Abstract
Dexamethasone (dex) induces apoptosis in multiple myeloma (MM) cells and is a frontline treatment for this disease. However resistance to dex remains a major challenge and novel treatment approaches are needed. We hypothesized that dex utilizes translational pathways to promote apoptosis in MM and that specific targeting of these pathways could overcome dex-resistance. Global unbiased profiling of mRNA translational profiles in MM cells treated with or without dex revealed that dex significantly repressed eIF2 signaling, an important pathway for regulating ternary complex formation and protein synthesis. We demonstrate that dex induces the phosphorylation of eIF2α resulting in the translational upregulation of ATF4, a known eIF2 regulated mRNA. Pharmacologic induction of eIF2α phosphorylation via activation of the heme-regulated eIF2α kinase (HRI) induced apoptosis in MM cell lines and in primary MM cells from patients with dex-resistant disease. In addition, co-culture with marrow stroma failed to protect MM cells from apoptosis induced by targeting the eIF2 pathway. Combination therapy with rapamycin, an mTOR inhibitor, and BTdCPU, an activator of HRI, demonstrated additive effects on apoptosis in dex-resistant cells. Thus, specific activation of the eIF2α kinase HRI is a novel therapeutic target in MM that can augment current treatment strategies.
Keywords: dexamethasone, multiple myeloma, eIF2 signaling, apoptosis
1. Introduction
Glucocorticoids (GC) such as dex have been used to treat MM for over 50 years and remain a backbone of treatment for this disease. However, as a single agent, dex demonstrates response rates of only 41–43% in newly diagnosed patients 1,2 and 18% in relapsed patients. Less than 1% of new or relapsed patients experience a complete remission with dex alone 3. Dex-resistance occurs by both cell-intrinsic and cell-extrinsic mechanisms. Cell-intrinsic mechanisms include the expression of a variant glucocorticoid receptor (GR) with decreased GC responsiveness 4, as well as overexpression of heat shock protein 27, which inhibits mitochondrial release of the pro-apoptotic protein Smac 5. Cell-extrinsic mechanisms include interactions between tumor cells and stromal cells 6, cell adhesion mediated-resistance 7 and the secretion of soluble factors such as IL-6 8. While the use of higher doses of dex may partially overcome dex resistance, high dose delivery of dex is limited by excess toxicities including venous thrombosis and infections 9.
Since plasma cells are highly translationally active 10, and nearly half of MM patients harbor mutations affecting protein translation pathway genes 11 we sought to better understand how dex affects translational pathways in MM to promote apoptosis. As alterations in gene expression at the level of mRNA translation would be missed by standard transcriptional profiling approaches 12, we analyzed polysome-associated mRNA profiles in the presence or absence of dex to identify dex-dependent changes in translationally-regulated pathways. We then evaluate whether targeting of specific translational pathways promotes apoptosis in MM cells.
2. Materials and methods
2.1 Patient Samples
Bone marrow specimens were obtained following written informed consent, under Institutional Review Board approved protocols at the Fred Hutchinson Cancer Research Center and Siteman Cancer Center and in accordance with the Declaration of Helsinki.
2.2. Cell culture and reagents
Multiple myeloma cell lines were purchased from ATCC. Human umbilical vein endothelial cells (HUVEC) were purchased from Lonza. Primary CD138- stromal cells were isolated from bone marrow of MM patients. Dex was purchased from Sigma. BTdCPU and NCPdCPU were purchased from EMD Millipore. I-18 was kindly provided by Dr. Bertal Aktas at Harvard Medical School.
2.3 Polysome profiles
Cells were extracted in polysome lysis buffer (Supplemental Table S1). Equal OD (260) units of extract (typically in the range of 1 to 1.5 OD units) were loaded onto 5–50% sucrose gradients and centrifuged at 39,000 RPM in an SW41 rotor for 2 hours at 4C, or 45,000 RPM in a SW55 rotor for 1.5 hours at 4C. Gradients were fractionated with continuous UV monitoring at 254 nm.
2.4 Translational profiling
RNA fractions were collected across the gradient. For each sucrose pool, equal aliquots of ERCC spike-in RNAs (Life Technologies, Grand Island, NY) (10 μl of 1:100 dilution) or pre-mixed bacterial poly-adenylated control RNAs (Affymetrix, Santa Clara, CA) (10 μl of 1:50,000 dilution) were added. RNA was purified by phenol:chloroform extraction. For total RNA specimens, cell pellets were lysed directly in TRIzol (Invitrogen, Grand Island, NY).
2.5 Microarrays Analysis
RNA integrity was assessed using an Agilent 2200 TapeStation (Agilent Technologies, Inc., Santa Clara, CA) and was quantified using a Trinean DropSense96 spectrophotometer (Caliper Life Sciences, Hopkinton, MA). High quality RNA samples were converted to cDNA and biotin-labeled for microarray analysis using Ambion’s Illumina TotalPrep RNA Amplification Kit (Life Technologies, Grand Island, NY). Labeled cRNAs were processed on a Human HT-12 Expression BeadChip (Illumina, Inc., San Diego, CA) and imaged using an Illumina iScan system.
2.6 Analysis of mRNA Translation
Isolated RNA was assessed for quality, followed by loess normalization using the Bioconductor package affy 13. The probes corresponding to the spiked-in ERCC controls were used to define the normalization parameters, which were subsequently applied across all the probes on a given array. Probes were flagged and removed from further analysis by applying the same intensity and variance filter as used for mRNA expression analysis. Changes in mRNA translation comparing 0 hours versus 4 hours of dex treatment were measured in the following manner: Pairwise log2(ratio) values were calculated for gradient fractions (A, B, and C) for a given time point (i.e., 0B/0A, 0C/0A, 4B/4A, 4C/4A). These values were then used to determine translational differences resulting from dex exposure {i.e., (4B–4A)-(0B–0A), (4C–4A)-(0C–0A)} using the Bioconductor package limma 14. Changes in mRNA translation were defined as |log2FC| ≥ 0.585 with a p-value ≤ 0.01. We further restricted this list of probes that showed translational differences to exclude differential mRNA expression as described in the text. Microarray data was deposited in the Gene Expression Omnibus, accession number GSE59080.
2.7 RT-qPCR
RNA was reverse-transcribed using SuperScript III with oligoDT priming (Invitrogen). Real-time qPCR was performed using iTaq Universal SYBR Green Supermix (BIO-RAD, Hercules, CA) on an ABI 7900HT Real Time PCR System. Primer sequences are listed in Supplemental Table S2.
2.8 Antibodies and Immunoblotting
Cells were lysed in RIPA buffer containing 1X PBS, 0.5% DOC, 1% NP40, 0.2% SDS, 1X Complete mini protease inhibitors, pepstatin and pefablock. For phospho-specific blots, phosphatase inhibitors were added, including 5 mM sodium fluoride, 1 mM sodium vanadate and 10 mM beta-glycerophosphate. Antibody reagents and conditions are listed in Supplemental Table S3.
3. Results
3.1 Dex alters polysome profiles in multiple myeloma cells
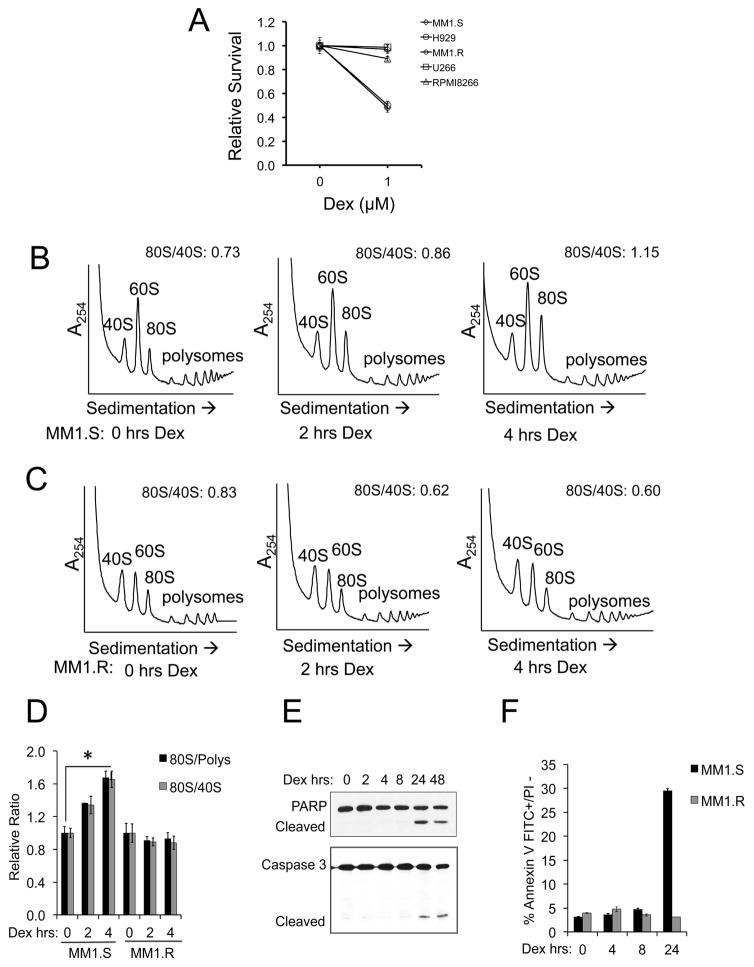
The MM cell lines MM1.S and H929 are sensitive to dex-induced apoptosis, while MM1.R, U266 and RPMI 8266 MM cell lines demonstrate dex-resistance (Figure 1A). We focused our attention on the MM1.S and MM1.R cell lines, since these cell lines are derived from the same patient and are specifically differentiated by their sensitivity or resistance to glucocorticoids 15. MM1.S and MM1.R cells were treated with 1 μM dex for 0–4 hours, and cell lysates were loaded onto 5–50% sucrose density gradients. Resultant polysome profiles were analyzed at 254 nm absorbance. Dex treatment resulted in increased 80S monomer abundance in MM1.S (Figure 1B), but not MM1.R cells (Figure 1C) in a time-dependent manner (Figure 1D). Similar polysome profile changes were found in dex-sensitive H929 cells but not in dex-resistant U266 cells (Supplemental Figure S1). To determine whether changes in polysome profiles preceded dex-induced apoptosis, cleavage of PARP and Caspase 3 were assessed following dex treatment. PARP and Caspase 3 cleavage was not detected until 24 hours post-dex treatment in MM1.S cells (Figure 1E). Apoptosis was also assessed by flow cytometry, and again no appreciable increase in apoptosis was noted until 24 hours of dex treatment (Figure 1F). No induction of apoptosis was seen in MM1.R cells treated with dex. These results indicate that early dex-induced changes in polysome profiles preceded apoptosis in dex-sensitive MM cells.
Figure 1. Dexamethasone induces rapid polysome profile alterations.
(A) Dex-sensitive (MM1.S, H929) and dex-resistant (MM1.R, U266, RPMI 8266) cell lines were treated with 1 μM Dex for 48 hours and assessed for cytotoxicity using an MTS assay. (B) MM1.S or (C) MM1.R cells were treated for 0, 2 or 4 hours (hrs) with 1 μM Dex. Cell lysates were sedimented through 5–50% sucrose gradients to obtain polysome profiles. 40S, 60S, 80S and polysome peaks were identified and the area under the curve was quantitated for each. The ratio of 80S/40S is depicted for each individual profile and the average results of three independent experiments are depicted in a bar graph in (D). Error bars represent the standard deviation of the three replicates. *p<0.05 (E) MM1.S cells were treated with 1 μM Dex for 0–48 hrs and cell lysates were immunoblotted for PARP and Caspase 3, revealing full length and cleaved products. (F) MM1.S or MM1.R cells were treated with 1 μM Dex for 0–24 hrs and evaluated for apoptosis by flow cytometry using Annexin V-FITC and propidium iodide (PI).
3.2 Dex alters mRNA translation in MM cells independent of transcription
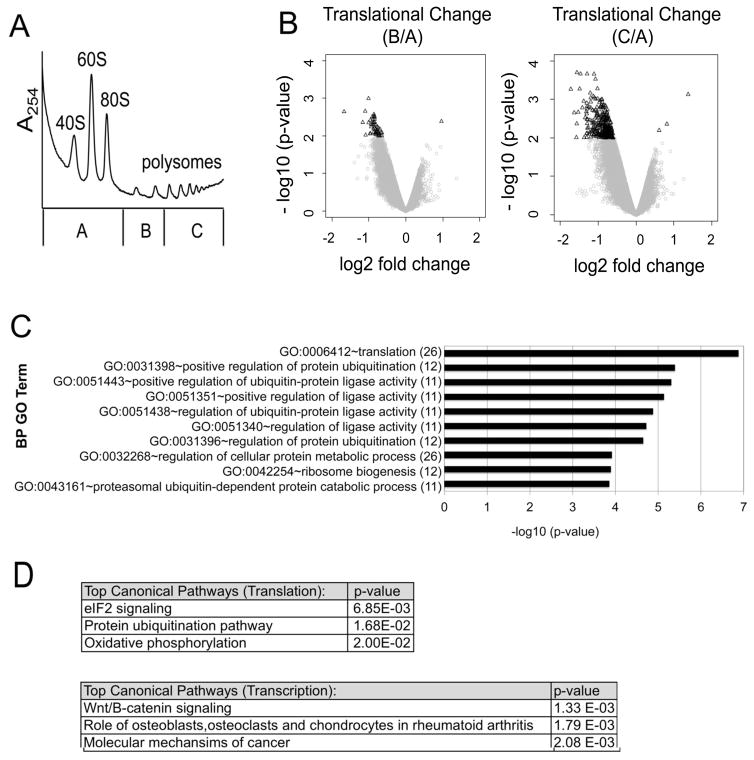
To investigate whether dex-dependent translational alterations contribute to dex sensitivity, we performed translational array profiling in MM1.S cells ± dex. Translational array profiling is an unbiased method to assess changes in polysome association of specific mRNAs. mRNA transcripts that are well translated are enriched in polysome fractions, while poorly translated or non-translated mRNAs are enriched in non-polysome fractions. MM1.S cells were treated with 1 μM dex for 0 or 4 hours and cell lysates were fractionated through sucrose gradients. The sucrose gradient fractions were then pooled into three distinct aliquots (pools A, B and C). Pool A included mRNA transcripts from the top of the gradient up to and including the 80S monomer, pool B included mRNA transcripts associated with 2 or 3 ribosomes, and pool C included mRNA transcripts associated with ≥ 4 ribosomes (Figure 2A). As an internal standard to control for potential variations in extraction efficiency, each fraction was spiked with an equal amount of a pre-mixed RNA control containing 92 pre-mixed polyadenylated RNAs. RNA was then purified from each pool and analyzed on Illumina Human Bead Chips (HT-12 v4). Total cellular RNA was extracted concurrently from a parallel aliquot of cells for each condition.
Figure 2. Dexamethasone alters the translation of specific mRNA subsets.
MM1.S cells were treated with 1 μM Dex for 0 or 4 hours. Cell lysates were fractionated by sucrose density ultracentrifugation. (A) For each polysome profile, three distinct gradient fractions (A,B,C) were collected. A representative polysome profile is shown. (B) Volcano plots were generated by comparing translational changes at 4 hours versus 0 hours of Dex treatment. A change in translation was defined as a shift in mRNA transcript distribution between pool B versus pool A (left) or pool C versus pool A (right). The log2 fold change in translation is plotted against the corresponding –log 10 (p-values). A filter of ≤ 0.01 p-value and |log2 fold change| ≥ 0.585 (1.5 fold) was used to define translationally altered genes for each condition (black triangles). To minimize confounding by concomitant transcriptional changes, mRNAs with a |log2 fold change| ≥ 0.585 in transcription were excluded from this analysis. (C) Translationally altered mRNAs from the profiling screen [pool C versus pool A] were subject to gene ontology (GO) analysis and the results are plotted against their corresponding −log 10 (p-values). The number of gene hits in each category is labeled in parenthesis. (D) Ingenuity Pathway Analysis (IPA) was performed to identify the top canonical pathways modulated by Dex at the level of translation versus transcription. The top hits are listed with their corresponding p-values.
Shifts in transcript abundance between the pooled fractions were used to calculate translation coefficients, similar to prior studies utilizing polysome fractionation 16. The translation coefficient was defined as the abundance of mRNA transcripts in polysome fractions (pool B or C) divided by the mRNA transcripts in non-polysome fractions (pool A). Translation coefficients were calculated and defined as the log2 fold changes (FC) in B/A or C/A, and are presented graphically in volcano plots (Figure 2B). Significant results were defined as those mRNAs with a |log2 FC| ≥ 0.585 (1.5 fold) in translation, with a p-value of ≤ 0.01 (Figure 2B, black triangles). To avoid confounding by concomitant transcriptional changes, those mRNAs that demonstrated changes in total transcript levels, as defined as |log2 FC| ≥ 0.585, with a false discovery rate of 5%, were filtered out of the translational analysis.
Gene ontology (GO) analysis revealed that specific GO groups were altered by dex at the level of translation, including networks involved with protein translation and protein homeostasis (Figure 2C). In addition, Ingenuity Pathway Analysis (IPA) identified distinct networks altered by dex at the level of translation versus transcription (Figure 2D). The eIF2 translation initiation pathway was the top translationally repressed pathway following dex treatment (Figure 2D). Together these data demonstrate that dex translationally represses specific mRNA subsets independent of its effect on transcription.
3.3 Dex represses eIF2 dependent translational pathways in MM cells
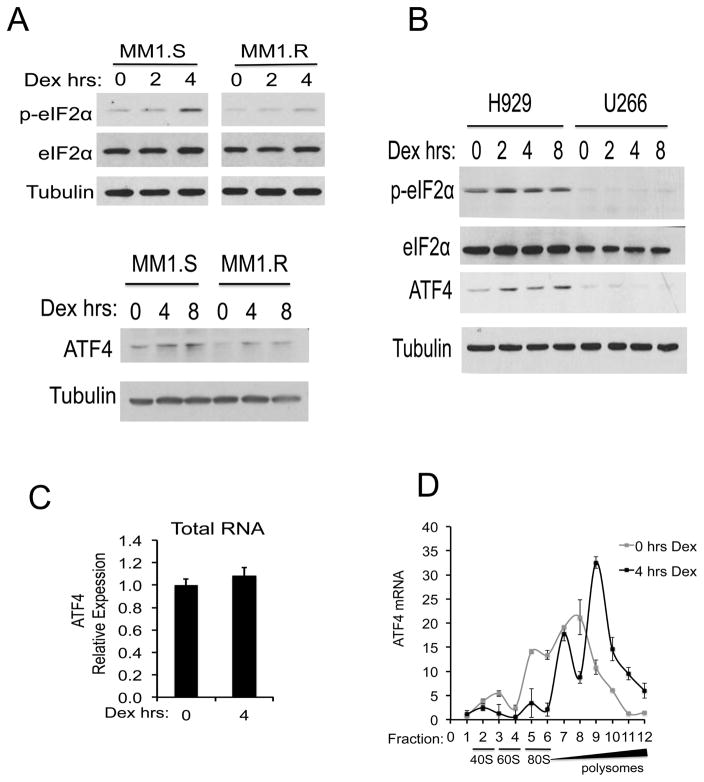
Of the translational pathways altered by dex, the eIF2 translation initiation pathway was the most significantly repressed in MM1.S cells. The eIF2-signaling pathway regulates translation initiation via formation and recycling of the eIF2-ternary complex 17. Phosphorylation of the alpha subunit of eIF2 on serine 51 blocks guanine nucleotide exchange that is necessary for translation re-initiation 17. To address the possibility that dex activates eIF2α kinases to promote apoptosis, lysates from MM1.S or MM1.R cells treated with 1 μM dex for 0–4 hours were immunoblotted for phosphorylated eIF2α (Ser51), total eIF2α, and tubulin. Dex treatment resulted in the phosphorylation of eIF2α in MM1.S cells (Figure 3A). In contrast, no significant change in eIF2α phosphorylation was observed in MM1.R cells. Similarly, dex induced the phosphorylation of eIF2α in dex-sensitive H929 cells but not dex-resistant U266 cells (Figure 3B).
Figure 3. Dexamethasone induces phosphorylation of eIF2α and upregulates ATF4.
(A) MM1.S and MM1.R cells were treated with 1 μM Dex for 0, 2, 4 or 8 hours. Western blots for phospho-eIF2α (p-eIF2α) and total eIF2α (top panel), ATF4 (bottom panel) and Tubulin are shown. (B) H929 and U266 cells were treated with 1 μM Dex for 0, 2, 4 or 8 hours. Cell lysates were immunoblotted for phospho-eIF2α, total eIF2α, ATF4 and Tubulin. (C) MM1.S cells were treated with 0 or 4 hours of Dex and total RNA was extracted. Expression levels of ATF4, relative to GAPDH, were analyzed by RT-qPCR for each condition. (D) MM1.S cells were treated with 1 μM Dex for 0 or 4 hours and lysates were fractionated by sucrose density ultracentrifugation. 12 equal fractions were collected across the gradient. Levels of ATF4 mRNA in each fraction were quantitated relative to a spike-in control RNA. The corresponding locations of the 40S, 60S, 80S and polysome fractions are shown.
The phosphorylation of eIF2α results in an upregulation of specific genes, such as ATF4, at the level of translation. ATF4 encodes a transcription factor that activates genes involved with remediating stress or alternatively promoting apoptosis 18–20. Consistent with the observed phosphorylation of eIF2α, dex treatment of MM1.S cells resulted in a shift of ATF4 mRNA into polysome-laden fractions without changes in total ATF4 transcript levels (Figure 3C and 3D). ATF4 protein levels were concurrently increased in dex-sensitive but not dex-resistant cells (Figure 3A and 3B). Together these data show that eIF2 translation pathways are modulated by dex.
3.4 Activation of the eIF2α kinase HRI promotes apoptosis in dex-resistant cells
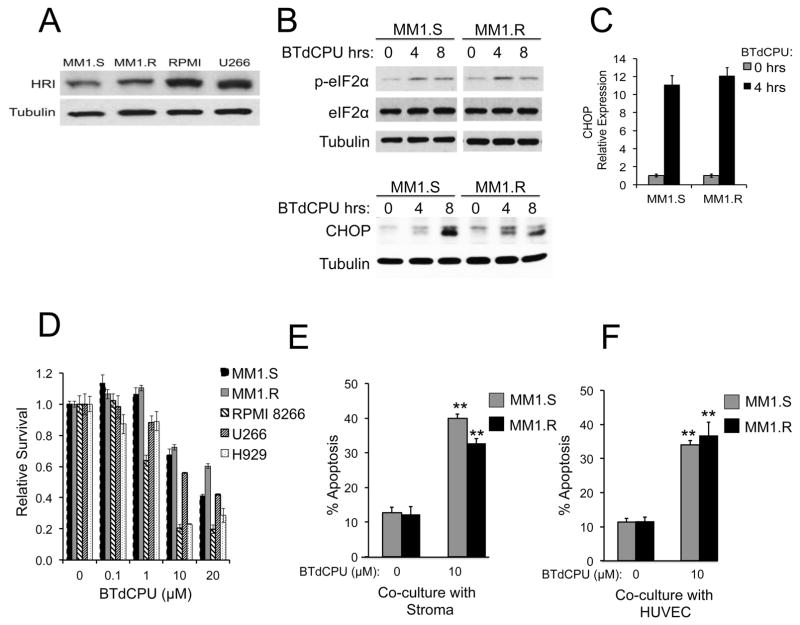
The translational inhibition of the eIF2 pathway in dex-sensitive but not dex-resistant MM cells raised the possibility that pharmacologic inhibition of this pathway might induce apoptosis in dex-resistant cells. To further investigate whether phosphorylation of eIF2α promotes apoptosis, MM cells were treated with the diarylurea compound BTdCPU, an activator of the heme-regulated inhibitor (HRI) eIF2α kinase 21, which is expressed in MM cells (Figure 4A). BTdCPU treatment induced phosphorylation of eIF2α by 4–8 hours (Figure 4B) and upregulated mRNA and protein levels of the pro-apoptotic protein CHOP (Figure 4B and 4C), a known downstream target of eIF2α phosphorylation 22. BTdCPU induced cell death in both dex-sensitive (MM1.S, H929) and dex-resistant (MM1.R, RPMI8266, U266) MM cells (Figure 4D). A second diarylurea compound, I-18, another activator of HRI 23, also significantly induced MM cell death (Supplemental Figure S2) compared to an inactive diarylurea control, NCPdCPU.
Figure 4. BTdCPU induces phosphorylation of eIF2α and promotes cell death in MM cells.
(A) Levels of the heme-regulated inhibitor kinase (HRI) relative to Tubulin were assessed by Western blotting of lysates of Dex-sensitive (MM1.S) and Dex-resistant (MM1.R, RPMI 8266, U266) MM cells. (B) MM1.S or MM1.R cells were treated with 10 μM BTdCPU for 0, 4 or 8 hours and immunoblotted for expression of phospho-eIF2α, total eIF2α, CHOP and Tubulin. (C) MM1.S or MM1.R cells were treated with 10 μM BTdCPU for 0 or 4 hours. Expression levels of total CHOP mRNA were quantitated relative to GAPDH. (D) Dex sensitive and resistant cell lines were treated with 0 to 20 μM BTdCPU for 48 hours. Cell viability was measured using an MTS assay and relative survival levels are graphed. (E,F) MM1.S or MM1.R cells were co-cultured with bone marrow stroma (E) or HUVEC (F) with or without 10 μM BTdCPU for 24 hours and apoptosis was measured with AnnexinV/PI. **p <0.01.
The bone marrow microenvironment also plays an important role in MM resistance to dex 24. To test whether BTdCPU could overcome this resistance mechanism, MM1.S or MM1.R cells co-cultured with bone marrow stromal cells or with HUVEC were treated with BTdCPU for 24 hours and monitored for apoptosis by flow cytometry for Annexin V. Co-culture with marrow stroma or HUVEC failed to protect either MM1.S or MM1.R cells from the cytotoxic effects of BTdCPU under conditions that mimic the bone marrow microenvironment (Figure 4E and 4F).
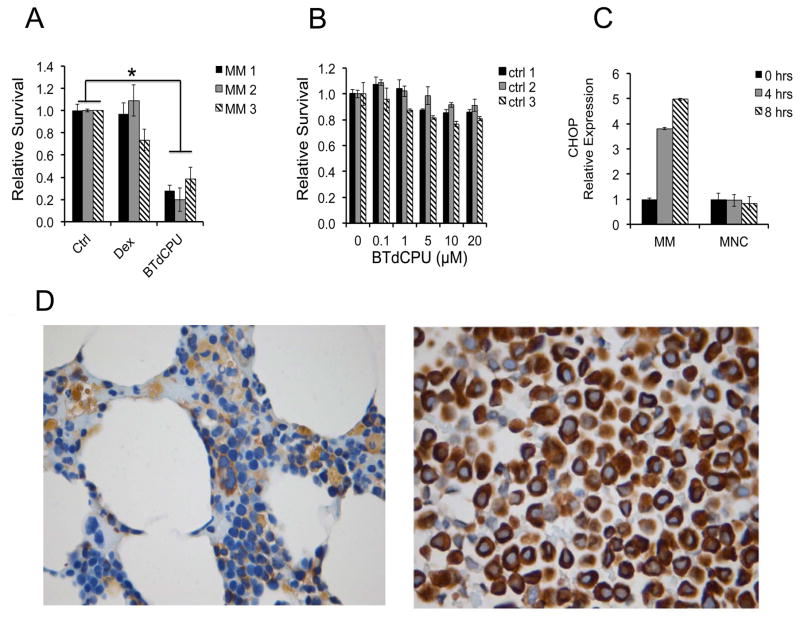
We next extended our analysis of the eIF2 pathway to primary MM patient samples. CD138+ purified plasma cells from three different relapsed/refractory MM patients were treated with 1 μM dex or 10 μM BTdCPU for 24 hours and assayed for cell viability. BTdCPU exhibited significant cytotoxicity towards MM patient samples, which were resistant to dex (Figure 5A). In contrast, minimal toxicity was noted against healthy donor bone marrow cells following treatment with BTdCPU (Figure 5B). Additionally, BTdCPU induced early expression of CHOP in MM patient cells, but not in healthy bone marrow mononuclear cells (MNC) (Figure 5C). Thus, BTdCPU is cytotoxic for dex-resistant primary MM cells with relative sparing of normal cells perhaps in part due to the elevated levels of eIF2α in MM primary cells compared to normal bone marrow cells (Figure 5D).
Figure 5. MM patient cells are sensitive to BTdCPU.
(A) Bone marrow purified CD138+ cells from three relapsed/refractory MM patients (MM 1,2,3) were treated with 1 μM Dex, 10 μM BTdCPU or control for 24 hours and relative survival was assessed. (B) Three healthy donor bone marrow mononuclear control samples (ctrl 1,2,3) were treated with increasing doses of BTdCPU and relative survival was assessed. (C) MM patient cells or healthy donor marrow mononuclear cells (MNC) were treated with 10 μM BTdCPU for 0, 4 or 8 hours. Total RNA expression levels of CHOP relative to GAPDH were quantitated. (D) Bone marrow biopsies from healthy donors (left panel) or MM patients (right panel) were stained for eIF2α expression by immunohistochemistry. Normal hematopoietic elements show weak staining, while MM patient cells demonstrate uniformly strong staining for eIF2α. Images are 630X magnification, taken on a Leica DM3000 microscope.
3.5 Dex suppresses both eIF2 and mTOR translational pathways
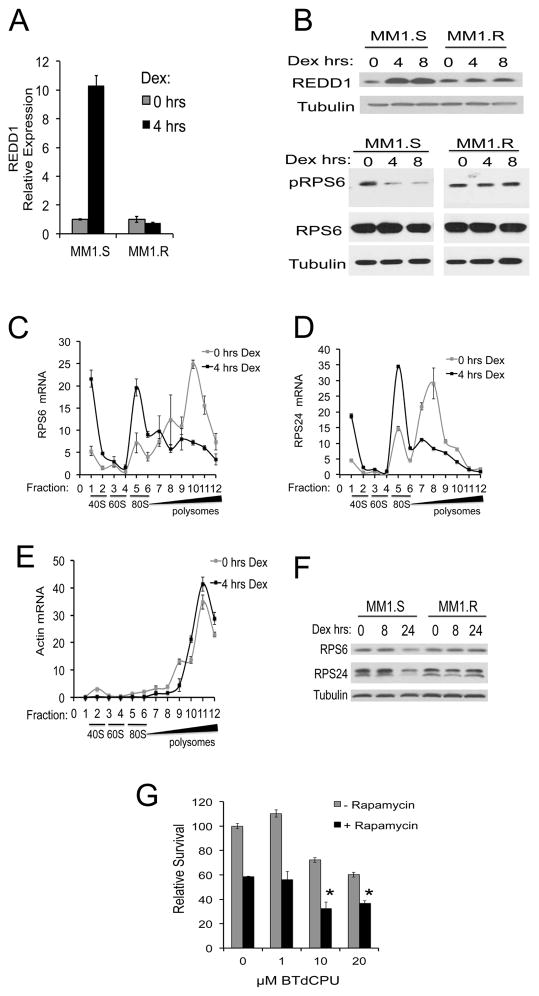
In skeletal muscle, dex represses mTOR signaling through induction of REDD1 25–27. Similarly, we found that dex upregulated REDD1 RNA and protein levels in MM1.S but not in MM1.R cells (Figure 6A and 6B). Dex treatment also reduced the phosphorylation of the mTOR target RPS6 in dex-sensitive but not dex-resistant cells (Figure 6B).
Figure 6. Dexamethasone upregulates REDD1 and represses mTOR targets.
(A) MM1.S or MM1.R cells were treated with 1 μM Dex for 0 or 4 hours. Total RNA was isolated and mRNA expression levels of REDD1 were quantitated relative to GAPDH. (B) MM1.S and MM1.R cells were treated with 1 μM Dex for 0, 4 or 8 hours. Cell lysates were analyzed by Western blotting for REDD1 (top panel) or phospho-RPS6 (pRPS6) and total RPS6 (bottom panel), and Tubulin. (C–E) MM1.S cells were treated with 1 μM of Dex for 0 or 4 hours. Polysome gradients were prepared and 12 equal fractions were collected. mRNA levels of RPS6 (C), RPS24 (D) or Actin (E) were evaluated by RT-qPCR. The relative levels of mRNA in each fraction are graphed. Results were normalized to a representative RNA spike-in control. The corresponding positions of the 40S, 60S, 80S and polysome fractions are shown. (F) MM1.S or MM1.R cells were treated with 1 μM Dex for 0, 8 or 24 hours. Cell lysates were analyzed by Western blotting for RPS6, RPS24 and Tubulin. (G) MM1.R cells were treated with 0–20 μM BTdCPU ± 10 nM of rapamcyin for 48 hours and relative survival was assessed by MTS assay. * p <0.05 compared to rapamycin alone.
Inhibition of mTOR rapidly suppresses the translation of mRNAs involved in protein synthesis, including ribosomal subunit proteins and translation initiation factors 28. A common feature of many of these translation factors is the presence of a 5′ terminal oligopyrimidine (TOP) motif 28–30. The effects of dex on the translational control of the 5′TOP mRNAs encoding RPS6 and RPS24 were explored in MM cells. In MM1.S cells, RPS6 and RPS24 mRNAs shifted from polysome-loaded fractions into non-polysome fractions following four hours of dex treatment (Figure 6C,D and Supplemental Figure S3). In contrast, no shift in polysome loading of these ribosomal protein mRNAs was observed in the dex-resistant cell line MM1.R and there was no change in total RNA levels (Supplemental Figure S3). Another heavily translated non-5′TOP mRNA, actin, demonstrated no shift in polysome loading (Figure 6E). In concordance with the decreased RPS6 and RPS24 translation following dex treatment, protein levels of RPS6 and RPS24 were also decreased in MM1.S but not MM1.R cells (Figure 6F) by 24 hours. The delayed effect on suppressing protein levels may be reflective of the high abundance and slow turnover rate of ribosomal proteins, which have been estimated at over 30 hours 31. Thus, dex-induced translational changes in MM cells include the repression of mTOR targets.
The results of early phase clinical trials utilizing mTOR inhibitors in MM have been modest thus far 32,33. Pre-clinical studies have demonstrated improved efficacy using dual TORC1/2 inhibitors and by targeting feedback mechanisms, including the up-regulation of IGF1R phosphorylation and PI3K/AKT activation 34–36. We reasoned that since dex represses both mTOR and eIF2 signaling, dual targeting of mTOR and eIF2 might have additive effects on promoting cell death in dex-resistant MM cells. To address this hypothesis, we treated the dex-resistant cell line MM1.R with 0–20 μM BTdCPU with or without 10 nM rapamycin for 48 hours. BTdCPU and rapamcyin demonstrated additive effects on apoptosis (Figure 6G). Thus, dual targeting of mTOR and eIF2 signaling poses a potential novel therapeutic combination for dex-resistant MM.
4. Discussion
Translational (polysome) profiling is a method used to fractionate mRNAs based on the number of associated ribosomes to interrogate changes in translational activity 16,37,38. Our study demonstrated that dex, a cornerstone of MM treatment, rapidly altered polysome profiles at early time points in dex-sensitive but not dex-resistant cells, preceding apoptosis. Using translational array profiling we demonstrated that dex specifically repressed the translation of mRNA networks involved with protein translation, and in particular eIF2-signaling. The eIF2 complex is composed of α, β and γ subunits, and in the presence of GTP, assembles with Met-tRNA, the 40S ribosomal subunit and eIF3 to form the 43S pre-initiation complex 39. The regulation of translational components at the translational level has been described under various conditions, including growth arrest and nutrient depletion, possibly as a way for the cell to reduce energy consumption during physiologic stress 29. Ribosomal protein mRNAs, in particular, are under growth-dependent translational control 40 and deficiencies in ribosomal proteins can result in the altered translation of mRNAs in a transcript-specific context 37,38.
The translational regulatory effects of dex on ribosomal proteins and translation initiation factors are similar to those observed with mTOR inhibitors 28. An important distinction however is the ability of dex to repress both mTOR targets and eIF2 signaling (Figure 7). This is of potential therapeutic interest since protein synthesis is regulated by multiple mechanisms including the repression of mTOR and the activation of eIF2α kinases 41,42. Moreover, the phosphorylation of eIF2α has effects on protein translation that are distinct from mTOR inhibition 43. Since mTOR inhibitors are already in clinical development in myeloma, we focused our attention on eIF2 signaling as a potential therapeutic target. Of the known eIF2α kinases involved in eIF2 signaling 44, the heme-regulated inhibitor (HRI) was of particular interest, since activation of HRI has been demonstrated to induce cancer regression in mouse breast tumor models 21. Moreover, in these studies, activation of HRI resulted in no significant organ toxicity or effects on blood parameters. In our study, we found that MM cells express HRI, and that activation of this kinase using BTdCPU promoted eIF2α phosphorylation and induced apoptosis in dex-resistant cells, with relative sparing of normal bone marrow cells. Importantly, co-culture with bone marrow stromal cells did not protect MM cells from drug-induced apoptosis. In addition, dual targeting of both mTOR and HRI showed additive effects on promoting apoptosis in MM cells, suggesting that the eIF2α kinase HRI plays an important role in promoting apoptosis beyond repression of mTOR. As a novel target within the eIF2 signaling pathway, activation of the HRI kinase offers a potential avenue for the treatment of steroid resistant MM. Further insights into dex-induced translational control will be important for developing the next generation of steroid-sparing agents.
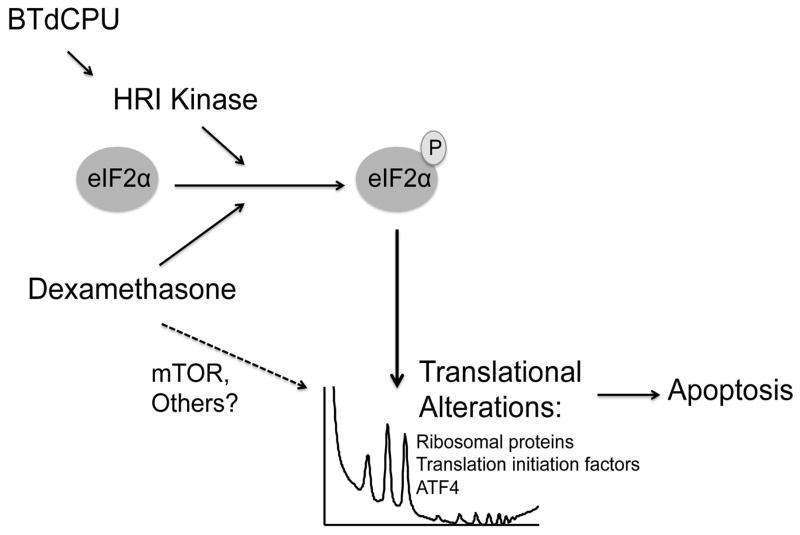
Figure 7. Dexamethasone and BTdCPU promote apoptosis in MM through the modulation of protein translation.
Multiple Myeloma cells express high levels of eIF2α. Both BTdCPU and dexamethasone treatment result in phosphorylation of eIF2α (solid arrows) and translational alterations including downregulation of translational machinery components and upregulation of ATF4, promoting apoptosis. Dex may also promote apoptosis through additional translational pathways, including mTOR (dashed arrow).
Supplementary Material
Highlights.
Dexamethasone alters mRNA translation independent of transcription in myeloma.
Dexamethasone treatment leads to translational repression of the eIF2 pathway.
Activation of the eIF2α kinase HRI promotes apoptosis of dex-resistant myeloma.
Inhibition of both mTOR and eIF2 pathways additively promotes apoptosis.
Acknowledgments
We gratefully acknowledge Dr. David Morris and Dr. Takehito Furuyama for helpful discussions and thoughtful review of the manuscript. We thank Tracy Goodpaster, Dr.Julie Randolph-Habecker, and Dr. Keith Loeb for assistance with immunohistochemistry studies. This work was supported by an NIH/NHLBI 5 R01 HL079582-11 (AS), NIH/NIDDK 5 P30 DK056465 (AS), K12 Career Development Award in Pediatric and Medical Oncology (NB), and a Multiple Myeloma Opportunities for Research and Education (MMORE) award (NB).
Footnotes
AUTHORSHIP CONTRIBUTIONS: NB, PP, AA, MS, and TN performed the experiments and analyzed the data. MYZ, TSH, MR, JJD, VLM analyzed the data. The manuscript was written by NB and AS. AS oversaw the design and analysis of this project.
DISCLOSURE OF CONFLICTS OF INTEREST: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the eastern cooperative oncology group. J Clin Oncol. 2006;24(3):431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 2.Alexanian R, Dimopoulos MA, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80(4):887–890. [PubMed] [Google Scholar]
- 3.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. 352/24/2487 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Krett NL, Pillay S, Moalli PA, Greipp PR, Rosen ST. A variant glucocorticoid receptor messenger RNA is expressed in multiple myeloma patients. Cancer Res. 1995;55(13):2727–2729. [PubMed] [Google Scholar]
- 5.Chauhan D, Li G, Hideshima T, et al. Hsp27 inhibits release of mitochondrial protein smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102(9):3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- 6.McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: Challenges and opportunities. Nat Rev Drug Discov. 2013;12(3):217–228. doi: 10.1038/nrd3870. [DOI] [PubMed] [Google Scholar]
- 7.Neri P, Bahlis NJ. Targeting of adhesion molecules as a therapeutic strategy in multiple myeloma. Curr Cancer Drug Targets. 2012;12(7):776–796. doi: 10.2174/156800912802429337. CCDT-EPUB-20120605-1 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Hardin J, MacLeod S, Grigorieva I, et al. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84(9):3063–3070. [PubMed] [Google Scholar]
- 9.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5(3):230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 11.Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471(7339):467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan D, Auclair D, Robinson EK, et al. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21(9):1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- 13.Gautier L, Cope L, Bolstad BM, Irizarry RA. Affy--analysis of affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 14.Gentleman VCR, Dudoit S, Irizarry R, Huber W. “Limma: Linear models for microarray data,” bioinformatics and computational biology solutions using R and bioconductor. Springer; new york, NY USA: 2005. pp. 397–420. [Google Scholar]
- 15.Greenstein S, Krett NL, Kurosawa Y, et al. Characterization of the MM.1 human multiple myeloma (MM) cell lines: A model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol. 2003;31(4):271–282. doi: 10.1016/s0301-472x(03)00023-7. S0301472X03000237 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Sampath P, Pritchard DK, Pabon L, et al. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;2(5):448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- 18.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 19.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 20.Lange PS, Chavez JC, Pinto JT, et al. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205(5):1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Ozel D, Qiao Y, et al. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol. 2011;7(9):610–616. doi: 10.1038/nchembio.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286(13):10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T, Takrouri K, Hee-Hwang S, et al. Explorations of substituted urea functionality for the discovery of new activators of the heme-regulated inhibitor kinase. J Med Chem. 2013;56(23):9457–9470. doi: 10.1021/jm400793v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigorieva I, Thomas X, Epstein J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Exp Hematol. 1998;26(7):597–603. [PubMed] [Google Scholar]
- 25.Britto FA, Begue G, Rossano B, et al. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab. 2014;307(11):E983–93. doi: 10.1152/ajpendo.00234.2014. [DOI] [PubMed] [Google Scholar]
- 26.Kimball SR, Do AN, Kutzler L, Cavener DR, Jefferson LS. Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J Biol Chem. 2008;283(6):3465–3475. doi: 10.1074/jbc.M706643200. M706643200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem. 2006;281(51):39128–39134. doi: 10.1074/jbc.M610023200. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh AC, Liu Y, Edlind MP, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267(21):6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- 30.Jefferies HB, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci U S A. 1994;91(10):4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boisvert FM, Ahmad Y, Gierlinski M, et al. A quantitative spatial proteomics analysis of proteome turnover in human cells. Mol Cell Proteomics. 2012;11(3) doi: 10.1074/mcp.M111.011429. M111.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunther A, Baumann P, Burger R, et al. Activity of everolimus (RAD001) in relapsed and/or refractory multiple myeloma: A phase I study. Haematologica. 2015;100(4):541–547. doi: 10.3324/haematol.2014.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee AJ, Hari P, Marcheselli R, et al. Outcomes in patients with relapsed or refractory multiple myeloma in a phase I study of everolimus in combination with lenalidomide. Br J Haematol. 2014;166(3):401–409. doi: 10.1111/bjh.12909. [DOI] [PubMed] [Google Scholar]
- 34.Cirstea D, Santo L, Hideshima T, et al. Delineating the mTOR kinase pathway using a dual TORC1/2 inhibitor, AZD8055, in multiple myeloma. Mol Cancer Ther. 2014;13(11):2489–2500. doi: 10.1158/1535-7163.MCT-14-0147. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4(10):1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. 4/10/1533 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Baumann P, Schneider L, Mandl-Weber S, Oduncu F, Schmidmaier R. Simultaneous targeting of PI3K and mTOR with NVP-BGT226 is highly effective in multiple myeloma. Anticancer Drugs. 2012;23(1):131–138. doi: 10.1097/CAD.0b013e32834c8683. [DOI] [PubMed] [Google Scholar]
- 37.Horos R, Ijspeert H, Pospisilova D, et al. Ribosomal deficiencies in diamond-blackfan anemia impair translation of transcripts essential for differentiation of murine and human erythroblasts. Blood. 2012;119(1):262–272. doi: 10.1182/blood-2011-06-358200. [DOI] [PubMed] [Google Scholar]
- 38.Kondrashov N, Pusic A, Stumpf CR, et al. Ribosome-mediated specificity in hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145(3):383–397. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pestova TV, Kolupaeva VG, Lomakin IB, et al. Molecular mechanisms of translation initiation in eukaryotes. Proc Natl Acad Sci U S A. 2001;98(13):7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geyer PK, Meyuhas O, Perry RP, Johnson LF. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 42.Caldarola S, Amaldi F, Proud CG, Loreni F. Translational regulation of terminal oligopyrimidine mRNAs induced by serum and amino acids involves distinct signaling events. J Biol Chem. 2004;279(14):13522–13531. doi: 10.1074/jbc.M310574200. [DOI] [PubMed] [Google Scholar]
- 43.Gao X, Wan J, Liu B, Ma M, Shen B, Qian SB. Quantitative profiling of initiating ribosomes in vivo. Nat Methods. 2015;12(2):147–153. doi: 10.1038/nmeth.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: Their structures and functions. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.