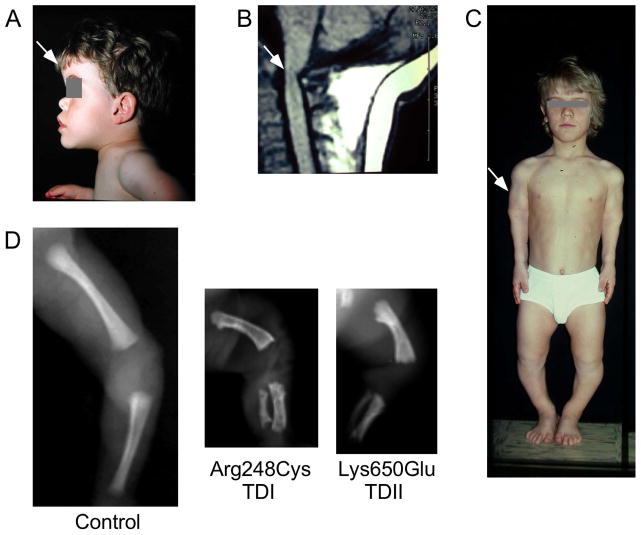
Figure 3. Clinical features of skeletal disorders resulting from activating mutations in FGFR3.
A. The head of a patient with Ach is characterized by macrocephaly, frontal bossing (arrow), and hypoplasia of the midface. B. MRI (magnetic resonance imaging) showing the cervicomedullary compression at the foramen magnum (arrow). C. Rhizomelic short stature (arrow) of a patient with Ach (image courtesy of Dr. G. Finidori). D. X-rays of the lower limb (femur and tibia) of a 24 week-old normal fetus (control) and fetuses with TDI (p.Arg248Cyst) and TDII (p.Lys650Glu) FGFR3 mutations. Note the short and curved femur compared to the age-matched control.
The Ach gene locus was mapped to FGFR3 in 1994 (Le Merrer et al., 1994; Velinov et al., 1994). Over 97% of cases result from an autosomal dominant missense mutation (p.Gly380Arg) localized in the transmembrane domain of FGFR3 (Figure 4) (Shiang et al., 1994; Wilkin et al., 1998; Vajo et al., 2000). Ach patients that do not have a p.Gly380Arg mutation are usually found to have other less common FGFR3 mutations such as p.Ser217Cys, Ser279Cys, p.Ser344Cys and p.Gly375Cys (Superti-Furga et al., 1995; Zhang et al., 2007; Xue et al., 2014; Takagi et al., 2015). These less common mutations in FGFR3, that add a cysteine residue, are likely to result in constitutive receptor activation, similar to that seen in TDI; however, their mechanism of action will need to be further investigated. Ach mutations show a penetrance of 100 percent. Rare homozygous cases of Ach are lethal with phenotypes resembling that of TD (Stanescu et al., 1990; Tavormina et al., 1995).
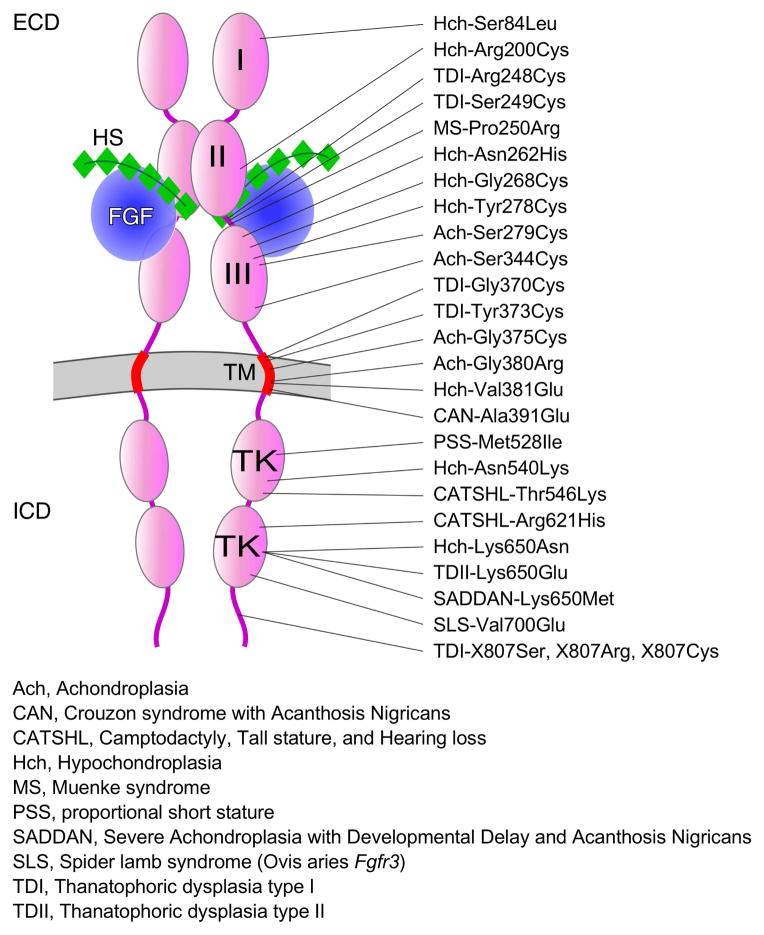
Figure 4. The mutational spectrum of FGFR3.
The relative location of gain-of-function and loss-of-function mutations causing genetic skeletal disease in humans is shown distributed over the entire FGFR3 coding region. Abbreviations for different types of genetic diseases are shown. FGF ligands are shown in blue and heparan sulfate co-factors are shown in green. Some of the mutations in FGFR3 change the affinity or specificity of the receptor for different FGF ligands, while others affect tyrosine kinase activity or receptor internalization and degradation. ECD, extracellular domain; ICD, intracellular domain; HS, heparan sulfate; I, II, III, immunoglobulin-like domains; TK, tyrosine kinase domains; TM, transmembrane domain (red).
Mutation analysis of Ach patients showed that nearly all mutations arise on the paternal chromosome. The paternal origin of Ach mutations in FGFR3 correlates with advanced paternal age in all cases examined (Wilkin et al., 1998). The paternal origin of activating mutations in FGF receptors is attributed to positive selection and clonal expansion of spermatogonial stem cells with age (Goriely and Wilkie, 2012; Shinde et al., 2013).
Mutations causing Ach result in activation of FGFR3 and its signaling pathways that can be further enhanced in the presence of FGF ligands (Naski et al., 1996; Webster and Donoghue, 1996; Komla-Ebri et al., 2016). Increased activity may result from impaired receptor internalization and degradation (Monsonego-Ornan et al., 2000; Cho et al., 2004). Biochemical analysis shows that the Ach mutations increase the efficiency of receptor phosphorylation in the absence of ligand (He et al., 2012). Ach phenotypes have been modeled in mice by expressing the mutant Fgfr3 in chondrocytes or directly introducing Ach mutations into the Fgfr3 gene (Naski et al., 1998; Chen et al., 1999; Wang et al., 1999; Pannier et al., 2009a).
Thanatophoric Dysplasia type I and II
Thanatophoric Dysplasia type I and II (TDI and TDII) are sporadic more severe forms of dwarfism that are usually lethal. TD is characterized by short limbs (Figure 3), narrow thorax with short ribs, macrocephaly, and brain malformation with temporal lobe enlargement (Rousseau et al., 1995; Tavormina et al., 1995). The radiologic features that distinguish TDII are the frequent observation of straight femurs and a cloverleaf skull.
TDI and TDII are attributed to various mutations in FGFR3 (Figure 4). The most frequent (75%) TDI missense mutations introduce a cysteine residue in the extracellular (p.Arg248Cys, p.Ser249Cys) or transmembrane (p.Tyr373Cys, p.Gly370Cys) domain of the receptor. Less commonly, mutations that introduce a stop codon (p.X807Ser, X807Arg, X807Cys) have been observed in 20% of case of TDI (Rousseau et al., 1995). TDII results from a FGFR3 mutation (p.Lys650Glu) localized in the tyrosine kinase domain of the receptor. Both TDI and TDII mutations result in ligand-independent constitutive activation of the receptor (Naski et al., 1996); however, only the TDII mutation impedes complete maturation of FGFR3 and induces premature phosphorylation of the receptor (Lievens and Liboi, 2003; Gibbs and Legeai-Mallet, 2007). Analysis of downstream signaling showed that the TDI mutation strongly activates ERK1/2 and STAT1 (Legeai-Mallet et al., 2004; Krejci et al., 2008). Mouse models expressing TDI and TDII mutations all display a severe dwarf phenotype (Li et al., 1999; Iwata et al., 2001; Pannier et al., 2009b).
Hypochondroplasia
Hypochondroplasia (Hch) is a relatively mild form of dwarfism that shares many phenotypic features with Ach. Most cases of Hch develop as de novo mutations in the FGFR3 gene, but in some cases there is a positive family history for this condition. In the sporadic cases, the diagnosis of this milder form of dwarfism is frequently not made at birth but later during childhood when an inflection in the growth curve is observed. Hch is caused by the FGFR3 missense mutation, p.Asn540Lys, localized in tyrosine kinase domain I and is the most common Hch mutation, occurring in ~60% of cases (Figure 4). Other less common missense mutations have been identified in the tyrosine kinase domain II of FGFR3 (e.g. p.Lys650Asn) (Bellus et al., 1995; Tavormina et al., 1995; Bonaventure et al., 1996; Bellus et al., 2000) and in the extracellular domain (Heuertz et al., 2006). In vitro analyses of the p.Lys650Asn mutation showed weak activation of the FGFR3 kinase domain (Lievens et al., 2004; Gibbs and Legeai-Mallet, 2007). Analysis of the p.Asn540Lys mutation showed activation of ERK1/2 but not STAT1 (Krejci et al., 2008).
SADDAN syndrome and Platyspondylic lethal skeletal dysplasia, San Diego type (PLSD-SD)
Severe Achondroplasia with Developmental Delay and Acanthosis Nigricans (SADDAN) and Platyspondylic Lethal Skeletal Dysplasia, San Diego Type (PLSD-SD) are very rare lethal chondrodysplasias that are accompanied by acanthosis nigricans (hyperpigmentation and thickening of the skin), and brain malformations. These syndromes and classical TDI are all caused by a p.Lys650Met mutation in FGFR3 (Figure 4) (Bellus et al., 1999; Brodie et al., 1999; Tavormina et al., 1999; Farmakis et al., 2015). Analysis of the p.Lys650Met mutation showed strong activation of ERK1/2 and STAT1 (Krejci et al., 2008). A mouse model expressing the SADDAN mutation displays a phenotype similar to the human pathology in SADDAN syndrome (Iwata et al., 2001).
Proportionate short stature
A dominant mutation (p.Met528Ile) that causes proportionate short stature (PSS) was identified in FGFR3 (Figure 4) (Kant et al., 2015). Functional studies suggest that this mutation is activating, similar to that of the p.Gly380Arg mutation that causes Ach; however, the mechanisms that determine proportionate vs. rhizomelic limb shortening are not known.
Patients with tall stature
Rare pathogenic FGFR3 mutations cause tall stature. CATSHL (camptodactyly, tall stature, and hearing loss) syndrome results from a dominant FGFR3 loss of function mutation (p.Arg621His) (Figure 4). These patients are characterized by skeletal overgrowth, sensorineural hearing loss and microcephaly (Toydemir et al., 2006; Makrythanasis et al., 2014; Escobar et al., 2016). It is hypothesized that this mutation results in loss of function or expression of a dominant negative protein. A rare recessive FGFR3 loss of function mutation (p.Thr546Lys) was also reported in patients that exhibited tall stature, microcephaly, moderate hearing loss and intellectual disability (Makrythanasis et al., 2014).
These phenotypes are consitent with those of mice that lack Fgfr3, which show skeletal overgrowth (Colvin et al., 1996; Deng et al., 1996; Eswarakumar and Schlessinger, 2007) and deafness (Colvin et al., 1996), and sheep with a recessive mutation in FGFR3 (p.Val700Glu) that results in spider lamb syndrome (SLS), characterized by long limbs, kyphoscoliosis, malformed ribs and sternebrae, Roman nose, lack of body fat, and muscular atrophy (Beever et al., 2006). Heterozygous sheep with this mutation show mild increased skeletal growth (Smith et al., 2006).
Craniosynostosis and hearing loss associated with FGFR3 mutations
Pathogenic dominant FGFR3 mutations also cause craniosynostosis (premature fusion of cranial sutures). Muenke syndrome (MS) is the most common craniosynostosis syndrome (Sabatino et al., 2004). This autosomal dominant disorder is characterized by premature fusion of the coronal sutures, hearing loss, developmental delay and intellectual disability (Kruszka et al., 2016). Muenke syndrome is caused by a missense mutation (p.Pro250Arg) localized in the extracellular domain of FGFR3 in the linker between immunoglobulin-like domains II and III (Figure 4) (Bellus et al., 1996; Gripp et al., 1998). Interestingly, this mutation changes the specificity of both the FGFR3b and FGFR3c splice variants, allowing activation by FGF10 (Mansour et al., 2013). This is similar to the effects of corresponding mutations in FGFR2c that cause Apert syndrome (Yu et al., 2000). Paternal origin associated with advanced paternal age is also reported in Muenke syndrome (Rannan-Eliya et al., 2004). Mouse models with the p.Pro244Arg mutation also display craniosynostosis and hearing loss (Mansour et al., 2009; Twigg et al., 2009; Laurita et al., 2011; Nah et al., 2012; Mansour et al., 2013).
Crouzon syndrome associated with acanthosis nigricans (CAN) is a rare syndrome characterized by craniosynostoses, ocular ptosis, midface hypoplasia and hyperkeratosis, and hyperpigmentation of the skin. Patients with this syndrome carry a dominant missense mutation (p.Ala391Glu) in FGFR3 (Meyers et al., 1995; Wilkes et al., 1996). This mutation is localized in the transmembrane domain of the receptor distal to the recurrent Ach mutation (p.Gly380Arg). Differences in phenotypes (craniosynostoses vs chondrodysplasia) of the p.Ala391Glu and p.Gly380Arg mutations may be attributed to relative increased formation of FGFR3 heterodimers with the p.Ala391Glu mutation (He et al., 2011).
Therapeutic approaches
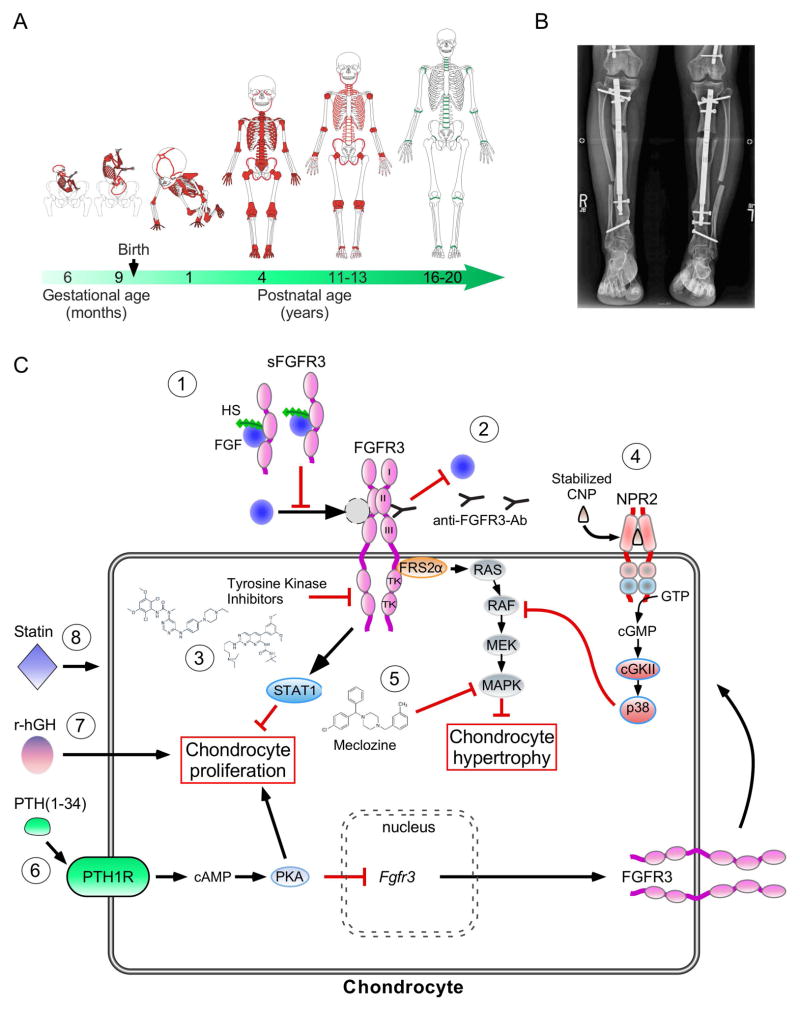
In 1994, the chondrodysplasia research field made significant progress with the discovery that activating mutations in the FGFR3 gene are the etiology of a broad clinical spectrum of chondrodysplasias, including Hch, Ach, SADDAN and TD. Potential therapeutic approaches to treat these conditions have been emerging over the past decade. To be effective, therapies for Ach need to be administered within a time window extending from birth to puberty (Figure 5A).
Figure 5. Therapeutic approaches for FGFR3-related disorders.
A. Schematic representation of key milestones in bone and growth plate activity during skeletal development. The location of the active growth plates and bone sutures are shown in red, according to age. As skeletal development progresses, growth plates and skull sutures fuse (green). B. Tibia intramedullary lengthening in sixteen-year-old girl with Ach using the PRECICE system (image courtesy of Dr. D. Paley). C. Schematic representation of therapeutic approaches for Ach that are currently being evaluated. 1) Soluble FGFR3 bind and sequester FGF ligands, 2) Anti-FGFR3 antibodies block ligand binding to the receptor and subsequent downstream signaling pathways. 3) Tyrosine kinase inhibitors block receptor phosphorylation of substrates. 4) Stabilized CNP (BMN-111) antagonizes RAF activation through the activation of the natriuretic peptide receptor 2 (NPR2), a guanylyl cyclase. cGMP activates cyclic GMP-dependent protein kinase II (cGKII) and p38 MAPK. 5) Meclozine, an anti-emetic drug, suppresses high ERK1/2 phosphorylation. 6) PTH(1-34) treatment leads to increased chondrocyte proliferation and suppression of Fgfr3 expression. 7) Indirect effect of r-hGH on bone growth 8) Statin promotes degradation of FGFR3.
Surgical approaches
Surgical intervention is a common form of therapy for both proportional and disproportional dwarfism (e.g. Ach, Hch). Surgical limb lengthening classically uses the Ilizerov procedure in which cortical long bones are cut (osteotomy), external fixators are placed proximal and distal to the osteotomy and distraction is applied gradually over many months to extend bone length (Paley, 1988; Schiedel and Rodl, 2012). The average length gained is ~20.5 cm after multiple procedures (applied to the femurs and tibias) (Kim et al., 2014; Donaldson et al., 2015). This surgical treatment allows functional gains and quality of life improvements. However, this procedure is painful and is associated with complications that include infection, muscle contractures, and increased risk of fracture (Paley, 1990; Donaldson et al., 2015). Recent innovations, such as the use of intramedullary fixation (Figure 5B), may improve outcome and lessen risk (Paley, 2015). Limb lengthening, involving the surgical breaking of a bone, fixation, and distraction during the healing process remains controversial and is associated with a high degree of risk. A pre-operative psychological assessment is required before surgery to evaluate the high risk of complications vs. the improvement of short stature. In the future, the combination of surgical limb lengthening with pharmacological strategies (see below) could further improve outcomes.
Approaches to treat Hypochondroplasia
The primary therapies that are proposed to patients with Hch include treatment with recombinant human growth hormone (r-hGH) or surgical intervention (see surgical approaches section) (Tanaka et al., 2003; Kim et al., 2014; Burghardt et al., 2015; Massart et al., 2015). R-hGH is indicated for the treatment of short stature in children with other skeletal dysplasias, such as Léri-Weill dyschondrosteosis and Idiopathic Short Stature, which are associated with mutations in the SHOX gene (Fukami et al., 2016). In clinical trials, treatment with r-hGH improved growth velocity in these patients (Blum et al., 2007; Blum et al., 2013). R-hGH therapy is also effective for Hch patients and the benefits of this treatment are reported in many studies (Ramaswami et al., 1998; Tanaka et al., 2003). R-hGH is well tolerated and effective in improving growth in Hch patients, particularly when started early (Pinto et al., 2014; Massart et al., 2015). The mechanism of action of r-hGH does not directly act on FGFR3 signaling pathways; rather, r-hGH stimulates the growth of the cartilage through its pro-anabolic properties (Figure 5C-7) (Wang et al., 2004). Additional studies are necessary to establish safety of r-hGH and its benefits to achieving adult height and body proportion.
Approaches to treat Achondroplasia
Treatment of the developmental complications of Ach involves symptomatic management, surgical intervention, and lifelong follow-up care. Health problems commonly associated with Ach include: cervico medullary compression, which can present in the first few months of life due to a reduced size of the foramen magnum; recurrent otitis media, which is common in young patients and needs to be treated to prevent conductive hearing loss; restrictive respiratory insufficiency, due to small chest size; and in adults, lumbar spinal compression (Figure 3).
To treat the short stature and the impairment of linear growth, several surgical procedures (described above) have been used, and nonsurgical strategies are being evaluated. The first therapeutic strategy offered to Ach patients was treatment with r-hGH. An increase in growth (height) velocity was reported following short term r-hGH treatment, but no clear benefit was established for long term treatment (Miccoli et al., 2016). However, the effect on body proportion is still unknown and currently the use of r-hGH to treat Ach is not routinely recommended. Current pharmacological approaches are aimed at directly blocking FGFR3 activation or regulating other signaling pathways that control chondrocyte proliferation and differentiation.
Therapies aimed at FGFR3 signaling
Many nonsurgical strategies aimed at reducing excessive activation of FGFR3 have been proposed to stimulate linear bone growth in Ach. Many strategies have been borrowed conceptually from the oncological field, which is not surprising because the genetic lesions leading to FGFR3-related skeletal disorders are identical to those found in FGFR3-driven cancers (e.g. bladder tumors, multiple myeloma) (Chesi et al., 1997; Cappellen et al., 1999; Turner and Grose, 2010; Patani et al., 2016). Several studies have focused on FGFR-selective small molecule tyrosine kinase inhibitors (TKI) to directly reduce the high tyrosine kinase activity resulting from mutations in FGFR3 (Figure 5C-3). Therapeutic efficacy of the TKI CHIR-258 was demonstrated in a xenograft mouse model of FGFR3-induced multiple myeloma (MM) (Trudel et al., 2005) and A31 was effective in increasing the growth of femur explants from FGFR3(p.Tyr367Cys) mutant mice (Jonquoy et al., 2012). Two other FGFR TKIs, PD173074 and SU5402, are also able to inhibit the growth and induce apoptosis of MM cells. However, these TKIs are not selective for FGFR3 (Mohammadi et al., 1997; Dimitroff et al., 1999). Recently, NVP-BGJ398, a TKI more selective for FGFR3 over others FGFRs (Gudernova et al., 2016) was used in preclinical murine models for treating several FGFR-related cancers such as malignant rhabdoid tumors (Wohrle et al., 2013b), hepatocellular carcinoma (Scheller et al., 2015) and skeletal disorders including FGF23-mediated hypophosphatemic rickets (Wohrle et al., 2013a) and Ach (Komla-Ebri et al., 2016). Importantly, NVP-BGJ398 was shown in vivo to reduce FGFR3(p.Tyr367Cys) activation and improve the skeletal phenotype of Ach-like mice (Komla-Ebri et al., 2016). Following safety and pharmacokinetic studies, this compound may be appropriate for evaluation in clinical trials with Ach patients.
Another approach to inhibit FGFR3 consists of using monoclonal antibodies to target the extracellular part of the receptor to block ligand binding or to use soluble decoy receptors which can bind and sequester FGF ligands, preventing them from interacting with endogenous receptors (Figure 5C-2). Several studies demonstrated that FGFR3-specific monoclonal antibodies were highly efficient in slowing the growth of various bladder cancer cell lines and were able to reduce the growth of FGFR3-dependent tumors in mice and FGFR3-expressing tumor xenografts (Rauchenberger et al., 2003; Martinez-Torrecuadrada et al., 2005; Trudel et al., 2006; Gorbenko et al., 2009; Qing et al., 2009; Gust et al., 2013; Yin et al., 2016). FGFR3-specific monoclonal antibodies have not yet been evaluated in vivo in mouse models for Ach.
Soluble FGFR3 extracellular domain decoy receptors (sFGFR3) were recently designed with the objective of binding and sequestering available FGF to compete with endogenous FGFR3 binding to FGF ligands that functionally regulate chondrogenesis (Figure 5C-1) (Liu et al., 2002; Ohbayashi et al., 2002; Hung et al., 2007; Liu et al., 2007; Garcia et al., 2013). Subcutaneous injections of recombinant sFGFR3 into a transgenic mouse model for Ach (Col2a1 promoter driving expression of FGFR3(p.Gly380Arg), Fgfr3Ach/+ mice) (Naski et al., 1998), was found to decrease mortality and improve skeletal growth (Garcia et al., 2013).
Targeting non-FGF signaling pathways that control chondrocyte proliferation and differentiation
Many signaling molecules and transcription factors are involved during growth plate development and maturation stages (Figure 2). In Ach, the balance between chondrocyte proliferation and differentiation is severely disrupted. Among the factors playing a crucial role, PTH/PTHrP (PTHLH) is a well-studied regulator of growth plate chondrocyte proliferation and differentiation (Figure 5C-6). To correct the proliferation and differentiation defect in Ach, systemic intermittent PTH (1-34) injections were administered to Fgfr3K544E/+ mice. These pre-clinical studies showed rescue of the retarded skeletal development in these mice (Xie et al., 2012). However, clinical use of PTH (1-34) (Teriparatide) is limited to two years in humans for treatment of osteoporosis (Hodsman et al., 2005). Use of Teriparatide humans to treat Ach will require long-term administration and thus new clinical trials to evaluate safety and efficacy.
Recently, others strategies have emerged using drugs currently used for non-skeletal disorders. The first example is Meclozine, an over the-counter H1 receptor inhibitor used to treat motion sickness. In various cell lines, Meclozine is able to promote chondrocyte proliferation and differentiation and attenuate ERK1/2 phosphorylation (Figure 5C-5) (Matsushita et al., 2013). In ex vivo culture, Meclozine increases longitudinal growth of embryonic normal and Fgfr3Ach/+ tibiae explants. Oral administration of Meclozine to Fgfr3Ach/+ mice increased longitudinal bone growth but failed to increase the size of the foramen magnum and lumbar spinal canal (Matsushita et al., 2015). Future studies will require histological analyses of the growth plate to confirm rescue of the growth plate defect. A second example is statins, a class of cholesterol-lowering drugs (Figure 5C-8). Addition of statins to culture media rescued the defective chondrogenesis seen in chondrocytes derived from induced pluripotent stem cells (iPS) from Ach patients, and corrected the skeletal phenotype of Fgfr3Ach/+ mice in vivo (Yamashita et al., 2014). However, controversy remains regarding the use of statins as a therapeutic approach for Ach, as recent studies showed that statin treatment retarded cartilage development and reduced the expression of the principal regulators of growth plate cartilage (Wu and De Luca, 2004; Woods et al., 2009). The mechanism by which statins could modify bone growth in Ach needs further investigation (Bush et al., 2015).
C-type natriuretic peptide
The most promising therapy thus far for treatment of Ach is the use of a stabilized form of C-type natriuretic peptide (CNP) called BMN-111 (Lorget et al., 2012; Wendt et al., 2015). CNP and its receptor, natriuretic peptide receptor B (Npr2, guanylyl cyclase B) are recognized as important regulators of longitudinal bone growth (Chusho et al., 2001). Loss-of-function mutations in Npr2 are responsible for acromesomelic dysplasia Maroteaux type, a disproportionate dwarfism in humans (Bartels et al., 2004) and heterozygous inactivating mutations in Npr2 are associated with short stature (Olney et al., 2006). Mutant mice with a disruption of CNP (Nppc−/−) also show disproportionate dwarfism with short limbs (Chusho et al., 2001). Conversely, tall stature has been reported in a patient heterozygous for an activating NPR2 mutation (Hannema et al., 2013) and skeletal overgrowth has been observed in patients that overexpress CNP caused by a balanced translocation (Bocciardi et al., 2007; Moncla et al., 2007). The same phenotype was reported in transgenic mice overexpressing brain natriuretic peptide (BNP) (Suda et al., 1998). Interestingly, CNP over-expression in cartilage or continuous delivery of CNP through intravenous infusion normalizes the dwarfism of Fgfr3Ach/+ mice (Yasoda et al., 2004; Yasoda et al., 2009), suggesting that CNP administration is a potential strategy to treat Ach.
CNP signals through NPR2 in chondrocytes and inhibits the MAPK signaling pathway at the level of RAF1 (Figure 5C-4) (Yasoda et al., 2004; Krejci et al., 2005; Geister et al., 2013). The role of the MAPK pathway in mediating FGFR3 activity is illustrated by the dwarfism of mice with constitutive activation of extracellular signal regulated kinases 1 (ERK1/MEK1) and conversely by the overgrowth of long bones of mice with ERK1/2 inactivation (Sebastian et al., 2011). Several studies have attempted to explain the signaling cascades triggered by CNP in the growth plate. NPR2/CNP-induced cGMP activates cyclic GMP-dependent protein kinase II (cGKII, encoded by PRKG2) and p38 (MAPK14). MAPK14 functionally antagonizes RAF1 activation of MEK (MAP2K1), which is a critical pathway that regulates chondrocyte hypertrophy (Murakami et al., 2004; Ozasa et al., 2005; Agoston et al., 2007; Hutchison, 2012; Peake et al., 2014). Signaling by FGF ligands through FGFR3 is functionally antagonized by CNP (BMN-111) signaling through NPR2, which decreases ERK1/2 phosphorylation in human chondrocytes and enhances the rate of chondrocyte hypertrophy and skeletal growth in a mouse model of Ach (Fgfr3Y367C/+) (Lorget et al., 2012). The putative hemodynamic effects of BMN-111 were tested in normal juvenile cynomolgus monkeys. Echocardiographic parameters were unaffected at any dose of BMN-111, and there were no clinical signs of hypotension or distress at any time during the treatment (Wendt et al., 2015). A phase 2 clinical trial with BMN-111 (Vosoritide) is currently underway for the treatment of Ach (https://clinicaltrials.gov/ct2/show/NCT02055157).
Conclusion and future directions
Considerable progress has been made during the past twenty years in understanding FGFR3-related disorders as well in developing a rationale for effective therapeutic strategies to treat FGFR3-associated bone growth defects. Although there has been some success in developing therapies, a clear challenge for the future will be to further improve the care and treatment of children and adults with Ach. As reviewed here, there are several novel therapeutic strategies that need to be considered in the future. Additionally, it will be important to investigate the potential for synergy of two or more pharmacological inhibitors of FGFR3 and its signaling pathways, which could lead to more effective treatments for Ach patients. Progress in developing therapies for Ach will also contribute to the treatment of other diseases such as cancer (multiple myeloma, lung adenocarcinoma, bladder, gastric, colorectal cancers), ostheoarthitis, and aging that result from activation of FGF signaling pathways.
Further analyses and understanding of FGFR3 downstream signaling pathways in the growth plate, of mechanisms that regulate communication between cortical and trabecular bone and the growth plate, and mechanisms by which endocrine signals interact with FGFR3 signaling pathways will likely lead to additional therapeutic strategies. Finally, studies of the role of FGFR3 in extra skeletal tissue (e.g. heart, inner ear, lung) could explain some of the clinical features associated with mutations in FGFR3 and will need to be considered during clinical trials for Ach.
Acknowledgments
This work was supported by NIH grant HD049808 to DMO and the European Community’s Seventh Framework Programme Under grant agreement no. 602300 (Sybil) to LLM.
We thank the large number of researchers who contributed to the work reviewed here. We apologize to the investigators whose work could not be cited due to space limitations and our inadvertent omissions. We thank I. Boime for critically reading the manuscript. This work was supported by NIH grant HD049808 to DMO and the European Community’s Seventh Framework Programme Under grant agreement no. 602300 (Sybil) to LLM.
References
- Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, Beier F. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Dev Biol. 2007;7:18. doi: 10.1186/1471-213X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa T, Segre GV, Lee K. Fibroblast growth factor inhibits chondrocytic growth through induction of p21 and subsequent inactivation of cyclin E-Cdk2. J Biol Chem. 2001;276:29347–29352. doi: 10.1074/jbc.M101859200. [DOI] [PubMed] [Google Scholar]
- Barnard JC, Williams AJ, Rabier B, Chassande O, Samarut J, Cheng SY, Bassett JH, Williams GR. Thyroid hormones regulate fibroblast growth factor receptor signaling during chondrogenesis. Endocrinology. 2005;146:5568–5580. doi: 10.1210/en.2005-0762. [DOI] [PubMed] [Google Scholar]
- Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al-Gazali LI, Kant S, Cole T, Morton J, Cormier-Daire V, Faivre L, Lees M, Kirk J, Mortier GR, Leroy J, Zabel B, Kim CA, Crow Y, Braverman NE, van den Akker F, Warman ML. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JH, Williams GR. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr Rev. 2016;37:135–187. doi: 10.1210/er.2015-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baujat G, Legeai-Mallet L, Finidori G, Cormier-Daire V, Le Merrer M. Achondroplasia. Best Practice & Research in Clinical Rheumatology. 2008;22:3–18. doi: 10.1016/j.berh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Beever JE, Smit MA, Meyers SN, Hadfield TS, Bottema C, Albretsen J, Cockett NE. A single-base change in the tyrosine kinase II domain of ovine FGFR3 causes hereditary chondrodysplasia in sheep. Anim Genet. 2006;37:66–71. doi: 10.1111/j.1365-2052.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- Bellus GA, Bamshad MJ, Przylepa KA, Dorst J, Lee RR, Hurko O, Jabs EW, Curry CJ, Wilcox WR, Lachman RS, Rimoin DL, Francomano CA. Severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN): phenotypic analysis of a new skeletal dysplasia caused by a Lys650Met mutation in fibroblast growth factor receptor 3. Am J Med Genet. 1999;85:53–65. [PubMed] [Google Scholar]
- Bellus GA, Gaudenz K, Zackai EH, Clarke LA, Szabo J, Francomano CA, Muenke M. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nat Genet. 1996;14:174–176. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- Bellus GA, McIntosh I, Smith EA, Aylsworth AS, Kaitila I, Horton WA, Greenhaw GA, Hecht JT, Francomano CA. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat Genet. 1995;10:357–359. doi: 10.1038/ng0795-357. [DOI] [PubMed] [Google Scholar]
- Bellus GA, Spector EB, Speiser PW, Weaver CA, Garber AT, Bryke CR, Israel J, Rosengren SS, Webster MK, Donoghue DJ, Francomano CA. Distinct missense mutations of the FGFR3 lys650 codon modulate receptor kinase activation and the severity of the skeletal dysplasia phenotype. Am J Hum Genet. 2000;67:1411–1421. doi: 10.1086/316892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov AA, Mohammadi M. Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb Perspect Biol. 2013;5:1–24. doi: 10.1101/cshperspect.a015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blick C, Ramachandran A, Wigfield S, McCormick R, Jubb A, Buffa FM, Turley H, Knowles MA, Cranston D, Catto J, Harris AL. Hypoxia regulates FGFR3 expression via HIF-1alpha and miR-100 and contributes to cell survival in non-muscle invasive bladder cancer. Br J Cancer. 2013;109:50–59. doi: 10.1038/bjc.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum WF, Crowe BJ, Quigley CA, Jung H, Cao D, Ross JL, Braun L, Rappold G. Growth hormone is effective in treatment of short stature associated with short stature homeobox-containing gene deficiency: Two-year results of a randomized, controlled, multicenter trial. J Clin Endocrinol Metab. 2007;92:219–228. doi: 10.1210/jc.2006-1409. [DOI] [PubMed] [Google Scholar]
- Blum WF, Ross JL, Zimmermann AG, Quigley CA, Child CJ, Kalifa G, Deal C, Drop SL, Rappold G, Cutler GB., Jr GH treatment to final height produces similar height gains in patients with SHOX deficiency and Turner syndrome: results of a multicenter trial. J Clin Endocrinol Metab. 2013;98:E1383–1392. doi: 10.1210/jc.2013-1222. [DOI] [PubMed] [Google Scholar]
- Bocciardi R, Giorda R, Buttgereit J, Gimelli S, Divizia MT, Beri S, Garofalo S, Tavella S, Lerone M, Zuffardi O, Bader M, Ravazzolo R, Gimelli G. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum Mutat. 2007;28:724–731. doi: 10.1002/humu.20511. [DOI] [PubMed] [Google Scholar]
- Bonaventure J, Rousseau F, Legeai-Mallet L, Le Merrer M, Munnich A, Maroteaux P. Common mutations in the fibroblast growth factor receptor 3 (FGFR 3) gene account for achondroplasia, hypochondroplasia, and thanatophoric dwarfism. Am J Med Genet. 1996;63:148–154. doi: 10.1002/(SICI)1096-8628(19960503)63:1<148::AID-AJMG26>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Bouderlique T, Vuppalapati KK, Newton PT, Li L, Barenius B, Chagin AS. Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann Rheum Dis. 2016;75:627–631. doi: 10.1136/annrheumdis-2015-207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breur GJ, VanEnkevort BA, Farnum CE, Wilsman NJ. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res. 1991;9:348–359. doi: 10.1002/jor.1100090306. [DOI] [PubMed] [Google Scholar]
- Brewer JR, Mazot P, Soriano P. Genetic insights into the mechanisms of Fgf signaling. Genes Dev. 2016;30:751–771. doi: 10.1101/gad.277137.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie SG, Kitoh H, Lachman RS, Nolasco LM, Mekikian PB, Wilcox WR. Platyspondylic lethal skeletal dysplasia, San Diego type, is caused by FGFR3 mutations. Am J Med Genet. 1999;84:476–480. [PubMed] [Google Scholar]
- Burghardt RD, Yoshino K, Kashiwagi N, Yoshino S, Bhave A, Paley D, Herzenberg JE. Bilateral double level tibial lengthening in dwarfism. J Orthop. 2015;12:242–247. doi: 10.1016/j.jor.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JR, Berube NG, Beier F. A new prescription for growth? Statins, cholesterol and cartilage homeostasis. Osteoarthritis Cartilage. 2015;23:503–506. doi: 10.1016/j.joca.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Pechak DG. The cellular and molecular embryology of bone formation. In: Peck WA, editor. Bone and Mineral Research. New York: Elsevier Science Publishers; 1987. pp. 117–183. [Google Scholar]
- Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- Chau M, Forcinito P, Andrade AC, Hegde A, Ahn S, Lui JC, Baron J, Nilsson O. Organization of the Indian hedgehog--parathyroid hormone-related protein system in the postnatal growth plate. J Mol Endocrinol. 2011;47:99–107. doi: 10.1530/JME-10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Adar R, Yang X, Monsonego EO, Li C, Hauschka PV, Yayon A, Deng CX. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J Clin Invest. 1999;104:1517–1525. doi: 10.1172/JCI6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li C, Qiao W, Xu X, Deng C. A Ser(365)-->Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum Mol Genet. 2001;10:457–465. doi: 10.1093/hmg/10.5.457. [DOI] [PubMed] [Google Scholar]
- Chesi M, Nardini E, Brents LA, Schrock E, Ried T, Kuehl WM, Bergsagel PL. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat Genet. 1997;16:260–264. doi: 10.1038/ng0797-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Guo C, Torello M, Lunstrum GP, Iwata T, Deng C, Horton WA. Defective lysosomal targeting of activated fibroblast growth factor receptor 3 in achondroplasia. Proc Natl Acad Sci U S A. 2004;101:609–614. doi: 10.1073/pnas.2237184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque L, Forrester A, Bartolomeo R, Svelto M, Venditti R, Montefusco S, Polishchuk E, Nusco E, Rossi A, Medina DL, Polishchuk R, De Matteis MA, Settembre C. FGF signalling regulates bone growth through autophagy. Nature. 2015;528:272–275. doi: 10.1038/nature16063. [DOI] [PubMed] [Google Scholar]
- Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, Harlow E, Beach D, Weinberg RA, Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Dailey L, Laplantine E, Priore R, Basilico C. A network of transcriptional and signaling events is activated by FGF to induce chondrocyte growth arrest and differentiation. J Cell Biol. 2003;161:1053–1066. doi: 10.1083/jcb.200302075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frutos CA, Vega S, Manzanares M, Flores JM, Huertas H, Martinez-Frias ML, Nieto MA. Snail1 is a transcriptional effector of FGFR3 signaling during chondrogenesis and achondroplasias. Dev Cell. 2007;13:872–883. doi: 10.1016/j.devcel.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Delezoide AL, Benoist-Lasselin C, Legeai-Mallet L, Le Merrer M, Munnich A, Vekemans M, Bonaventure J. Spatio-temporal expression of FGFR 1, 2 and 3 genes during human embryo-fetal ossification. Mech Dev. 1998;77:19–30. doi: 10.1016/s0925-4773(98)00133-6. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Di Rocco F, Biosse Duplan M, Heuze Y, Kaci N, Komla-Ebri D, Munnich A, Mugniery E, Benoist-Lasselin C, Legeai-Mallet L. FGFR3 mutation causes abnormal membranous ossification in achondroplasia. Hum Mol Genet. 2014;23:2914–2925. doi: 10.1093/hmg/ddu004. [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Klohs W, Sharma A, Pera P, Driscoll D, Veith J, Steinkampf R, Schroeder M, Klutchko S, Sumlin A, Henderson B, Dougherty TJ, Bernacki RJ. Anti-angiogenic activity of selected receptor tyrosine kinase inhibitors, PD166285 and PD173074: implications for combination treatment with photodynamic therapy. Invest New Drugs. 1999;17:121–135. doi: 10.1023/a:1006367032156. [DOI] [PubMed] [Google Scholar]
- Donaldson J, Aftab S, Bradish C. Achondroplasia and limb lengthening: Results in a UK cohort and review of the literature. J Orthop. 2015;12:31–34. doi: 10.1016/j.jor.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar LF, Tucker M, Bamshad M. A second family with CATSHL syndrome: Confirmatory report of another unique FGFR3 syndrome. Am J Med Genet A. 2016 doi: 10.1002/ajmg.a.37676. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Schlessinger J. Skeletal overgrowth is mediated by deficiency in a specific isoform of fibroblast growth factor receptor 3. Proc Natl Acad Sci U S A. 2007;104:3937–3942. doi: 10.1073/pnas.0700012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmakis SG, Shinawi M, Miller-Thomas M, Radmanesh A, Herman TE. FGFR3-related condition: a skeletal dysplasia with similarities to thanatophoric dysplasia and SADDAN due to Lys650Met. Skeletal Radiol. 2015;44:441–445. doi: 10.1007/s00256-014-1983-6. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Xing L, Zhang JH, Zhao M, Horn D, Chan J, Boyce BF, Harris SE, Mundy GR, Chen D. NF-kappaB specifically activates BMP-2 gene expression in growth plate chondrocytes in vivo and in a chondrocyte cell line in vitro. J Biol Chem. 2003;278:29130–29135. doi: 10.1074/jbc.M212296200. [DOI] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenichel I, Evron Z, Nevo Z. The perichondrial ring as a reservoir for precartilaginous cells. In vivo model in young chicks’ epiphysis. Int Orthop. 2006;30:353–356. doi: 10.1007/s00264-006-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami M, Seki A, Ogata T. SHOX Haploinsufficiency as a Cause of Syndromic and Nonsyndromic Short Stature. Mol Syndromol. 2016;7:3–11. doi: 10.1159/000444596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia S, Dirat B, Tognacci T, Rochet N, Mouska X, Bonnafous S, Patouraux S, Tran A, Gual P, Le Marchand-Brustel Y, Gennero I, Gouze E. Postnatal soluble FGFR3 therapy rescues achondroplasia symptoms and restores bone growth in mice. Sci Transl Med. 2013;5:203ra124. doi: 10.1126/scitranslmed.3006247. [DOI] [PubMed] [Google Scholar]
- Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O. Genetic analysis reveals different functions for the products of the thyroid hormone receptor alpha locus. Mol Cell Biol. 2001;21:4748–4760. doi: 10.1128/MCB.21.14.4748-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geister KA, Brinkmeier ML, Hsieh M, Faust SM, Karolyi IJ, Perosky JE, Kozloff KM, Conti M, Camper SA. A novel loss-of-function mutation in Npr2 clarifies primary role in female reproduction and reveals a potential therapy for acromesomelic dysplasia, Maroteaux type. Hum Mol Genet. 2013;22:345–357. doi: 10.1093/hmg/dds432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germiller JA, Goldstein SA. Structure and function of embryonic growth plate in the absence of functioning skeletal muscle. J Orthop Res. 1997;15:362–370. doi: 10.1002/jor.1100150308. [DOI] [PubMed] [Google Scholar]
- Gibbs L, Legeai-Mallet L. FGFR3 intracellular mutations induce tyrosine phosphorylation in the Golgi and defective glycosylation. Biochim Biophys Acta. 2007;1773:502–512. doi: 10.1016/j.bbamcr.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Hill DJ, Logan A, Maher PA, Baird A. Distribution of fibroblast growth factor (FGF)-2 and FGF receptor-1 messenger RNA expression and protein presence in the mid-trimester human fetus. Pediatr Res. 1996;39:375–385. doi: 10.1203/00006450-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Gorbenko O, Ovcharenko G, Klymenko T, Zhyvoloup O, Gaman N, Volkova D, Gout I, Filonenko V. Generation of monoclonal antibody targeting fibroblast growth factor receptor 3. Hybridoma (Larchmt) 2009;28:295–300. doi: 10.1089/hyb.2009.0018. [DOI] [PubMed] [Google Scholar]
- Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90:175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, McDonald-McGinn DM, Gaudenz K, Whitaker LA, Bartlett SP, Glat PM, Cassileth LB, Mayro R, Zackai EH, Muenke M. Identification of a genetic cause for isolated unilateral coronal synostosis: a unique mutation in the fibroblast growth factor receptor 3. J Pediatr. 1998;132:714–716. doi: 10.1016/s0022-3476(98)70366-x. [DOI] [PubMed] [Google Scholar]
- Gudernova I, Vesela I, Balek L, Buchtova M, Dosedelova H, Kunova M, Pivnicka J, Jelinkova I, Roubalova L, Kozubik A, Krejci P. Multikinase activity of fibroblast growth factor receptor (FGFR) inhibitors SU5402, PD173074, AZD1480, AZD4547 and BGJ398 compromises the use of small chemicals targeting FGFR catalytic activity for therapy of short-stature syndromes. Hum Mol Genet. 2016;25:9–23. doi: 10.1093/hmg/ddv441. [DOI] [PubMed] [Google Scholar]
- Gust KM, McConkey DJ, Awrey S, Hegarty PK, Qing J, Bondaruk J, Ashkenazi A, Czerniak B, Dinney CP, Black PC. Fibroblast growth factor receptor 3 is a rational therapeutic target in bladder cancer. Mol Cancer Ther. 2013;12:1245–1254. doi: 10.1158/1535-7163.MCT-12-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK, Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol (Berl) 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- Hannema SE, van Duyvenvoorde HA, Premsler T, Yang RB, Mueller TD, Gassner B, Oberwinkler H, Roelfsema F, Santen GW, Prickett T, Kant SG, Verkerk AJ, Uitterlinden AG, Espiner E, Ruivenkamp CA, Oostdijk W, Pereira AM, Losekoot M, Kuhn M, Wit JM. An activating mutation in the kinase homology domain of the natriuretic peptide receptor-2 causes extremely tall stature without skeletal deformities. J Clin Endocrinol Metab. 2013;98:E1988–1998. doi: 10.1210/jc.2013-2358. [DOI] [PubMed] [Google Scholar]
- Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, Schlund B, Bosl MR, Hess A, Surmann-Schmitt C, von der Mark H, de Crombrugghe B, von der Mark K. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 2010;137:901–911. doi: 10.1242/dev.045203. [DOI] [PubMed] [Google Scholar]
- Havens BA, Velonis D, Kronenberg MS, Lichtler AC, Oliver B, Mina M. Roles of FGFR3 during morphogenesis of Meckel’s cartilage and mandibular bones. Dev Biol. 2008;316:336–349. doi: 10.1016/j.ydbio.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Serrano C, Niphadkar N, Shobnam N, Hristova K. Effect of the G375C and G346E Achondroplasia Mutations on FGFR3 Activation. PLoS One. 2012;7:e34808. doi: 10.1371/journal.pone.0034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Shobnam N, Wimley WC, Hristova K. FGFR3 heterodimerization in achondroplasia, the most common form of human dwarfism. J Biol Chem. 2011;286:13272–13281. doi: 10.1074/jbc.M110.205583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuertz S, Le Merrer M, Zabel B, Wright M, Legeai-Mallet L, Cormier-Daire V, Gibbs L, Bonaventure J. Novel FGFR3 mutations creating cysteine residues in the extracellular domain of the receptor cause achondroplasia or severe forms of hypochondroplasia. Eur J Hum Genet. 2006;14:1240–1247. doi: 10.1038/sj.ejhg.5201700. [DOI] [PubMed] [Google Scholar]
- Hirai T, Chagin AS, Kobayashi T, Mackem S, Kronenberg HM. Parathyroid hormone/parathyroid hormone-related protein receptor signaling is required for maintenance of the growth plate in postnatal life. Proc Natl Acad Sci U S A. 2011;108:191–196. doi: 10.1073/pnas.1005011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162–172. doi: 10.1016/S0140-6736(07)61090-3. [DOI] [PubMed] [Google Scholar]
- Hung IH, Schoenwolf GC, Lewandoski M, Ornitz DM. A combined series of Fgf9 and Fgf18 mutant alleles identifies unique and redundant roles in skeletal development. Dev Biol. 2016;411:72–84. doi: 10.1016/j.ydbio.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IH, Yu K, Lavine KJ, Ornitz DM. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev Biol. 2007;307:300–313. doi: 10.1016/j.ydbio.2007.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28:505–519. doi: 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987;69:162–173. [PubMed] [Google Scholar]
- Hutchison MR. BDNF alters ERK/p38 MAPK activity ratios to promote differentiation in growth plate chondrocytes. Mol Endocrinol. 2012;26:1406–1416. doi: 10.1210/me.2012-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami D, Akiyama H, Suzuki A, Nakamura T, Nakano T, Yoshikawa H, Tsumaki N. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development. 2011;138:1507–1519. doi: 10.1242/dev.057802. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ohta H, Konishi M. Endocrine FGFs: Evolution, Physiology, Pathophysiology, and Pharmacotherapy. Front Endocrinol (Lausanne) 2015;6:154. doi: 10.3389/fendo.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata T, Chen L, Li C, Ovchinnikov DA, Behringer RR, Francomano CA, Deng CX. A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet. 2000;9:1603–1613. doi: 10.1093/hmg/9.11.1603. [DOI] [PubMed] [Google Scholar]
- Iwata T, Li CL, Deng CX, Francomano CA. Highly activated Fgfr3 with the K644M mutation causes prolonged survival in severe dwarf mice. Hum Mol Genet. 2001;10:1255–1264. doi: 10.1093/hmg/10.12.1255. [DOI] [PubMed] [Google Scholar]
- Jonquoy A, Mugniery E, Benoist-Lasselin C, Kaci N, Le Corre L, Barbault F, Girard AL, Le Merrer Y, Busca P, Schibler L, Munnich A, Legeai-Mallet L. A novel tyrosine kinase inhibitor restores chondrocyte differentiation and promotes bone growth in a gain-of-function Fgfr3 mouse model. Hum Mol Genet. 2012;21:841–851. doi: 10.1093/hmg/ddr514. [DOI] [PubMed] [Google Scholar]
- Kant SG, Cervenkova I, Balek L, Trantirek L, Santen GW, de Vries MC, van Duyvenvoorde HA, van der Wielen MJ, Verkerk AJ, Uitterlinden AG, Hannema SE, Wit JM, Oostdijk W, Krejci P, Losekoot M. A novel variant of FGFR3 causes proportionate short stature. Eur J Endocrinol. 2015;172:763–770. doi: 10.1530/EJE-14-0945. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Thornemo M, Henriksson HB, Lindahl A. Identification of a stem cell niche in the zone of Ranvier within the knee joint. J Anat. 2009;215:355–363. doi: 10.1111/j.1469-7580.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Developmental Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Karuppaiah K, Yu K, Lim J, Chen J, Smith C, Long F, Ornitz DM. FGF signaling in the osteoprogenitor lineage non-autonomously regulates postnatal chondrocyte proliferation and skeletal growth. Development. 2016;143:1811–1822. doi: 10.1242/dev.131722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Pierce W, Sabharwal S. The etiology of short stature affects the clinical outcome of lower limb lengthening using external fixation. A systematic review of 18 trials involving 547 patients. Acta Orthop. 2014;85:181–186. doi: 10.3109/17453674.2014.899856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Murao H, Yamamoto K, Deng JM, Behringer RR, Nakamura T, Akiyama H. Generation of transgenic mice for conditional overexpression of Sox9. J Bone Miner Metab. 2011;29:123–129. doi: 10.1007/s00774-010-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolupaeva V, Daempfling L, Basilico C. The B55alpha regulatory subunit of protein phosphatase 2A mediates fibroblast growth factor-induced p107 dephosphorylation and growth arrest in chondrocytes. Mol Cell Biol. 2013;33:2865–2878. doi: 10.1128/MCB.01730-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolupaeva V, Laplantine E, Basilico C. PP2A-mediated dephosphorylation of p107 plays a critical role in chondrocyte cell cycle arrest by FGF. PLoS One. 2008;3:e3447. doi: 10.1371/journal.pone.0003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komla-Ebri D, Dambroise E, Kramer I, Benoist-Lasselin C, Kaci N, Le Gall C, Martin L, Busca P, Barbault F, Graus-Porta D, Munnich A, Kneissel M, Di Rocco F, Biosse-Duplan M, Legeai-Mallet L. Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model. J Clin Invest. 2016;126:1871–1884. doi: 10.1172/JCI83926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142:817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci P, Krakow D, Mekikian PB, Wilcox WR. Fibroblast growth factors 1, 2, 17, and 19 are the predominant FGF ligands expressed in human fetal growth plate cartilage. Pediatr Res. 2007;61:267–272. doi: 10.1203/pdr.0b013e318030d157. [DOI] [PubMed] [Google Scholar]
- Krejci P, Masri B, Fontaine V, Mekikian PB, Weis M, Prats H, Wilcox WR. Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J Cell Sci. 2005;118:5089–5100. doi: 10.1242/jcs.02618. [DOI] [PubMed] [Google Scholar]
- Krejci P, Salazar L, Kashiwada TA, Chlebova K, Salasova A, Thompson LM, Bryja V, Kozubik A, Wilcox WR. Analysis of STAT1 activation by six FGFR3 mutants associated with skeletal dysplasia undermines dominant role of STAT1 in FGFR3 signaling in cartilage. PLoS One. 2008;3:e3961. doi: 10.1371/journal.pone.0003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszka P, Addissie YA, Yarnell CM, Hadley DW, Guillen Sacoto MJ, Platte P, Paelecke Y, Collmann H, Snow N, Schweitzer T, Boyadjiev SA, Aravidis C, Hall SE, Mulliken JB, Roscioli T, Muenke M. Muenke syndrome: An international multicenter natural history study. Am J Med Genet A. 2016;170a:918–929. doi: 10.1002/ajmg.a.37528. [DOI] [PubMed] [Google Scholar]
- Laplantine E, Rossi F, Sahni M, Basilico C, Cobrinik D. FGF signaling targets the pRb-related p107 and p130 proteins to induce chondrocyte growth arrest. J Cell Biol. 2002;158:741–750. doi: 10.1083/jcb.200205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurita J, Koyama E, Chin B, Taylor JA, Lakin GE, Hankenson KD, Bartlett SP, Nah HD. The Muenke syndrome mutation (FgfR3P244R) causes cranial base shortening associated with growth plate dysfunction and premature perichondrial ossification in murine basicranial synchondroses. Dev Dyn. 2011;240:2584–2596. doi: 10.1002/dvdy.22752. [DOI] [PubMed] [Google Scholar]
- Le Merrer M, Rousseau F, Legeai-Mallet L, Landais JC, Pelet A, Bonaventure J, Sanak M, Weissenbach J, Stoll C, Munnich A, et al. A gene for achondroplasia-hypochondroplasia maps to chromosome 4p. Nat Genet. 1994;6:318–321. doi: 10.1038/ng0394-318. [DOI] [PubMed] [Google Scholar]
- Legeai-Mallet L, Benoist-Lasselin C, Delezoide AL, Munnich A, Bonaventure J. Fibroblast growth factor receptor 3 mutations promote apoptosis but do not alter chondrocyte proliferation in thanatophoric dysplasia. J Biol Chem. 1998;273:13007–13014. doi: 10.1074/jbc.273.21.13007. [DOI] [PubMed] [Google Scholar]
- Legeai-Mallet L, Benoist-Lasselin C, Munnich A, Bonaventure J. Overexpression of FGFR3, Stat1, Stat5 and p21Cip1 correlates with phenotypic severity and defective chondrocyte differentiation in FGFR3-related chondrodysplasias. Bone. 2004;34:26–36. doi: 10.1016/j.bone.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Li C, Chen L, Iwata T, Kitagawa M, Fu XY, Deng CX. A Lys644Glu substitution in fibroblast growth factor receptor 3 (FGFR3) causes dwarfism in mice by activation of STATs and ink4 cell cycle inhibitors. Hum Mol Genet. 1999;8:35–44. doi: 10.1093/hmg/8.1.35. [DOI] [PubMed] [Google Scholar]
- Li X, Wang C, Xiao J, McKeehan WL, Wang F. Fibroblast growth factors, old kids on the new block. Semin Cell Dev Biol. 2016;53:155–167. doi: 10.1016/j.semcdb.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens PM, Liboi E. The thanatophoric dysplasia type II mutation hampers complete maturation of fibroblast growth factor receptor 3 (FGFR3), which activates signal transducer and activator of transcription 1 (STAT1) from the endoplasmic reticulum. J Biol Chem. 2003;278:17344–17349. doi: 10.1074/jbc.M212710200. [DOI] [PubMed] [Google Scholar]
- Lievens PM, Mutinelli C, Baynes D, Liboi E. The kinase activity of fibroblast growth factor receptor 3 with activation loop mutations affects receptor trafficking and signaling. J Biol Chem. 2004;279:43254–43260. doi: 10.1074/jbc.M405247200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lavine KJ, Hung IH, Ornitz DM. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev Biol. 2007;302:80–91. doi: 10.1016/j.ydbio.2006.08.071. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Hill DJ, Gonzalez AM. Expression and release of basic fibroblast growth factor by epiphyseal growth plate chondrocytes. Ann N Y Acad Sci. 1991;638:459–462. doi: 10.1111/j.1749-6632.1991.tb49069.x. [DOI] [PubMed] [Google Scholar]
- Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol. 2013;5:a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Lorget F, Kaci N, Peng J, Benoist-Lasselin C, Mugniery E, Oppeneer T, Wendt DJ, Bell SM, Bullens S, Bunting S, Tsuruda LS, O’Neill CA, Di Rocco F, Munnich A, Legeai-Mallet L. Evaluation of the Therapeutic Potential of a CNP Analog in a Fgfr3 Mouse Model Recapitulating Achondroplasia. Am J Hum Genet. 2012;91:1108–1114. doi: 10.1016/j.ajhg.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Wan Y, Cao J, Zhu X, Yu J, Zhou R, Yao Y, Zhang L, Zhao H, Li H, Zhao J, He L, Ma G, Yang X, Yao Z, Guo X. Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification. Bone. 2013;53:566–574. doi: 10.1016/j.bone.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Lui JC, Nilsson O, Baron J. Recent research on the growth plate: Recent insights into the regulation of the growth plate. J Mol Endocrinol. 2014;53:T1–9. doi: 10.1530/JME-14-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C. Signaling pathways effecting crosstalk between cartilage and adjacent tissues: Seminars in cell and developmental biology: The biology and pathology of cartilage. Semin Cell Dev Biol. 2016;S1084-9521:30131–30138. doi: 10.1016/j.semcdb.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Makrythanasis P, Temtamy S, Aglan MS, Otaify GA, Hamamy H, Antonarakis SE. A novel homozygous mutation in FGFR3 causes tall stature, severe lateral tibial deviation, scoliosis, hearing impairment, camptodactyly, and arachnodactyly. Hum Mutat. 2014;35:959–963. doi: 10.1002/humu.22597. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Li C, Urness LD. Genetic rescue of Muenke syndrome model hearing loss reveals prolonged FGF-dependent plasticity in cochlear supporting cell fates. Genes Dev. 2013;27:2320–2331. doi: 10.1101/gad.228957.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour SL, Twigg SR, Freeland RM, Wall SA, Li C, Wilkie AO. Hearing loss in a mouse model of Muenke syndrome. Hum Mol Genet. 2009;18:43–50. doi: 10.1093/hmg/ddn311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torrecuadrada J, Cifuentes G, Lopez-Serra P, Saenz P, Martinez A, Casal JI. Targeting the extracellular domain of fibroblast growth factor receptor 3 with human single-chain Fv antibodies inhibits bladder carcinoma cell line proliferation. Clin Cancer Res. 2005;11:6280–6290. doi: 10.1158/1078-0432.CCR-05-0282. [DOI] [PubMed] [Google Scholar]
- Massart F, Miccoli M, Baggiani A, Bertelloni S. Height outcome of short children with hypochondroplasia after recombinant human growth hormone treatment: a meta-analysis. Pharmacogenomics. 2015;16:1965–1973. doi: 10.2217/pgs.15.129. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hasegawa S, Kitoh H, Mori K, Ohkawara B, Yasoda A, Masuda A, Ishiguro N, Ohno K. Meclozine promotes longitudinal skeletal growth in transgenic mice with achondroplasia carrying a gain-of-function mutation in the FGFR3 gene. Endocrinology. 2015;156:548–554. doi: 10.1210/en.2014-1914. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Kitoh H, Ohkawara B, Mishima K, Kaneko H, Ito M, Masuda A, Ishiguro N, Ohno K. Meclozine Facilitates Proliferation and Differentiation of Chondrocytes by Attenuating Abnormally Activated FGFR3 Signaling in Achondroplasia. PLoS One. 2013;8:e81569. doi: 10.1371/journal.pone.0081569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DG, Green RP, Naski MC, Towler DA, Ornitz DM. Fibroblast growth factor receptor 3 gene transcription is suppressed by cyclic adenosine 3′,5 ′-monophosphate - Identification of a chondrocytic regulatory element. J Biol Chem. 1999;274:30934–30942. doi: 10.1074/jbc.274.43.30934. [DOI] [PubMed] [Google Scholar]
- McEwen DG, Ornitz DM. Regulation of the fibroblast growth factor receptor 3 promoter and intron I enhancer by Sp1 family transcription factors. J Biol Chem. 1998;273:5349–5357. doi: 10.1074/jbc.273.9.5349. [DOI] [PubMed] [Google Scholar]
- Meyers GA, Orlow SJ, Munro IR, Przylepa KA, Jabs EW. Fibroblast growth factor receptor 3 (FGFR3) transmembrane mutation in Crouzon syndrome with acanthosis nigricans. Nat Genet. 1995;11:462–464. doi: 10.1038/ng1295-462. [DOI] [PubMed] [Google Scholar]
- Miccoli M, Bertelloni S, Massart F. Height Outcome of Recombinant Human Growth Hormone Treatment in Achondroplasia Children: A Meta-Analysis. Horm Res Paediatr. 2016;86:27–34. doi: 10.1159/000446958. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Moncla A, Missirian C, Cacciagli P, Balzamo E, Legeai-Mallet L, Jouve JL, Chabrol B, Le Merrer M, Plessis G, Villard L, Philip N. A cluster of translocation breakpoints in 2q37 is associated with overexpression of NPPC in patients with a similar overgrowth phenotype. Hum Mutat. 2007;28:1183–1188. doi: 10.1002/humu.20611. [DOI] [PubMed] [Google Scholar]
- Monsonego-Ornan E, Adar R, Feferman T, Segev O, Yayon A. The transmembrane mutation G380R in fibroblast growth factor receptor 3 uncouples ligand-mediated receptor activation from down-regulation. Mol Cell Biol. 2000;20:516–522. doi: 10.1128/mcb.20.2.516-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero A, Okada Y, Tomita M, Ito M, Tsurukami H, Nakamura T, Doetschman T, Coffin JD, Hurley MM. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J Clin Invest. 2000;105:1085–1093. doi: 10.1172/JCI8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugniery E, Dacquin R, Marty C, Benoist-Lasselin C, de Vernejoul MC, Jurdic P, Munnich A, Geoffroy V, Legeai-Mallet L. An activating Fgfr3 mutation affects trabecular bone formation via a paracrine mechanism during growth. Hum Mol Genet. 2012;21:2503–2513. doi: 10.1093/hmg/dds065. [DOI] [PubMed] [Google Scholar]
- Murakami S, Balmes G, McKinney S, Zhang Z, Givol D, De Crombrugghe B. Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev. 2004;18:290–305. doi: 10.1101/gad.1179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah HD, Koyama E, Agochukwu NB, Bartlett SP, Muenke M. Phenotype profile of a genetic mouse model for Muenke syndrome. Childs Nerv Syst. 2012;28:1483–1493. doi: 10.1007/s00381-012-1778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naski MC, Colvin JS, Coffin JD, Ornitz DM. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development. 1998;125:4977–4988. doi: 10.1242/dev.125.24.4977. [DOI] [PubMed] [Google Scholar]
- Naski MC, Wang Q, Xu J, Ornitz DM. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat Genet. 1996;13:233–237. doi: 10.1038/ng0696-233. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Parker EA, Hegde A, Chau M, Barnes KM, Baron J. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol. 2007;193:75–84. doi: 10.1677/joe.1.07099. [DOI] [PubMed] [Google Scholar]
- Noonan KJ, Hunziker EB, Nessler J, Buckwalter JA. Changes in cell, matrix compartment, and fibrillar collagen volumes between growth-plate zones. J Orthop Res. 1998;16:500–508. doi: 10.1002/jor.1100160416. [DOI] [PubMed] [Google Scholar]
- Nowlan NC, Bourdon C, Dumas G, Tajbakhsh S, Prendergast PJ, Murphy P. Developing bones are differentially affected by compromised skeletal muscle formation. Bone. 2010;46:1275–1285. doi: 10.1016/j.bone.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea PJ, Harvey CB, Suzuki H, Kaneshige M, Kaneshige K, Cheng SY, Williams GR. A thyrotoxic skeletal phenotype of advanced bone formation in mice with resistance to thyroid hormone. Mol Endocrinol. 2003;17:1410–1424. doi: 10.1210/me.2002-0296. [DOI] [PubMed] [Google Scholar]
- Ohbayashi N, Shibayama M, Kurotaki Y, Imanishi M, Fujimori T, Itoh N, Takada S. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16:870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab. 2006;91:1229–1232. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015;29:1463–1486. doi: 10.1101/gad.266551.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozasa A, Komatsu Y, Yasoda A, Miura M, Sakuma Y, Nakatsuru Y, Arai H, Itoh N, Nakao K. Complementary antagonistic actions between C-type natriuretic peptide and the MAPK pathway through FGFR-3 in ATDC5 cells. Bone. 2005;36:1056–1064. doi: 10.1016/j.bone.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Paley D. Current techniques of limb lengthening. J Pediatr Orthop. 1988;8:73–92. doi: 10.1097/01241398-198801000-00018. [DOI] [PubMed] [Google Scholar]
- Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990:81–104. [PubMed] [Google Scholar]
- Paley D. PRECICE intramedullary limb lengthening system. Expert Rev Med Devices. 2015;12:231–249. doi: 10.1586/17434440.2015.1005604. [DOI] [PubMed] [Google Scholar]
- Pan F, Liu XG, Guo YF, Chen Y, Dong SS, Qiu C, Zhang ZX, Zhou Q, Yang TL, Guo Y, Zhu XZ, Deng HW. The regulation-of-autophagy pathway may influence Chinese stature variation: evidence from elder adults. J Hum Genet. 2010;55:441–447. doi: 10.1038/jhg.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit SG, Govindraj P, Sasse J, Neame PJ, Hassell JR. The fibroblast growth factor receptor, FGFR3, forms gradients of intact and degraded protein across the growth plate of developing bovine ribs. Biochem J. 2002;361:231–241. doi: 10.1042/0264-6021:3610231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannier S, Couloigner V, Messaddeq N, Elmaleh-Berges M, Munnich A, Romand R, Legeai-Mallet L. Activating Fgfr3 Y367C mutation causes hearing loss and inner ear defect in a mouse model of chondrodysplasia. Biochim Biophys Acta. 2009a;1792:140–147. doi: 10.1016/j.bbadis.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Pannier S, Martinovic J, Heuertz S, Delezoide AL, Munnich A, Schibler L, Serre V, Legeai-Mallet L. Thanatophoric dysplasia caused by double missense FGFR3 mutations. Am J Med Genet A. 2009b;149a:1296–1301. doi: 10.1002/ajmg.a.32880. [DOI] [PubMed] [Google Scholar]
- Pannier S, Mugniery E, Jonquoy A, Benoist-Lasselin C, Odent T, Jais JP, Munnich A, Legeai-Mallet L. Delayed bone age due to a dual effect of FGFR3 mutation in Achondroplasia. Bone. 2010;47:905–915. doi: 10.1016/j.bone.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Parafioriti A, del Bianco S, Barisani D, Armiraglio E, Peretti G, Albisetti W. Increased p21 expression in chondrocytes of achondroplasic children independently from the presence of the G380R FGFR3 mutation. J Orthop Sci. 2009;14:623–630. doi: 10.1007/s00776-009-1355-6. [DOI] [PubMed] [Google Scholar]
- Park J, Gebhardt M, Golovchenko S, Perez-Branguli F, Hattori T, Hartmann C, Zhou X, deCrombrugghe B, Stock M, Schneider H, von der Mark K. Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biol Open. 2015;4:608–621. doi: 10.1242/bio.201411031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patani H, Bunney TD, Thiyagarajan N, Norman RA, Ogg D, Breed J, Ashford P, Potterton A, Edwards M, Williams SV, Thomson GS, Pang CS, Knowles MA, Breeze AL, Orengo C, Phillips C, Katan M. Landscape of activating cancer mutations in FGFR kinases and their differential responses to inhibitors in clinical use. Oncotarget. 2016;7:24252–24268. doi: 10.18632/oncotarget.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake NJ, Hobbs AJ, Pingguan-Murphy B, Salter DM, Berenbaum F, Chowdhury TT. Role of C-type natriuretic peptide signalling in maintaining cartilage and bone function. Osteoarthritis Cartilage. 2014;22:1800–1807. doi: 10.1016/j.joca.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Farquharson C, Ahmed SF. The role of sex steroids in controlling pubertal growth. Clin Endocrinol (Oxf) 2008;68:4–15. doi: 10.1111/j.1365-2265.2007.02960.x. [DOI] [PubMed] [Google Scholar]
- Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Pinto G, Cormier-Daire V, Le Merrer M, Samara-Boustani D, Baujat G, Fresneau L, Viaud M, Souberbielle JC, Pineau JC, Polak M. Efficacy and safety of growth hormone treatment in children with hypochondroplasia: comparison with an historical cohort. Horm Res Paediatr. 2014;82:355–363. doi: 10.1159/000364807. [DOI] [PubMed] [Google Scholar]
- Polanska UM, Fernig DG, Kinnunen T. Extracellular interactome of the FGF receptor-ligand system: Complexities and the relative simplicity of the worm. Dev Dyn. 2009 doi: 10.1002/dvdy.21757. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Qi H, Jin M, Duan Y, Du X, Zhang Y, Ren F, Wang Y, Tian Q, Wang X, Wang Q, Zhu Y, Xie Y, Liu C, Cao X, Mishina Y, Chen D, Deng CX, Chang Z, Chen L. FGFR3 induces degradation of BMP type I receptor to regulate skeletal development. Biochim Biophys Acta. 2014;1843:1237–1247. doi: 10.1016/j.bbamcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K, Ross S, Stinson S, Dornan D, French D, Wang QR, Stephan JP, Wu Y, Wiesmann C, Ashkenazi A. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest. 2009;119:1216–1229. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami U, Rumsby G, Hindmarsh PC, Brook CG. Genotype and phenotype in hypochondroplasia [see comments] J Pediatr. 1998;133:99–102. doi: 10.1016/s0022-3476(98)70186-6. [DOI] [PubMed] [Google Scholar]
- Rannan-Eliya SV, Taylor IB, De Heer IM, Van Den Ouweland AM, Wall SA, Wilkie AO. Paternal origin of FGFR3 mutations in Muenke-type craniosynostosis. Hum Genet. 2004;115:200–207. doi: 10.1007/s00439-004-1151-5. [DOI] [PubMed] [Google Scholar]
- Ranvier L. Quelques faits relatifs au developpement du tissu osseux. Comptes Rend. Acad. Sciences. 1873;77:1105–1109. [Google Scholar]
- Ranvier L. Traité technique d’histologie. Paris: Savy; 1889. Developpement du Tissu Osseux; pp. 339–365. [Google Scholar]
- Raucci A, Laplantine E, Mansukhani A, Basilico C. Activation of the ERK1/2 and p38 mitogen-activated protein kinase pathways mediates fibroblast growth factor-induced growth arrest of chondrocytes. J Biol Chem. 2004;279:1747–1756. doi: 10.1074/jbc.M310384200. [DOI] [PubMed] [Google Scholar]
- Rauchenberger R, Borges E, Thomassen-Wolf E, Rom E, Adar R, Yaniv Y, Malka M, Chumakov I, Kotzer S, Resnitzky D, Knappik A, Reiffert S, Prassler J, Jury K, Waldherr D, Bauer S, Kretzschmar T, Yayon A, Rothe C. Human combinatorial Fab library yielding specific and functional antibodies against the human fibroblast growth factor receptor 3. J Biol Chem. 2003;278:38194–38205. doi: 10.1074/jbc.M303164200. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Naski MC. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. J Biol Chem. 2007;282:3653–3663. doi: 10.1074/jbc.M608995200. [DOI] [PubMed] [Google Scholar]
- Robinson D, Hasharoni A, Cohen N, Yayon A, Moskowitz RM, Nevo Z. Fibroblast growth factor receptor-3 as a marker for precartilaginous stem cells. Clin Orthop Relat Res. 1999:S163–175. doi: 10.1097/00003086-199910001-00018. [DOI] [PubMed] [Google Scholar]
- Rosello-Diez A, Joyner AL. Regulation of Long Bone Growth in Vertebrates; It Is Time to Catch Up. Endocr Rev. 2015;36:646–680. doi: 10.1210/er.2015-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Saugier P, Le Merrer M, Munnich A, Delezoide AL, Maroteaux P, Bonaventure J, Narcy F, Sanak M. Stop codon FGFR3 mutations in thanatophoric dwarfism type 1. Nat Genet. 1995;10:11–12. doi: 10.1038/ng0595-11. [DOI] [PubMed] [Google Scholar]
- Sabatino G, Di Rocco F, Zampino G, Tamburrini G, Caldarelli M, Di Rocco C. Muenke syndrome. Childs Nerv Syst. 2004;20:297–301. doi: 10.1007/s00381-003-0906-y. [DOI] [PubMed] [Google Scholar]
- Scheller T, Hellerbrand C, Moser C, Schmidt K, Kroemer A, Brunner SM, Schlitt HJ, Geissler EK, Lang SA. mTOR inhibition improves fibroblast growth factor receptor targeting in hepatocellular carcinoma. Br J Cancer. 2015;112:841–850. doi: 10.1038/bjc.2014.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiedel F, Rodl R. Lower limb lengthening in patients with disproportionate short stature with achondroplasia: a systematic review of the last 20 years. Disabil Rehabil. 2012;34:982–987. doi: 10.3109/09638288.2011.631677. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Matsushita T, Kawanami A, Mackem S, Landreth GE, Murakami S. Genetic inactivation of ERK1 and ERK2 in chondrocytes promotes bone growth and enlarges the spinal canal. J Orthop Res. 2011;29:375–379. doi: 10.1002/jor.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Fujimori T, Savagner P, Hata A, Aikawa T, Ogata N, Nabeshima Y, Kaechoong L. Mouse Snail family transcription repressors regulate chondrocyte, extracellular matrix, type II collagen, and aggrecan. J Biol Chem. 2003;278:41862–41870. doi: 10.1074/jbc.M308336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao YY, Wang L, Welter JF, Ballock RT. Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone. 2012;50:79–84. doi: 10.1016/j.bone.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro F, Holtrop ME, Glimcher MJ. Organization and cellular biology of the perichondrial ossification groove of ranvier: a morphological study in rabbits. J Bone Joint Surg Am. 1977;59:703–723. [PubMed] [Google Scholar]
- Shapiro IM, Layfield R, Lotz M, Settembre C, Whitehouse C. Boning up on autophagy: the role of autophagy in skeletal biology. Autophagy. 2014;10:7–19. doi: 10.4161/auto.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Shinde DN, Elmer DP, Calabrese P, Boulanger J, Arnheim N, Tiemann-Boege I. New evidence for positive selection helps explain the paternal age effect observed in achondroplasia. Hum Mol Genet. 2013;22:4117–4126. doi: 10.1093/hmg/ddt260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O’Keefe RJ, Hilton MJ, Wang Y, Chen D. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shung CY, Ota S, Zhou ZQ, Keene DR, Hurlin PJ. Disruption of a Sox9-beta-catenin circuit by mutant Fgfr3 in thanatophoric dysplasia type II. Hum Mol Genet. 2012;21:4628–4644. doi: 10.1093/hmg/dds305. [DOI] [PubMed] [Google Scholar]
- Smith BN, Burton LJ, Henderson V, Randle DD, Morton DJ, Smith BA, Taliaferro-Smith L, Nagappan P, Yates C, Zayzafoon M, Chung LW, Odero-Marah VA. Snail promotes epithelial mesenchymal transition in breast cancer cells in part via activation of nuclear ERK2. PLoS One. 2014;9:e104987. doi: 10.1371/journal.pone.0104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Dally MR, Sainz RD, Rodrigue KL, Oberbauer AM. Enhanced skeletal growth of sheep heterozygous for an inactivated fibroblast growth factor receptor 3. J Anim Sci. 2006;84:2942–2949. doi: 10.2527/jas.2006-255. [DOI] [PubMed] [Google Scholar]
- Stanescu R, Stanescu V, Maroteaux P. Homozygous Achondroplasia: Morphologic and Biochemical Study of Cartilage. Am J Med Genet. 1990;37:412–421. doi: 10.1002/ajmg.1320370323. [DOI] [PubMed] [Google Scholar]
- Su N, Li X, Tang Y, Yang J, Wen X, Guo J, Tang J, Du X, Chen L. Deletion of FGFR3 in Osteoclast Lineage Cells Results in Increased Bone Mass in Mice by Inhibiting Osteoclastic Bone Resorption. J Bone Miner Res. 2016;31:1676–1687. doi: 10.1002/jbmr.2839. [DOI] [PubMed] [Google Scholar]
- Su N, Sun Q, Li C, Lu X, Qi H, Chen S, Yang J, Du X, Zhao L, He Q, Jin M, Shen Y, Chen D, Chen L. Gain-of-function mutation in FGFR3 in mice leads to decreased bone mass by affecting both osteoblastogenesis and osteoclastogenesis. Hum Mol Genet. 2010;19:1199–1210. doi: 10.1093/hmg/ddp590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WC, Kitagawa M, Xue N, Xie B, Garofalo S, Cho J, Deng C, Horton WA, Fu XY. Activation of Stat1 by mutant fibroblast growth-factor receptor in thanatophoric dysplasia type II dwarfism. Nature. 1997;386:288–292. doi: 10.1038/386288a0. [DOI] [PubMed] [Google Scholar]
- Suda M, Ogawa Y, Tanaka K, Tamura N, Yasoda A, Takigawa T, Uehira M, Nishimoto H, Itoh H, Saito Y, Shiota K, Nakao K. Skeletal overgrowth in transgenic mice that overexpress brain natriuretic peptide. Proc Natl Acad Sci U S A. 1998;95:2337–2342. doi: 10.1073/pnas.95.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Marcello M, Li Y, Mason D, Levy R, Fernig DG. Selectivity in glycosaminoglycan binding dictates the distribution and diffusion of fibroblast growth factors in the pericellular matrix. Open Biol. 2016:6. doi: 10.1098/rsob.150277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Chen Q, Yang S, Pan Q, Ma J, Wan Y, Chang CH, Hong A. Remodeling of chromatin structure within the promoter is important for bmp-2-induced fgfr3 expression. Nucleic Acids Res. 2009;37:3897–3911. doi: 10.1093/nar/gkp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga A, Eich G, Bucher HU, Wisser J, Giedion A, Gitzelmann R, Steinmann B. A glycine 375-to-cysteine substitution in the transmembrane domain of the fibroblast growth factor receptor-3 in a newborn with achondroplasia. Eur J Pediatr. 1995;154:215–219. doi: 10.1007/BF01954274. [DOI] [PubMed] [Google Scholar]
- Takagi M, Kouwaki M, Kawase K, Shinohara H, Hasegawa Y, Yamada T, Fujiwara I, Sawai H, Nishimura G, Hasegawa T. A novel mutation Ser344Cys in FGFR3 causes achondroplasia with severe platyspondyly. Am J Med Genet A. 2015;167A:2851–2854. doi: 10.1002/ajmg.a.37231. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Katsumata N, Horikawa R, Tanaka T. The comparison of the effects of short-term growth hormone treatment in patients with achondroplasia and with hypochondroplasia. Endocr J. 2003;50:69–75. doi: 10.1507/endocrj.50.69. [DOI] [PubMed] [Google Scholar]
- Tavormina PL, Bellus GA, Webster MK, Bamshad MJ, Fraley AE, McIntosh I, Szabo J, Jiang W, Jabs EW, Wilcox WR, Wasmuth JJ, Donoghue DJ, Thompson LM, Francomano CA. A novel skeletal dysplasia with developmental delay and acanthosis nigricans is caused by a Lys650Met mutation in the fibroblast growth factor receptor 3 gene. Am J Hum Genet. 1999;64:722–731. doi: 10.1086/302275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina PL, Shiang R, Thompson LM, Zhu YZ, Wilkin DJ, Lachman RS, Wilcox WR, Rimoin DL, Cohn DH, Wasmuth JJ. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet. 1995;9:321–328. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- Toydemir RM, Brassington AE, Bayrak-Toydemir P, Krakowiak PA, Jorde LB, Whitby FG, Longo N, Viskochil DH, Carey JC, Bamshad MJ. A novel mutation in FGFR3 causes camptodactyly, tall stature, and hearing loss (CATSHL) syndrome. Am J Hum Genet. 2006;79:935–941. doi: 10.1086/508433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, Reece D, Heise C, Stewart AK. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2005;105:2941–2948. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- Trudel S, Stewart AK, Rom E, Wei E, Li ZH, Kotzer S, Chumakov I, Singer Y, Chang H, Liang SB, Yayon A. The inhibitory anti-FGFR3 antibody, PRO-001, is cytotoxic to t(4;14) multiple myeloma cells. Blood. 2006;107:4039–4046. doi: 10.1182/blood-2005-10-4179. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Twigg SR, Healy C, Babbs C, Sharpe JA, Wood WG, Sharpe PT, Morriss-Kay GM, Wilkie AO. Skeletal analysis of the Fgfr3(P244R) mouse, a genetic model for the Muenke craniosynostosis syndrome. Dev Dyn. 2009;238:331–342. doi: 10.1002/dvdy.21790. [DOI] [PubMed] [Google Scholar]
- Vajo Z, Francomano CA, Wilkin DJ. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, Muenke craniosynostosis, and Crouzon syndrome with acanthosis nigricans. Endocr Rev. 2000;21:23–39. doi: 10.1210/edrv.21.1.0387. [DOI] [PubMed] [Google Scholar]
- Velinov M, Slaugenhaupt SA, Stoilov I, Scott CI, Jr, Gusella JF, Tsipouras P. The gene for achondroplasia maps to the telomeric region of chromosome 4p. Nat Genet. 1994;6:314–317. doi: 10.1038/ng0394-314. [DOI] [PubMed] [Google Scholar]
- Villemure I, Stokes IA. Growth plate mechanics and mechanobiology. A survey of present understanding. J Biomech. 2009;42:1793–1803. doi: 10.1016/j.jbiomech.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalapati KK, Bouderlique T, Newton PT, Kaminskyy VO, Wehtje H, Ohlsson C, Zhivotovsky B, Chagin AS. Targeted Deletion of Autophagy Genes Atg5 or Atg7 in the Chondrocytes Promotes Caspase-Dependent Cell Death and Leads to Mild Growth Retardation. J Bone Miner Res. 2015;30:2249–2261. doi: 10.1002/jbmr.2575. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004;180:247–255. doi: 10.1677/joe.0.1800247. [DOI] [PubMed] [Google Scholar]
- Wang X, Qi H, Wang Q, Zhu Y, Wang X, Jin M, Tan Q, Huang Q, Xu W, Li X, Kuang L, Tang Y, Du X, Chen D, Chen L. FGFR3/fibroblast growth factor receptor 3 inhibits autophagy through decreasing the ATG12-ATG5 conjugate, leading to the delay of cartilage development in achondroplasia. Autophagy. 2015;11:1998–2013. doi: 10.1080/15548627.2015.1091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Spatz MK, Kannan K, Hayk H, Avivi A, Gorivodsky M, Pines M, Yayon A, Lonai P, Givol D. A mouse model for achondroplasia produced by targeting fibroblast growth factor receptor 3. Proc Natl Acad Sci U S A. 1999;96:4455–4460. doi: 10.1073/pnas.96.8.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MK, Donoghue DJ. Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO J. 1996;15:520–527. [PMC free article] [PubMed] [Google Scholar]
- Wen X, Li X, Tang Y, Tang J, Zhou S, Xie Y, Guo J, Yang J, Du X, Su N, Chen L. Chondrocyte FGFR3 Regulates Bone Mass by Inhibiting Osteogenesis. J Biol Chem. 2016;291:24912–24921. doi: 10.1074/jbc.M116.730093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt DJ, Dvorak-Ewell M, Bullens S, Lorget F, Bell SM, Peng J, Castillo S, Aoyagi-Scharber M, O’Neill CA, Krejci P, Wilcox WR, Rimoin DL, Bunting S. Neutral endopeptidase-resistant C-type natriuretic peptide variant represents a new therapeutic approach for treatment of fibroblast growth factor receptor 3-related dwarfism. J Pharmacol Exp Ther. 2015;353:132–149. doi: 10.1124/jpet.114.218560. [DOI] [PubMed] [Google Scholar]
- Wilkes D, Rutland P, Pulleyn LJ, Reardon W, Moss C, Ellis JP, Winter RM, Malcolm S. A recurrent mutation, ala391glu, in the transmembrane region of FGFR3 causes Crouzon syndrome and acanthosis nigricans. J Med Genet. 1996;33:744–748. doi: 10.1136/jmg.33.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin DJ, Szabo JK, Cameron R, Henderson S, Bellus GA, Mack ML, Kaitila I, Loughlin J, Munnich A, Sykes B, Bonaventure J, Francomano CA. Mutations in fibroblast growth-factor receptor 3 in sporadic cases of achondroplasia occur exclusively on the paternally derived chromosome. Am J Hum Genet. 1998;63:711–716. doi: 10.1086/302000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14:927–936. doi: 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- Wohrle S, Henninger C, Bonny O, Thuery A, Beluch N, Hynes NE, Guagnano V, Sellers WR, Hofmann F, Kneissel M, Graus Porta D. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res. 2013a;28:899–911. doi: 10.1002/jbmr.1810. [DOI] [PubMed] [Google Scholar]
- Wohrle S, Weiss A, Ito M, Kauffmann A, Murakami M, Jagani Z, Thuery A, Bauer-Probst B, Reimann F, Stamm C, Pornon A, Romanet V, Guagnano V, Brummendorf T, Sellers WR, Hofmann F, Roberts CW, Graus Porta D. Fibroblast growth factor receptors as novel therapeutic targets in SNF5-deleted malignant rhabdoid tumors. PLoS One. 2013b;8:e77652. doi: 10.1371/journal.pone.0077652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, James CG, Wang G, Dupuis H, Beier F. Control of chondrocyte gene expression by actin dynamics: a novel role of cholesterol/Ror-alpha signalling in endochondral bone growth. J Cell Mol Med. 2009;13:3497–3516. doi: 10.1111/j.1582-4934.2009.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, De Luca F. Role of cholesterol in the regulation of growth plate chondrogenesis and longitudinal bone growth. J Biol Chem. 2004;279:4642–4647. doi: 10.1074/jbc.M305518200. [DOI] [PubMed] [Google Scholar]
- Xie Y, Su N, Jin M, Qi H, Yang J, Li C, Du X, Luo F, Chen B, Shen Y, Huang H, Xian CJ, Deng C, Chen L. Intermittent PTH (1–34) injection rescues the retarded skeletal development and postnatal lethality of mice mimicking human achondroplasia and thanatophoric dysplasia. Hum Mol Genet. 2012;21:3941–3955. doi: 10.1093/hmg/dds181. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhou S, Chen H, Du X, Chen L. RECENT RESEARCH ON THE GROWTH PLATE: Advances in fibroblast growth factor signaling in growth plate development and disorders. J Mol Endocrinol. 2014;53:T11–t34. doi: 10.1530/JME-14-0012. [DOI] [PubMed] [Google Scholar]
- Xue Y, Sun A, Mekikian PB, Martin J, Rimoin DL, Lachman RS, Wilcox WR. FGFR3 mutation frequency in 324 cases from the International Skeletal Dysplasia Registry. Mol Genet Genomic Med. 2014;2:497–503. doi: 10.1002/mgg3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: Lessons from mouse models. Growth Horm IGF Res. 2016;28:26–42. doi: 10.1016/j.ghir.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Morioka M, Kishi H, Kimura T, Yahara Y, Okada M, Fujita K, Sawai H, Ikegawa S, Tsumaki N. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature. 2014;513:507–511. doi: 10.1038/nature13775. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhu L, Hou N, Lan Y, Wu XM, Zhou B, Teng Y, Yang X. Osteogenic fate of hypertrophic chondrocytes. Cell Res. 2014a;24:1266–1269. doi: 10.1038/cr.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014b;111:12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoda A, Kitamura H, Fujii T, Kondo E, Murao N, Miura M, Kanamoto N, Komatsu Y, Arai H, Nakao K. Systemic administration of C-type natriuretic peptide as a novel therapeutic strategy for skeletal dysplasias. Endocrinology. 2009;150:3138–3144. doi: 10.1210/en.2008-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10:80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- Yeung Tsang K, Wa Tsang S, Chan D, Cheah KS. The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res C Embryo Today. 2014;102:52–73. doi: 10.1002/bdrc.21060. [DOI] [PubMed] [Google Scholar]
- Yin Y, Ren X, Smith C, Guo Q, Malabunga M, Guernah I, Zhang Y, Shen J, Sun H, Chehab N, Loizos N, Ludwig DL, Ornitz DM. Inhibition of fibroblast growth factor receptor 3-dependent lung adenocarcinoma with a human monoclonal antibody. Dis Model Mech. 2016;9:563–571. doi: 10.1242/dmm.024760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Herr AB, Waksman G, Ornitz DM. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc Natl Acad Sci U S A. 2000;97:14536–14541. doi: 10.1073/pnas.97.26.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SR, Zhou XQ, Ren X, Wang TT, Yuan MX, Wang Q, Liu JY, Liu MG. Ser217Cys mutation in the Ig II domain of FGFR3 in a Chinese family with autosomal dominant achondroplasia. Chin Med J (Engl) 2007;120:1017–1019. [PubMed] [Google Scholar]
- Zhang Z, Kamiya N, Tsuji T, Takeda H, Scott G, Rajderkar S, Ray MK, Mochida Y, Allen B, Lefebvre V, Hung IH, Ornitz DM, Kunieda T, Mishina Y. Elevated Fibroblast Growth Factor signaling is critical for the pathogenesis of the dwarfism in Evc2 mutant mice. PLOS Genetics. 2016 doi: 10.1371/journal.pgen.1006510. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Xie Y, Tang J, Huang J, Huang Q, Xu W, Wang Z, Luo F, Wang Q, Chen H, Du X, Shen Y, Chen D, Chen L. FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling. PLoS Genet. 2015a;11:e1005214. doi: 10.1371/journal.pgen.1005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10:e1004820. doi: 10.1371/journal.pgen.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZQ, Ota S, Deng C, Akiyama H, Hurlin PJ. Mutant activated FGFR3 impairs endochondral bone growth by preventing SOX9 downregulation in differentiating chondrocytes. Hum Mol Genet. 2015b;24:1764–1773. doi: 10.1093/hmg/ddu594. [DOI] [PMC free article] [PubMed] [Google Scholar]