Abstract
Abdominal pain is one of the major symptoms in bowel obstruction (BO); its cellular mechanisms remain incompletely understood. We tested the hypothesis that mechanical stress in obstruction upregulates expression of nociception mediator nerve growth factor (NGF) in gut smooth muscle cells (SMC), and NGF sensitizes primary sensory nerve to contribute to pain in BO. Partial colon obstruction was induced with a silicon band implanted in the distal bowel of Sprague-Dawley rats. Colon-projecting sensory neurons in the dorsal root ganglia (DRG, T13 to L2) were identified for patch clamp and gene expression studies. Referred visceral sensitivity was assessed by measuring withdrawal response to stimulation by von Frey filaments (VFF) in the lower abdomen. Membrane excitability of colon-projecting DRG neurons was significantly enhanced, and the withdrawal response to VFF stimulation markedly increased in BO rats. The expression of NGF mRNA and protein was increased in a time-dependent manner (day 1 to day 7) in colonic SMC, but not in mucosa/submucosa of the obstructed colon. Mechanical stretch in vitro caused robust NGF mRNA and protein expression in colonic SMC. Treatment with anti-NGF antibody attenuated colon neuron hyper-excitability and referred hypersensitivity in BO rats. Obstruction led to significant increases of tetrodotoxin-resistant (TTX-r) Na+ currents and mRNA expression of Nav1.8, but not Nav1.6 and Nav1.7 in colon neurons; these changes were abolished by anti-NGF treatment. In conclusion, mechanical stress-induced upregulation of NGF in colon SMC underlies the visceral hypersensitivity in BO through increased gene expression and activity of TTX-resistant Na+ channels in sensory neurons.
Keywords: Lumen distension, Visceral sensitivity, Mechano-transcription, Dorsal root ganglia, Sensory neurons
INTRODUCTION
Obstructive bowel disorders (OBD) are characterized by lumen distention due to mechanical or functional obstruction in the gut. OBD represent a significant health challenge in adults and children [32, 45]. Mechanical bowel obstruction (BO) is one of the most common causes of acute abdomen [29, 32, 45], and accounts for 300,900 hospital admissions per year in the US alone; the aggregate cost for hospital stay is more than $2.7 billion annually, topping all other gastrointestinal (GI) conditions [29]. Abdominal pain and motility dysfunction are the main complaints in BO [33, 36, 42]. While surgical resolution is the treatment of choice for many patients with mechanical BO, about 43% of patients receive non-surgical conservative management [10, 46]. Abdominal pain is a major focus in conservative management, especially among those with inoperable or malignant obstruction [18, 34, 35]. Mechanical BO occurs in up to 25% of patients with colon cancer and 42% with ovarian cancer [33, 35]. High dose opioids are the primary analgesic treatment for BO-associated pain in such cases [18, 33–35]. However, opioids are known to further cause opioid-induced bowel dysfunction, i.e. constipation and narcotic bowel syndrome [14, 19]. In addition, distention-associated abdominal pain and discomfort are major symptoms in chronic functional obstruction, such as intestinal pseudo-obstruction and Hirschsprung’s disease [8, 16, 30]. The mechanisms of distension-associated abdominal pain in mechanical and functional obstructions remain unknown, and no specific analgesics are available to target distension-associated pain.
Visceral sensitization is a well-recognized contributor to abdominal pain [1, 3, 12, 28]. The nerve endings of primary afferent neurons sense chemical and mechanical stimuli in the gut [5, 44] and transduce the signals to the neuronal cell body located in dorsal root ganglia (DRG), which relays the signals to the second order neurons in the spinal cord to initiate central processing of sensory information for perception [1, 3, 11, 12]. Several peripheral mediators including neurotrophins (NT), such as nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF), may sensitize afferent neurons [9, 31, 52]. This mechanism of peripheral sensitization is critical to the development of abdominal pain [1, 3, 9, 11, 31, 52]. Recent studies found that the firing threshold of colon-projecting afferent neurons and the pain threshold to abdominal stimulation were decreased in colon obstruction mice [17]. These data suggest that visceral sensitivity is increased in BO. However, what accounts for the increased visceral sensitivity in obstruction remains largely unknown.
Mechanical distention is a cardinal feature in OBD [32, 36, 42, 45]. Mechanical stress was found to increase NGF expression in vascular smooth muscle cells (SMC) in vitro [7]. We tested a hypothesis in the present study that lumen distention associated mechanical stress induces gene expression (mechano-transcription) [22, 25, 27, 39, 50] of NGF in colonic SMC, and that mechanical stress-induced NGF from SMC sensitizes afferent neurons and contributes to abdominal pain in OBD. We found that NGF expression in colonic smooth muscle was significantly up-regulated by mechanical stress in distended colon segment in a rat model of bowel obstruction and in the primary culture of rat colonic SMC in vitro. Peripheral treatment with anti-NGF antibody by intra-peritoneal injections attenuated visceral hypersensitivity in BO. We further investigated the cellular mechanisms of hypersensitivity in the colon-projecting DRG neurons in obstruction.
METHODS
Rat model of bowel obstruction
Sprague-Dawley male rats weighing 200–275 g, aged between 8 to 9 weeks, were purchased from Harlan Sprague Dawley (Indianapolis, IN). The rats were housed in a controlled environment (22°C, 12-h light-dark cycle) and allowed food and water ad libitum. The Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch (UTMB) at Galveston approved all procedures performed on the animals.
The rat model of partial colon obstruction was prepared by following procedures described previously [22, 25, 39]. In brief, rats were anesthetized with 2% isoflurane inhalation by an E-Z Anesthesia vaporizer (Palmer, PA). After midline laparotomy, a distal colon segment 4 cm proximal to the end of colon was carefully exposed. A small mesenteric opening (5 × 5 mm2) was made next to the exposed colon segment. A 3-mm wide medical grade silicon ring was placed around the colon wall through the small mesenteric opening. The size of the silicon ring (21 mm in length) was ~1–2 mm greater than the outer circumference of the colon when the colon segment was filled with fecal pellets, allowing partial obstruction. The procedure to implement the silicon ring was completed within 2 min. The sham control rats underwent the same surgical procedure except that the ring was removed immediately after the 2-min procedure. Rats were euthanized at different time points up to 7 days following partial obstruction. A 3-cm-long colon segment starting at 1 cm oral to the site of obstruction was collected as distended tissue, and a 2-cm-long colon segment starting at 0.5 cm aboral to the obstruction was taken as non-distended internal control. These tissues were used for histological, biochemical, and molecular studies.
In a separate experiment in vivo, anti-NGF antibody (20 µg/kg, i.p. daily) [9, 49] was administered to sham and BO rats starting on day 1. Rats were euthanized on day 7. Colon tissue and DRGs were isolated for further molecular and electrophysiological studies.
Colon tissue collection
The colon segments oral and aboral to the obstruction site were collected in fresh carbogenated Krebs buffer (in mmol/l: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1 NaH2PO4, 1.2 MgCl2, 11 D-glucose, and 25 NaHCO3). The segments were cleansed, opened along the mesenteric border, and pinned flat in a petri dish with Sylgard base. The mucosal/submucosal (M/SM) and muscularis externa (ME) layers were separated by microdissection as described previously [22–27, 39, 50].
Collection of conditioned media and ex vivo study
To determine if stretch-induced mediators in colonic smooth muscle lead to the increase of afferent neuron excitability in BO, we developed an ex vivo study protocol as described previously [26] with modifications. In brief, muscle strips of 20 mg were isolated from the colon segment oral and aboral to BO (day 3), and from the sham controls. The strips were incubated in 1 mL of DMEM (+1% FBS) for 24 h, and the conditioned media were collected. Normal colon projecting DRG neurons were isolated from T13-L2 of naïve rats, and cultured for 24 h in DMEM(+1% FBS) in 1:2 dilution with the conditioned media in the presence of NGF neutralizing antibody (0.2 µg/mL) or control IgG, respectively. We then carried out patch clamp recordings of the cultured neurons to determine neuron cell excitability.
Primary culture of RCCSMC and in vitro stretch of RCCSMC in culture
Rat colonic circular SMCs (RCCSMC) were isolated as described previously [22, 38–41]. In brief, the circular muscle tissue pieces in 0.5 × 0.5 cm2 size were incubated in sterile HEPES buffer (in mmol/l: 120 NaCl, 2.6 KH2SO4, 4 KCl, 2 CaCl2, 0.6 MgCl2, 25 HEPES, 14 glucose, and 2.1% essential amino acid mixture, pH 7.4) with 1.5 mg/ml collagenase (type II, 319 U/mg; Worthington, Freehold, NJ) and 1.0 mg/ml soybean trypsin inhibitor (Sigma-Aldrich) for 45 min at 31°C. At the end of digestion, tissue pieces were incubated in fresh buffer without digestion enzymes. The spontaneously dispersed cells were collected and cultured in DMEM supplemented with 10% fetal bovine serum in the presence of 100 U/ml of penicillin G, 100 µg/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B (Invitrogen). The culture medium was changed every 3 days. Immunofluorescence staining showed that more than 95% of the cultured cells stained positive for smooth muscle-specific α-actin [22, 38–41].
Primary culture of RCCSMC was allowed to grow for 8–10 days until it was confluent. The cells were then seeded at 8 × 104 cells/well in six-well BioFlex culture plates coated with type I collagen (Flexcell, Hillsborough, NC), grown to ~80% confluence, and then subjected to DMEM/1% FBS for 24 h prior to stretch. Cells were subjected to stretch via a FX-4000 Flexercell Tension Plus System (Flexcell). This computer-regulated bioreactor applies multiaxial strain to cultured cells [22, 39]. Through vacuum pressure, cultured cells are deformed on flexible membrane plates. To mimic tonic lumen distention as in BO, cells were subjected to static, rather than cyclic, stretch at 18% elongation in all the experiments of this study. Cells incubated in parallel under identical conditions but without exposure to stretch served as controls.
Measurement of referred visceral sensitivity
Direct assessment of visceral sensitivity by measuring visceromotor response (VMR) to colorectal distension with a balloon [6, 23, 47, 51] is not feasible during colon obstruction. As visceral pain has the unique feature that a painful sensation is found in a referred somatic region, we measured referred visceral sensitivity in our model with Von Frey filament (VFF) test as described elsewhere [11, 17]. Rats were shaved in the abdomen, and a 3 × 3 cm2 area of the lower abdomen along the midline was marked for VFF test. Rats were kept in a translucent cage (3.5 in × 7.0 in × 3.5 in) for 30 min for pre-test adaptation and during VFF test. The Von Frey filaments for each of the forces (0.1, 0.2, 0.5, 1.0, 2.0, 4.0 and 8.0 g) were applied to the marked lower abdomen 10 times (each for 2 seconds at 10-second interval) in an ascending order of forces. Care was taken to avoid touching the midline of the abdomen where incision was made during laparotomy operation. The numbers of withdrawal responses including sharp abdominal retraction, licking or scratching of the filament, or immediate movement or jumping were recorded.
Labeling of colon specific sensory neurons in DRG for patch clamp study and for mRNA detection
Colon specific neurons in DRG were labeled for patch-clamp recordings by injecting 1,1′-dioleyl-3,3,3′,3-tetramethylindocarbocyanine methanesulfonate (DiI, Invitrogen, Carlsbad, CA) into the colon wall as described previously [6, 23]. In brief, animals were anesthetized by 2% isoflurane with an E-Z anesthesia vaporizer. After a midline laparotomy, 5 µl of DiI (50 mg/ml in methanol) was injected into the muscle layer of the gut wall at 6~8 sites of the colon segment (~4 cm in length) oral to obstruction in BO rats or the middle colon in sham controls. Animals were returned to normal housing until euthanasia for patch-clamp recordings 7~10 days after DiI injection.
To determine gene expression in colon specific neurons, cholera toxin B subunit (CTB, 40 µg in 20 µL PBS per rat) was injected to the colon wall (6 sites). After 7~10 days, rats were euthanized, and CTB-labed colon specific DRG neurons were isolated by laser capture micro-dissection [6, 49]. The expression of mRNA for Nav channels in these neurons were quantitated with real-time qPCR.
DRG neuron dispersion and patch-clamp study
DRG neurons from sham and obstruction rats were isolated as described previously [6, 23, 24, 48]. Briefly, rats were euthanized by decapitation. The spinal cord was removed and transferred to ice-cold, oxygenated fresh dissecting solution containing (in mmol/l) 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES, pH 7.2 (osmolarity = 305 mosM). Thoracolumbar DRG (T13–L2) were obtained bilaterally. The ganglia were digested in dissecting solution containing collagenase D (~1.5 mg/ml; Roche, Indianapolis, IN) and trypsin (~1.2 mg/ml; Sigma, St. Louis, MO) at 34.5°C for 1.5 h. The samples were washed in enzyme-free solution and triturated repetitively with glass pipettes to obtain single cell suspension.
Cells were plated onto acid-cleaned glass coverslips and perfused with normal external solution containing (in mmol/L) 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH (295–300 mOsM). Recording pipettes, pulled from borosilicate glass tubing, had resistance of 4–7 MΩ and filled with pipette solution containing (in mM): 100 KmeSO3, 40 KCl, and 10 HEPES, pH 7.3 adjusted with KOH (290 mOsm). DiI-labeled small- to medium-sized DRG neurons (< 30 µm in diameter) were accepted for analysis if they had a stable resting membrane potential (> −45 mV) and displayed overshooting action potentials. The small- to medium-sized neurons are involved in the transmission of nociception in rat colon [3, 5, 23]. Composition of the voltage-clamp solutions for isolating Na+ currents were (in mM): 55 NaCl, 80 Choline Cl, 1 CaCl2, 1 MgCl2, 0.1 CdCl2, 10 HEPES, and 5 glucose, pH adjusted to 7.4 by using NaOH. The patch electrode was filled with the pipette solution containing (in mM): 110 CsCl, 1 MgCl2, 11 EGTA, 10 NaCl, 10 glucose, and 10 HEPES (pH 7.3, adjusted with CsOH; 285–295 mOsm). DiI-labeled colon-specific DRG neurons (bright red) were identified by using a fluorescence microscope (Olympus, Tokyo, Japan) with a rhodamine filter (excitation 546 mm, barrier filter at 580 mm). Whole-cell current and voltage were recorded by a Dagan 3911 patch-clamp amplifier (Dagan, Minneapolis, MN) as described [6, 23]. Capacitive transients were corrected by using capacitive cancellation circuitry on the amplifier that yielded the whole-cell capacitance and access resistance. Up to 90% of the series resistance was compensated electronically. The currents were filtered at ~2–5 kHz and sampled at 50 or 100 µs per point. Data were acquired and stored on a Dell computer for later analysis by using pCLAMP 9.2 (Axon Instruments, Sunnyvale, CA).
Western blot
Colonic ME and M/S samples were homogenized on ice in lysis buffer supplemented with protease inhibitor cocktails (Sigma-Aldrich, St. Louis, MO) as described previously [32–36]. The compositions of lysis buffer are (in mmol/l) 20 Tris·HCl, pH 7.5, 150 NaCl, 1 EDTA, 1 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 2.5 sodium pyrophosphate, 1 β-glycerolphosphate, 1 Na3VO4, 1% Triton X-100, and 1 µg/ml leupeptin. After spinning at 12,000 g at 4°C for 15 min, the supernatant proteins were collected and resolved by a standard immunoblotting method [22–27, 39, 50]. Equal quantities (20 µg) of total protein were run on premade 4–12% Bis-Tris SDS-PAGE (Invitrogen, Carlsbad, CA). The primary antibody to NGF (1:200) was purchased from Santa Cruz (Santa Cruz, CA). β-actin antibody (1:5,000, Sigma) was used as loading control. The protein detection was performed using ODYSSEY Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Immunohistochemistry study
Immunohistochemical staining of NGF protein was performed on formalin-fixed, paraffin-embedded colon segments (5–6 cm from the anus) isolated from rats in sham control and with obstruction, as described previously [39]. Sections at 4-µm thickness were blocked with 5% normal goat serum in PBS for 20 min at room temperature, and incubated with the rabbit anti-NGF antibody (1:200, Santa Cruz Biotech, CA) and a biotin-conjugated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA). After being incubated with avidin-biotin complex (Vector kit, Vector Laboratories), the sections were stained in diaminobenzidine tetrahydrochloride with 0.03% hydrogen peroxide. As a negative control, sections of the same specimens were processed by the same method but omitting anti-NGF primary antibody.
Enzyme immunoassay of NGF
Rat colonic tissues (ME) were homogenized in cold PBS (in mmol/l 137 NaCl, 2.7 KCl, 10 Na2HPO4, KH2PO4, pH 7.4). NGF levels in the homogenates were measured with the NGF rat ELISA kit by following the manufacturer's protocols (Abcam, Cambridge, MA).
RNA preparation and quantitative RT-PCR
Total RNA was extracted from colon ME and M/SM tissues, and from CBT-labeled colon specific DRG neurons by using the Qiagen RNeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was reverse-transcribed with the SuperScript III First-Strand Synthesis System (Invitrogen) for quantitative RT-PCR with the Applied Biosystems 7000 real-time PCR system (Foster City, CA) [22, 25, 27, 39, 50]. The assay IDs for TaqMan detection of rat NGF, Nav1.6, Nav1.7, Nav1.8, and Nav1.9 mRNAs are Rn01533872_m1, Rn00591020_m1, Rn00570506_m1, Rn00568393_m1, and Rn00570487_m1, respectively (Applied Biosystems). For relative quantification of mRNA expression, real-time qPCR was performed with 40 ng of cDNA for the target gene and for the endogenous control 18S rRNA (Part no. 4352930E, Applied Biosystems).
Statistical analysis
All data points are expressed as means ± SEM. Statistical analysis was performed by analysis of variance with nonrepeated measures (by Student-Newman-Keuls test) for comparisons of multiple groups and Student's t-test for comparisons of two groups. A P value of ≤0.05 was considered statistically significant.
RESULTS
1) Colon projecting DRG neurons are sensitized in BO
To determine if afferent sensory nerves are sensitized in our model of partial colon obstruction, we recorded the electrophysiological properties of colon projecting DRG neurons (colon neurons, < 30 µm in diameter) of T13 to L2 segment by patch clamp [6, 23, 51]. We found that cell excitability of the colon neurons was significantly augmented in BO on day 3 (n = 5 /26, number of animals/number of neurons) and day 7 (n = 5/31), compared to sham controls (n = 11/51) (Fig. 1, Table 1). As shown in Fig. 1, both the resting membrane potential (RP) and rheobase were significantly decreased in BO at days 3 and 7 (both P < 0.05 vs. sham for PP and rheobase). The numbers of action potentials (AP) evoked by 3× rheobase stimulation (for 0.3 s) were 2.1(±0.25) and 3.8(±0.32) -fold greater in BO (days 3 and 7, respectively) than in sham rats (for both, P < 0.05 vs. sham). The numbers of AP evoked by 2× rheobase stimulation for 0.3 s were also increased in BO (Table 1). However, colon neurons did not exhibit hyper-excitability in rats with BO on day 1 post obstruction (n = 6/57, P > 0.05 vs. sham in RP, rheobase, and AP) (Fig. 1). BO did not significantly alter other electrophysiological characteristics of colon neurons including cell size (diameter), capacitance, input resistance, action potential amplitude, duration, threshold, and overshoot (Table 1).
Fig. 1.
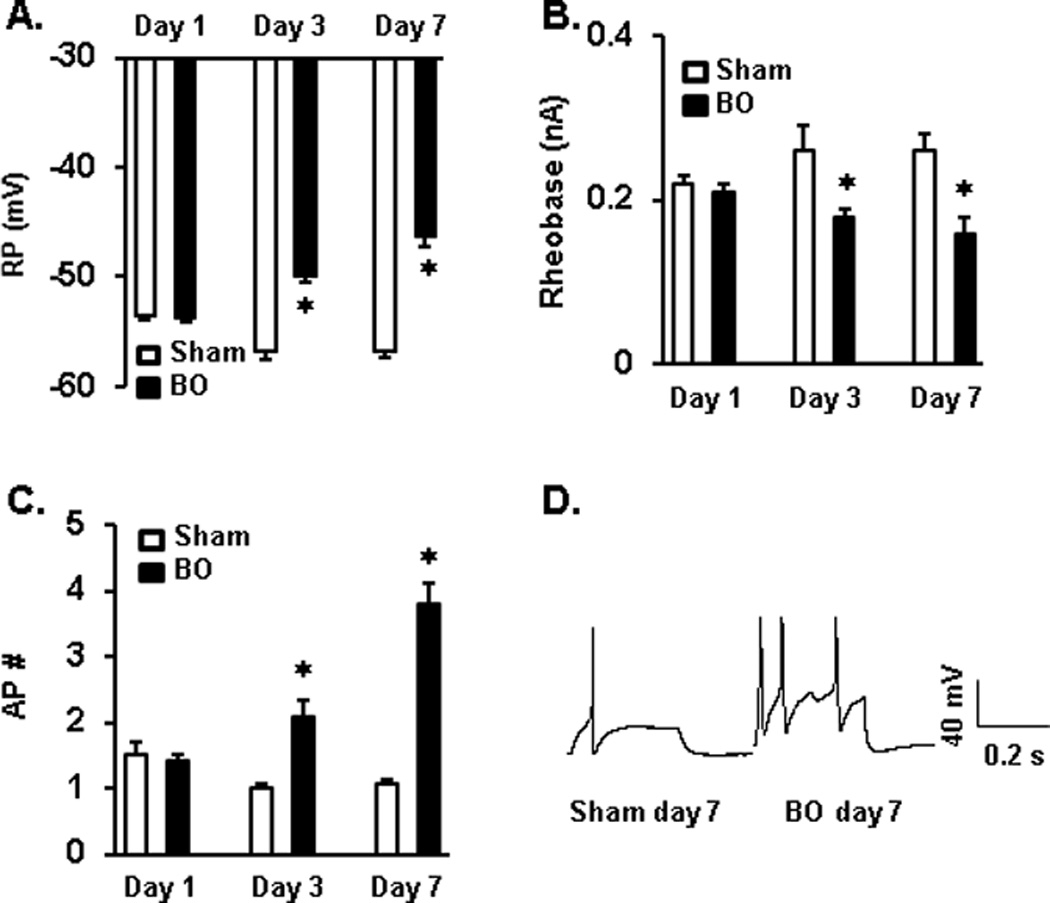
Patch clamp study on colon projecting DRG neurons. The colon-specific DRG neurons were highly excited in rats with bowel obstruction (BO) on day 3 and day 7, with decreased resting membrane potential (A) and rheobase (B), and increased number of action potentials in response to stimulation of 3× rheobase (C). The neuron excitability was not significantly changed 1 day after obstruction. The panel insert (D) shows representative tracing of patch clamp recordings in sham and BO (day 7). n = 11/51 (numbers of rats/neurons) in sham; n = 6/57 for BO day 1; n = 5/26 for BO day 3; n = 5/31 for BO day 7. *P < 0.05 vs. sham of the group.
Table 1. Changes of cell membrane electrical properties of colon projecting DRG neurons in bowel obstruction.
Shown here are parameters of cell membrane electrical properties other than shown in Fig. 1.
| Size (µm) |
Cm (pF) |
Rin (MΩ) |
AP (2× rheobase) (# in 300 ms) |
Threshold (mV) |
Amplitude (mV) |
Overshoot (mV) |
Duration (ms) |
||
|---|---|---|---|---|---|---|---|---|---|
| Sham | 28.1±0.64 | 50.3±1.49 | 446.5±26.07 | 1.1±0.03 | −32.1±1.19 | 95.6±2.34 | 44.5±1.98 | 2.6±0.13 | |
| BO | 1 d | 27.2±0.60 | 47.7±1.38 | 514.3±30.14 | 1.2±0.05 | −32.9±1.04 | 91.1±1.79 | 43.7±1.98 | 2.4±0.08 |
| 3 d | 27.3±0.75 | 49.3±1.42 | 451.4±42.92 | 1.5±0.10 * | −32.0±1.23 | 91.4±3.11 | 42.7±3.05 | 2.6±0.09 | |
| 7 d | 28.5±0.68 | 51.7±1.68 | 450.5±30.01 | 2.2±0.15 * | −30.2±1.81 | 92.9±3.44 | 45.2±2.92 | 2.7±0.18 | |
Size, neuron diameter; Cm, cell capacitance; Rin, input resistance; AP, action potential; Threshold, AP threshold; Amplitude, AP amplitude; Overshoot, AP overshoot; Duration, AP duration. Neurons recorded in each group: n = 11/51 (numbers of rats/neurons) in sham; n = 6/57 for BO day 1 (1 d); n = 5/26 for BO day 3 (3 d); n = 5/31 for BO day 7 (7 d). Data is expressed as mean ± SEM.
P < 0.05 vs. sham.
2) Withdrawal response to VFF stimulation in the lower abdomen was increased in BO rats
Because animals with visceral pain exhibit referred somatic hyperalgesia, we measured rat withdrawal response to VFF stimulations (0.1 to 8 g) on the referred somatic region (lower abdomen) [26, 30]. We found that the withdrawal response to VFF stimulation was significantly increased in BO rats compared to sham control rats on days 3 and 7 (Fig. 2). The 8 g VFF stimulation evoked immediate response in 76.3 (±14.2)% and 80.3 (±14.6)% of tests in BO rats on day 3 (n = 7, P < 0.05 vs. sham) and 7 (n = 8, P < 0.05 vs. sham), respectively, compared to 31.8 (±7.4)% and 32.4 (±7.8)% in day 3 and day 7 sham controls (n = 8) (Fig. 2).
Fig. 2.
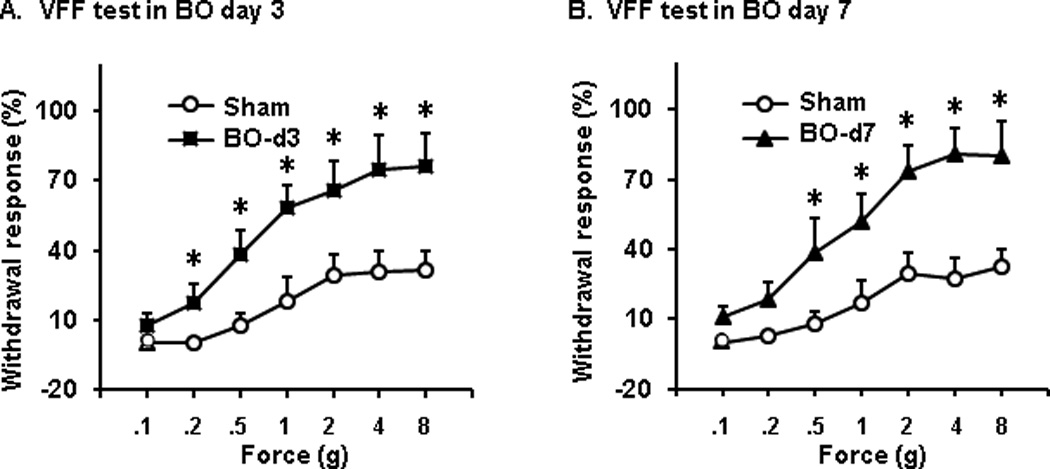
VFF test showed that the referred sensitivity to mechanical stimulation to abdominal wall was significantly increased in obstruction (BO) on day 3 (A) and day 7 (B). n = 8 for sham and BO day 7; n = 7 for BO day 3. *P < 0.05 vs. sham.
3) Mechano-transcription of NGF in colonic SMC in BO rats
As previously reported [22, 39], partial obstruction in the distal colon led to marked distention of the colon proximal to the site of obstruction. The external circumference of the middle colon changed from 19.1 (±0.5) mm in sham control (when filled with fecal pellets) to 30.6 (±0.6) mm in rats with obstruction (day 7) (n = 5 rats in each group, P < 0.01). To determine if mechanical stress upregulates NGF expression in smooth muscle in obstruction, we compared the levels of NGF mRNA and protein in both muscularis externa and mucosa/submucosa tissues from sham and BO rats in the oral dilated and aboral non-dilated colon segments. Our results showed that NGF mRNA expressions in colonic muscularis externa were significantly increased in BO rats in the oral dilated segment (P < 0.05 vs. sham), but not in the non-dilated aboral segment (P > 0.05 vs. sham) (Fig. 3A), indicating that upregulation of NGF expression is due to mechanical stress in obstruction. The obstruction-associated upregulation of NGF mRNA and protein did not happen in the mucosa/submucosa layers (P > 0.05 vs. sham) (Fig. 3 B, C). Time course study showed that the expression of NGF mRNA in the muscularis externae was not significantly increased at 12 h of obstruction [1.49 (±0.31)], compared to sham controls [1.0 (±0.24), P > 0.05, n = 6]. However, both NGF mRNA and protein expression levels in colonic muscularis externae were increased on days 1, 3 and 7 (n = 5 or 6 for sham, and BO days 1, 3, 7) (Fig. 3 A–D). Quantitative EIA analysis showed that NGF protein levels in the muscle layer increased from 99.7 ± 27.8 pg/mg in sham (n = 6) to 274.5 ± 29.6 pg/mg of protein in obstruction (day 7, n = 6) (P < 0.01). Immunohistochemistry analysis in 4 sham and 4 BO samples (Fig. 3E) showed that NGF (in brown) was detectable only in the myenteric plexus in the muscularis externae of normal colon (a), but in the smooth muscle cells as well in BO (b).
Fig. 3.
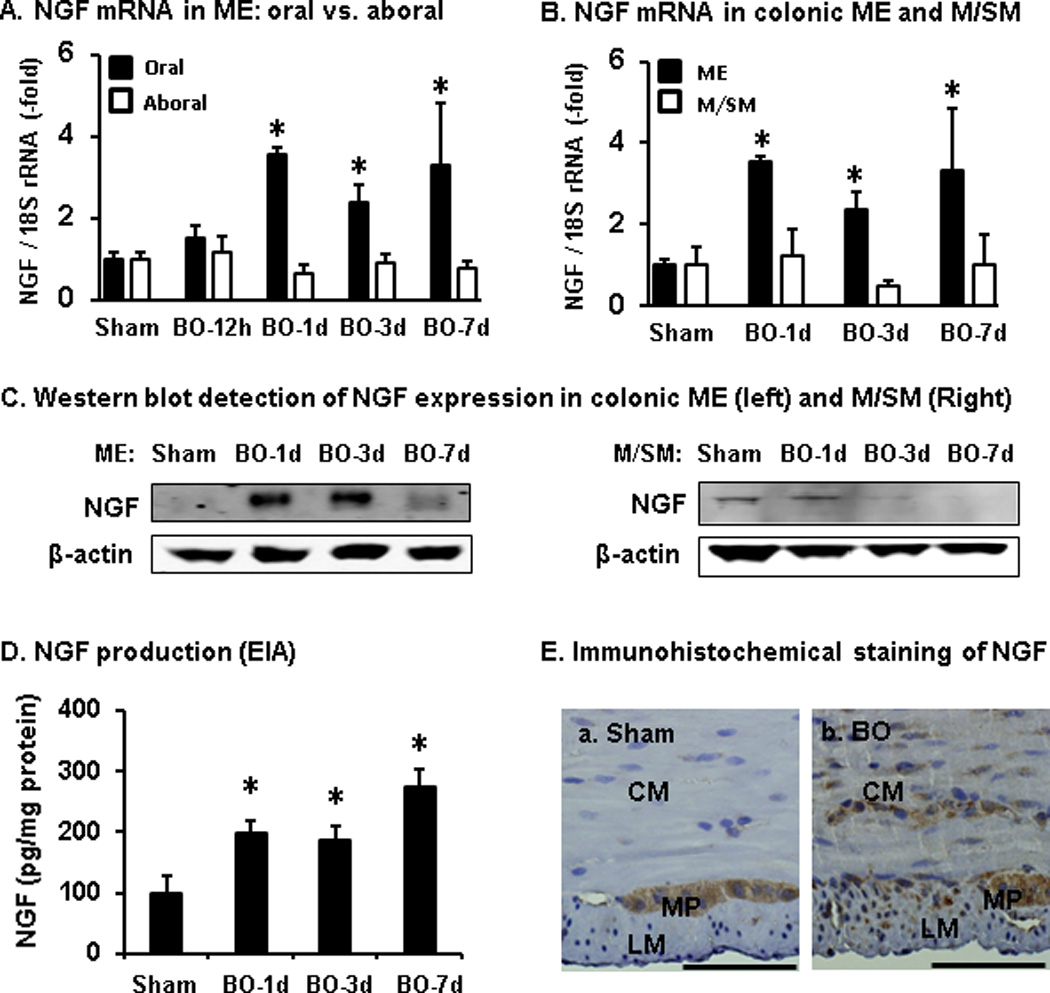
Expression of NGF mRNA and protein in the colon in bowel obstruction (BO) detected by qPCR (A, B), Western blot (C), enzyme immunoassay (EIA, D), and immunohistochemistry (E). Note that NGF expression was up-regulated in the distended oral, but not non-distended aboral segment (A, n = 5 each group). The increased NGF expression in the distended colon occurred in the muscularis externae (ME), but not mucosa/submucosa layer (M/SM) (B–D, n = 5 or 6 in each group). Immunohistochemical staining of NGF (in brown, E) in the ME of sham colon (a) and in BO (day 7, b). Bar, 50 µm). CM, circular muscle; LM, longitudinal muscle; MP, myenteric plexus. Images are representative of 4 sham and 4 BO samples. *P < 0.05 vs sham.
4) In vitro stretch of primary culture of colonic SMC induced NGF mRNA and protein expression
To further determine if the expression of NGF in colonic SMC is inducible by mechanical stress, we applied 18% static mechanical stretch to primary culture of rat colonic SMC in vitro [22, 39]. We found that mechanical stretch increased NGF gene expression in a time dependent manner (Fig. 4). Compared to non-stretch control (n = 6), the stretched samples started to show a significant increase of NGF mRNA when stretched for 30 min or longer (P < 0.05 vs. non-stretch control) (Fig. 4A). The NGF mRNA (detected 3 h after stretch, n = 6) and protein (detected 24 h after stretch, n = 5) were increased by 2.2(±0.14)-fold and 3.5(±0.39) –fold (both P < 0.05 vs. non-stretch control), respectively, with the static stretch for 60 min (Fig. 4A and 4B).
Fig. 4.
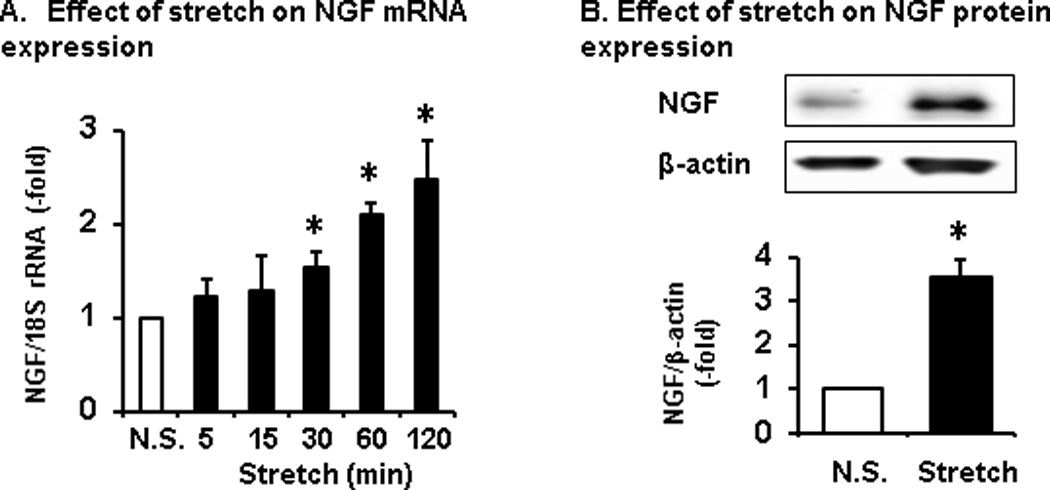
Effect of mechanical stretch on NGF expression in primary culture of rat colonic SMC. (A) Expression of NGF mRNA started to increase significantly when cells were stretched at 18% elongation for 30 min. All the samples were harvested in 3 h after the start of stretch. (B) Stretch induced expression of NGF protein in cultured rat colonic SMC. To detect NGF in western blot study, cells were stretched at 18% for 60 min, and samples were harvested 24 h later. n = 5 or 6 experiments for each time point or group. *P < 0.05 vs non-stretch (N.S.).
5) Role of mechanical stress-induced SMC-derived NGF in afferent neuron sensitization: ex vivo study
In the ex vivo study, we found that DRG neuron excitability was enhanced significantly (P < 0.05 vs. sham control) by the treatment with the media collected from the muscle strips of the oral distended segment of BO rats, but not by media from sham controls or the aboral non-distended segment of BO rats (n = 4 or 5 independent experiments, 13 to 18 neurons were recorded in each group) (Fig. 5). Moreover, the increased cell excitability was significantly attenuated by anti-NGF antibody (0.2 µg/mL) (P < 0.05 vs. obstruction control, n = 13 neurons in 4 independent experiments) (Fig. 5), suggesting that mechanical stress-induced smooth muscle-derived NGF plays a critical role in colon neuron sensitization in BO.
Fig. 5.
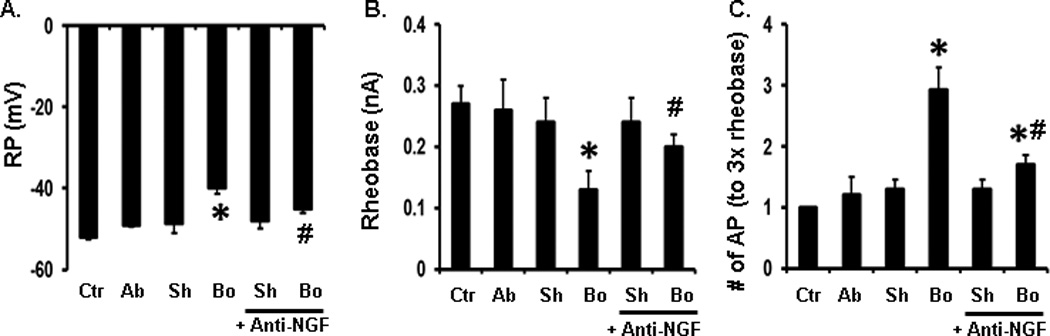
Effects on DRG neuron excitability of conditioned media collected from different sources of smooth muscle tissue. Shown are patch clamp results of RP (A), rheobase (B), and AP (C). Note that the conditioned media from the obstructed (Bo) oral segment increased DRG neuron excitability, but the media control (Ctr), or media from the aboral segment (ab) or sham (sh) tissues did not. Neutralization of NGF with specific anti-NGF antibody partially blocked the effect of the Bo media. Conditioned media were pooled from 4 sham and 4 obstruction rats. n = 4 or 5 independent experiments, and 13 to 18 neurons were recorded in each group. *P < 0.05 vs. sh control, #p<0.05 vs. Bo.
6) In vivo administration of anti-NGF antibody attenuated VHS in BO rats
To determine if mechanical stress-induced NGF in colonic SMC plays a role in visceral hypersensitivity in obstruction in vivo, we treated sham and BO rats with serum control and anti-NGF antibody (20 µg/kg, i.p., daily). VFF test was carried out and colon neuron excitability was studied on day 7. We found that anti-NGF treatment (n = 7/38) partly but significantly normalized colon neuron excitability (P < 0.05 vs. BO control) (Fig. 6A–C) and attenuated VFF-evoked withdrawal response in BO (n = 8 in BO control, and n = 7 in BO with anti-NGF treatment) (Fig. 6D).
Fig. 6.
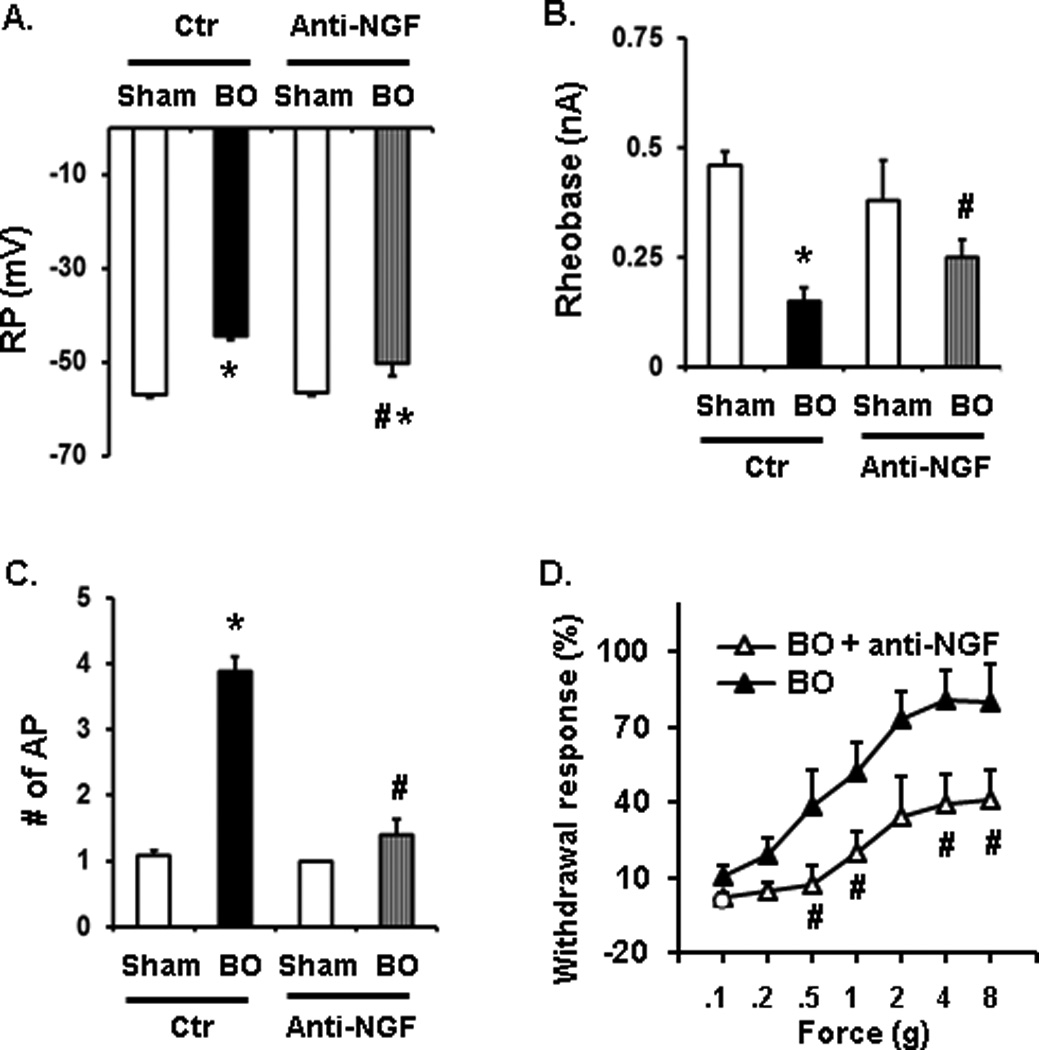
Beneficial effect of anti-NGF antibody administration on afferent neuron excitability (A–C, patch clamp studies) and referred sensitivity to abdominal stimulation (D, VFF test) in bowel obstruction (BO, day 7). For patch clamp studies, n = 6/19 and 7/38 for anti-NGF treated sham and BO (day 7). For VFF tests, n = 8 in BO and 7 in BO with anti-NGF treatment. *P < 0.05 vs. sham in the group; #p < 0.05 vs. BO (day 7).
7) Cellular mechanisms of colon neuron hyper-excitability in BO
To explore the molecular mechanisms of sensory neuron hyper-excitability in BO, we studied expression and activity of Nav channels in colon neurons. To determine Na+ channel activity, we recorded total Na+ current, tetrodotoxin-resistant (TTX-r) and TTX-sensitive (TTX-s) Na+ currents [3, 13]. Currents were evoked by stepwise depolarizations between −80 and 50 mV from a holding potential of −70 mV. Fast TTX-s Nav was obtained by digital subtraction of slow TTX-r Nav from the total Na+ currents. We found that the total and TTX-r, but not TTX-s, Na+ currents were significantly increased in BO rats (n = 6/30 in sham and BO) (Fig. 7A). However, anti-NGF antibody treatment (n = 6/26) almost completely blocked this effect, suggesting a critical role of NGF in Na+ current changes in BO (Fig. 7A).
Fig. 7.
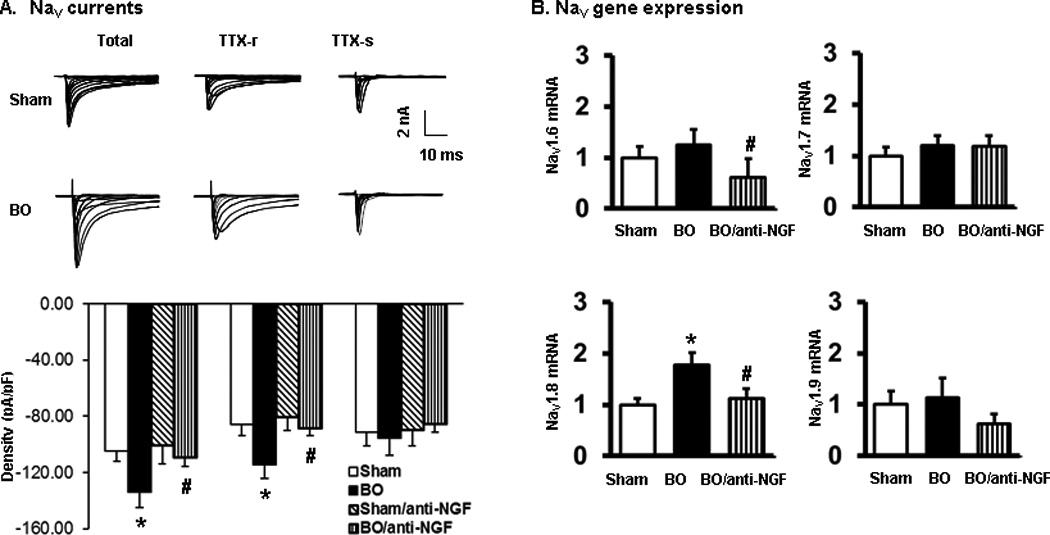
Nav currents and gene expression of colon projecting DRG neurons in BO (day 7). A, Nav currents. Top panel shows representative traces of Nav currents in sham and BO (day 7). Bottom panel shows the summarized peak Nav current density including total, TTX-resistant (TTX-r), and TTX-sensitive (TTX-s) currents in sham and BO rats without and with anti-NGF antibody administration. n = 6/30 sham, n = 6/30 BO, n = 4/10 in sham with anti-NGF, and n = 6/26 in BO treated with anti-NGF. *P < 0.05 vs. sham. #P < 0.05 vs. BO. B, Expression of Nav1.6, Nav1.7, Nav1.8 and Nav1.9 mRNAs in colon projecting DRG neurons isolated by laser capture microdissection followed by qPCR detection. n = 4 or 5. *P < 0.05 vs. sham. #P < 0.05 vs. BO.
We also detected gene expression of Na+ channel alpha subunits Nav1.6, Nav1.7, Nav1.8, and Nav1.9 [13] in colon projecting DRG neurons isolated by laser capture microdissection followed by qPCR detection. We found that the mRNA expression of Nav1.6 and Nav1.7 (responsible for TTX-s currents) is not altered in BO (P > 0.05, n = 5 sham and 4 BO rats). However, the mRNA expression of Nav1.8 (produces TTX-r currents) is significantly increased by 1.8 (±0.23)-fold in BO (P < 0.05 vs. sham). This increase was abolished with anti-NGF treatment (n = 5) (Fig. 7A and B). Although Nav1.9 is considered to also contribute to TTX-r currents in DRG neurons [12], its mRNA expression was not altered in BO.
DISCUSSION
Abdominal pain is one of the primary symptoms in acute and chronic obstructions. In acute complete or complicated obstructions, surgical release is the treatment of choice; distention associated abdominal pain in these patients may disappear soon after surgical resolution. However, distension associated pain is a major concern in the conservative management for partial mechanical obstruction and in palliative treatments for inoperable and malignant obstructions [18, 34, 35]. It is reported that 92% of patients with advanced malignant obstruction have distension associated abdominal pain [2, 31]. Our study in a pre-clinical model of partial colon obstruction found that colon projecting DRG neurons demonstrated significantly higher excitability in BO than in sham controls. The increase of cell excitability in sensory neurons indicates a heightened visceral sensitivity [3, 11, 12]. Moreover, we found that the referred visceral sensitivity measured by withdrawal response to abdominal stimulation was significantly increased in obstruction. The increased visceral sensitivity may contribute to abdominal pain in obstruction. Furthermore, we found that mechanical stress in obstruction induced up-regulation of NGF expression in colonic SMC. Our ex vivo and in vivo studies demonstrate that mechanical stress-induced NGF may play a critical role in the development of visceral hypersensitivity in obstruction; neutralizing antibody against NGF significantly attenuated visceral hypersensitivity in obstructed rats. Thus peripheral NGF and the pathway leading to mechano-transcription of NGF in the colon may represent potential therapeutic targets for distention associated abdominal pain.
NGF is known for its role in the peripheral mechanisms of visceral sensitization in inflammatory disorders in the gut such as pancreatitis and alimentary tract inflammation [9, 31, 47, 51]. In these conditions, NGF is released from inflammatory cells in the tissues. NGF has also been shown to contribute to VHS through a central sensitization mechanism in functional bowel disorders [43]. However, our study found that up-regulation of NGF expression is induced in gut smooth muscle cells by mechanical stress in obstruction. The increase of NGF expression was not detected in the mucosa/submucosa layer of the distended segment, nor in the muscularis externae of the non-distended aboral segment in BO rats. These results, together with our strictly executed sham controls in whom obstruction ring was placed but removed immediately [22, 23, 39], exclude any possibility that up-regulation of NGF was due to surgery-related inflammation. Our in vitro study provides further evidence that expression of NGF in colon SMC is inducible by mechanical stretch. Up-regulation of NGF expression in distended bowel may also occur in chronic functional obstruction [20, 24]. Kuroda et al [20] observed that NGF mRNA expression in colon smooth muscle was increased in the dilated colon proximal to the aganglioned distal bowel in patients with Hirschsprung’s disease as well as in piebald lethal mouse [20]. Interestingly, they found that the expression of NGF mRNA in the aganglioned distal bowel was not increased compared with normal subjects. These observations further support our conclusion that the up-regulation of NGF in obstruction is due to mechano-transcription.
A previous study found that lumen distention with a balloon in the colon could induce mechano-transcription of cyclo-oxygenase-2 (COX-2) and led to persistent visceral hypersensitivity for at least three days [23]. Mechano-transcription of COX-2 was also induced in the model of mechanical obstruction [22, 25–27, 39]. We observed that lumen distention led to upregulation of COX-2 [25] and NGF (unpublished study by Lin, Fu, and Shi) in the small intestine similarly as in the colon. These data suggest that mechano-transcription of nociceptive molecules in gut SMC may be a common underlying mechanism in the development of distention associated pain in the GI tract. The pathway in which mechanical stress-induced expression of nociceptive mediators in gut SMC sensitize afferent neurons and contribute to visceral pain highlights an important role of gut SMC in gut-nerve interaction. While it has long been established that extrinsic and enteric nervous systems regulate gut SMC activity [37], our studies demonstrate that gut SMC may modulate sensory nerve activity through mechano-transcription of nociceptive mediators.
Mechanical stimulation may lead to immediate activation of sensory nerves [3, 11, 12] directly via mechanosensitive channels, i.e. certain members of transient receptor potential (TRP) family of ion channels, K+ channels, Piezo channels [4, 15, 21]. This may be involved in nociceptive transmission in obstruction, especially in early hours of obstruction. However, our study suggests that the increase of sensory neuron excitability in obstruction did not result from a direct effect of mechanical stress on the neurons. First, dispersed DRG neurons were recorded in the patch clamp experiments hours after DRG were isolated from the body. Steps involved in dispersion and equilibration of neurons take 3 to 4 hr. Second, our ex vivo study used the conditioned medium from muscle strips of sham and obstruction rats to incubate DRG neurons isolated from naïve rats before the neurons were recorded. These neurons were completely off any direct effect of mechanical stress associated with obstruction. Nevertheless, augmented excitability was found only in the neurons incubated with conditioned medium derived from the distended muscle strips, not in neurons incubated with media from sham rats or from non-distended colon. Importantly, the enhanced neuron excitability was attenuated by anti-NGF antibody. These data suggest that DRG neurons are sensitized by mediators released from stretched colon muscle strips, and that NGF is a key molecule from the smooth muscle which is involved in the sensitization of the DRG neurons. What other mechanisms are involved in visceral hypersensitivity in BO remains to be determined. As mechanosensitive TRP channels, K+ channels, Piezo channels are expressed in DRG sensory neurons [4, 15, 21], it would be of interest to investigate whether the mechanosensitive channels’ expression and function are altered in obstruction, and if so, whether these changes contribute to DRG neuron hyper-excitability and visceral hypersensitivity in BO.
Our time course study showed that colon neuron excitability was significantly augmented at days 3 and 7, but not affected at day 1 of obstruction (Fig. 1). The lack of changes of cell excitability on day 1 of obstruction may be attributed to the time lag between mechano-transcription of NGF in colon SMC and the changes of gene expression and channel function in DRG neurons secondary to peripheral increase of NGF. We found that NGF expression did not increase until 24 h after obstruction. It will certainly take more time for colon smooth muscle-derived NGF to act on nerve endings, be transported to neuron cell body, and further regulate gene expression in DRG neurons [3, 31].
We further investigated the cellular mechanisms of sensory neuron hyper-excitability in BO. In the present study, we focused on voltage-gated Na+ channels (Nav), as neuron cell excitability is determined in large part by Nav [3, 13]. Ten pore-forming subunits (α-subunits) of Nav have been identified (Nav1.1 to Nav1.9) [13]. TTX-s currents are produced mainly by channels with Nav1.6 and Nav1.7; and TTX-r channels include these with Nav1.8 and Nav1.9 in DRG neurons. We found that the total and TTX-r Na+ current activities are significantly increased, but TTX-s Na+ current remain unchanged in obstruction. These changes in Nav function may be due at least partly to gene expression of the respective α-subunits, as studies on laser capture microscopy isolated colon projecting DRG neurons suggest that TTX-r Nav 1.8 was significantly up-regulated in obstruction, whereas the expression of Nav1.6, Nav1.7, and Nav1.9 was not altered. More importantly, anti-NGF treatment completely abolished the changes of Nav currents and gene expression, suggesting that NGF-dependent enhancement of Na+ currents is, at least partly, through up-regulation of Nav1.8 gene expression in colon neurons.
In summary, our study demonstrates that mechano-transcription of NGF in colonic smooth muscle plays a critical role in visceral hypersensitivity in bowel obstruction, and this may be through the increased gene expression and activity of TTX-r Na+ channel (i.e. Nav1.8) in colon neurons. Thus, peripheral inhibition of mechano-transcription of NGF and central modulation of Nav 1.8 channel may represent potential therapeutic strategies in the management of distension associated abdominal pain in OBD.
Acknowledgments
We thank Dr. Feng Li for his help in some of the cell culture experiments. This study was funded by a grant from NIH/NIDDK (R01DK102811, XZS).
Footnotes
Conflict of Interest
The authors report no conflict of interest.
Some of the results in the paper were presented as an abstract in the Digestive Disease Week 2016 in San Diego, CA, May 21–24, 2016.
References
- 1.Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(Suppl):62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 2.Baines M, Oliver DJ, Carter RL. Medical management of intestinal obstruction in patients with advanced malignant disease: A clinical and pathological study. Lancet. 1985;326(8462):990–993. doi: 10.1016/s0140-6736(85)90534-3. [DOI] [PubMed] [Google Scholar]
- 3.Bielefeldt K. Neurochemical and molecular basis of peripheral sensitization. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain. Informa Healthcare; 2006. pp. 67–83. [Google Scholar]
- 4.Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezochannel activity by depleting membrane phosphoinositides. Sci Signal. 2005;8(363):ra15. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brierley SM, Blackshaw . The neurobiology of visceral nociceptors. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain. Informa Healthcare; 2006. pp. 45–66. [Google Scholar]
- 6.Chen J, Winston JH, Fu Y, Guptarak J, Jensen KL, Shi XZ, Green TA, Sarna SK. Genesis of anxiety, depression, and ongoing abdominal discomfort in ulcerative colitis-like colon inflammation. Am J Physiol Regul Integr Comp Physiol. 2015 Jan 1;308(1):R18–R27. doi: 10.1152/ajpregu.00298.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemow DB, Steers WD, Tuttle JB. Stretch-activated signaling of nerve growth factor secretion in bladder and vascular smooth muscle cells from hypertensive and hyperactive rats. J Cell Physiol. 2000;183(3):289–300. doi: 10.1002/(SICI)1097-4652(200006)183:3<289::AID-JCP1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.De Giorgio R, Cogliandro RF, Barbara G, Corinaldesi R, Stanghellini V. Chronic intestinal pseudo-obstruction: Clinical features, diagnosis, and therapy. Gastroenterol Clin North Am. 2011;40(4):787–807. doi: 10.1016/j.gtc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Delafoy L, Raymond F, Doherty AM, Eschalier A, Diop L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain. 2003 Oct;105(3):489–497. doi: 10.1016/S0304-3959(03)00266-5. [DOI] [PubMed] [Google Scholar]
- 10.Fraser SA, Shrier I, Miller G, Gordon PH. Immediate postlaparotomy small bowel obstruction: a 16-year retrospective analysis. Am Surg. 2002 Sep;68(9):780–782. [PubMed] [Google Scholar]
- 11.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol gastrointest Liver Physiol. 2000;278(6):G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 12.Gold MS. Overview of pain and sensitization. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain. Informa Healthcare; 2006. pp. 17–32. [Google Scholar]
- 13.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels. Neuron. 2000 Nov;28(2):365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 14.Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007 Oct;5(10):1126–1139. doi: 10.1016/j.cgh.2007.06.013. quiz 1121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Y, Gu C. Physiological and pathological functions of mechanosensitive ion channels. Mol Neurobiol. 2014;50(2):339–347. doi: 10.1007/s12035-014-8654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanauer SB, Wald A. Acute and chronic megacolon. Curr Treat Options Gastroenterol. 2007;10(3):237–247. doi: 10.1007/s11938-007-0017-z. [DOI] [PubMed] [Google Scholar]
- 17.Huang TY, Hanani M. Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G670–G678. doi: 10.1152/ajpgi.00028.2005. [DOI] [PubMed] [Google Scholar]
- 18.Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol. 2004 Jul-Aug;2(4):323–334. discussion 334-7. [PubMed] [Google Scholar]
- 19.Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep. 2013 Sep;15(9):344. doi: 10.1007/s11894-013-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroda T, Ueda M, Nakano M, Saeki M. Altered production of nerve growth factor in aganglionic intestines. J Pediatr Surg. 1994 Feb;29(2):288–292. doi: 10.1016/0022-3468(94)90334-4. discussion 292-3. [DOI] [PubMed] [Google Scholar]
- 21.La JH, Gebhart GF. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2011 Jul;301(1):G165–G174. doi: 10.1152/ajpgi.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Lin YM, Sarna SK, Shi XZ. Cellular mechanism of mechano-transcription in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2012 Sep;303(5):G670–G679. doi: 10.1152/ajpgi.00440.2011. PMID: 22700825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YM, Fu Y, Wu CC, Xu GY, Huang LY, Shi XZ. Colon distention induces persistent visceral hypersensitivity by mechano-transcription of pain mediators in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2015 Mar 1;308(5):G434–G441. doi: 10.1152/ajpgi.00328.2014. PMID: 25540231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YM, Fu Y, Sarna SK, Radhakrishnan R, Shi XZ. Lumen Distension Induced Gene Expression and Bowel Dysfunction in Chronic Functional Obstruction (Abstract). 2nd Federation of Neurogastroenterology & Motility Meeting; Aug 25th–28th, 2016; San Fransisco, CA. [Google Scholar]
- 25.Lin YM, Li F, Shi XZ. Mechano-transcription of COX-2 is a common response to lumen dilation of the rat gastrointestinal tract. Neurogastroenterol Motil. 2012 Jul;24(7):670–679. doi: 10.1111/j.1365-2982.2012.01918.x. PMID: 22489918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YM, Li F, Shi XZ. Mechanical stress is a pro-inflammatory stimulus in the gut: in vitro, in vivo and ex vivo evidence. PLoS One. 2014 Sep 2;9(9):e106242. doi: 10.1371/journal.pone.0106242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YM, Sarna SK, Shi XZ. Prophylactic and therapeutic benefits of COX-2 inhibitor on motility dysfunction in bowel obstruction: roles of PGE2 and EP receptors. Am J Physiol Gastrointest Liver Physiol. 2012 Jan;302(2):G267–G275. doi: 10.1152/ajpgi.00326.2011. PMID: 22038825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994 Jul;107(1):271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 29.Milenkovic M, Russo CA, Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD, USA: Agency for Health Care Research and Quality; 2006. Sep, Hospital Stays for Gastrointestinal Diseases, 2004: Statistical Brief 12. PMID: 21938841. [PubMed] [Google Scholar]
- 30.Nunez R, Blesa E, Cabrera R. Hirschsprung’s Disease: Clinical features. In: Nunez RN, Lopez-Alonso M, editors. Hirschsprung’s Disease: Diagnosis and treatment. New York: Nova Science Publishers; 2009. [Google Scholar]
- 31.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 32.Postier RG, Squires RA. Chap 45, Acute abdomen. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston Textbook of Surgery. 18th. Philadelphia, PA: Saunders Elsevier; 2007. [Google Scholar]
- 33.Ripamonti C, Mercadante S. Pathophysiology and management of malignant bowel obstruction. In: Doyle D, Hanks G, et al., editors. Oxford Textbook of Palliative Medicine. 3rd. Vol. 8. Oxford: Oxford University Press; 2003. p. 496. ISBN: 0192620282. [Google Scholar]
- 34.Ripamonti C, Twycross R, Baines M, Bozzetti F, Capri S, De Conno F, Gemlo B, Hunt TM, Krebs H-B, Mercadante S, Schaerer R, Wilkinson P. Clinical-practice recommendations for the management of bowel obstruction with end-stage cancer. Support Care Cancer. 2001;9:223–233. doi: 10.1007/s005200000198. [DOI] [PubMed] [Google Scholar]
- 35.Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep. 2009 Jul;11(4):298–303. doi: 10.1007/s11912-009-0042-2. [DOI] [PubMed] [Google Scholar]
- 36.Russell JC, Welch JP. Pathophysiology of Bowel Obstruction. In: Welch JP, editor. Bowel Obstruction. Philadelphia, PA: W.B. Saunders; 1990. pp. 28–58. [Google Scholar]
- 37.Sarna SK, Shi XZ. Function and regulation of colonic contractions in health and disease. In: Johnson LR, editor. Physiology of the gastrointestinal tract. Vol. 1. Amsterdam: Elsevier Academic Press; 2006. pp. 965–993. [Google Scholar]
- 38.Shi XZ, Choudhury B, Pasricha JP, Sarna SK. A novel role of VIP in colonic motility function: Induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology. 2007 Apr;132(4):1388–1400. doi: 10.1053/j.gastro.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Shi XZ, Lin YM, Powell DW, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol. 2011 Jan;300(1):G99–G108. doi: 10.1152/ajpgi.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology. 2005 Nov;129(5):1518–1532. doi: 10.1053/j.gastro.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 41.Shi XZ, Sarna SK. Cell culture retains contractile phenotype but epigenetically modulates cell-signaling proteins of excitation-contraction coupling in colon smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2013 Feb 15;304(4):G337–G345. doi: 10.1152/ajpgi.00369.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Summers RW. Chapter 39, Approach to the patient with ileus and obstruction. In: Yamada Alpers, Laine Owyang, Powell, editors. Textbook of Gastroenterology. Vol. 1. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. pp. 842–858. [Google Scholar]
- 43.Tsang SW, Zhao M, Wu J, Sung JJ, Bian ZX. Nerve growth factor-mediated neuronal plasticity in spinal cord contributes to neonatal maternal separation-induced visceral hypersensitivity in rats. Eur J Pain. 2012 Apr;16(4):463–472. doi: 10.1016/j.ejpain.2011.07.005. Eur J Pain 2012. [DOI] [PubMed] [Google Scholar]
- 44.Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp. Neurol. 2000;421:302–324. [PubMed] [Google Scholar]
- 45.Welch JP. Chap 3, General considerations and mortality. In: Welch JP, editor. Bowel obstruction. Philadelphia, PA: Saunders; 1990. pp. 59–95. [Google Scholar]
- 46.Williams SB, Greenspon J, Young HA, Orkin BA. Small bowel obstruction: conservative vs. surgical management. Dis Colon Rectum. 2005 Jun;48(6):1140–1146. doi: 10.1007/s10350-004-0882-7. [DOI] [PubMed] [Google Scholar]
- 47.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterol. 2007;132(2):615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001 Jan;89(2–3):181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 49.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology. 2010 Jan;138(1):294.e3–304.e3. doi: 10.1053/j.gastro.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu CW, Lin YM, Gao J, Winston JH, Cheng L, Shi XZ. Are interstitial cells of Cajal involved in mechanical stress-induced gene expression and impairment of smooth muscle contractility in bowel obstruction? PLoS One. 2013 Sep;8(9) doi: 10.1371/journal.pone.0076222. e76222 (1–) PMID: 24098782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu GY, Winston J, Shenoy M, Yin H, Pendyala S, Pasricha PJ. TRPV1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterol. 2007;133(4):1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, Wang P, Li YQ. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012 May;61(5):685–694. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]


