Abstract
Purpose
Slow-accruing clinical trials delay the translation of basic biomedical research, contribute to increasing healthcare costs, and may prohibit trials from reaching their original goals.
Experimental Design
We analyzed a prospectively maintained institutional database that tracks all clinical studies at the MD Anderson Cancer Center. Inclusion criteria were: activated phase I-III trials, maximum projected accrual ≥10 participants, and activation prior to March 25, 2011. The primary outcome was slow accrual, defined as <2 participants/year. Correlations of trial characteristics with slow accrual were assessed with logistic regression.
Results
4,269 clinical trials meeting inclusion criteria. Trials were activated between January 5, 1981, and March 25, 2011, with a total of 145,214 participants enrolled. Median total enrollment was 16 (interquartile range [IQR]: 5-34), with an average enrollment rate of 8.7 participants/year (IQR: 3.3-17.7). There were 755 (18%) trials classified as slow accruing.
On multivariable analysis, slow accrual exhibited robust associations with national cooperative group trials (odds ratio [OR] =4.16, P<0.0001 vs. industry sponsored), time from trial activation to first enrollment (OR=1.13 per month, P<0.0001), and maximum targeted accrual (OR=0.16 per Log10 increase, P<0.0001). Recursive partitioning analysis identified trials requiring more than 70 days (2.3 months)between activation and first participant enrollment as having higher odds of slow accrual (23% vs. 5%, OR=5.56, P<0.0001).
Conclusions
We identified factors associated with slow trial accrual. Given the lack of data on clinical trials at the institutional level, these data will help build a foundation from which targeted initiatives may be developed to improve the clinical trial enterprise.
Keywords: Clinical trial design, clinical trial performance, phase I-III clinical trials
Introduction
The cancer clinical trial enterprise is increasingly challenged by constrained financial resources, extensive regulatory requirements, protracted drug approval times, and mismatches between available participants and trials(1-3). The last decades have produced an exponential increase in the understanding of cancer biology and a concurrent outcry over rising healthcare costs and the prolonged times required to translate bench discovery to clinic(4-6). A significant hurdle in advancing the translation of cancer discoveries is slow trial accrual, which may lead to premature trial closures, overutilization of scarce clinical resources, and loss of relevance of the original research question(s)(1,4,7-10). A recent article by Stensland et al. identified the most frequent reason for a clinical trial to be classified as “failed to complete” was poor patient accrual(11). The need for efficient clinical investigation is illustrated by reports that only a small percentage of major clinical guidelines are supported by prospective evidence (12,13).
Despite the need to enhance cancer clinical trial development, few studies have identified predictors of slow trial accrual and even fewer still have focused on institutional level analyses. Analyses of accrual to the National Cancer Institute (NCI) cooperative group and Cancer Therapy Evaluation Program (CTEP) studies have identified a long trial concept review times (median 1.5-2.5 years) and a high frequency of poor accrual (up to 71%)(7-9,14). In 2008, the Operational Efficiency Working Group was commissioned to develop guidelines for the NCI trial development processes (14). Despite these efforts on the national level, few data and no guidelines exist at the institutional level.
The goal of this study was to collect enrollment and trial characteristics from phase I-III clinical trials activated in a major academic cancer center and to assess factors associated with slow accrual. Based on these results, our institution has initiated clinical research initiatives designed to address identified deficits.
Materials and Methods
The Clinical Oncology REsearch (CORe) database is a prospectively maintained institutional registry of all clinical studies proposed and conducted at MD Anderson. Since 1984, all clinical research studies are required to be registered in CORe and longitudinally tracked for milestones of regulatory reviews, approval, participant accrual, and study closure/termination. As of March 25, 2015, a total of 17,632 registered clinical research studies were identified within CORe. Studies were removed because they were non-phase I-III trials, had missing key information, low (< 10 participant) or missing information on maximum projected accrual. Clinical trials included in the primary analysis were activated between January 5, 1981, and March 25, 2011 to ensure adequate (at least 3 year) trial follow-up time (n=4,269, combined enrollment 145,214). For a sensitivity analysis we include trials activated up to December 31, 2014 (n=5,021) (Supplemental Fig. 1). This study was reviewed and deemed IRB-exempt.
Statistical analysis
Achievement of slow accrual was analyzed as the primary outcome. Slow accruing trials were those that enrolled fewer than 2 participants per year. Univariate and multivariable logistic regression were utilized. Variables with P<0.05 on univariate analysis were initially entered into a multivariable model and retained only if P<0.05 in the final model after backward elimination. P<0.05 was considered significant. All analysis was conducted with SAS ver. 9.4 and JMP Pro ver. 11 (both SAS institute Inc., Cary, NC).
Results
Trial characteristics
Trial characteristics are presented in Table 1 as defined by the study investigation team. The median total accrual was 16 participants (interquartile range [IQR] 5-34) with a median accrual rate of 8.7 participants per year (IQR 3.3-17.7). Among all trials, 755 (18%) accrued fewer than 2 participants per year including 394 (9%) that accrued 0 participants.
Table 1. Clinical trial characteristics for the primary cohort (n=4,269).
| Characteristics | All Protocols; n=4,269 | Slow Accruing (<2 participants / year); n=755 | Not Slow Accruing (≥2 participants / year); n=3,514 |
|---|---|---|---|
| Trial Source* | |||
| Externally Peer-Reviewed | 388 (10%) | 41 (6%) | 347 (11%) |
| Industry | 2017 (52%) | 288 (43%) | 1729 (54%) |
| Institutional | 1106 (29%) | 168 (25%) | 938 (29%) |
| National Cooperative Group | 368 (9%) | 178 (26%) | 190 (6%) |
| Trial Phase Designation | |||
| Phase I | 903 (21%) | 120 (16%) | 783 (22%) |
| Phase I-II | 434 (10%) | 46 (6%) | 388 (11%) |
| Phase II | 2,040 (48%) | 331 (44%) | 1,709 (49%) |
| Phase II-III | 46 (1%) | 11 (1%) | 35 (1%) |
| Phase III | 846 (20%) | 247 (33%) | 599 (17%) |
| Trial Timing (median time[mo], IQR) | |||
| CORe Registration to activation | 4.8 (3.1-8.0) | 5.2 (3.2-9.0) | 4.8 (3.1-7.8) |
| CORe Registration to IRB approval | 1.3 (1.0-1.9) | 1.4 (1.0-2.3) | 1.3 (1.0-1.9) |
| IRB approval to activation | 3.1 (1.5-5.8) | 3.2 (1.5-6.2) | 3.0 (1.5-5.8) |
| Activation to first participant** | 0.8 (0.2-2.1) | 3.0 (0.9-7.2) | 0.7 (0.2-1.8) |
| First participant to final “closure to new participant entry (CNPE)”**,† | 23.2 (14.0-37.5) | 26.2 (15.6-43.3) | 21.0 (11.0-41.0) |
| Patient Enrollment (median, IQR) | |||
| Maximum anticipated accrual | 40 (25-75) | 30 (15-60) | 41 (30-75) |
| Total participants enrolled per trial† | 16 (5-34) | 0 (0-2) | 22 (12-44) |
| Total participants enrolled all trials | 145,214 | 1,285 | 143,929 |
| Enrollment rate (participants/year) | 8.7 (3.3-17.7) | 0 (0-1.2) | 11.3 (6.1-20.3) |
Abbreviations: IRB, Institutional Review Board; IQR, interquartile range; CNPE, closed to new patient enrollment
As defined by study investigator team; externally peer-reviewed are those funded from external non-industry or federal sources (e.g., National Institutes of Health [NIH], Department of Defense [DoD], National Cancer Institute [NCI], and Cancer Therapy Evaluation Program [CTEP]); 390 trials missing study trial source information.
Includes only trials that accrued at least one participant. In the absence of a date for CNPE then study termination date was used instead.
Includes only trials achieving final termination (n=3,735).
Trial activation year
The number of activated trials was observed to increase over time (Fig. 1a). This increase in the number of activated trials was generally reflected in all trial phases (Fig. 1b). The proportion of slow-accruing trials generally decreased over time (OR=0.95, P=0.04 per every 5 years) (Table 2; Fig. 1a). However, the magnitude of this association was relatively weak, with variations in the frequency of slow-accruing trials across time periods: 1986-1990 (16%), 1991-1996 (22%), 1996-2000 (18%), 2001-2005 (19%), and 2006-2010 (15%) (Fig. 1a).
Figure 1.
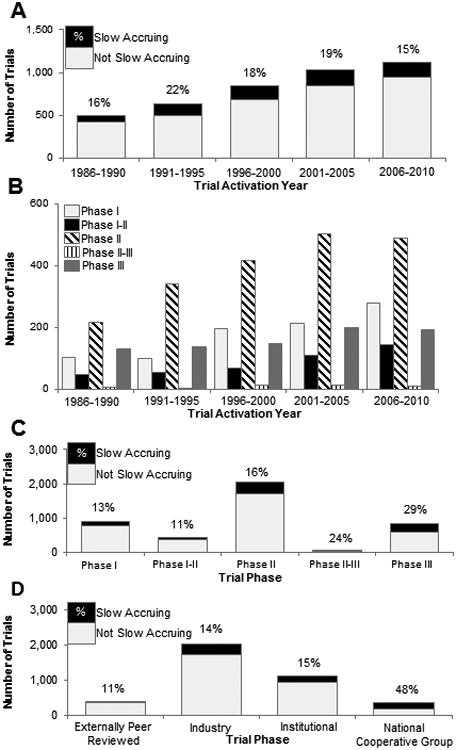
Frequency distribution of total trials and slow accruing trials (fewer than 2 participants per year). (A) Trial activation over time with the proportion of slow accruing trials displayed. (B) Frequency of total trials by phase and trial activation year. (C) Slow-accruing trials by trial phase. (D) slow-accruing trials by source of trial support.
Table 2. Univariate and multivariable logistic regression for a slow accruing trial (less than 2 patients/year) for the primary cohort (n=4,269).
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristics | OR | 95% CI | P | OR | 95% CI | P |
| Trial activation year (per 5 years) | 0.95 | 0.90-1.00 | 0.04 | NI | ||
| Trial activated 2000 or later (vs. prior to 2000) | 0.83 | 0.71-0.98 | 0.02 | NI | ||
| Max anticipated accrual (per Log10) | 0.48 | 0.38-0.59 | <0.0001 | 0.19 | 0.13-0.27 | <0.0001 |
| Trial Phase* | ||||||
| Phase I | 1 | Reference | 1 | Reference | ||
| Phase I-II | 0.77 | 0.54-1.11 | 0.16 | 0.98 | 0.58-1.67 | 0.95 |
| Phase II | 1.26 | 1.01-1.58 | 0.04 | 1.40 | 0.99-1.98 | 0.06 |
| Phase II-III | 2.05 | 1.01-4.15 | 0.05 | 3.10 | 1.18-8.12 | 0.02 |
| Phase III | 2.69 | 2.11-3.43 | <0.0001 | 1.98 | 1.31-2.98 | 0.001 |
| Trial Source* | ||||||
| Industry | 1 | Reference | 1 | Reference | ||
| Externally peer reviewed | 0.71 | 0.50-1.00 | 0.046 | 0.72 | 0.43-1.22 | 0.22 |
| Institutional | 1.08 | 0.88-1.32 | 0.49 | 1.30 | 0.96-1.75 | 0.09 |
| National Cooperative Group | 5.63 | 4.43-7.15 | <0.0001 | 4.59 | 3.22-6.54 | <0.0001 |
| Trial Timing (per month) | ||||||
| CORe Registration to IRB approval | 1.11 | 1.05-1.17 | 0.0004 | NI | ||
| IRB approval to study activation | 1.01 | 1.00-1.02 | 0.13 | NI | ||
| Activation to first patient | 1.09 | 1.07-1.11 | <0.0001 | 1.08 | 1.06-1.10 | <0.0001 |
| Total number of trials within a clinical department during the study period (department total trial activation)* | ||||||
| High (251+ trials) | 1 | Reference | 1 | Reference | ||
| Moderate (80-250 trials) | 2.73 | 2.28-3.27 | <0.0001 | 1.32 | 1.00-1.73 | 0.048 |
| Low (1-79 trials) | 2.49 | 1.96-3.17 | <0.0001 | 1.42 | 0.97-2.08 | 0.07 |
| Number of trials activated within the same year in a clinical department (department annual trial activation rate)* | ||||||
| High (17+ trials in a year) | 1 | Reference | NI | |||
| Moderate (7-16 trials in a year) | 2.25 | 1.77-2.86 | <0.0001 | NI | ||
| Low (0-6 trials in a year) | 1.62 | 1.29-2.04 | <0.0001 | NI | ||
Abbreviations: IRB, Institutional Review Board; OR, Odds Ratio; 95% CI, Confidence Interval; NI, Not Included.
Included into the multivariable analysis if the variable as an overall class met a significance threshold of p<0.05.
Trial phases and sources
The highest frequency of slow-accruing trials were in phase III (29%) and II-III (24%) trials, while the lowest frequencies were among phase I (13%) and I-II (11%) trials (Fig. 1c). When compared with phase I trials, both phase II (OR=1.26, P=0.04 vs. phase I), II-III (OR=2.05, P=0.05), and III (OR=2.69, P<0.0001) trials exhibited a significantly higher odds of slow accrual on univariate analysis. On multivariable analysis, a significant association was observed for phase II-III (OR=3.10, P=0.02) and III (OR=1.98, P=0.001) with slow accrual (Table 2).
Among trial sources, the highest frequency of slow accrual was observed among national cooperative group trials (48%), while the lowest frequency was among externally peer-reviewed trials (11%). Trials designated as externally peer-reviewed are those funded from external non-industry or federal sources (e.g., National Institutes of Health [NIH], NCI, and CTEP). National cooperative group (OR=5.63, P<0.0001 vs. Industry) were significantly associated with slow accrual, while externally peer-reviewed trials (OR=0.71, P=0.046) were inversely associated with slow accrual. On multivariable analysis only national cooperative group trials maintained significance (OR=4.59, P<0.0001) (Table 2).
Timelines for trial activation and early participant enrollment
After a protocol has been written, the regulatory and scientific review and approval process is initiated by protocol submission for review and approval sequentially by a Clinical Research Committee (CRC) followed by the Institutional Review Board (IRB) (Fig. 2a). The median time from protocol submission to IRB approval was 1.3 months (IQR 1.0 to 1.9) and from IRB approval to study activation was 3.1 months (IQR 1.5 to 5.8). The median time from study activation to first participant enrolled was 0.8 months (IQR 0.2-2.1) (Table 1). Slow accruing trials exhibited longer times from study registration in CORe to activation (median: 5.2 mo vs. 4.8 mo, P=0.006), study activation to first participant enrollment (median: 3.0 mo vs. 0.7 mo, P<0.0001), and first participant registration to final closure to new participant entry (median 26.2 mo. vs 21.0 mo, P=0.005) (Fig. 2b-d).
Figure 2.
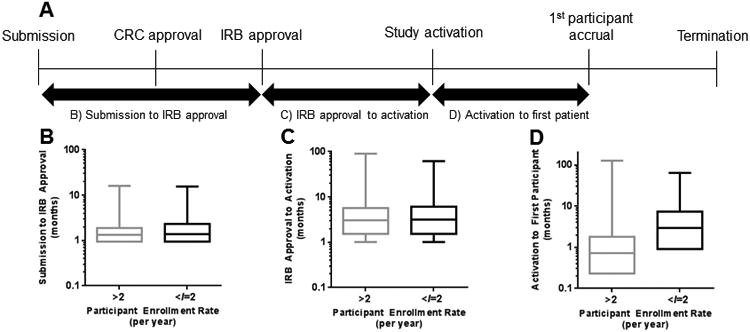
(A) Schematic of the trial approval process at MD Anderson. (B) Time from protocol submission to institutional review board (IRB) approval, (C) time from IRB approval to study activation, and (D) time from trial activation to first participant enrolled stratified by participant enrollment rate.
Time between protocol submission to IRB approval (OR=1.11 per month, P=0.0004) and between study activation and first participant enrollment (OR=1.09, P<0.0001) were significantly associated with slow accrual (Table 2), while time between IRB approval and study activation was not (P=0.13). On multivariable analysis, only longer time between study activation and first participant enrollment was significantly associated with slow accrual (OR=1.08, P<0.0001). A recursive partitioning analysis determined 70 days (2.3 months) to be an optimal cut-point for first patient accrual (OR=5.56, P<0.0001). Trials with first patient enrolled beyond 70 days were significantly associated with slow accrual.
Department-specific trial activity
To assess differences in accrual rates across clinical departments, we stratified based on the total number of trials activated within a department. A total of 59 departments had activated trials during the study period, with a median of 19 trials (range 1 to 646) per department. Examination of the distribution of trial activation identified 3 groupings: low (1-79 trials, 43 departments), moderate (80-250 trials, 10 departments), and high (>250 trials, 6 departments) total trial activations. Trials from departments with moderate or low total trial activations had significantly higher odds of slow-accrual rates compared with trials from departments with high total trial activations (OR=2.73, P<0.0001, and OR=2.49, P<0.0001, respectively) (Table 2; Fig. 3a).
Figure 3.
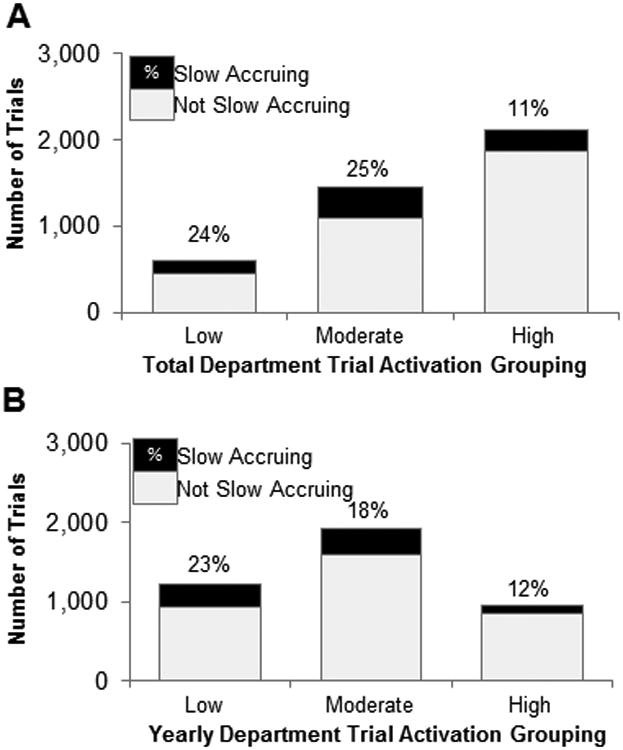
Frequency of slow-accruing trials (fewer than 2 participants per year) with respect to (A) the total number of trials activated in that department or (B) number of trials activated within the same year in the same department. Proportion of slow-accruing trials is shown at the top of each bar.
As total department trial activation is a function of the yearly rate of trial activation and number of years that department has been active, we assessed the impact of annual trial activation rates. For each trial, we calculated the total number of additional trials activated within the same department in the same year. The median annual activation rate was 3 additional trials per year (range 1-60). Analyzing by tertiles revealed that trials in departments with moderate (second tertile: 7-16 trials/year; OR=2.25, P<0.0001 vs. third tertile) or low (first tertile: 0-6 trials/year, OR=1.62, P<0.0001 vs. third tertile) annual activation rates exhibited a significantly higher odds of being slow-accruing compared with trials in departments with high total annual activation rates (third tertile: >16 trials/year). Significance was not maintained on multivariable analysis (all P>0.05) (Table 2; Fig. 3b).
Over the course of the analysis 616 principle investigators activated clinical trials (median of 3 trials per investigator). We stratified trials by the number of total trials opened by that principle investigator as follows: high-volume (activated >3 clinical trials; n=278), moderate-volume (activated 2-3 clinical trials; n=139), and low-volume (activated 1 clinical trial; n=199). This analysis identified lower accrual rates among low-volume principle investigators (median accrual: high-volume: 8.8 participants/year, moderate-volume: 8.0, and low-volume 6.9, ANOVA P=0.002). In a subset of trials with available data (n=630), the association of Investigational New Drug (IND) status with accrual rate was analyzed. This analysis revealed similar accrual rates in trials in which IND status was exempt versus non-exempt (median accrual rate: 12.2 versus 12.1 participants/year; P=0.46).
Sensitivity analysis: slow trial accrual
We conducted a sensitivity analysis defining slow trial accrual at <6 participants (38% of total trials) per year (Supplemental Table 1), univariate and multivariable analyses revealed similar results to those observed with the prior multivariable analysis (Table 2). When analyzing with a cutpoint of <6 participants per year, Recursive partitioning analysis identified an optimal cut point for time to first participant enrollment to be 60 days (OR=4.29, P<0.0001). Similarly, modifying trial inclusion criteria to include trials activated up to December 31, 2014 (n=5,021) (Supplemental Table 2) revealed similar results. Out of 809 trials activated between 2011 and 2014, 86 (11%) were slow accruing, which was lower than the 18% rate of slow accruing trials observed among trials activated between 1981 and 2011.
Trial publications as a surrogate measure of trial success
We assessed the association of accrual rates with frequency of produced peer-reviewed publications, as both metrics may be viewed as indicators of trial “success”. Peer-reviewed publications resulting from a subset of 100 randomly selected protocols that accrued fewer than 2 participants per year as well as a set of 100 protocols that accrued at least 2 participants per year, matched by trial source, phase, year of activation, and maximum accrual size were identified via searches in PubMed and Google Scholar using the related ClinicalTrials.gov number (when available), trial title, and names of principal investigators. Among slow-accruing trials, 14 (14%) produced at least one publication (range 1-3), with a total of 18 publications. In contrast, among trials accruing at least 2 participants per year, 69 (69%) produced at least one associated publication (range 1-16), with a total of 147 publications.
Institutional initiatives to improve trial accrual
As noted in our analysis, national cooperative group trials are associated with slow participant accrual, a finding noted in other analyses(15). We speculate that the lack of incentives for local investigators may have contributed to slow accrual, as authorship on the resulting paper is often not guaranteed and capitated funding rarely supports the total trial costs. To enhance accrual, MD Anderson has provided subsidized funding for these trials since 2010, providing an additional $2000 per participant towards support of clinical research personnel within the enrolling department. To evaluate the impact of this program, a preliminary analysis noted a decrease in slow accruing trials after 2010 (Supplemental Fig. 2).
In addition to supplemental funding for national cooperative group trials, other institutional funding sources have been created to defray trial costs. Since 2013, underfunded novel, IRB-approved, investigator-initiated trials may apply to the High Impact Clinical Research Support Program (HI-CRSP) program, which funds 3-5 applications per year for up to $100,000 per year for 1 to 2 years. Twelve HI-CRSP trials have enrolled patients after obtaining this funding, of which only 1 trial (8%) exhibiting an accrual rate of fewer than 2 participants per year.
Finally, since 1999, MD Anderson has conducted a semi-annual institutional review by the electronic Protocol Accrual Auditing Committee (ePAAC) to flag protocols meeting the following criteria: has previously enrolled participants but accrued fewer than 3 during the past 6 months, IRB approval for at least 6 months but not yet activated, and activation for at least 6 months with zero participants accrued. Flagged trials are reviewed and the investigators are required to provide efforts for increasing accrual. Upon two or more reviews, if the trial is judged unlikely to meet accrual goals by the committee, these trials are closed. From 2007 to 2014, 6562 clinical studies have been reviewed by ePAAC resulting in 939 studies (14%) temporarily or permanently closed.
Discussion
This study provides an overview of phase I-III clinical trial characteristics and an analysis of the predictors of slow trial accrual at a large tertiary cancer center. We believe that this analysis is representative of national trends and provides insight into the clinical trial enrollment given the concordance with similar analyses(16-18). It should be emphasized that as trials activated after 2011 were not analyzed in the primary analysis. However, a sensitivity analysis was performed extending to the trials enrolled until 2014. Nevertheless, the presented data may not reflect the full spectrum of most contemporary trials.
We found that the majority of protocols conducted at MD Anderson were phase II (48%) and industry-sponsored (52%), similar to other tertiary cancer referral centers. An analysis of 83 lung cancer trials from Washington University School of Medicine (WUSM) and 218 oncology trials from Vanderbilt-Ingram Cancer Center and its affiliated network sites (VICC/VICCAN) also identified the majority of trials to be phase II (WUSM: 72%; VICC/VICCAN: 43%) and industry-sponsored (WUSM: 53%; VICC/VICCAN: 62%)(16,17). In addition, our study identified a median accrual of 16 participants across all trials, which is higher than the medians identified at VICC/VICCA (8.7) and WUSM (7.4)(16,17). However, our analysis did not consider 188 studies with maximum projected accrual of <10 participants.
Analyses of CTEP-sponsored trials by Cheng et al. identified trial development time of <12 months(9) and time from activation to first participant enrollment of fewer than 2 months(7) to be predictive of attaining accrual goals. We analyzed multiple steps of the trial activation process at MD Anderson and found that the timeframes identified here were similar to those reported from other large academic institutions (Table 1). For example, median time from protocol submission to trial activation was 146 days (4.8 months) at MD Anderson, compared to 172 days reported for VICC/VICCAN, 163 days for WUSM, and 112.5 days for the University of Torino(16,17). However, it should be noted that over time, regulatory hurdles have generally increased(1,19) and that many of the trials analyzed here were activated prior to those in other analyses. With regard to trial development, on multivariable analysis only time from activation to first participant enrollment maintained a significance association with slow accrual (Table 2). Furthermore, our identified cut points for first participant enrollment of 60 or 70 days are similar to the 2-month cut point identified by Cheng et al.(7) A recent analysis by Bennette et al. presented a multivariable model associating trial characteristics with low accrual in cooperative group sponsored phase II and III trials(18). This group identified phase III trials, rarity of the condition treated, and specific therapeutic modalities to be associated with low accrual. The analysis presented notes a similar association between phase III studies and slow accrual, but generally presents a group of trial characteristics that are non-overlapping and thus complementary to those presented by Bennette et al. A final model to identify trials at risk for slow accrual will likely require parameters presented in both analyses. Factors of potential importance that could not be analyze included: protocol design (e.g. precision medicine/biomarker driven protocols), protected time of principle investigators, drug accessibility outside of a protocol, protocol staffing, patient population characteristics, and FDA approval status of the investigational agent.
With regards to protocol design, biomarker-driven studies are of particular contemporary relevance given the rise in molecular testing. We have assessed accrual rates in 4 biomarker driven trials: Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE)(20) (accrual rate: 107 participants/year), BATTLE-2 (accrual rate: 88 participants/year), BATTLE-front line (BATTLE-FL) (accrual rate: 19 participants/year), and the Investigation of Serial Studies to Predict Your Therapeutic Response through Imaging and Molecular Analysis 2 (I-SPY 2) (accrual rate: 88 participants/year)(21,22). Although a more thorough analysis is warranted, despite a general increase in trial complexity for biomarker driven trials and more stringent eligibility criteria, an analysis of this limited sampling showed that accrual rates are not adversely affected. In fact, these trials accrued better than comparable trials which could contribute by cutting-edge science, novel trial designs, and potentially better treatments.
In addition to providing additional funding for national cooperative group trials and inadequately funded novel trials via HI-CRSP, a number of institutional initiatives have also been designed to improve the trial development process. These include an annual 3-day intensive clinical trial method and design workshop for junior faculty initiated in 2014 that seeks to enhance the quality of clinical trials conducted at MD Anderson. MD Anderson is now negotiating large strategic agreements with pharmaceutical companies for the development of multiple trials within a single contract to facilitate more rapid trial activation and robust accrual. Finally, our institution has opened a number of regional care centers throughout the Houston metropolitan area. Participants from these centers generally reflect a less treatment-refractory population that may be more suitable for enrollment into phase III and national cooperative group trials. In addition to institutional initiatives, trial participant engagement can also be promoted through multiple diverse channels(10). These include web-based educational platforms to enhance participant knowledge, attitudes, and preparation for trial enrollment(23,24). Most recently there has been interest in leveraging social media tools to promote self-referral(25-27). Furthermore, individual departments within our institution have evolved research-focused infrastructures worth further discussion. The Investigational Cancer Therapeutics (ICT) department is a department that has been tasked with conducting all-comers early phase clinical trials. The impact of such infrastructure elements has been outlined in a recent publication by our group(28).
Various weaknesses of our analysis deserve mention. In analyzing factors predictive of slow accrual, we focused our primary outcome on slow accrual defined as fewer than 2 participants per year. We removed very small trials (maximum projected accrual <10) as low accrual rates might have been acceptable for such trials. As a cut point of fewer than 2 participants per year could be considered arbitrary, we conducted a sensitivity analysis at fewer than 6 participants per year. We also evaluated another potential “marker” for trial impact or success, the rate of associated publications, in matched samples of 100 slow-accruing and non-slow-accruing trials. This analysis identified almost a 5-fold difference in the rate of peer-reviewed publications from slow-accruing and non-slow-accruing trials (14% vs 69% of trials producing at least one publication, respectively). Other weaknesses of the current analysis include the possibility of selection biases in excluded trials as trials exclusion for missing data may have occurred in a non-random way. The large sample size utilized in this study has the potential to highlight clinically insignificant differences with statistically significant p-values. Finally, the presented analysis represents a single institution experience, and thus must be validated in an external dataset. These results may be indicative of our patient population, which is enriched with patients who are motivated to seek newer targeted agents and thus preferentially enroll on early phase trials and not on phase III or national cooperative group trials that test more established therapeutics.
In conclusion, we have reported our clinical trial characteristics and conducted an analysis associating trial factors with slow participant accrual. Analysis of clinical trial performance on the institutional level is lacking and sorely needed. Prior to the current report we only identified two prior publications focusing on this topic(16,17). Thus, the goals of the current analyses are to build the foundation to assess the current clinical trial landscape, generate evidence-based guidelines for trial design and monitoring, and identify weaknesses in the clinical trial enterprise. Based on our analysis we believe that common themes of fast accruing trials include the following: momentum, quick identification of the first trial participants sets the pace for continued robust accrual and investigator incentives, the potential for greater academic credit for the institutional principle investigator likely facilitates faster participant accrual (e.g. institutional or industry trials and phase I or phase II trials).
Supplementary Material
Statement of Translational Relevance.
Limited resources mandate the careful allocation of assets for clinical research. Given the exponential rise in biomedical discovery there is an urgent need to streamline the clinical trial process. We analyze a prospectively-collected clinical study registry at MD Anderson Cancer Center and present our clinical trial experience over a 30 year period. Furthermore, we conduct a detailed analysis of factors influence slow participant accrual, identifying factors including trial sponsorship and longer development times to be associated with slow accrual. We believe that trends and shortcomings identified in this analysis are applicable to the broader oncologic community. Finally, we present recent institutional initiatives designed to mitigate the factors identified in this analysis.
Acknowledgments
The research was supported in part by grant from the National Cancer Institute CA016672. The authors would like to acknowledge Maria Mercado and Alberto Renteria for their assistance in compiling the analyzed data and Jiaomin Ouyang for figure preparation.
Footnotes
No conflicts of interest.
References
- 1.Sung NS, Crowley WF, Jr, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–87. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 2.DeMets DL, Califf RM. A historical perspective on clinical trials innovation and leadership: where have the academics gone? JAMA. 2011;305(7):713–4. doi: 10.1001/jama.2011.175. [DOI] [PubMed] [Google Scholar]
- 3.Ho J, Pond GR, Newman C, Maclean M, Chen EX, Oza AM, et al. Barriers in phase I cancer clinical trials referrals and enrollment: five-year experience at the Princess Margaret Hospital. BMC cancer. 2006;6:263. doi: 10.1186/1471-2407-6-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doroshow JH. Timely completion of scientifically rigorous cancer clinical trials: an unfulfilled priority. J Clin Oncol. 2013;31(27):3312–4. doi: 10.1200/JCO.2013.51.3192. [DOI] [PubMed] [Google Scholar]
- 5.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. Journal of the National Cancer Institute. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. The New England journal of medicine. 2009;360(6):626–33. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 7.Cheng SK, Dietrich MS, Dilts DM. Predicting accrual achievement: monitoring accrual milestones of NCI-CTEP-sponsored clinical trials. Clin Cancer Res. 2011;17(7):1947–55. doi: 10.1158/1078-0432.CCR-10-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroen AT, Petroni GR, Wang H, Thielen MJ, Gray R, Benedetti J, et al. Achieving sufficient accrual to address the primary endpoint in phase III clinical trials from U.S. Cooperative Oncology Groups. Clin Cancer Res. 2012;18(1):256–62. doi: 10.1158/1078-0432.CCR-11-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng SK, Dietrich MS, Dilts DM. A sense of urgency: Evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clin Cancer Res. 2010;16(22):5557–63. doi: 10.1158/1078-0432.CCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267–76. doi: 10.1200/JOP.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stensland KD, McBride RB, Latif A, Wisnivesky J, Hendricks R, Roper N, et al. Adult cancer clinical trials that fail to complete: an epidemic? Journal of the National Cancer Institute. 2014;106(9) doi: 10.1093/jnci/dju229. [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Vielemeyer O. Analysis of overall level of evidence behind Infectious Diseases Society of America practice guidelines. Arch Intern Med. 2011;171(1):18–22. doi: 10.1001/archinternmed.2010.482. [DOI] [PubMed] [Google Scholar]
- 13.Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC., Jr Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301(8):831–41. doi: 10.1001/jama.2009.205. [DOI] [PubMed] [Google Scholar]
- 14.Abrams JS, Mooney MM, Zwiebel JA, Korn EL, Friedman SH, Finnigan SR, et al. Implementation of timeline reforms speeds initiation of National Cancer Institute-sponsored trials. Journal of the National Cancer Institute. 2013;105(13):954–9. doi: 10.1093/jnci/djt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilts DM, Cheng SK, Crites JS, Sandler AB, Doroshow JH. Phase III clinical trial development: a process of chutes and ladders. Clin Cancer Res. 2010;16(22):5381–9. doi: 10.1158/1078-0432.CCR-10-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang-Gillam A, Williams K, Novello S, Gao F, Scagliotti GV, Govindan R. Time to activate lung cancer clinical trials and patient enrollment: a representative comparison study between two academic centers across the atlantic. J Clin Oncol. 2010;28(24):3803–7. doi: 10.1200/JCO.2010.28.1824. [DOI] [PubMed] [Google Scholar]
- 17.Dilts DM, Sandler AB. Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24(28):4545–52. doi: 10.1200/JCO.2005.05.0104. [DOI] [PubMed] [Google Scholar]
- 18.Bennette CS, Ramsey SD, McDermott CL, Carlson JJ, Basu A, Veenstra DL. Predicting Low Accrual in the National Cancer Institute 's Cooperative Group Clinical Trials. Journal of the National Cancer Institute. 2016;108(2) doi: 10.1093/jnci/djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Infectious Diseases Society of A. Grinding to a halt: the effects of the increasing regulatory burden on research and quality improvement efforts. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(3):328–35. doi: 10.1086/605454. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GR, Jr, Tsao A, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1(1):44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rugo HS, Olopade OI, DeMichele A, Yau C, van 't Veer LJ, Buxton MB, et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. The New England journal of medicine. 2016;375(1):23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JW, Liu MC, Yee D, Yau C, van 't Veer LJ, Symmans WF, et al. Adaptive Randomization of Neratinib in Early Breast Cancer. The New England journal of medicine. 2016;375(1):11–22. doi: 10.1056/NEJMoa1513750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller SM, Hudson SV, Egleston BL, Manne S, Buzaglo JS, Devarajan K, et al. The relationships among knowledge, self-efficacy, preparedness, decisional conflict, and decisions to participate in a cancer clinical trial. Psycho-Oncology. 2013;22(3):481–9. doi: 10.1002/pon.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meropol NJ, Wong YN, Albrecht T, Manne S, Miller SM, Flamm AL, et al. Randomized Trial of a Web-Based Intervention to Address Barriers to Clinical Trials. J Clin Oncol. 2016;34(5):469–78. doi: 10.1200/JCO.2015.63.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heywood J, Evangelou M, Goymer D, Kennet J, Anselmiova K, Guy C, et al. Effective recruitment of participants to a phase I study using the internet and publicity releases through charities and patient organisations: analysis of the adaptive study of IL-2 dose on regulatory T cells in type 1 diabetes (DILT1D) Trials. 2015;16:86. doi: 10.1186/s13063-015-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson MA. Social media in clinical trials. Am Soc Clin Oncol Educ Book. 2014:e101–5. doi: 10.14694/EdBook_AM.2014.34.e101. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Calfas KJ, Marshall SJ, Robinson TN, Rock CL, Huang JS, et al. Clinical trial management of participant recruitment, enrollment, engagement, and retention in the SMART study using a Marketing and Information Technology (MARKIT) model. Contemp Clin Trials. 2015;42:185–95. doi: 10.1016/j.cct.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang C, Hess KR, Sanders D, Davis SE, Buzdar AU, Kurzrock R, et al. Modifying the clinical research infrastructure at a dedicated clinical trials unit: assessment of trial development, activation, and participant accrual. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


