1. Introduction
Motor vehicle collision (MVC) results in fifty million injuries worldwide and almost four million US emergency department (ED) visits each year.[32,46] In the US, more than 90% of individuals presenting to the ED after MVC are discharged to home after evaluation.[33] A substantial proportion of these individuals develop persistent musculoskeletal pain,[29] and the most common and morbid location of such pain is the axial region (neck, shoulders, and/or back).[8]
Posttraumatic stress disorder (PTSD) symptoms including re-experiencing the MVC, avoidance of reminders of the MVC, and hyperarousal symptoms are also common in this population and have been hypothesized to contribute to pain persistence via a number of mechanisms.[28,38] Re-experiencing may disrupt cognitive efforts to reduce pain perception and facilitate recovery, or may drive the activation of stress systems which alter neurosensory processing.[30,36,37] Avoidance and associated withdrawal from usual activities may promote disuse and disability, contributing to pain persistence. [23,36,44] Hyperarousal may lead to alterations in muscle use and persistent biomechanical changes which result in persistent pain,[23,27] and may drive the activation of stress systems which alter neurosensory processing.[30] Attentional bias due to hyperarousal may also directly augment pain experiences.[3,23,36]
In this study we used a path analytic approach to evaluate whether longitudinal relationships between post-traumatic stress and axial pain symptoms after MVC support the hypothesis that one or more PTSD symptom clusters promote axial pain persistence. To our knowledge, such an analysis has not previously been performed. Based on existing evidence from other settings that hyperarousal and re-experiencing symptoms have the greatest influence on pain outcomes and that such symptoms may partially mediate the association between acute and chronic pain,[21,22,26] we hypothesized that hyperarousal and re-experiencing symptoms would partially mediate post-MVC axial pain persistence. In addition, based on the concept that hyperarousal symptoms reflect and/or drive continued stress system activation, we hypothesized that increased hyperarousal symptoms would more strongly predict increased pain among individuals with increased genetic vulnerability to stress-induced pain. To evaluate this hypothesis, we explored the relationship between hyperarousal and axial pain persistence among individuals with glucocorticoid receptor co-chaperone FK506 binding protein 51 (FKBP5) haplotypes that are known to increase vulnerability to chronic post-MVC pain.[9] In secondary analyses we also explored path differences over time among those with and without substantial early and persistent PTSD or axial pain symptoms.
2. Methods
2.1 Design and setting
This prospective longitudinal study enrolled patients presenting to the ED within 24 hours of MVC. Data were collected at eight EDs in four no-fault MVC litigation/insurance states (Michigan, Massachusetts, New York, and Florida) between February 2009 and October 2011. The study was approved by the institutional review boards of all participating hospitals, and each participant provided written informed consent. Complete information regarding study design, procedures, and methods has previously been described.[34]
2.2 Participant eligibility criteria and study sites
Patients aged 18 to 65 who presented to the ED within 24 hours after a MVC and were unlikely to require hospitalization were screened for eligibility. Patients who were admitted to the hospital, had fractures other than phalangeal fractures, had more than 4 lacerations requiring sutures or a single laceration more than 20 cm in length, or had intracranial or spinal injuries were excluded. Spinal injury was defined by the presence of a fracture, dislocation, or new neurologic deficit. Enrollment was limited to non-Hispanic whites (the most common ethnicity at study sites) because the study included the collection of genetic data and genetic analyses are potentially biased by population stratification.[13] Patients who were not alert and oriented were also excluded, as were pregnant patients, prisoners, patients unable to read and understand English, patients taking a β-adrenoreceptor antagonist, or patients taking opioids above a total daily dose of 20 mg of oral morphine or equivalent.
2.3 Study procedures
Eligible and consenting participants completed ED interview evaluations that included an assessment of baseline demographic characteristics and current symptoms. These assessments were conducted by research assistants using a web-based survey with explicit definitions of variables. Before enrolling patients in the ED, each research assistant completed a study training module followed by an interview with a standardized mock ED patient. Comparison of mock ED patient data across research assistants demonstrated an error rate of 1.3%. Injury characteristics and medications administered in the ED were extracted from the medical record. Six weeks, six months and one year after the MVC, participants completed a follow up interview online, by telephone, or via mail. Participants were compensated $80 for completing the ED interview, $50 for completing the 6-week interview, $60 for completing the 6-month interview, and $70 for completing the 12-month interview.
2.4 Measures
Study measures are described below, and complete study measures are described in full elsewhere.[34]
2.4.1 Participant demographics
Participant demographic characteristics (including age, gender, income, height, weight, and educational attainment) were obtained from participant self-report and the ED medical record.
2.4.2 Pain assessments and pain outcome definitions
Pain extent and severity was assessed in the ED and at 6 weeks, 6 months and 1 year. In each of 19 discrete body regions in which the participant reported pain, pain severity was assessed using a verbal 0-to-10 numeric rating scale (NRS). If a participant reported pain in a body region (NRS score of ≥ 1), then they were also asked whether the pain was due to the MVC. Axial pain severity was defined as the maximum pain reported in the neck, left shoulder, right shoulder, upper back region or lower back region. Axial pain severity ≥ 4 was defined as moderate or severe. Participants that experienced non-MVC moderate/severe axial pain were excluded from the analyses to reduce any potential influence of non-MVC related axial pain on PTSD symptoms.
2.4.3 Psychological symptoms
Psychological distress in the ED was assessed using the Peritraumatic Distress Inventory (PDI), which measures peritraumatic distress symptom clusters (life threat, helplessness/anger, loss of control, and guilt/shame). This measure has been found to have high internal consistency (standardized coefficient alpha of 0.75-0.76) and test-retest reliability (coefficient of 0.74) within a general civilian population.[10] A PDI cut-off score of ≥23 was used to define marked distress symptoms (“distress”).[31]
Post-traumatic stress disorder (PTSD) symptoms (intrusion, avoidance, and hyperarousal) were assessed at 6-weeks, 6-months, and 1-year using the Impact of Events Scale – Revised (IES-R).[4] An IES-R score of >30 was used to define the presence of substantial PTSD symptoms.[16]
2.4.4 DNA collection and genotyping
Study personnel collected blood samples at the time of enrollment using PAXgene DNA tubes. Following DNA purification (PAXgene blood DNA kit, QIAGEN, Valencia, CA, USA), genotyping using the Sequenom platform (Sequenom, San Diego, CA, USA) was performed at 33 single nucleotide polymorphisms (SNPs) selected to cover haplotype diversity at the FKBP5 gene locus (Figure 1). Two Hapmap samples and two repeat samples were included in each genotyping batch (96 samples). FKBP5 tag SNPs previously identified using the Tagger procedure in Haploview (r2 ≥ 0.8) were selected for gene-by-environment interaction analyses.[1,12]
Figure 1.
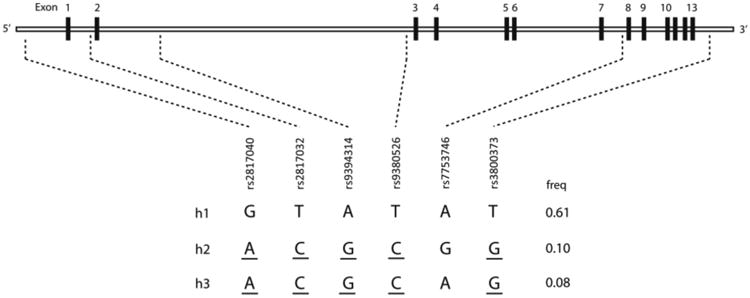
FKBP5 risk haplotype was defined by the presence of one or more copies of the h2 or h3 haplotype (combined frequency 0.18). Reproduced from A.V. Bortsov et al. Pain 154.8 (2013): 1419-1426
2.5 Statistical analyses
Confirmatory factor analyses (CFA) and longitudinal path analyses were performed using Mplus version 7 (Muthén and Muthén, Los Angeles, CA). The root mean square error of approximation (RMSEA) with 90% confidence intervals and the Comparative Fit Index (CFI) were used to assess model fit. CFA results indicated acceptable fit for the three IES-R subscales (RMSEA=0.051 [0.047, 0.055]; CFI=0.958). Cronbach's alpha confirmed IES-R scale internal validity (0.90 for intrusion, 0.85 for avoidance, and 0.86 for hyperarousal, and 0.95 overall) and PDI scale internal validity (0.81 overall). We first fit CFA models to assess PDI and IES-R subscales in our samples. We then fit a longitudinal path analysis using these subscales and axial pain outcome data from each timepoint (i.e., 52 total free paths). All variables measured at the same timepoint were allowed to correlate freely, as were repeated measurements of the same variable over time. Path models were fit using maximum likelihood methods to allow inclusion of all observed data for those participants who provided incomplete data. After fitting the full model, paths for which p ≥ 0.05 were sequentially removed (i.e., restricted to be zero) to obtain the reduced model.
For comparing the model across subgroups of interest (described in section 3.7), we fit multiple-group path analyses, in which we initially allowed all path coefficients and other model parameters to vary freely between groups (the completely unrestricted model). We then fit a series of two restricted models to explore differences between subgroups: 1) restricting the 6 paths from the PTSD symptom clusters to axial pain severity at the subsequent visit to be the same across the subgroups; 2) restricting the same paths in (1) plus the 6 paths from axial pain severity to the PTSD symptom clusters at subsequent visits to be the same across subgroups. We compared the restricted models to the unrestricted model using chi-squared difference tests with 6 or 12 degrees of freedom, respectively. In the event that either chi-squared difference test produced a p<0.05, providing evidence that the less restricted model fit the data better than the more restricted model, we then explored which specific paths differed across subgroups using additional chi-squared difference tests, each conducted at the 0.05 significance level.
3. Results
3.1 Sample characteristics
A total of 10,629 patients were screened, 1,416 were eligible, 969 consented to study participation and 948 were enrolled. The median time between MVC and ED presentation was 1.2 hours. Consistent with eligibility criteria, all patients were discharged home after ED evaluation and did not have life-threatening injury. Ninety-nine percent of participants had an Abbreviated Injury Scale score of 1. Retention rates at 6 weeks, 6 months, and 1 year were 859/948 (91%), 840/948 (89%), and 861/948 (91%), respectively. Study participant characteristics are shown in Table 1.
Table 1. Characteristics of CRASH Study Participants (ED = emergency department; MVC = motor vehicle collision).
| N = 948 | |
|---|---|
| Individual (n, %) | |
| Age, years | |
| 18-27 | 350 (36.9) |
| 28-41 | 284 (30.0) |
| 42-65 | 314 (33.1) |
| Sex | |
| Female | 575 (60.7) |
| Male | 373 (39.3) |
| Education | |
| High school or less | 226 (23.9) |
| Some college or trade school | 369 (39.0) |
| College/post-graduate degree | 351 (37.1) |
| Income, annual | |
| <$20,000 | 117 (13.9) |
| $20,000-40,000 | 176 (20.9) |
| $40,000-80,000 | 277 (32.9) |
| >$80,000 | 273 (32.4) |
| Works full time | |
| No | 395 (41.7) |
| Yes | 553 (58.3) |
| Body mass index (BMI) | |
| <25 | 356 (38.7) |
| 25 – 30 | 287 (31.2) |
| >30 | 277 (30.1) |
| ED MVC-related axial pain | |
| None (NRS = 0) | 137 (14.7) |
| Mild (NRS = 1-3) | 136 (14.6) |
| Moderate (NRS = 4-7) | 294 (31.5) |
| Severe (NRS = 8-10) | 366 (39.2) |
| rs9394314 | |
| Homozygous for major allele | 449 (47.5) |
| Other | 497 (52.5) |
| Presence of ED Peritraumatic Distress | 355 (38.0) |
3.2 Genotyping
Blood was obtained from each of the 948 participants, and DNA was extracted from 946/948 (99.8%). Call rates for genotyping were >99%. Selected repeated genotyping demonstrated >98% call agreement, and all SNPs were in Hardy-Weinberg equilibrium.
3.3 Pain and posttraumatic stress disorder (PTSD) symptoms over time
Most participants who reported pain status had moderate or severe axial pain in the ED (660/933, 71%), and greater than 1 in 3 who completed the PDI assessment experienced peritaumatic distress (355/934, 38%) (Table 1). At 6 weeks, moderate or severe axial pain was present in 437/809 participants (54%) and 241/856 participants (28%) had substantial PTSD symptoms. At 6 months, 305/759 participants (40%) had moderate or severe axial pain and 145/834 participants (17%) had substantial PTSD symptoms. At one year, 276 /723 participants (38%) had moderate or severe axial pain and 140/859 participants (16%) had substantial PTSD symptoms. (Note: group sizes for these proportions include only those individuals with non-missing responses at the timepoint for that measure.)
3.4 Relationship between pain and PDI/PTSD symptoms over time
The correlation between PTSD symptoms (assessed using the PDI in the ED, and IES-R at subsequent timepoints) and MVC-related axial pain symptoms increased steadily over time: ED r = .12, 6 week r = .28, 6 month r = .42, 12 month r = .43 (p < .01 at all time points). Across all timepoints, moderate or severe axial pain was more common among those with substantial PTSD symptoms than substantial PTSD symptoms were among those with moderate or severe axial pain (Figure 2).
Figure 2.
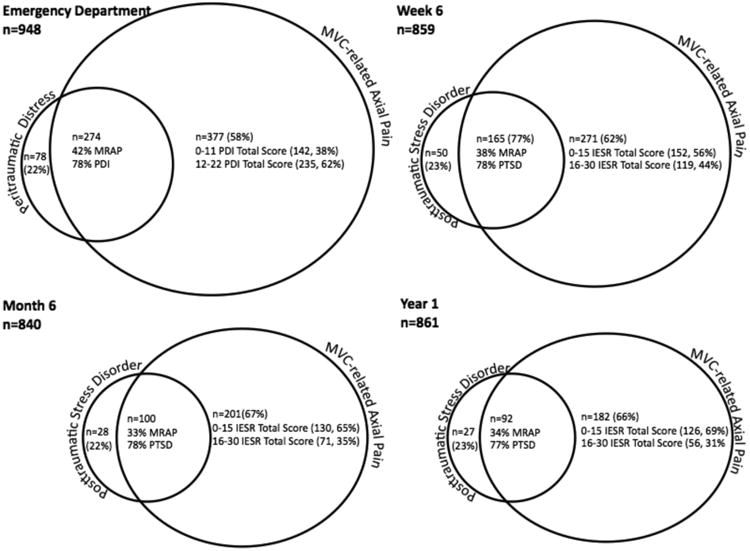
Proportion of overlap between moderate/severe motor vehicle collision (MVC) related axial pain symptoms (MRAP), psychological distress (as measured by Peritraumatic Distress Inventory (PDI)) and post-traumatic distress disorder (PTSD) symptoms (as measured by the Impact of Event Scale-Revised (IES-R)) among study participants in the emergency department and at six weeks, six months and one-year follow-up timepoints.
3.5 Path analysis
Path analyses evaluated the relationship between peritraumatic axial pain and distress symptoms and PTSD symptom cluster and axial pain severity over time (Figure 3).
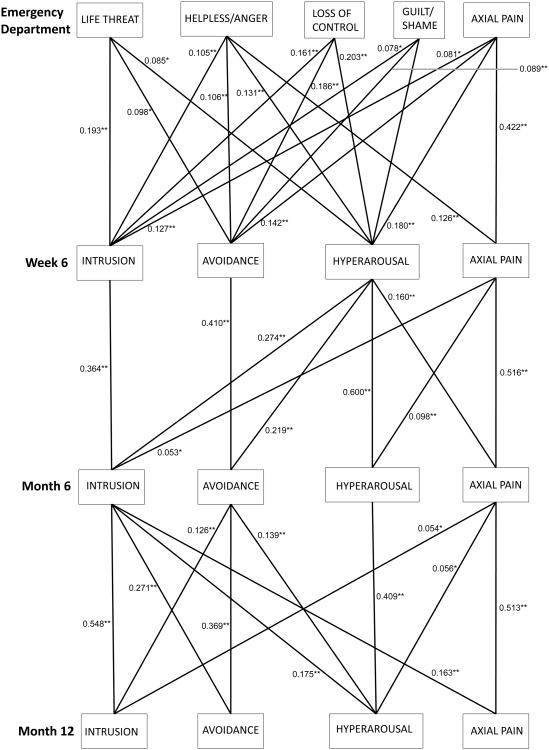
Figure 3.
Significant pathways between peritraumatic distress symptom cluster severity (assessed in the ED as life threat, helplessness, loss of control, and guilt/shame) and post-traumatic stress disorder symptom cluster severity (assessed at subsequent timepoints as intrusion, avoidance, and hyperarousal) and motor vehicle collision (MVC)-related axial pain severity across ED, 6 week, 6 month, and 1 year timepoints after MVC. *p < 0.05, **p<0.01
3.5.1 Associations between axial pain severity and subsequent PTSD symptom cluster severity
Axial pain severity generally predicted PTSD symptom clusters at subsequent timepoints (Figure 3). Axial pain consistently predicted hyperarousal symptoms at subsequent timepoints, and was more strongly predictive of hyperarousal symptoms than other PTSD symptom clusters (higher β values, data not shown). Axial pain was also consistently linked to intrusive symptoms at subsequent timepoints. Axial pain severity in the ED predicted avoidance symptoms at 6 weeks, but not at later timepoints.
3.5.2 Associations between PTSD symptom cluster severity and subsequent axial pain severity
Peritraumatic helplessness and anger symptoms predicted axial pain 6 weeks after MVC, but peritraumatic life threat, loss of control, and guilt/shame symptoms did not (Figure 3). Six week hyperarousal symptoms predicted axial pain severity at 6 months, as well as all PTSD symptom clusters, whereas six week intrusion and avoidance symptoms did not. In contrast, at six months intrusive rather than hyperarousal symptoms predicted axial pain at 12 months, and hyperarousal and avoidance symptoms did not. Intrusion and avoidance symptoms at 6 months also predicted PTSD symptom clusters at 12 months.
3.5.3 PTSD symptom clusters partially mediating axial pain persistence
Hyperarousal symptoms assessed at six weeks partially mediated the relationship between axial pain symptoms in the ED and axial pain symptoms at six months (Figure 3). The estimated standardized indirect effect of ED axial pain on 6-month axial pain through 6-week axial pain is 0.218 (p < 0.001) and the standardized indirect effect through hyperarousal at six weeks is 0.029 (p < 0.001), which indicates significant partial mediation through hyperarousal. Similarly, intrusive symptoms partially mediated the relationship between axial pain at six weeks and axial pain at 12 months. The standardized indirect effect of 6-week axial pain on 1-year axial pain through 6-month axial pain is 0.265 (p < 0.001), and the standardized indirect effect through 6-month intrusion is 0.009 (p=0.034), which indicates significant partial mediation through intrusion.
3.6 Influence of PTSD symptoms on post-MVC axial pain among individuals with genetic vulnerability to stress-induced pain
Among the 460/949 (48%) study participants with increased genetic vulnerability to stress-induced pain (individuals with one or more copies of the FKBP5 risk haplotype[9]), six week hyperarousal symptoms more strongly mediated the relationship between acute axial pain severity and axial pain severity at 6 months (Figure 4).
Figure 4.
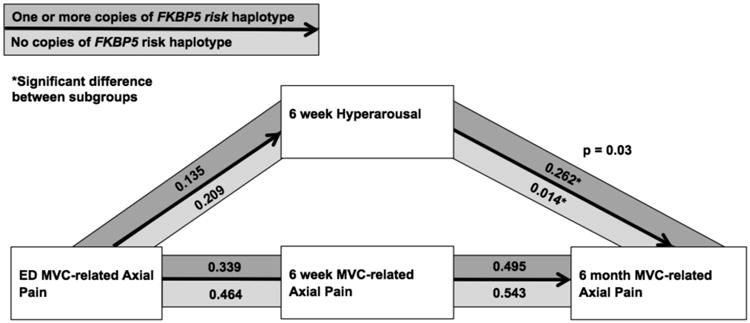
Among study participants with one or more copies of the FKBP5 risk haplotype (18%), hyperarousal symptoms had a greater influence on mediating the relationship between acute axial pain and axial pain at 6 months than among other participants (p=0.03)
3.7. Secondary analyses
Among study participants with substantial peritraumatic distress in the ED (62%), hyperarousal symptoms were less influential in the persistence of axial pain symptoms at one year, whereas avoidance symptoms were more influential (Figure 5). No differences in the relationship between PTSD symptoms and axial pain were observed among the other subgroups analyzed: those with versus without moderate or severe axial pain in the ED, those with and without persistent PTSD at year 1, those with versus without moderate or severe axial pain at year 1, and in men versus women.
Figure 5.
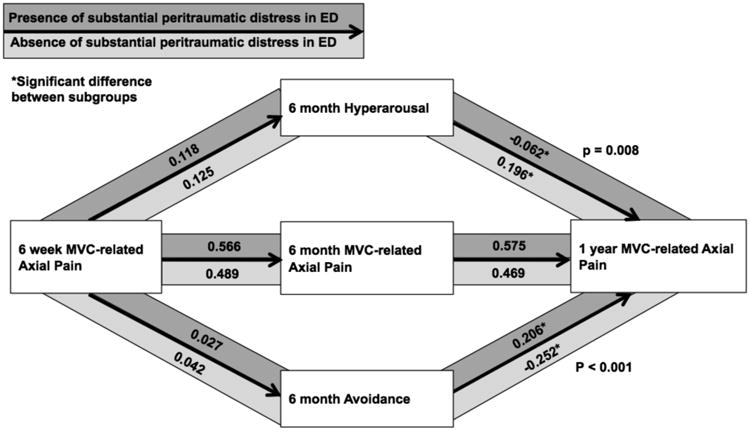
Among study participants with substantial peritraumatic distress, hyperarousal symptoms had a lesser influence on mediating the relationship between axial pain at 6 weeks and axial pain at 1 year than among other participants (p=0.008), and avoidance symptoms had a greater influence on mediating the relationship between axial pain at 6 weeks and axial pain at 1 year (p < 0.001) than among other participants.
4. Discussion
In this study we evaluated longitudinal associations between post-traumatic stress symptoms and axial pain outcomes in a large cohort of individuals experiencing MVC. Both substantial PTSD symptoms and moderate or severe axial pain symptoms were common in the cohort, with more than 1 in 4 participants having substantial PTSD symptoms and 1 in 2 having moderate or severe axial pain symptoms at six weeks. The correlation between PTSD and axial pain symptoms increased steadily over time, from 0.12 in the ED to 0.43 at one year. Path analysis results support the hypothesis that axial pain after MVC consistently promotes the maintenance of hyperarousal and intrusive symptoms from the early weeks post-injury through 1 year. In contrast, while one or more PTSD symptom clusters had an influence on axial pain outcomes throughout the year after MVC, the different symptom clusters were influential at different time points, with helplessness and anger most influencing axial pain severity in the initial weeks after MVC, hyperarousal symptoms most influencing (and partially mediating) axial pain persistence in the initial months after MVC, and intrusive symptoms partially mediating the persistence of axial pain at one year. In addition, PTSD symptoms (specifically hyperarousal symptoms) had a greater influence on pain outcomes among individuals with increased genetic vulnerability to stress-induced pain, and relationships between pain and PTSD symptoms vary among those with substantial peritraumatic distress.
Our study findings are consistent with previous studies of other trauma populations that found that PTSD symptoms in general, and hyperarousal and intrusive/re-experiencing symptoms in particular, contribute to the development of chronic musculoskeletal pain. Kimerling et al. examined the relationship between war-zone stress exposure, PTSD symptom clusters, and physical symptoms (including neck pain and back pain) among 52 female Vietnam veterans evaluated many years after military service.[21] In this study, PTSD symptoms were found to mediate the relationship between trauma exposure and physical symptom outcomes, with hyperarousal symptoms showing the strongest association. McFarlane et al. assessed a group of Australian firefighters 42 months after a brushfire disaster, and found that individuals with PTSD reported significantly more physical symptoms, including musculoskeletal pain.[26] In this study, re-experiencing symptoms was most strongly associated with physical symptom outcomes.[26] Jenewein et al. assessed 323 hospitalized trauma patients five days, six months, and twelve months after injury and found that PTSD symptoms influenced pain intensity across time points.[19] Liedl et al. assessed 824 hospitalized trauma patients one week, three months, and twelve months after trauma and found that pain severity three and twelve months after trauma was predicted by re-experiencing and hyperarousal at previous time points. In addition, three month hyperararousal symptoms mediated the relationship between baseline and twelve month pain.[22]
To our knowledge, our study is the first to use a path analytic approach to evaluate longitudinal relationships between specific PTSD symptom clusters and axial pain symptoms after time after MVC. Our results build upon the findings of Sterling and colleagues, who first identified PTSD symptoms in the early aftermath of MVC as an important predictor of chronic pain.[39-41] Sterling and colleagues also found that individuals with chronic pain after MVC who have substantial comorbid PTSD symptoms have greater disability, negative affect, pain, arousal, and lower pain thresholds than those without PTSD.[15] Our study design provided the opportunity to evaluate the influence of individual symptom clusters over time, and are broadly consistent with the mutual maintenance model, which proposes that PTSD and pain symptoms are mutually enhancing.[22] However, as noted above, while pain symptoms had a consistent augmenting effect on PTSD symptoms across time, our results indicate that the relative influence of different PTSD symptom clusters may be time-dependent. Hyperarousal symptoms most influenced axial pain persistence in the initial months after MVC, and may be most important to target with interventions during this time period, with intrusive symptoms playing a greater role in maintaining/augmenting pain after chronic pain has developed.
Importantly, our study results also indicate that constitutional (genetic) factors affect the influence of PTSD symptom clusters on chronic pain pathogenesis. Forty eight percent of study participants had a genetic variant in the FKBP5 gene that results in increased FKBP5 levels [7], reduced glucocorticoid receptor sensitivity [5,6], and relatively elevated glucocorticoid levels.[18,42] Among such individuals, the influence of hyperarousal symptoms on pain was much greater. Indeed, among individuals without such a risk allele hyperarousal symptoms had only a negligible effect on pain (Figure 4). Animal data indicate that persistently elevated levels of cortisol sensitize primary afferents,[20] and that elevated spinal FKBP5 levels contribute to hypersensitivity and intracellular changes/switches that cause the cellular effects of glucocorticoids to change from analgesic to hyperalgesic.[24] Such molecular switches that cause stress molecules (e.g., glucocorticoids) to change from analgesic to hyperalgesic are fundamental to the development of chronic post-traumatic pain. In such individuals, hyperarousal symptoms, a dominant PTSD symptom cluster [25,35] known to drive the further activation of stress systems including hypothalamic-pituitary-adrenal and catecholaminergic systems,[45] may promote the cellular changes that underline chronic pain. In human studies, the above described FKBP5 genetic variants have been associated with vulnerability to chronic pain development after several different traumatic stress exposures;[9] enhancing the ability of PTSD symptoms to shape chronic pain outcomes may be one mechanism by which these polymorphisms increase susceptibility to chronic pain. Such an effect may be increased among those living in more stressful, low socioeconomic status environments.[43]
Our findings add to evidence that interventions to reduce PTSD symptoms in the aftermath of MVC may improve chronic axial pain outcomes. This hypothesis is further supported by evidence that among individuals with chronic axial pain and substantial PTSD symptoms after MVC, interventions targeting PTSD symptoms also improve pain symptoms.[14] Recently, several cognitive-behavioral interventions have been developed to target PTSD symptoms in the early aftermath of MVC, to determine if such interventions can reduce both pain and PTSD symptoms.[2,11] Such interventions offer exciting new opportunities for interventions to reduce pain and disability after MVC, and may be most efficacious among individuals subgroups of individuals with specific constitutional characteristics and/or among individuals identifiable by peritraumatic phenotype.
Several limitations must be considered when interpreting our study findings. First, the study population was limited to European Americans (the most common ethnicity at study sites) to avoid potential bias in genetic analyses due to population stratification. The generalizability of our results to other racial/ethnic groups is unknown. Second, to reduce confounding, we excluded data from study participants who reported no MVC-related axial pain ≥ 4 but did report non MVC-related axial pain ≥ 4. The exclusion of axial pain that was not MVC-related was based on the hypothesis that presence of any axial pain may influence PTSD symptoms, whereas this analysis was focused solely on pain resulting from MVC. However, this choice of methodology may have introduced other forms of bias into the study. Also, posttraumatic stress symptoms were assessed using the PDI during the peritraumatic period, whereas the IES-R was used at six weeks, six months, and twelve months. Thus we are not able to evaluate the influence of PTSD symptom clusters using a common assessment method from the time of trauma. Our study also did not explore the potential role that individual cognitive factors, such as pain catastrophizing, self-rated physical health, and beliefs and expectations around recovery may have had on the associations studied. Literature suggests that such factors contribute to the recovery process following trauma[17,29], and they may influence the relationship between pain and PTSD symptoms in the aftermath of trauma exposure. In addition, our study only focused on the influence of post-traumatic stress symptoms on pain symptoms, and did not evaluate the association between post-traumatic stress and pain-related disability or function. Finally, our study focused on the relationship between pain and PTSD symptoms only during the first year after MVC.
The results of this study suggest that axial pain after MVC consistently promotes the maintenance of PTSD symptoms, from the early weeks post-injury through 1 year, and that one or more PTSD symptom clusters had an influence on axial pain outcomes throughout the year after MVC, with hyperarousal symptoms most influencing axial pain persistence in the initial months after MVC. The influence of hyperarousal symptoms on pain persistence was only present among those with genetic vulnerability to stress-induced pain, suggesting specific mechanisms by which hyperarousal symptoms may lead to hyperalgesia and allodynia. Further studies are needed to better understand the interplay of pain and PTSD symptoms during the aftermath of trauma. Such understanding will allow the development of optimal interventions to reduce the incidence of both of these common, highly morbid outcomes.
Acknowledgments
We would like to thank the participants for taking part in this study. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR056328. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: Research reported in this publication was supported by Award Number 5-R01-AR056328 from the National Institutes of Health. Mark Weaver was supported by Grant 1UL1TR001111-01 from the National Center for Advancing Translational Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Scientific Meeting Presentation: Feinberg RK, Weaver MA, Liberzon I, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, McLean SA. Stress-Related Psychological Symptoms Contribute to Axial Pain Persistence after Motor Vehicle Collision: Path Analysis Results from A Prospective Longitudinal Study. Poster presented at: The American Pain Society Annual Meeting; April 2014; Tampa, FL.
Conflict of interest statement: The authors report no conflict of interest.
References
- 1.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 2.Andersen TE, Ravn SL, Roessler KK. Value-based cognitive-behavioural therapy for the prevention of chronic whiplash associated disorders: protocol of a randomized controlled trial. BMC musculoskeletal disorders. 2015;16:232. doi: 10.1186/s12891-015-0687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum C, Huber C, Schneider R, Lautenbacher S. Prediction of experimental pain sensitivity by attention to pain-related stimuli in health individuals 1, 2. Percept Mot Skills. 2011;112:926–946. doi: 10.2466/02.09.22.PMS.112.3.926-946. [DOI] [PubMed] [Google Scholar]
- 4.Beck JG, Grant DM, Read JP, Clapp JD, Coffey SF, Miller LM, Palyo SA. The Impact of Event Scale-Revised: Psychometric properties in a sample of motor vehicle accident survivors. J Anxiety Disord. 2008;22:187–198. doi: 10.1016/j.janxdis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–392. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 6.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, Papiol S, Seaman S, Lucae S, Kohli MA. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 8.Bortsov AV, Platts-Mills TF, Peak DA, Jones JS, Swor RA, Domeier RM, Lee DC, Rathlev NK, Hendry PL, Fillingim RB. Effect of pain location and duration on life function in the year after motor vehicle collision. Pain. 2014;155:1836–1845. doi: 10.1016/j.pain.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bortsov AV, Smith JE, Diatchenko L, Soward AC, Ulirsch JC, Rossi C, Swor RA, Hauda WE, Peak DA, Jones JS. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain. 2013;154:1419–1426. doi: 10.1016/j.pain.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, Fagan J, Marmar CR. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158:1480–1485. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- 11.Campbell L, Kenardy J, Andersen T, McGregor L, Maujean A, Sterling M. Trauma-focused cognitive behaviour therapy and exercise for chronic whiplash: protocol of a randomised, controlled trial. Journal of physiotherapy. 2015;61:218. doi: 10.1016/j.jphys.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 12.de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 13.Diatchenko L, Slade GD, Nackley AG, Maixner W. Responses to Drs. Kim and Dionne regarding comments on Diatchenko, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–24. doi: 10.1016/j.pain.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pain. 2007;129:366–2370. [Google Scholar]
- 14.Dunne RL, Kenardy J, Sterling M. A randomized controlled trial of cognitive-behavioral therapy for the treatment of PTSD in the context of chronic whiplash. Clin J Pain. 2012;28:755–765. doi: 10.1097/AJP.0b013e318243e16b. [DOI] [PubMed] [Google Scholar]
- 15.Dunne-Proctor RL, Kenardy J, Sterling M. The Impact of Posttraumatic Stress Disorder on Physiological Arousal, Disability and Sensory Pain Thresholds in Patients with Chronic Whiplash. Clin J Pain. 2015 doi: 10.1097/AJP.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 16.Favrole P, Jehel L, Levy P, Descombes S, Muresan I, Manifacier M, Alamowitch S. Frequency and predictors of post-traumatic stress disorder after stroke: A pilot study. J Neurol Sci. 2013;327:35–40. doi: 10.1016/j.jns.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Gopinath B, Jagnoor J, Nicholas M, Blyth F, Harris I, Casey P, Cameron I. Presence and predictors of persistent pain among persons who sustained an injury in a road traffic crash. European Journal of Pain. 2015;19:1111–1118. doi: 10.1002/ejp.634. [DOI] [PubMed] [Google Scholar]
- 18.Ising M, Depping A, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Müller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 19.Jenewein J, Wittmann L, Moergeli H, Creutzig J, Schnyder U. Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: a longitudinal study. J Trauma Stress. 2009;22:540–548. doi: 10.1002/jts.20453. [DOI] [PubMed] [Google Scholar]
- 20.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimerling R, Clum GA, Wolfe J. Relationships among trauma exposure, chronic posttraumatic stress disorder symptoms, and self-reported health in women: replication and extension. J Trauma Stress. 2000;13:115–128. doi: 10.1023/A:1007729116133. [DOI] [PubMed] [Google Scholar]
- 22.Liedl A, O'Donnell M, Creamer M, Silove D, McFarlane A, Knaevelsrud C, Bryant R. Support for the mutual maintenance of pain and post-traumatic stress disorder symptoms. Psychol Med. 2010;40:1215–1223. doi: 10.1017/S0033291709991310. [DOI] [PubMed] [Google Scholar]
- 23.Liedl A, Knaevelsrud C. Chronic pain and PTSD: the Perpetual Avoidance Model and its treatment implications. Torture. 2008;18:69–76. [PubMed] [Google Scholar]
- 24.Maiarù M, Tochiki KK, Cox MB, Annan LV, Bell CG, Feng X, Hausch F, Géranton SM. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Science translational medicine. 2016;8:325ra19–325ra19. doi: 10.1126/scitranslmed.aab3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall GN, Schell TL, Glynn SM, Shetty V. The role of hyperarousal in the manifestation of posttraumatic psychological distress following injury. J Abnorm Psychol. 2006;115:624. doi: 10.1037/0021-843X.115.3.624. [DOI] [PubMed] [Google Scholar]
- 26.McFarlane AC, Atchison M, Rafalowicz E, Papay P. Physical symptoms in post-traumatic stress disorder. J Psychosom Res. 1994;38:715–726. doi: 10.1016/0022-3999(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 27.McFarlane AC. Stress-related musculoskeletal pain. Best Practice & Research Clinical Rheumatology. 2007;21:549–565. doi: 10.1016/j.berh.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 28.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67:783–790. doi: 10.1097/01.psy.0000181276.49204.bb. [DOI] [PubMed] [Google Scholar]
- 29.McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM. Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. Pain. 2014;155:309–321. doi: 10.1016/j.pain.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean SA. The potential contribution of stress systems to the transition to chronic whiplash-associated disorders. Spine (Phila Pa 1976) 2011;36:S226–32. doi: 10.1097/BRS.0b013e3182387fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishi D, Matsuoka Y, Yonemoto N, Noguchi H, Kim Y, Kanba S. Peritraumatic Distress Inventory as a predictor of post-traumatic stress disorder after a severe motor vehicle accident. Psychiatry Clin Neurosci. 2010;64:149–156. doi: 10.1111/j.1440-1819.2010.02065.x. [DOI] [PubMed] [Google Scholar]
- 32.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report. 2010;(26):1–31. [PubMed] [Google Scholar]
- 33.Platts-Mills TF, Hunold KM, Esserman DA, Sloane PD, McLean SA. Motor Vehicle Collision–related Emergency Department Visits by Older Adults in the United States. Acad Emerg Med. 2012;19:821–827. doi: 10.1111/j.1553-2712.2012.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platts-Mills TF, Ballina L, Bortsov AV, Soward A, Swor RA, Jones JS, Lee DC, Peak DA, Domeier RM, Rathlev NK. Using emergency department-based inception cohorts to determine genetic characteristics associated with long term patient outcomes after motor vehicle collision: Methodology of the CRASH study. BMC emergency medicine. 2011;11:14. doi: 10.1186/1471-227X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schell TL, Marshall GN, Jaycox LH. All symptoms are not created equal: the prominent role of hyperarousal in the natural course of posttraumatic psychological distress. J Abnorm Psychol. 2004;113:189. doi: 10.1037/0021-843X.113.2.189. [DOI] [PubMed] [Google Scholar]
- 36.Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001;21:857–877. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 37.Smith TW, Aberger EW, Follick MJ, Ahern DK. Cognitive distortion and psychological distress in chronic low back pain. J Consult Clin Psychol. 1986;54:573. doi: 10.1037//0022-006x.54.4.573. [DOI] [PubMed] [Google Scholar]
- 38.Sterling M, Hendrikz J, Kenardy J. Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain. 2011;152:1272–1278. doi: 10.1016/j.pain.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 39.Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122:102–108. doi: 10.1016/j.pain.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Physical and psychological factors predict outcome following whiplash injury. Pain. 2005;114:141–148. doi: 10.1016/j.pain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Sterling M, Kenardy J, Jull G, Vicenzino B. The development of psychological changes following whiplash injury. Pain. 2003;106:481–489. doi: 10.1016/j.pain.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Büll DR, Ionescu IA, Heinzmann J, Knapman A, Siebertz A, Depping A. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Ulirsch JC, Weaver MA, Bortsov AV, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM. No man is an island: Living in a disadvantaged neighborhood influences chronic pain development after motor vehicle collision. Pain. 2014;155:2116–2123. doi: 10.1016/j.pain.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 45.Weston CS. Posttraumatic stress disorder: a theoretical model of the hyperarousal subtype. Front Psychiatry. 2014;5:37. doi: 10.3389/fpsyt.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. World Report on Road Traffic Injury Prevention; 2004. World Health Organization: Geneva; 2008. [Google Scholar]