Abstract
Mastocytosis is a term used to denote a heterogeneous group of conditions defined by expansion and accumulation of clonal (neoplastic) tissue mast cells in various organs. The classification of the World Health Organization (WHO) divides the disease into cutaneous mastocytosis (CM), systemic mastocytosis (SM), and localized mast cell tumors. Based on histomorphologic criteria, clinical parameters, and organ involvement, SM is further divided into indolent SM (ISM) and advanced SM variants, including aggressive SM (ASM) and mast cell leukemia (MCL). The clinical impact and prognostic value of this classification has been confirmed in numerous studies, and its basic concept remains valid. However, refinements have recently been proposed by the consensus group, the WHO, and the European Competence Network on Mastocytosis (ECNM). In addition, new treatment options are available for patients with advanced SM, including allogeneic hematopoietic stem cell transplantation and multi-kinase inhibitors directed against KIT D816V and other key signalling molecules. Our current article provides an overview of recent advances in the field of mastocytosis, with emphasis on classification, prognostication, and emerging new treatment options in advanced SM.
Keywords: mast cells, mastocytosis, KIT D816V, tryptase, prognostication, midostaurin
Introduction and Historical Overview
Mastocytosis is a heterogeneous group of neoplastic conditions characterized by expansion and accumulation of clonal (neoplastic) mast cells (MCs) in the skin and various internal organs, such as the bone marrow (BM), spleen, lymph nodes, and the gastrointestinal (GI) tract (1–4). Cutaneous involvement is found in most patients. A first description of the typical (pigmented) skin lesions was provided by Nettleship and Tay in 1869. A few years later, the term urticaria pigmentosa (UP) was coined, and following Paul Ehrlich’s description of MCs in 1879, the presence and accumulation of MCs in UP lesions was recognized by Unna in 1887 (Table 1). For many years, mastocytosis was believed to be a disease of the skin. However, in 1949, Ellis described a first case of systemic mastocytosis (SM) (Table 1). Over time since this observation, SM has become a well-recognized diagnostic entity, although other patients were found to have UP without systemic involvement. These patients, mostly children, are classified as cutaneous mastocytosis (CM) and have a good prognosis (5). A localized form of CM, termed mastocytoma of skin, has also been described (6,7). Overall, the basic classification of mastocytosis into cutaneous and systemic remains valid. However, during the past 30 years, a number of clinically and prognostically distinct sub-variants of CM and SM have been described (8–10). Between 1991 and 2000, novel robust criteria of SM were established (11–19). These criteria were discussed extensively before and during a Working Conference in 2000, from which an updated consensus classification was proposed by the EU-US consensus group (20). This proposal was adopted by the World Health Organization (WHO) in 2001 (21) and was up-dated and re-confirmed in 2008 (22).
Table 1.
Milestones in the History of Mast Cell- and Mastocytosis Research
| Year | Author(s) | Discovery – Achievement |
|---|---|---|
| 1869 | Edward Nettelship & Warren Tay | Rare form of persistent/pigmented urticaria described |
| 1878 | Alfred Sangster | Urticaria pigmentosa (UP) term proposed |
| 1879 | Paul Ehrlich | Tissue mast cells (MCs) described and named |
| 1887 | Paul Gerson Unna | MCs in UP lesions demonstrated |
| 1890~ | Ferdinand-Jean Darier | Darier’s sign described |
| 1949 | John M. Ellis | Systemic mastocytosis described (autopsy case) |
| 1966–1970 | Kimishige Ishizaka & Teruko Ishizaka | Reaginic antibody (IgE) detected and linked to IgE-dependent MC activation by allergens |
| 1967–1968 | S.G.O. Johansson & Hans Bennich | Discovery of an IgE myeloma and development of tests for measuring IgE levels in biological fluids |
| 1977 | Yukihiko Kitamura | MCs derive from hematopoietic stem cells |
| 1978/1979 | Yukihiko Kitamura | W/Wv and Sl/Sld mice are MC-deficient |
| 1979 | Karl Lennert | Kiel classification includes mastocytosis |
| 1987 | Lawrence B. Schwartz | Acute serum tryptase elevation indicates MC activation |
| 1988 | Joseph H. Butterfield | First human MC line, HMC-1, established |
| 1988 | Multiple Researchers | KIT is encoded by W locus of mice |
| 1989 | Peter Valent | Specific immunophenotype of human MCs |
| 1990 | Multiple Researchers | SCF is encoded by Sl locus of mice |
| 1991 | Dean Metcalfe | Consensus classification of mastocytosis |
| 1992 | Multiple Researchers | SCF is a growth factor for human MCs |
| 1993 | Takuma Furitsu & Yukihiko Kitamura | KIT D816V detected in HMC-1 cells |
| 1995 | Hiroshi Nagata & Dean D. Metcalfe | KIT D816V detected in patients with SM |
| 1995 | Lawrence B. Schwartz | Basal serum tryptase level reflects MC burden |
| 1998 | Hans-Peter Horny | Tryptase immunohistochemistry to detect neoplastic MC infiltrates in bone marrow |
| 1998 | Luis Escribano | Abnormal MC phenotype (CD2+ and CD25+) on MCs in patients with SM |
| 2000 | Multiple Researchers | Year 2000 working conference on mastocytosis: consensus criteria & classification proposed |
| 2001 | WHO Group | WHO classification of mastocytosis established based on year 2000 working conference proposal |
| 2001 | Wolfgang R. Sperr | Morphologic subsets of neoplastic mast cells |
| 2002 | Peter Valent | European Competence Network on Mastocytosis (ECNM) founded |
| 2003–2007 | Consensus Group | Standardization and response criteria for mastocytosis established and updated |
| 2008 | WHO Group | Updated WHO classification |
| 2010 | Cem Akin | Definition and criteria for mast cell activation syndrome (MCAS) proposed |
| 2011 | Karl Sotlar | CD30 expression in neoplastic mast cells |
| 2012 | Consensus Group | Global consensus classification of mast cell disorders including MCAS |
| 2016 | WHO Group | Updated WHO classification |
| 2016 | Jason Gotlib | First successful trial with a multi-kinase/KIT inhibitor (midostaurin) in patients with advanced SM including MCL |
MCs, mast cells; IgE, immunoglobulin E; WHO, World Health Organization; IgE-R, IgE receptor; SM, systemic mastocytosis
WHO Criteria and Classification of Mastocytosis 2001–2016
The WHO classification divides CM into maculopapular CM (MPCM), also known as UP, diffuse cutaneous mastocytosis (DCM), and localized mastocytoma of skin. Specific criteria of CM have been defined and published by the consensus group (23,24). Most patients with CM are children. By contrast, in most adult patients, SM is detected. The major criterion of SM is the multifocal accumulation and clustering of MCs (at least 15/cluster) in the BM or another extra-cutaneous organ (20–22). Minor SM criteria confirm the clonal (neoplastic) nature of the disease and include an abnormal MC morphology (spindling), expression of CD2 and/or CD25 in MCs in extracutaneous organs, expression of an activating mutation in codon 816 of KIT (usually KIT D816V) in extra-cutaneous cells, and a basal serum tryptase level exceeding 20 ng/ml (Supplemental Table S1) (20–22). When the major SM criterion and at least one minor SM criterion or 3 minor SM criteria are fulfilled, the diagnosis of SM is established (Supplemental Table S1).
A number of attempts have been made to define and standardize diagnostic markers and laboratory approaches in CM and SM and to develop diagnostic algorithms (23–27). Recommended parameters to screen for SM include an elevated baseline serum tryptase and detection of KIT codon 816 mutations in peripheral blood (PB) leukocytes using a highly sensitive allele-specific PCR test (23,26–31). A thorough BM investigation is recommended in adult patients who have a clearly elevated tryptase, a KIT (codon 816) mutation, or typical clinical symptoms, such as pruritus, flushing, GI cramping, diarrhoea, anaphylaxis to bee or wasp venom, or osteoporosis (23,26). An accumulation of such findings increases the likelihood of SM. A diagnostic algorithm incorporating such observations and parameters has been published recently by the consensus group (26).
SM is further divided into indolent SM (ISM), SM with an associated clonal hematologic non-MC-lineage disease (SM-AHNMD), aggressive SM (ASM), and MC leukemia (MCL) (Supplemental Table S2) (20–22). In indolent SM, two provisional sub-variants have been described: i) a variant where the disease is essentially restricted to the BM and the tryptase level is low or normal, termed isolated BM mastocytosis (BMM); and ii) a variant where the burden of MCs in internal organs is high and the neoplastic (KIT mutant-triggered) process expands to other myeloid lineages, termed smoldering SM (SSM) (20–22). In 2007, SSM was designated as a separate variant of SM by the consensus group (23), and since 2016, SSM is also a separate WHO category of SM (32,33). Repeated studies have confirmed the prognostic impact of the WHO classification regarding survival of patients with SM (19,34–37). Thus, whereas patients with ISM have a normal or near normal life-expectancy, the prognosis (survival) is poor in advanced SM. With the exception of skin mastocytomas, localized MC neoplasms, including MC sarcomas (MCS), are extremely rare (38–41).
WHO Update 2016 and Justification
Several adjustments have been introduced in the 2016-update of the WHO classification of mastocytosis (Table 2 and Supplemental Table S3) (32,33). First, the smoldering subtype of SM (SSM), a provisional entity of ISM in previous WHO proposals, is now considered a distinct category of SM by the WHO. The prognosis in SSM regarding progression-free and overall survival is better than that in ASM or MCL, but is still poor when compared to typical ISM (excluding SSM). It is noteworthy that each B-Finding (criteria of SSM) by itself, including organomegaly, high tryptase level (>200 ng/ml), and evidence of clonal involvement of non-MC lineages, indicates a poor prognosis (35,42–46).
Table 2.
Updated WHO Classification of Mastocytosis 2016
Cutaneous mastocytosis (CM)
|
Systemic mastocytosis (SM)
|
| Mast cell sarcoma (MCS) |
The previous term SM-AHNMD (SM with clonal hematologic non-mast cell-lineage disease) and the new term AHN can be used synonymously.
In the WHO classification 2016, the extremely rare ‘extra-cutaneous mastocytoma’ has been removed (Table 2 and Supplemental Table S3) (32,33) as with one recent exception (38), no such cases have been published during the past 20 years.
The term AHNMD has been accepted and used for many years, although sometimes thought to be unnecessarily descriptive (20–23). Therefore, the WHO update of 2016 allows and recommends the use of the following new synonym: associated hematologic neoplasm = AHN (Table 2 and Supplemental Table S3) (32,33). Both terms, AHN and AHNMD, may be used interchangeably.
In patients with ASM, the percentage of MCs in the BM smear is of prognostic significance, and those who have 5–19% MCs in their BM smears may at some point transform to overt MCL (19). Therefore, the consensus group proposed in 2014 that ASM be divided into untransformed disease and ASM in transformation to MCL (47). The proposed terminology for transformed ASM is ‘ASM-t’, and is defined by a MC count of 5–19% in BM smears (47). The updated WHO classification of mastocytosis is shown in Table 2.
Most Recent Advances in the Classification and Criteria defining CM and SM
1. Delineation of Variants of Childhood MPCM (UP)
Cutaneous lesions of mastocytosis are present in CM and SM regardless of age. Most adult patients with typical skin lesions have SM. In children, CM is usually diagnosed. In many cases, cutaneous lesions disappear before or during puberty. However, in some patients skin lesions persist into adulthood (5,24). More recently, two distinct forms of childhood MPCM have been recognized: a variant characterized by monomorphic small-sized lesions, and a second variant defined by polymorphic (often larger) lesions (24,48). Only the monomorphic form is found in adults, suggesting that only this variant persists into adulthood whereas polymorphic lesions usually resolve (24,48). Unexpectedly, there is only a weak correlation between monomorphic lesions and KIT D816V (48). In fact, KIT D816V is also detected in some cases with polymorphic lesions that may resolve by adulthood, and there are other patients with monomorphic skin infiltrates in whom no KIT D816V mutation is found (48).
2. Diagnostic Potential of ‘Non-Codon-816’ KIT Mutations
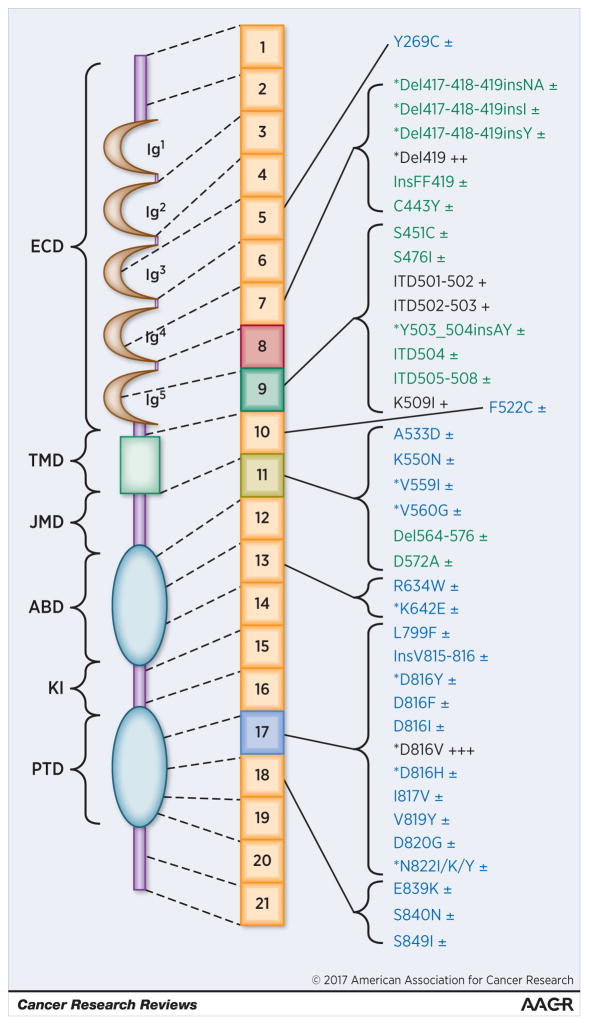
A number of novel KIT mutations have been detected in patients with CM and SM (Figure 1) (27,49–52). Some of these mutations, such as K509I, are more common in pediatric cases, sometimes being detected in germline DNA (49–52). Interestingly, several ‘atypical’ KIT mutations may facilitate transformation to more advanced disease. Indeed, advanced SM has been associated with such ‘atypical’ non-D816V KIT mutations (Figure 1, Supplemental Table S4). These KIT mutations may play a similar role in disease evolution as KIT D816V. Therefore, these mutations may serve as minor SM criterion in future proposals to diagnose and classify CM and SM. Another important issue is the method applied to detect KIT D816V in the PB and BM. A generally accepted recommendation is to employ a highly sensitive, allele-specific, PCR method and to quantify the KIT D816V mutant allele burden in all SM patients if possible (27–30,43,44).
Figure 1.
Overview of KIT mutations described in patients with mastocytosis
Mutations described primarily in pediatric cases with cutaneous mastocytosis (CM) are depicted in green color and those found preferentially in adults with systemic mastocytosis (SM) in blue color. The most frequently detected KIT mutations are marked with dark colors (CM: dark-green; SM: dark-blue). In addition, the frequency of mutations is defined by the following score: ±: found in less than 1% of the pediatric or adult patients; +: found in 1 to 5% of pediatric patients; ++: found in 5 to 20% of pediatric patients; +++ (KIT D816V): mutation found in around 30% of pediatric patients and in > 80% of all adult patients. Abbreviations: ABD: ATP-binding domain; Del: deletion; ECD: Extracellular domain; Ins: Insertion; ITD: Internal tandem duplication; JMD: Juxtamembrane domain; KI: Kinase insert; PTD: Phosphotransferase domain; TMD: Transmembrane domain. The asterisk (*) denotes KIT mutations that have also been described in other neoplasms such as gastro-intestinal stromal tumors (GIST), acute myeloid leukemia (AML), lymphomas, or seminomas. These neoplasms may also present with other KIT mutants not yet described in mastocytosis (not shown in this figure).
3. Does BM Mastocytosis (BMM) qualify as a separate Variant of SM?
A number of recent studies have shown that BMM is a clinically relevant entity that is often overlooked in clinical practice. In many cases, severe recurrent anaphylaxis, sometimes associated with an IgE-mediated allergy to bee or wasp venom, or unexplained osteoporosis are found (53–55). Cutaneous lesions of mastocytosis are typically absent and basal tryptase levels are normal or slightly elevated. Moreover, in most patients with BMM, the KIT D816V allele burden in the peripheral blood is low. A BM examination usually reveals small-sized clusters and aggregates of MCs. Sometimes, a well-differentiated (WD) MC morphology is seen. Regarding progression to advanced SM, the prognosis is good. However, severe or even fatal anaphylaxis may occur. Based on these considerations, BMM - now regarded a provisional sub-category of SM - should probably become a separate variant of SM. While no detailed diagnostic criteria for BMM have been proposed or agreed upon by a formal consensus conference, we are of the opinion that i) absence of skin lesions, ii) a low or normal serum tryptase level, and iii) absence of B-Findings and C-Findings, would be reasonable diagnostic criteria to propose for BMM.
4. Well-Differentiated (WD) Morphology of MCs
A unique histopathologic variant of SM displaying mature MC morphology was described in 2004 (56). Since then, a number of studies have reported a well-differentiated morphology of MCs in a subset of patients with SM (52,57–59). Some of the authors have reasoned that this finding should be the basis for a separate category of SM. However, a well-differentiated MC morphology can occur in many WHO variants of SM, including BMM, ISM, and MCL. Therefore, it may be appropriate that the well-differentiated morphology be applied broadly as an adjunct to the SM variant defined by the WHO. Likewise, a patient with ISM with a ‘WD-morphology’ of MCs would be called ISM-WD (ISMWD) or ISM-WDSM (ISMWDSM). The justification to add WD to denote a sub-category rather than a separate category is that the clinical course and prognosis are determined by the WHO designated variant rather than the WD morphology of MCs. Nevertheless, in patients with a WD-sub-category of mastocytosis, unique laboratory and clinical features are detected, supporting the notion that the WD morphology should be reported. First, in most WD patients, no KIT D816V mutation is found. Rather other mutations in KIT, such as K509I or F522C, are detected (52,56–58). As a result, some of these patients may respond to imatinib (60), which is not the case in patients who have KIT D816V+ SM. Second, the phenotype of MCs in the WDSM group is different from that found in patients with KIT D816V. Notably, in most WD-patients, MCs lack CD2 and CD25 (52,56–59). However, MCs in WDSM often express CD30 which may be of diagnostic significance (59).
5. Potential Value of CD30 as Diagnostic Criterion of SM
Recent studies have shown that CD30, also known as Ki-1 antigen, is aberrantly expressed in the cytoplasm and on the surface of neoplastic MCs in SM (59,61,62). However, CD30 is not expressed in MCs in all patients. In initial investigations, CD30 was found to be primarily expressed in MCs in advanced SM (62). However, although a correlation does exist, CD30 is not an absolute marker of advanced SM (ASM, MCL, SM-AHN), but is also detectable in MCs in many patients with ISM (59,63). CD30 may thus not be an optimal grading-marker in SM, but may qualify as a new minor criterion of SM, similar to CD25. It is noteworthy in this regard that CD30 is usually detectable on MCs in patients with SMWDSM (SMWD) (59). This is of importance as other minor SM criteria may be absent in WD-patients. Therefore, our consensus group recommends the inclusion of CD30 as a new minor SM criterion in the future. By contrast, the value of CD2 as a minor SM criterion is limited, which may support replacement of CD2 by CD30 as criterion. In fact, CD30 is a more sensitive parameter both in flow cytometry and immunohistochemistry.
6. Refinements in B-Findings and C-Findings
Whereas B-Findings are generally indicative of a huge MC burden and involvement of multiple lineages in SM, the presence of C-Findings is indicative of organ damage caused by the ‘invasive’ MC infiltrate (20–22,64). However, it is often difficult to define the relationship between SM and organ damage or SM and organomegaly, especially in cases with SM-AHN and patients with (other) co-morbidities. In these patients, tissue biopsies may be necessary to document involvement by SM and thus B- or C-Findings (65). Imaging techniques (e.g. volumetric techniques) may be helpful to quantify organomegaly (45). It has also been described that multi-color flow cytometry and cell sorting improves evaluation of lineage involvement in SM (25,46,66) and that multi-lineage involvement in ISM/SSM is of prognostic significance (35,46,66). Moreover, it has been described that a certain KIT D816V allele burden in the PB (5–10%) reflects multi-lineage involvement (30,46). Therefore, we are of the opinion that i) molecular studies revealing KIT D816V in various sorting leukocyte subsets and ii) a KIT D816V allele burden of >5% in unfractionated peripheral blood leukocytes are robust indicators of multi-lineage involvement in SM and should thus be nominated as potential new B Findings. Whether these parameters will finally be accepted as novel B Findings, and thereby can be used to predict or define the smoldering state in future classifications or mastocytosis, remains at present unknown.
7. Impact of Somatic Mutations in Other Genes (apart from KIT)
A number of recent studies have shown that in addition to activating mutations in KIT, additional mutations in other genes may occur in SM (67–71). Most of these patients have SM-AHN, ASM, or MCL. In patients with SM-AHN, such additional lesions are often detectable in AHN cells. Mutations are commonly found in TET2, SRSF2, ASXL1, CBL, RUNX1, and RAS (Supplemental Table S4) (67–72). Less common mutations include JAK2 V617F and RUNX1-RUNX1T1. All these mutations may be co-expressed with KIT D816V in the same cells or may be expressed in other myeloid cells but not MCs, especially in SM-AHN. Based on colony-assay studies, acquisition of KIT D816V may be a late event (71). Overall, the type and number of lesions (mutations) detectable in patients with multi-mutated SM correlates with the clinical course and prognosis (72). Currently, the question remains whether additional molecular lesions detected in SM can qualify as B-Finding (2 or more B-Findings are required to diagnose SSM) or a criterion of an AHN. One reasonable approach may be to implement these mutations as a novel B-Finding provided that they do not lead to the diagnosis of an AHN. In other words, in cases with additional criteria for an AHN, the same mutations should qualify as criteria of an AHN.
8. Delineation of MCL into Sub-Variants
MCL was initially divided into the classical (leukemia) variant defined by at least 10% MCs (of all leukocytes) in the PB, and an aleukemic variant (<10% MCs in PB) (20–23). However, MCL is a heterogeneous disease, both in terms of clinical presentation and survival (47). In some patients, MCL develops rapidly without a recognized pre-phase and with massive organ damage, whereas in other (rare) patients, there is a more chronic disease process without rapid organ damage. Therefore, MCL is now divided into an acute variant (with organ damage) and a chronic variant (without organ damage = without C-Findings); and also into primary MCL and secondary MCL (47). Secondary MCL variants have been described after a pre-phase of ASM or MCS. In general, all patients with MCL have a poor prognosis and should be treated with intensive therapy. However, in chronic MCL, treatment can be delayed (at least some weeks) whereas this is not advisable in acute MCL. An important differential in the diagnosis of MCL is myelomastocytic leukemia (MML) (47,73).
Therapeutic Options for Patients with CM and ISM
A review of all treatment options in CM and ISM is beyond the scope of this article. With regard to specific therapeutic algorithms, we refer to the available literature (1,23,31,74,75). In patients with mediator-related symptoms, HR blockers are recommended. In SM cases with severe symptoms, additional pharmacologic agents such as corticosteroids, cromolyn sodium, ketotifen, or leukotriene antagonists, may be applied. Some SM patients with severe symptoms suffer from bee or wasp venom allergy. In these patients, specific immunotherapy should be administered life-long to ensure protection. If immunotherapy is not effective, (additional) IgE-depleting treatment with omalizumab or similar experimental therapies should be considered. Another clinical challenge in SM is osteoporosis. In all patients with SM in whom the T score arrives at −2, bisphosphonate therapy should be initiated (in the absence of contraindications). In drug-resistant cases, RANKL-inhibitor therapy may be considered.
First Line Therapy in Patients with Advanced SM
First line treatment of advanced SM is a challenging problem. Before planning treatment, the following considerations should be taken into account: First, is the patient young and fit and in a transplantable condition? And, are there any relevant comorbidities that may interfere with transplant tolerability? Second, is the disease progressing rapidly or slowly? Third, what molecular targets are expressed by neoplastic cells? Finally, what organ systems are involved? In unusual cases (rare KIT mutant-forms or wild type KIT) the disease may respond to imatinib (50,51,56,57,76). In a subgroup of ASM patients with slow progression, including those who present with isolated liver involvement (with recurrent ascites), low dose prednisolone and interferon alpha (IFN-A) may be efficacious (77–81). Cladribine (2CdA) is often recommended as first-line therapy in patients with advanced SM with multi-organ involvement and slow progression (82–85). A forthcoming new standard of therapy in advanced SM is midostaurin (PKC412) (86–88). For ASM/MCL patients with rapid progression and those who are resistant against 2CdA or midostaurin, poly-chemotherapy (protocols otherwise used for high-risk AML) is usually recommended (23,64,65). In patients who are young and fit and have a suitable donor, stem cell transplantation (SCT) should be considered after successful debulking. The outcome after allogeneic SCT is better in patients who have ASM or SM-AHN compared to MCL, and for those prepared with ablative conditioning compared to less-intensive (non-myeloablative) conditioning (89). In patients with ASM-AHN, remission of the AHN is often achieved, whereas the ASM component of the disease cannot be eradicated (89). The overall response rate of ASM after SCT is similar when comparing ASM with ASM-AHN recipients. However, overall survival is better in patients with either ASM or ASM-AHN compared to patients with MCL (89). In those who relapse or are resistant, experimental drugs or alternative chemotherapies are recommended. For patients with SM-AHN, separate treatment plans for the SM component and AHN component should be established; in such cases the AHN should be treated as if no SM was diagnosed, with recognition that any type of AHN counts as secondary: e.g. in SM-AML, AML is regarded secondary and thus poor-risk AML.
New Treatment Options for Patients with Advanced SM
During the past 15 years, a number of novel treatment concepts for MC-proliferative disorders have been established. Since mutant forms of KIT, especially KIT D816V, are critically involved in the pathogenesis of SM, attempts have been made to develop drugs that are directed against this target receptor. The most well-known example is midostaurin, a drug that inhibits the growth of neoplastic MCs exhibiting various mutant forms of KIT, including KIT D816V (90,91). In addition, in contrasting to other KIT-targeting drugs, midostaurin also inhibits IgE-dependent release of histamine (92,93). Finally, midostaurin has been reported to be efficacious in patients with advanced SM, including ASM and MCL (86–88). In particular, data from the global trial of midostaurin in advanced SM indicate that the drug exhibits high response rates and durable activity (86). These data are the basis of the current submission to the health authorities; and if the drug is approved for advanced SM, midostaurin can be regarded as standard front-line therapy of patients with advanced SM. The drug may also be useful for patients who need debulking prior to SCT or those who fail treatment with 2CdA or IFN-A. However, midostaurin does not induce complete hematologic remissions in patients with advanced SM. Therefore, future studies should consider evaluating the benefit of combining midostaurin with other drugs. Indeed, midostaurin and 2CdA exert strong synergistic anti-neoplastic in vitro effects on MCs carrying KIT D816V (90). It is also noteworthy that KIT D816V per se is not considered to act as a strong oncoprotein but rather as a differentiation-inducer in neoplastic cells (94), an assumption that is supported by the observation that KIT D816V is expressed in patients with ISM where life-expectancy is normal (19–23,34). Therefore, the current view is that additional, KIT-independent, pathways and pro-oncogenic hits and lesions are responsible for disease progression and resistance (67–72) which is supported by the observation that relapsing disease in KIT D816V+ SM during midostaurin may present as KIT D816V-negative leukemia (95). Based on these observations, it seems reasonable to suggest that additional pathways and effector molecules need to be blocked to achieve disease-eradication. Such target pathways and molecules include, among others, RAS, PI3-kinase, mTOR, STAT5, and members of the BCL-2 family. Some of these molecules are expressed (and activated) in neoplastic MCs in both a KIT-dependent and KIT-independent manner. It has also been shown that suppression of these targets is associated with growth inhibition and apoptosis of neoplastic MCs (96–98). However, these effects may be largely restricted to proliferating cells, whereas non-proliferating neoplastic stem cells are often resistant. Such MCL-initiating stem cells have recently been identified in advanced SM (98). One effective approach to kill such quiescent (stem) cells may be to apply antibody-based toxin conjugates or other targeted antibodies (63,99,100). The CD30 antibody-based drug brentuximab-vedotin has recently been considered for the treatment of patients with advanced SM (63) and a clinical trial in patients with CD30+ ASM and MCL has recently been initiated in the USA.
Concluding Remarks and Future Perspectives
Mastocytosis is a paradigmatic example of a rare disease with complex pathology, distinct subtypes, and highly variable clinical courses, ranging from asymptomatic with normal life expectancy to fatal within months or weeks. The management of these disorders requires a deep understanding of their molecular and cellular pathogenesis and a precise diagnostic evaluation. Unfortunately, mastocytosis remains incurable, and in those with advanced SM, the prognosis is still dismal. In other cases, the prognosis is good regarding survival, but mediator- and symptom burden can be considerable and the quality of life may be poor. However, in the past few years, novel treatment approaches have been developed. Most promising drugs are those that target mutant forms of KIT and other critical target molecules. For patients with advanced SM who are young and fit and have a suitable donor, allogeneic SCT is a realistic option and can be recommended in drug-refractory disease.
Supplementary Material
Acknowledgments
Supported by: Austrian Science Funds, SFB grant F47-04 (PV) and by the NIAID Division of Intramural Research (DM)
Funding
This study was supported by the Austrian Science Funds, SFB grant F47-04 (to P.V.), by the German Research Council, grant HA 2393/6-1 (to K.H.) and by the NIAID Division of Intramural Research (to D.M.).
We like to thank Barbara Peter, Ghaith Wedeh, and Emir Hadzijusufovic for their helpful support and discussions.
Footnotes
This manuscript is dedicated to the pioneering work and scientific achievements of Paul Ehrlich (1854–1915) on the occasion of the 100th anniversary of his death day
Conflicts of interest: Peter Valent: 1. Research grant: Deciphera 2. Advisory Board and Honoraria: Novartis, Deciphera; Cem Akin: Honoraria: Novartis, Deciphera, Patara; Karin Hartmann: Advisory Board: Novartis and Deciphera; Adreas Reiter: 1. Honoraria: Novartis and 2. Advisory Board: Novartis, Deciphera; Olivier Hermine: 1. Co-Founder and Stock Holder: AB Science and 2. Research grant: AB Science, Novartis, Celgene, Lipomed; Wolfgang R. Sperr: 1. Research Grant: Phadia, 2. Advisory Board: Novartis, Deciphera, and 3. Honoraria: Phadia, Novartis; Tracy I. George: 1. Research grant: Allakos and 2. Honoraria: Novartis, Blueprint; Hanneke C. Kluin-Nelemans: Advisory Board: Novartis and Deciphera; Jason Gotlib: 1. Research grant (funds for administration of clinical trials): Novartis, Blueprint, Pharmacyclics, and Seattle Genetics, 2. Advisory Board and Honoraria: Novartis, Deciphera, and 3. Reimbursement of travel expenses: Novartis; Lawrence B. Schwartz: 1. Receives royalties for the tryptase assay (ThermoFisher) and 2. Received a grant from Novartis as a site PI for their midostaurin in advanced mastocytosis clinical trial; Hans-Peter Horny: Advisory Board and Honoraria: Novartis, Deciphera; Michel Arock: 1. Research grants: Blueprint Medicine, Deciphera and 2. Honoraria: Deciphera. The authors declare that they have no other conflicts of interest to disclose in this project.
Disclosure
The authors have defined their conflicts of interest by completing and sending the electronic ‘conflict of interest form’ to Cancer Research.
References
- 1.Valent P, Akin C, Sperr WR, Horny HP, Arock M, Lechner K, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122:695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- 2.Horny HP, Sotlar K, Valent P. Mastocytosis: state of the art. Pathobiology. 2007;74:121–32. doi: 10.1159/000101711. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 5.Caplan RM. The natural course of urticaria pigmentosa: analysis and follow-up of 112 cases. Arch Dermatol. 1963;87:146–57. doi: 10.1001/archderm.1963.01590140008002. [DOI] [PubMed] [Google Scholar]
- 6.Chagrin L, Sachs P. Urticaria pigmentosa appearing as a solitary nodular lesion. Arch Dermatol Syphilol. 1954;69:345–55. doi: 10.1001/archderm.1954.01540150091008. [DOI] [PubMed] [Google Scholar]
- 7.Johnson WC, Helwig EB. Solitary mastocytosis (urticaria pigmentosa) Arch Dermatol. 1961;84:806–15. doi: 10.1001/archderm.1961.01580170100014. [DOI] [PubMed] [Google Scholar]
- 8.Lennert K, Parwaresch MR. Mast cells and mast cell neoplasia: a review. Histopathology. 1979;3:349–65. doi: 10.1111/j.1365-2559.1979.tb03017.x. [DOI] [PubMed] [Google Scholar]
- 9.Parwaresch MR, Horny HP, Lennert K. Tissue mast cells in health and disease. Pathol Res Pract. 1985;179:439–61. doi: 10.1016/s0344-0338(85)80184-9. [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol. 1991;96:2S–4S. [PubMed] [Google Scholar]
- 11.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–26. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz LB, Irani AM. Serum tryptase and the laboratory diagnosis of systemic mastocytosis. Hematol Oncol Clin North Am. 2000;14:641–57. doi: 10.1016/s0889-8588(05)70300-2. [DOI] [PubMed] [Google Scholar]
- 13.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci (USA) 1995;92:10560–64. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–14. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 15.Sotlar K, Marafioti T, Griesser H, Theil J, Aepinus C, Jaussi R, et al. Detection of c-kit mutation Asp 816 to Val in microdissected bone marrow infiltrates in a case of systemic mastocytosis associated with chronic myelomonocytic leukaemia. Mol Pathol. 2000;53:188–93. doi: 10.1136/mp.53.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P, et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113:357–64. doi: 10.1046/j.1365-2141.2001.02783.x. [DOI] [PubMed] [Google Scholar]
- 17.Horny HP, Sillaber C, Menke D, Kaiserling E, Wehrmann M, Stehberger B, et al. Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol. 1998;22:1132–40. doi: 10.1097/00000478-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Escribano L, Orfao A, Díaz-Agustin B, Villarrubia J, Cerveró C, López A, et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998;91:2731–36. [PubMed] [Google Scholar]
- 19.Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001;25:529–36. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 20.Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 21.Valent P, Horny H-P, Li CY, Longley JB, Metcalfe DD, Parwaresch RM, et al. Mastocytosis (mast cell disease) In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization (WHO) Classification of Tumours. Pathology & Genetics. Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2001. pp. 291–302. [Google Scholar]
- 22.Horny HP, Akin C, Metcalfe DD, Escribano L, Bennett JM, Valent P, et al. Mastocytosis (mast cell disease) In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization (WHO) Classification of Tumours. Pathology & Genetics. Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 54–63. [Google Scholar]
- 23.Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35–45. doi: 10.1016/j.jaci.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Escribano L, Diaz-Agustin B, López A, Núñez López R, García-Montero A, Almeida J, et al. Immunophenotypic analysis of mast cells in mastocytosis: When and how to do it. Proposals of the Spanish Network on Mastocytosis (REMA) Cytometry B Clin Cytom. 2004;58:1–8. doi: 10.1002/cyto.b.10072. [DOI] [PubMed] [Google Scholar]
- 26.Valent P, Escribano L, Broesby-Olsen S, Hartmann K, Grattan C, Brockow K, et al. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69:1267–74. doi: 10.1111/all.12436. [DOI] [PubMed] [Google Scholar]
- 27.Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29:1223–32. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen T, Vestergaard H, Moller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn. 2011;13:180–8. doi: 10.1016/j.jmoldx.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensen T, Broesby-Olsen S, Vestergaard H, Bindslev-Jensen C, Moller MB Mastocytosis Centre Odense University H. Circulating KIT D816V mutation-positive non-mast cells in peripheral blood are characteristic of indolent systemic mastocytosis. Eur J Haematol. 2012;89:42–6. doi: 10.1111/j.1600-0609.2012.01789.x. [DOI] [PubMed] [Google Scholar]
- 30.Jara-Acevedo M, Teodosio C, Sanchez-Muñoz L, Álvarez-Twose I, Mayado A, Caldas C, et al. Detection of the KIT D816V mutation in peripheral blood of systemic mastocytosis: diagnostic implications. Mod Pathol. 2015;28:1138–49. doi: 10.1038/modpathol.2015.72. [DOI] [PubMed] [Google Scholar]
- 31.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–72. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 32.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 33.Horny HP, Akin C, Arber D, Peterson LA, Tefferi A, Metcalfe DD, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization (WHO) Classification of Tumours. Pathology & Genetics. Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2016. in press. [Google Scholar]
- 34.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 35.Escribano L, Alvarez-Twose I, Sánchez-Muñoz L, Garcia-Montero A, Núñez R, Almeida J, et al. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009;124:514–21. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Pardanani A, Lim KH, Lasho TL, Finke C, McClure RF, Li CY, et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood. 2009;114:3769–72. doi: 10.1182/blood-2009-05-220145. [DOI] [PubMed] [Google Scholar]
- 37.Pardanani A, Lim KH, Lasho TL, Finke C, McClure RF, Li CY, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115(1):150–51. doi: 10.1182/blood-2009-10-249979. [DOI] [PubMed] [Google Scholar]
- 38.Ayadi L, Abid N, Makni S, Bahri I, Frikha I, Sellami-Boudawara T. An unusual tumour of the lung. Pathologica. 2015;107:14–18. [PubMed] [Google Scholar]
- 39.Horny HP, Parwaresch MR, Kaiserling E, Müller K, Olbermann M, Mainzer K, et al. Mast cell sarcoma of the larynx. J Clin Pathol. 1986;39:596–602. doi: 10.1136/jcp.39.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guenther PP, Huebner A, Sobottka SB, Neumeister V, Weissbach G, Todt H, et al. Temporary response of localized intracranial mast cell sarcoma to combination chemotherapy. J Pediatr Hematol Oncol. 2001;23:134–38. doi: 10.1097/00043426-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Schwaab J, Horny HP, Jonescheit J, Metzgeroth G, Schafhausen P, Gaiser T, et al. Mast cell sarcoma mimicking metastatic colon carcinoma. Ann Hematol. 2014;93:1067–69. doi: 10.1007/s00277-013-1931-x. [DOI] [PubMed] [Google Scholar]
- 42.Matito A, Morgado JM, Álvarez-Twose I, Sánchez-Muñoz L, Pedreira CE, Jara-Acevedo M, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:e76116. doi: 10.1371/journal.pone.0076116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erben P, Schwaab J, Metzgeroth G, Horny HP, Jawhar M, Sotlar K, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93:81–88. doi: 10.1007/s00277-013-1964-1. [DOI] [PubMed] [Google Scholar]
- 44.Hoermann G, Gleixner KV, Dinu GE, Kundi M, Greiner G, Wimazal F, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69:810–13. doi: 10.1111/all.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jawhar M, Schwaab J, Hausmann D, Clemens J, Naumann N, Henzler T, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. doi: 10.1038/leu.2016.190. in press. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Montero AC, Jara-Acevedo M, Alvarez-Twose I, Teodosio C, Sanchez-Muñoz L, Muñiz C, et al. KIT D816V-mutated bone marrow mesenchymal stem cells in indolent systemic mastocytosis are associated with disease progression. Blood. 2016;127:761–68. doi: 10.1182/blood-2015-07-655100. [DOI] [PubMed] [Google Scholar]
- 47.Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25:1691–700. doi: 10.1093/annonc/mdu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiechers T, Rabenhorst A, Schick T, Preussner LM, Förster A, Valent P, et al. Large maculopapular cutaneous lesions are associated with favorable outcome in childhood-onset mastocytosis. J Allergy Clin Immunol. 2015;136:1581–90. doi: 10.1016/j.jaci.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 49.Bodemer C, Hermine O, Palmérini F, Yang Y, Grandpeix-Guyodo C, Leventhal PS, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–15. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 50.Zhang LY, Smith ML, Schultheis B, Fitzgibbon J, Lister TA, Melo JV, et al. A novel K509I mutation of KIT identified in familial mastocytosis-in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30:373–78. doi: 10.1016/j.leukres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 51.de Melo Campos P, Machado-Neto JA, Scopim-Ribeiro R, Visconte V, Tabarroki A, Duarte AS, et al. Familial systemic mastocytosis with germline KIT K509I mutation is sensitive to treatment with imatinib, dasatinib and PKC412. Leuk Res. 2014;38:1245–51. doi: 10.1016/j.leukres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Álvarez-Twose I, Jara-Acevedo M, Morgado JM, García-Montero A, Sánchez-Muñoz L, Teodósio C, et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J Allergy Clin Immunol. 2016;137:168–78. doi: 10.1016/j.jaci.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Alvarez-Twose I, Zanotti R, González-de-Olano D, Bonadonna P, Vega A, Matito A, et al. Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J Allergy Clin Immunol. 2014;133:520–28. doi: 10.1016/j.jaci.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Zanotti R, Bonadonna P, Bonifacio M, Artuso A, Schena D, Rossini M, et al. Isolated bone marrow mastocytosis: an underestimated subvariant of indolent systemic mastocytosis. Haematologica. 2011;96:482–84. doi: 10.3324/haematol.2010.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manara M, Varenna M, Cantoni S, Parafioriti A, Gallazzi MB, Sinigaglia L. Osteoporosis with vertebral fractures in young males, due to bone marrow mastocytosis: a report of two cases. Clin Exp Rheumatol. 2010;28:97–100. [PubMed] [Google Scholar]
- 56.Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–25. doi: 10.1182/blood-2003-11-3816. [DOI] [PubMed] [Google Scholar]
- 57.Alvarez-Twose I, González P, Morgado JM, Jara-Acevedo M, Sánchez-Muñoz L, Matito A, et al. Complete response after imatinib mesylate therapy in a patient with well-differentiated systemic mastocytosis. J Clin Oncol. 2012;30:126–29. doi: 10.1200/JCO.2011.38.9973. [DOI] [PubMed] [Google Scholar]
- 58.Chan EC, Bai Y, Kirshenbaum AS, Fischer ER, Simakova O, Bandara G, et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J Allergy Clin Immunol. 2014;134:178–87. doi: 10.1016/j.jaci.2013.12.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgado JM, Perbellini O, Johnson RC, Teodósio C, Matito A, Álvarez-Twose I, et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013;63:780–87. doi: 10.1111/his.12221. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez-Twose I, Matito A, Morgado JM, Sánchez-Muñoz L, Jara-Acevedo M, García-Montero A, et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. doi: 10.18632/oncotarget.10711. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585–95. doi: 10.1038/modpathol.2010.224. [DOI] [PubMed] [Google Scholar]
- 62.Valent P, Sotlar K, Horny HP. Aberrant expression of CD30 in aggressive systemic mastocytosis and mast cell leukemia: a differential diagnosis to consider in aggressive hematopoietic CD30-positive neoplasms. Leuk Lymphoma. 2011;52:740–44. doi: 10.3109/10428194.2010.550072. [DOI] [PubMed] [Google Scholar]
- 63.Blatt K, Cerny-Reiterer S, Schwaab J, Sotlar K, Eisenwort G, Stefanzl G, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood. 2015;126:2832–41. doi: 10.1182/blood-2015-03-637728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27:635–41. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 65.Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010;116:5812–17. doi: 10.1182/blood-2010-08-292144. [DOI] [PubMed] [Google Scholar]
- 66.Teodosio C, García-Montero AC, Jara-Acevedo M, Alvarez-Twose I, Sánchez-Muñoz L, Almeida J, et al. An immature immunophenotype of bone marrow mast cells predicts for multilineage D816V KIT mutation in systemic mastocytosis. Leukemia. 2012;26:951–58. doi: 10.1038/leu.2011.293. [DOI] [PubMed] [Google Scholar]
- 67.Tefferi A, Levine RL, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. Frequent TET2 mutations in systemic mastocytosis: clinical, KITD816V and FIP1L1-PDGFRA correlates. Leukemia. 2009;23:900–904. doi: 10.1038/leu.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Traina F, Visconte V, Jankowska AM, Makishima H, O’Keefe CL, Elson P, et al. Single nucleotide polymorphism array lesions, TET2, DNMT3A, ASXL1 and CBL mutations are present in systemic mastocytosis. PLoS One. 2012;7:e43090. doi: 10.1371/journal.pone.0043090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson TM, Maric I, Simakova O, Bai Y, Chan EC, Olivares N, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–63. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–66. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 71.Jawhar M, Schwaab J, Schnittger S, Sotlar K, Horny HP, Metzgeroth G, et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia. 2015;29:1115–22. doi: 10.1038/leu.2015.4. [DOI] [PubMed] [Google Scholar]
- 72.Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30:136–43. doi: 10.1038/leu.2015.284. [DOI] [PubMed] [Google Scholar]
- 73.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–25. doi: 10.1159/000328760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81:677–90. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 75.Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage) Blood. 2013;121:3085–94. doi: 10.1182/blood-2013-01-453183. [DOI] [PubMed] [Google Scholar]
- 76.Valent P, Cerny-Reiterer S, Hoermann G, Sperr WR, Müllauer L, Mannhalter C, et al. Long-lasting complete response to imatinib in a patient with systemic mastocytosis exhibiting wild type KIT. Am J Blood Res. 2014;4:93–100. [PMC free article] [PubMed] [Google Scholar]
- 77.Kluin-Nelemans HC, Jansen JH, Breukelman H, Wolthers BG, Kluin PM, et al. Response to interferon alfa-2b in a patient with systemic mastocytosis. N Engl J Med. 1992;326:619–23. doi: 10.1056/NEJM199202273260907. [DOI] [PubMed] [Google Scholar]
- 78.Worobec AS, Kirshenbaum AS, Schwartz LB, Metcalfe DD. Treatment of three patients with systemic mastocytosis with interferon alpha-2b. Leuk Lymphoma. 1996;22:501–8. doi: 10.3109/10428199609054789. [DOI] [PubMed] [Google Scholar]
- 79.Casassus P, Caillat-Vigneron N, Martin A, Simon J, Gallais V, Beaudry P, et al. Treatment of adult systemic mastocytosis with interferon-alpha: results of a multicentre phase II trial on 20 patients. Br J Haematol. 2002;119:1090–97. doi: 10.1046/j.1365-2141.2002.03944.x. [DOI] [PubMed] [Google Scholar]
- 80.Hauswirth AW, Simonitsch-Klupp I, Uffmann M, Koller E, Sperr WR, Lechner K, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res. 2004;28:249–57. doi: 10.1016/s0145-2126(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 81.Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A. Cytoreductive therapy in 108 adults with systemic mastocytosis: Outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2-chlorodeoxyadenosine. Am J Hematol. 2009;84:790–94. doi: 10.1002/ajh.21561. [DOI] [PubMed] [Google Scholar]
- 82.Tefferi A, Li CY, Butterfield JH, Hoagland HC. Treatment of systemic mast-cell disease with cladribine. N Engl J Med. 2001;344:307–09. doi: 10.1056/NEJM200101253440415. [DOI] [PubMed] [Google Scholar]
- 83.Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, Van ‘t Wout JW, Verhoef G, Gerrits WB, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102:4270–76. doi: 10.1182/blood-2003-05-1699. [DOI] [PubMed] [Google Scholar]
- 84.Böhm A, Sonneck K, Gleixner KV, Schuch K, Pickl WF, Blatt K, et al. In vitro and in vivo growth-inhibitory effects of cladribine on neoplastic mast cells exhibiting the imatinib-resistant KIT mutation D816V. Exp Hematol. 2010;38:744–55. doi: 10.1016/j.exphem.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 85.Barete S, Lortholary O, Damaj G, Hirsch I, Chandesris MO, Elie C, et al. Long-term efficacy and safety of cladribine (2-CdA) in adult patients with mastocytosis. Blood. 2015;126:1009–16. doi: 10.1182/blood-2014-12-614743. [DOI] [PubMed] [Google Scholar]
- 86.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N Engl J Med. 2016;374:2530–41. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 87.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Durable responses and improved quality of life with midostaurin (PKC412) in advanced systemic mastocytosis (SM): updated stage 1 results of the global D2201 trial. Blood. 2013;122:106. [Google Scholar]
- 88.Chandesris MO, Damaj G, Canioni D, Brouzes C, Lhermitte L, Hanssens K, et al. Midostaurin in Advanced Systemic Mastocytosis. N Engl J Med. 2016;374:2605–07. doi: 10.1056/NEJMc1515403. [DOI] [PubMed] [Google Scholar]
- 89.Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264–74. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Böhm A, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–59. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 91.Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res. 2011;35:1143–52. doi: 10.1016/j.leukres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy. 2009;39:1711–20. doi: 10.1111/j.1365-2222.2009.03353.x. [DOI] [PubMed] [Google Scholar]
- 93.Peter B, Winter GE, Blatt K, Bennett KL, Stefanzl G, Rix U, et al. Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth. Leukemia. 2016;30:464–72. doi: 10.1038/leu.2015.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mayerhofer M, Gleixner KV, Hoelbl A, Florian S, Hoermann G, Aichberger KJ, et al. Unique effects of KIT D816V in BaF3 cells: induction of cluster formation, histamine synthesis, and early mast cell differentiation antigens. J Immunol. 2008;180:5466–76. doi: 10.4049/jimmunol.180.8.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gotlib J, Berubé C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865–70. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gleixner KV, Mayerhofer M, Cerny-Reiterer S, Hörmann G, Rix U, Bennett KL, et al. KIT-D816V-independent oncogenic signaling in neoplastic cells in systemic mastocytosis: role of Lyn and Btk activation and disruption by dasatinib and bosutinib. Blood. 2011;118:1885–98. doi: 10.1182/blood-2010-06-289959. [DOI] [PubMed] [Google Scholar]
- 97.Bibi S, Langenfeld F, Jeanningros S, Brenet F, Soucie E, Hermine O, et al. Molecular defects in mastocytosis: KIT and beyond KIT. Immunol Allergy Clin North Am. 2014;34:239–62. doi: 10.1016/j.iac.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 98.Peter B, Cerny-Reiterer S, Hadzijusufovic E, Schuch K, Stefanzl G, Eisenwort G, et al. The pan-Bcl-2 blocker obatoclax promotes the expression of Puma, Noxa, and Bim mRNA and induces apoptosis in neoplastic mast cells. J Leukoc Biol. 2014;95:95–104. doi: 10.1189/jlb.1112609. [DOI] [PubMed] [Google Scholar]
- 99.Eisenwort G, Peter B, Blatt K, Cerny-Reiterer S, Hoermann G, Sadovnik I, et al. Identification of a neoplastic stem cell in human mast cell leukemia. Blood. 2014;124:817. [Google Scholar]
- 100.Alvarez-Twose I, Martínez-Barranco P, Gotlib J, García-Montero A, Morgado JM, Jara-Acevedo M, et al. Complete response to gemtuzumab ozogamicin in a patient with refractory mast cell leukemia. Leukemia. doi: 10.1038/leu.2016.30. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.