Abstract
Purpose
Alternative lengthening of telomeres (ALT), a telomerase-independent telomere maintenance mechanism, is strongly associated with ATRX and DAXX alterations and occurs frequently in pancreatic neuroendocrine tumors (PanNETs).
Experimental design
In a Korean cohort of 269 surgically resected primary PanNETs and 19 sporadic microadenomas, ALT status and nuclear ATRX and DAXX protein expression were assessed and compared with clinicopathologic factors.
Results
In PanNETs, ALT or loss of ATRX/DAXX nuclear expression was observed in 20.8% and 19.3%, respectively, while microadenomas were not altered. ALT-positive PanNETs displayed a significantly higher grade, size, and pT classification (all, p<0.001). ALT also strongly correlated with lymphovascular (p<0.001) and perineural invasion (p=0.001), and the presence of lymph node (p<0.001) and distant metastases (p=0.002). Furthermore, patients with ALT-positive primary PanNETs had a shorter recurrence-free survival (HR=3.38, 95% CI=1.83–6.27; p<0.001). Interestingly, when limiting to patients with distant metastases, those with ALT-positive primary tumors had significantly better overall survival (HR=0.23, 95% CI=0.08–0.68; p=0.008). Similarly, tumors with loss of ATRX/DAXX expression were significantly associated with ALT (p<0.001), aggressive clinical behavior, and reduced recurrence-free survival (p<0.001). However, similar to ALT, when limiting to patients with distant metastases, loss of ATRX/DAXX expression was associated with better overall survival (p=0.003).
Conclusions
Both primary ALT-positive and ATRX/DAXX-negative PanNETs are independently associated with aggressive clinicopathologic behavior and displayed reduced recurrence-free survival. In contrast, ALT activation and loss of ATRX/DAXX are both associated with better overall survival in patients with metastases. Therefore, these biomarkers may be used as prognostic markers depending on the context of the disease.
Keywords: Pancreatic neuroendocrine tumors, ALT, ATRX, DAXX, Prognosis
INTRODUCTION
Pancreatic neuroendocrine tumors (PanNETs) are rare and comprise 1–3% of all pancreatic tumors (1, 2). Patients with PanNETs usually display a better survival than those with pancreatic ductal adenocarcinomas (3–6). However, PanNETs are still malignant, as evidenced by the fact that the 10-year survival rate for PanNET patients is only 40–50% (7–10). Although surgical resection is the only curative treatment option for patients diagnosed with a PanNET (11, 12), a growing number of medical therapies have been utilized in recent years for unresectable or metastatic PanNET patients, including somatostatin analogs, cytotoxic chemotherapies, and molecular targeted therapies, such as mTOR inhibitors (12, 13). To better predict clinical outcomes and to identify patients who may ultimately benefit from targeted therapies, a better understanding of the molecular mechanisms driving PanNETs is warranted.
Whole exome sequencing studies of PanNETs have demonstrated prevalent mutations in several genes, including ATRX, DAXX, MEN1, and mammalian target of rapamycin (mTOR) pathway genes (14). In particular, Jiao et al. reported that 43% of PanNETs harbored inactivating mutations in either the ATRX or DAXX gene; the presence of these mutations were mutually exclusive (14). Consequently, alterations in these two proteins are tightly associated with alternative lengthening of telomeres (ALT) in PanNETs (15, 16) and other tumor types (17–19).
ALT is a telomerase-independent mechanism of telomere maintenance, and ALT-positive tumors are thought to co-opt the homologous recombination machinery for telomere maintenance (20). Characteristics of ALT-positive tumors include the presence of large, ultra-bright telomeric DNA FISH signals, dramatic telomere length heterogeneity, the presence of extrachromosomal telomeric DNA, and increased chromosomal instability (21, 22). In previous studies, mostly including Caucasian patients, ALT was identified in a substantial fraction of PanNETs (48–61%), although a lower prevalence (15%) was observed in a small Korean cohort (15, 17, 21, 23). These studies have consistently observed a significant correlation between the presence of ALT and alterations in ATRX or DAXX. However, the prognostic effect of ALT and ATRX or DAXX alterations in PanNETs remains inconclusive. Previous studies reported that ALT activation is associated with better prognosis (14, 24), while another reported ALT is associated with an aggressive phenotype and a poor prognosis (21). In addition, recent studies have suggested that ATRX loss and/or ALT-positivity confers in vitro sensitivity to inhibition of the DNA damage mediator, ATR, as well as topoisomerase inhibitors and radiation (25, 26). Therefore, the study of ALT and ATRX or DAXX loss in PanNETs is warranted from a prognostic, as well as a potential therapeutic, standpoint.
Hence, we evaluated a well-characterized, large Korean cohort of primary PanNETs associated with or without synchronous or metachronous metastases. Using this cohort, we evaluated the presence of ALT and alterations in ATRX or DAXX expression and correlated these findings with clinicopathologic factors.
MATERIALS AND METHODS
Case selection
Two hundred sixty-nine primary PanNETs and 19 sporadic neuroendocrine microadenomas resected between January 1995 and March 2015 were retrieved from the Department of Pathology at the Asan Medical Center. Neuroendocrine microadenomas were defined as well-differentiated, non-functional neuroendocrine tumors <0.5 cm in diameter, while PanNETs were neuroendocrine neoplasms ≥0.5 cm in diameter (27, 28). All PanNETs were classified according to the 2010 WHO classification using mitotic activity and the Ki-67 labeling index (28). Poorly differentiated neuroendocrine carcinomas, including small cell carcinomas and large cell carcinomas were not included in this study. Clinical data, such as patients’ age, gender, symptoms, survival status, and survival time were reviewed. Pathologic data were extracted from the pathology reports, including tumor size, extension, lymph node and distant metastases, and perineural and lymphovascular tumor invasion. The protein expression profiles of specific peptide hormones, including insulin, glucagon-like peptide 1, glucagon, gastrin, serotonin, and somatostatin were determined previously (29). The extent of tumor was evaluated according to the T classification of the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging system (30).
Tissue microarray construction
Tissue microarrays were constructed from archived, formalin-fixed, paraffin-embedded tissue blocks with a manual tissue microarrayer (Uni TMA Co. Ltd, Seoul, Korea). Three cores from tumors and one core from normal pancreatic parenchyma with a diameter of 2.0 mm were punched from each PanNET or neuroendocrine microadenomas and placed into recipient blocks. From the tissue microarray, 4-µm-thick sections were cut.
ATRX and DAXX immunohistochemistry
Immunohistochemical labeling for ATRX and DAXX was performed as previously described (15). Anti-ATRX (HPA001906, Sigma-Aldrich, St Louis, MO, USA, 1:300) and anti-DAXX (HPA008736, Sigma-Aldrich, 1:100) antibodies were used. Briefly, 4-µm thick tissue sections were deparaffinized and hydrated in xylene and serially diluted in ethanol. For antigen retrieval, sections were steamed with EDTA for anti-ATRX, and citrate buffer for anti-DAXX for 50 min and 30 min, respectively. After cooling for 5 min, sections were blocked against endogenous peroxidase activity with dual endogenous enzyme blocking agent (Dako, Carpentaria, CA, USA) for 10 min. Sections were incubated with primary antibodies for 1 hour at room temperature followed by secondary antibody (Leica Microsystems) for 30 min and detected with 3,30-diaminobenzidine (Sigma-Aldrich) after 10 min. Wash steps were performed with phosphate buffered saline containing 0.1% Tween-20 for 5 min. Sections were counterstained with hematoxylin, rehydrated, and mounted. Immunohistochemical labeling was successfully performed on 264 of the 269 (98.1%) PanNETs on the tissue microarrays. Some cases (4 cases of ATRX and 5 cases of DAXX) were not immunolabeled successfully due to loss of tissue cores during the sectioning or labeling process.
Immunolabeling for ATRX and DAXX was considered positive if >5% of neoplastic cells had nuclear staining, as previously described (31). Neoplasms were scored as negative for ATRX or DAXX if the pattern was that of cytoplasmic accumulation with nuclear clearing, as long as the adequate internal controls (i.e., nuclear labeling of adjacent endothelial cells, lymphocytes, and/or islets of Langerhans) were present (14, 21). Cases with inadequate internal controls were validated by re-labeling whole sections of each case.
Telomere fluorescence in situ hybridization (FISH)
Briefly, deparaffinized slides were hydrated, steamed for 25 minutes in citrate buffer (Vector Laboratories, Burlingame, CA), dehydrated, and hybridized with a Cy3-labeled peptide nucleic acid (PNA) probe complementary to the mammalian telomere repeat sequence ([N-terminus to C-terminus] CCCTAACCCTAACCCTAA). As a positive control for hybridization efficiency, an Alexafluor-488 labeled PNA probe specific to human centromeric DNA repeats (ATTCGTTGGAAACGGGA; CENP-B binding sequence) was included in the hybridization solution. Following post-hybridization washes, the slides were counterstained with DAPI. Slides were imaged with a Nikon 50i epifluorescence microscope equipped with X-Cite series 120 illuminator (EXFO Photonics Solutions Inc., Ontario, CA) and appropriate fluorescence excitation/emission filters. Grayscale images were captured using Nikon NIS-Elements software and an attached Photometrics CoolsnapEZ digital camera, pseudo-colored and merged.
Characteristics of ALT-positive tumors in fixed tissue specimens are dramatic cell-to-cell telomere length heterogeneity and the presence of large, ultra-bright nuclear foci of telomere FISH signals marking ALT-associated telomeric DNA in interphase nuclei. As such, cases were visually assessed and classified as ALT-positive if they met the following criteria: 1) the presence of very bright nuclear foci of telomere FISH signals (e.g. total intensities for individual foci have been previously measured to be >10-fold that of mean integrated signal intensities for individual non-neoplastic stromal cells in the same cases)(15); and 2) ≥1% of tumor cells with ALT-associated telomeric foci. ALT-negativity was defined by the lack of ALT-associated telomeric foci in ≥1% of tumor cells, with at least 500 tumor cells evaluated (17). Areas exhibiting necrosis were excluded from consideration.
Statistical analysis
Statistical analysis was performed with χ2 and Fisher exact tests to identify correlations between ALT and other clinicopathologic factors. The overall survival and recurrence-free survival rate was analyzed by the Kaplan-Meier method, and significance was evaluated with the log-rank test. The Cox proportional hazards regression model was used to investigate the significance of ALT as a prognostic factor. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL).
RESULTS
Characteristics of cases
Patient characteristics and molecular features of the tumors are shown in Table 1. The mean age of the patients (127 men and 142 women) was 52.3 ± 0.8 years. According to the 2010 WHO classification scheme, 181 PanNETs were G1, 80 were G2, and 8 were G3 (well-differentiated neuroendocrine carcinomas). Overall, the mean tumor size was 3.0 ± 0.1 cm. While 113 cases were classified as pT1, the others were classified at a higher pT stage (109 as pT2, 44 as pT3, and 3 as pT4). Seventy cases (26.0%) had lymphovascular invasion, and 35 cases (13.0%) had perineural invasion. Lymph node metastasis occurred in 35 cases (13.0%), synchronous distant metastasis occurred in 10 cases (3.7%), and metachronous distant metastasis occurred in 32 cases (11.9%). Of the 269 total PanNETs, 265 were sporadic, and 2 were associated with Multiple Endocrine Neoplasia type 1 (MEN-1) syndrome and 2 with the von Hippel Lindau syndrome. Across the entire cohort, the median follow-up time was 28 months (range, 1–188 months).
Table 1.
Comparison of ALT and ATRX/DAXX expression status with clinicopathologic factors of PanNETs
| Clinicopathologic factors | ALT status | ATRX or DAXX expression | ||||||
|---|---|---|---|---|---|---|---|---|
| Number | Negative | Positive | P-value | Intact | Loss | P-value | ||
| Age (years) | ≤60 | 189 | 152 (80.4%) | 37 (19.6%) | 0.441 | 152 (82.2%) | 33 (17.8%) | 0.351 |
| >60 | 80 | 61 (76.2%) | 19 (23.8%) | 61 (77.2%) | 18 (22.8%) | |||
| Sex | Male | 127 | 94 (74.0%) | 33 (26.0%) | 0.048* | 97 (77.6%) | 28 (22.4%) | 0.229 |
| Female | 142 | 119 (83.8%) | 23 (16.2%) | 116 (83.5%) | 23 (16.5%) | |||
| Grade | Grade 1 | 181 | 158 (87.3%) | 23 (12.7%) | <0.001* | 157 (88.7%) | 20 (11.3%) | <0.001* |
| Grade 2 | 80 | 49 (61.2%) | 31 (38.8%) | 50 (63.3%) | 29 (36.7%) | |||
| Grade 3 | 8 | 6 (75.0%) | 2 (25.0%) | 6 (75.0%) | 2 (25.0%) | |||
| Ki-67 index | <3% | 195 | 167 (85.6%) | 28 (14.4%) | <0.001* | 166 (86.9%) | 25 (13.1%) | <0.001* |
| 3–20% | 66 | 40 (60.6%) | 26 (39.4%) | 41 (63.1%) | 24 (36.9%) | |||
| >20% | 8 | 6 (75.0%) | 2 (25.0%) | 6 (75.0%) | 2 (25.0%) | |||
| Size | ≤2 cm | 119 | 112 (94.1%) | 7 (5.9%) | <0.001* | 114 (96.6%) | 4 (3.4%) | <0.001* |
| >2 cm | 150 | 101 (67.3%) | 49 (32.7%) | 101 (68.2%) | 47 (31.8%) | |||
| pT classification | pT1 | 113 | 109 (96.5%) | 4 (3.5%) | <0.001* | 108 (98.2%) | 2 (1.8%) | <0.001* |
| pT2–pT4 | 156 | 104 (66.7%) | 52 (33.3%) | 105 (68.2%) | 49 (31.8%) | |||
| Lymphovascular invasion | Absence | 199 | 173 (86.9%) | 26 (13.1%) | <0.001* | 173 (88.7%) | 22 (11.3%) | <0.001* |
| Presence | 70 | 40 (57.1%) | 30 (42.9%) | 40 (58.0%) | 29 (42.0%) | |||
| Perineural invasion | Absence | 234 | 193 (82.5%) | 41 (17.5%) | 0.001* | 191 (83.4%) | 38 (16.6%) | 0.004* |
| Presence | 35 | 20 (57.1%) | 15 (42.9%) | 22 (62.9%) | 13 (37.1%) | |||
| Lymph node metastasis | Absence | 234 | 194 (82.9%) | 40 (17.1%) | <0.001* | 193 (84.3%) | 36 (15.7%) | <0.001* |
| Presence | 35 | 19 (54.3%) | 16 (45.7%) | 20 (57.1%) | 15 (42.9%) | |||
| Distant metastasis | Absence | 259 | 209 (80.7%) | 50 (19.3%) | 0.002* | 209 (82.3%) | 45 (17.7%) | 0.001* |
| Presence | 10 | 4 (40.0%) | 6 (60.0%) | 4 (40.0%) | 6 (60.0%) | |||
| Insulin expression | Absence | 124 | 86 (69.4%) | 38 (30.6%) | <0.001* | 82 (68.3%) | 38 (31.7%) | <0.001* |
| Presence | 43 | 43 (100.0%) | 0 (0.0%) | 42 (100.0%) | 0 (0.0%) | |||
| GLP-1 expression | Absence | 141 | 105 (74.5%) | 36 (25.5%) | 0.046* | 100 (73.5%) | 36 (26.5%) | 0.038* |
| Presence | 26 | 24 (92.3%) | 2 (7.7%) | 24 (92.3%) | 2 (7.7%) | |||
| Hormone expression | Absence | 94 | 59 (62.8%) | 35 (37.2%) | <0.001* | 55 (61.1%) | 35 (38.9%) | <0.001* |
| Presence | 73 | 70 (95.9%) | 3 (4.1%) | 69 (95.8%) | 3 (4.2%) | |||
Statistically significant at P<0.05.
The mean size of the neuroendocrine microadenomas was 0.3 ± 0.1cm and the mean age of these patients was 63.0 ± 6.4 years. Pancreatic lesions associated with microadenomas consisted of 8 ductal adenocarcinomas, 3 sporadic PanNETs, 3 chronic pancreatitis, 2 intraductal papillary mucinous neoplasms, 1 mucinous cystic neoplasms, 1 serous cystadenoma, and 1 type 1 autoimmune pancreatitis.
ALT activation in PanNETs
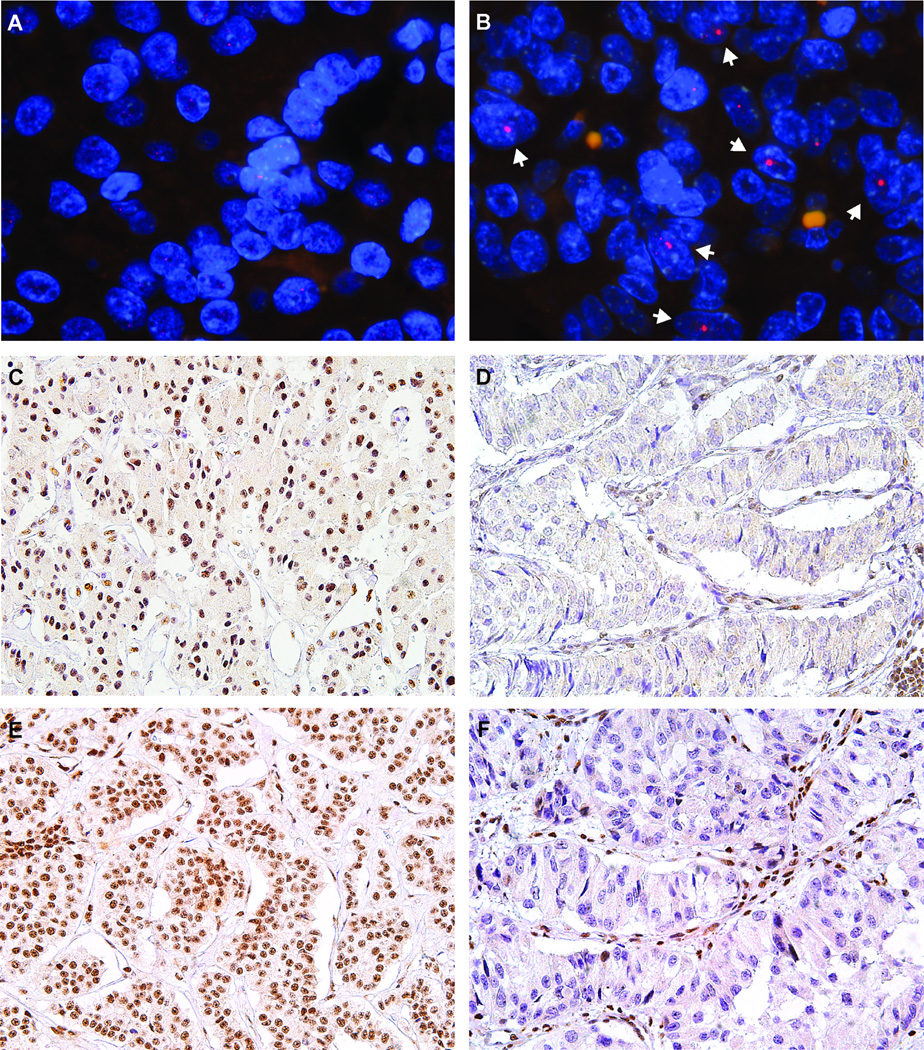
ALT was assessed in our cohort by telomere-specific FISH and representative images are depicted in Figure 1A–B. None of the 19 neuroendocrine microadenomas from sporadic PanNETs were ALT-positive (0%, 0/19 cases), while 20.8% (56/269 cases) of PanNETs were ALT-positive. All 56 ALT-positive tumors were sporadic PanNETs; none of the 4 hereditary PanNETs showed activation of ALT. As shown in Table 1, ALT-positive PanNETs were more commonly observed in tumors with higher grade, higher Ki-67 index, larger size, and higher pT classification (p<0.001 for all). ALT-positive PanNETs were also associated with the presence of lymphovascular (p<0.001) and perineural (p=0.001) invasion. Additionally, ALT-positive PanNETs were strongly associated lymph node metastasis (p<0.001), distant metastasis (p=0.002), absence of insulin expression (p<0.001), absence of GLP-1 expression (p=0.046), and without any hormone expression (p<0.001). Strikingly, the presence of ALT increased along with tumor size – larger tumors were more likely to display the ALT phenotype than smaller tumors (Figure 2A; p<0.001).
Figure 1.
Representative images of telomere-specific FISH and ATRX and DAXX immunostaining. (A) A PanNET that is ALT-negative and (B) a PanNET that is ALT-positive. The telomere foci (arrows) are indicative of ALT. For A and B; telomeres are depicted in red. (C) A PanNET with intact ATRX expression (40×) and (D) a PanNET with loss of nuclear ATRX expression. Tumor cells show loss of ATRX expression, while lymphocytes and endothelial cells demonstrate intact ATRX (40×). (E) A PanNET with intact DAXX expression (40×), and (F) a PanNET with loss of nuclear DAXX expression. Tumor cells show loss of nuclear DAXX expression, while lymphocytes and endothelial cells demonstrate intact DAXX expression (40×).
Figure 2.
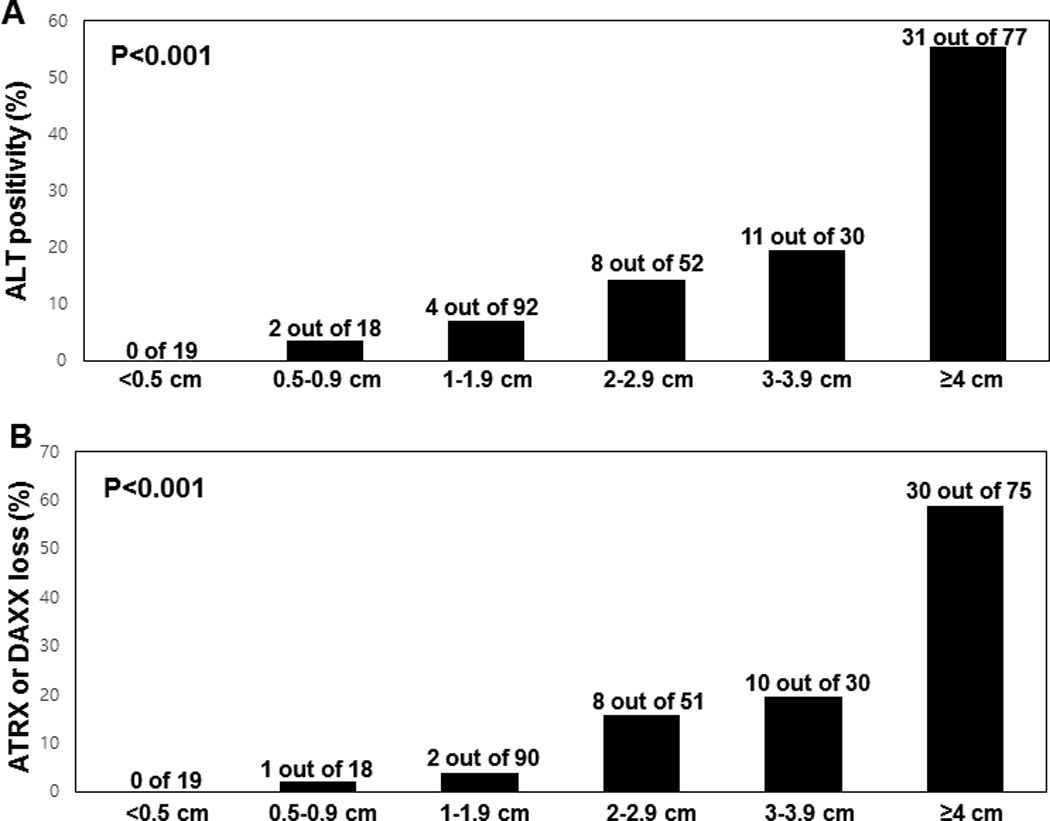
The correlation of ALT and ATRX and DAXX immunostaining with tumor size. The proportion of (A) ALT-positive or (B) ATRX- or DAXX-negative tumors increases with the size of the tumor (p<0.001).
ATRX and DAXX expression and correlation with ALT activation
Representative images of ATRX and DAXX immunostaining are depicted in Figure 1C–F. None of the 19 neuroendocrine microadenomas from sporadic PanNET cases showed loss of nuclear ATRX or DAXX expression (0%, 0/19 cases), while 8.6% (23/274) and 10.4% (28/273) of PanNETs showed loss of nuclear ATRX or DAXX expression, respectively. Altogether, 51 cases (19.0%) showed loss of nuclear expression of either ATRX or DAXX; this loss of protein expression was mutually exclusive. ALT was observed in 50 of 51 cases (98.0%) with loss of nuclear ATRX or DAXX expression, in addition to 6 cases (2.8%) with intact nuclear ATRX and DAXX expression (Supplementary Table S1). One case showed loss of nuclear ATRX expression, but was ALT-negative. Overall, ALT activation was significantly correlated with loss of nuclear ATRX or DAXX expression (p<0.001). Given the near perfect association between ALT-positivity and the loss of nuclear ATRX or DAXX observed, all correlations between ALT-positive PanNETs and clinicopathologic variables reported here are nearly identical when considering loss of ATRX or DAXX rather than ALT status (Table 1). Thus, similar to the ALT results, loss of nuclear ATRX or DAXX expression increased with tumor size (Figure 2B; p<0.001).
Recurrence-free survival analysis based on ALT status in PanNETs without metastasis
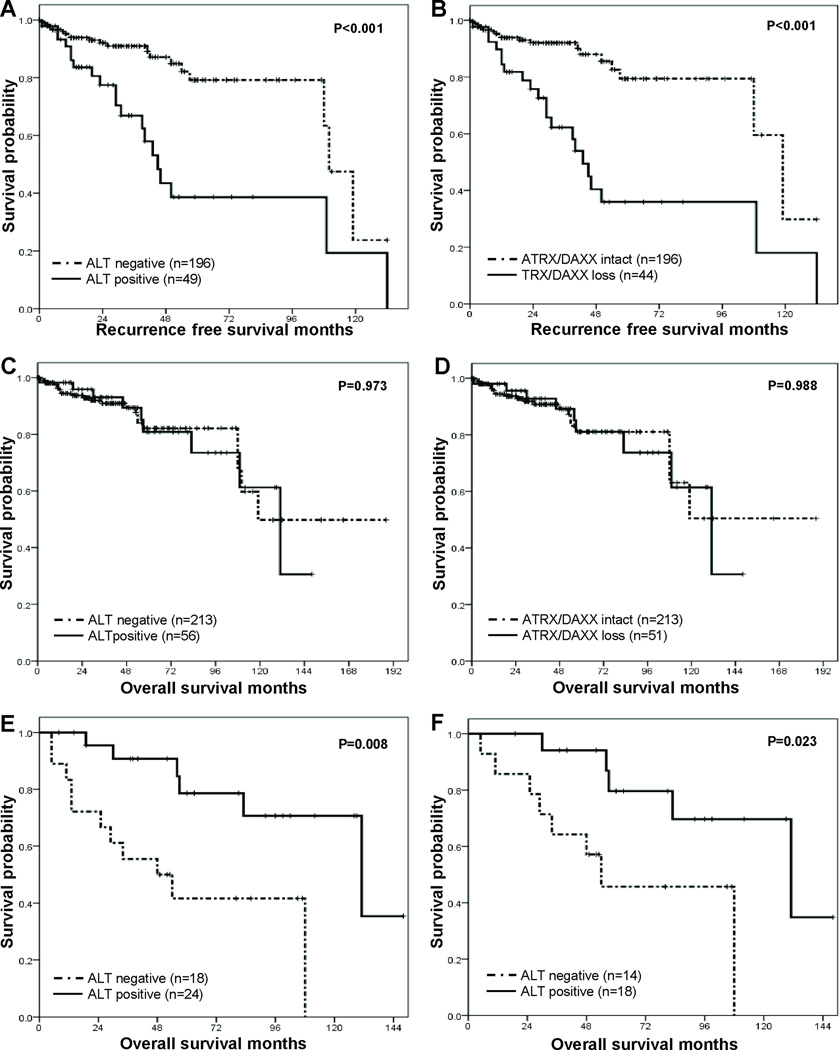
After excluding cases with R1 (N=14) resection or synchronous (N=10) distant metastases, the recurrence-free survival was analyzed for 245 primary PanNETs. The recurrence-free survival in patients with ALT-positive primary tumors was significantly worse than those with ALT-negative primary tumors (HR=3.38, 95% CI=1.83–6.27; p<0.001, Figure 3A). Similarly, patients with tumors demonstrated to have nuclear ATRX or DAXX loss had worse recurrence-free survival than those with intact ATRX and DAXX protein expression (HR=4.01, 95% CI=2.14–7.50; p<0.001, Figure 3B).
Figure 3.
Kaplan-Meier survival analyses. (A) The recurrence-free survival of ALT-positive primary PanNET patients was significantly worse than those with ALT-negative (5-year survival rate, 38.7% vs. 79.2%; p<0.001). (B) Primary PanNET patients with nuclear ATRX or DAXX loss had worse recurrence-free survival than those with intact ATRX and DAXX expression (5-year survival rate, 36.0% vs. 79.4%; p<0.001). (C) The overall survival in patients with ALT-positive primary PanNETs was not significantly different from those with ALT-negative tumors (5-year survival rate, 80.8% vs. 82.1%; p=0.973). (D) The overall survival in primary PanNET patients with ATRX or DAXX loss was not significantly different from those with ALT-negative tumors (5-year survival rate, 81.1% vs. 81.1%; p=0.988). (E) Synchronous and metachronous metastatic PanNET patients with ALT-positive tumors had significantly better overall survival time than those with ALT-negative tumors (5-year survival rate, 78.6% vs. 41.7%; p=0.008). (F) In metachronous metastatic PanNETs, the overall survival rate in metastatic PanNET patients with ALT-positivity was significantly better than those with ALT-negativity (5 year survival rate 79.6% vs. 45.7%; p=0.023).
Other clinicopathologic factors associated with poor recurrence-free survival were older age (>60 years), higher grade, higher Ki-67 index, large size, high T classification, presence of lymphovascular and perineural invasion, and lymph node metastasis (Table 2). In a multivariate analysis, presence of ALT (HR=1.97, 95% CI=1.02–3.78; p=0.043), older age (HR=2.28, 95% CI=1.18–4.41; p=0.014), and higher grade (HR=5.49, 95% CI=2.46–12.24; p<0.001) were independent poor prognostic factors (Table 2). Next, due to the robust correlation between the presence of ALT and loss of ATRX/DAXX expression, we substituted loss of ATRX/DAXX for ALT status in another model. In this multivariate analysis, loss of ATRX/DAXX expression (HR=2.26, 95% CI=1.17–4.39; p=0.016), older age (HR=2.18, 95% CI=1.12–4.27; p=0.022), and higher grade (HR=5.06, 95% CI=2.26–11.33; p<0.001) remained independent poor prognostic factors.
Table 2.
Recurrence-free survival in M0 PanNET patients
| Clinicopathologic factors | Univariate analysis (n=245) | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| ALT | Negative | 196 | 1.00 | 1.00 | ||||
| Positive | 49 | 3.38 | 1.83–6.27 | <0.001* | 1.97 | 1.02–3.78 | 0.043* | |
| ATRX/DAXX | Intact | 196 | 1.00 | |||||
| Loss | 44 | 4.01 | 2.14–7.50 | <0.001* | ||||
| Age | ≤60 | 173 | 1.00 | 1.00 | ||||
| >60 | 72 | 1.95 | 1.03–3.72 | 0.042* | 2.28 | 1.18–4.41 | 0.014* | |
| Sex | Male | 114 | 1.00 | |||||
| Female | 131 | 0.93 | 0.50–1.73 | 0.814 | ||||
| Grade | G1 | 173 | 1.00 | 1.00 | ||||
| G2 or G3 | 72 | 7.47 | 3.60–15.50 | <0.001* | 5.49 | 2.46–12.24 | <0.001* | |
| Ki-67 index | <3% | 183 | 1.00 | |||||
| ≥3% | 62 | 5.47 | 2.72–10.99 | <0.001* | ||||
| Size | ≤2cm | 114 | 1.00 | 1.00 | ||||
| >2 cm | 131 | 2.37 | 1.16–4.86 | 0.018* | 1.29 | 0.55–3.04 | 0.558 | |
| pT classification | pT1 | 108 | 1.00 | 1.00 | ||||
| pT2–4 | 137 | 2.25 | 1.10–4.62 | 0.026* | 0.38 | 0.05–2.75 | 0.339 | |
| Lymphovascular invasion | Absence | 187 | 1.00 | 1.00 | ||||
| Presence | 58 | 2.38 | 1.26–4.49 | 0.007* | 1.51 | 0.74–3.07 | 0.255 | |
| Perineural invasion | Absence | 219 | 1.00 | 1.00 | ||||
| Presence | 26 | 2.86 | 1.30–6.28 | 0.009* | 1.42 | 0.49–4.07 | 0.518 | |
| Lymph node metastasis | Absence | 220 | 1.00 | 1.00 | ||||
| Presence | 25 | 4.86 | 2.37–9.95 | <0.001* | 2.04 | 0.94–4.43 | 0.071 | |
Abbreviation: CI, confidence interval; HR, hazard ratio.
Statistically significant at P<0.05.
In the entire cohort (N=269), the overall survival in patients with ALT-positive tumors was not significantly different from those with ALT-negative tumors (HR=1.01, 95% CI=0.47–2.20; p=0.973, Figure 3C). Similarly, overall survival did not differ between patients with ATRX or DAXX loss and intact ATRX and DAXX expression (HR=1.01, 95% CI=0.46–2.21; p=0.988, Figure 3D).
Survival analysis of ALT in PanNETs with distant metastasis
Synchronous (N=10) or metachronous (N=32) metastatic PanNETs were observed, and within this subgroup, the patients with ALT-positive primary tumors had significantly better overall survival than those with ALT-negative tumors (HR=0.23, 95% CI=0.08–0.68; p=0.008, Figure 3E). Also, the patients with loss of ATRX or DAXX expression had better overall survival than those with intact ATRX and DAXX expression (HR=0.20, 95% CI=0.07–0.59; p=0.003). In addition, when limiting to only the metachronous metastatic PanNETs, the overall survival in patients with ALT-positive tumors remained significantly longer than those with ALT-negative tumors (HR=0.24, 95% CI=0.07–0.82; p=0.023, Figure 3F). Other clinicopathologic factors, including age, sex, grade, Ki-67 index, size, pT classification, lymphovascular invasion, perineural invasion, or lymph node metastasis were not significantly associated with overall survival of metastatic PanNETs (Table 3).
Table 3.
Overall survival in PanNET patients with synchronous and metachronous metastasis
| Clinicopathologic factors | No. | HR | 95% CI | P-value | |
|---|---|---|---|---|---|
| ALT | Negative | 18 | 1.00 | ||
| Positive | 24 | 0.23 | 0.08–0.68 | 0.008* | |
| ATRX/DAXX | Intact | 17 | 1.00 | ||
| Loss | 25 | 0.20 | 0.07–0.59 | 0.003* | |
| Age (years) | ≤60 | 29 | 1.00 | ||
| >60 | 13 | 2.14 | 0.82–5.60 | 0.121 | |
| Sex | Male | 24 | 1.00 | ||
| Female | 18 | 1.38 | 0.53–3.59 | 0.506 | |
| Grade | Grade 1 | 14 | 1.00 | ||
| Grade 2 or 3 | 28 | 2.28 | 0.72–7.17 | 0.160 | |
| Ki-67 index | <3% | 20 | 1.00 | ||
| ≥3% | 22 | 2.41 | 0.86–6.72 | 0.094 | |
| Size | ≤2 cm | 3 | 1.00 | ||
| >2 cm | 39 | 0.85 | 0.11–6.54 | 0.880 | |
| pT classification | pT1 | 4 | 1.00 | ||
| pT2–pT4 | 38 | 1.37 | 0.18–10.48 | 0.762 | |
| Lymphovascular invasion | Absence | 17 | 1.00 | ||
| Presence | 25 | 1.10 | 0.41–2.99 | 0.850 | |
| Perineural invasion | Absence | 29 | 1.00 | ||
| Presence | 13 | 1.47 | 0.50–4.28 | 0.484 | |
| Lymph node metastasis | Absence | 23 | 1.00 | ||
| Presence | 19 | 1.89 | 0.72–4.97 | 0.200 |
Abbreviation: CI, confidence interval; HR, hazard ratio.
Statistically significant at P<0.05.
Subgroup survival analysis according to clinicopathologic factors
Next, subgroup analyses based on clinicopathologic factors were performed. ALT-positive PanNET patients with lymph node metastasis had a tendency of better survival than ALT-negative PanNET patients with nodal metastasis (HR=0.31, 95% CI=0.08–1.19; p=0.088, Supplementary Figure S1A). In addition, ALT-positive PanNET patients with distant metastasis (overall 5-year survival rate, 75.0%) trended toward better survival than ALT-negative patients (25.0%), but this association was not statistically significant (p=0.118, Supplementary Figure S1B).
DISCUSSION
In most human cancers, neoplastic cells can proliferate unlimitedly by maintaining their telomere length via activating the enzyme telomerase; however, 5–10% of human malignancies activate the ALT pathway to maintain telomere lengths in neoplastic cells (24). ALT is a telomerase-independent mechanism to maintain telomere lengths that is thought to be mediated through homologous recombination (20). This process gives rise to dramatic nuclear foci containing large amounts of telomere DNA and significant telomere length heterogeneity within the neoplastic cells, features easily detected by telomere-specific FISH in archived specimens. ALT activation is closely associated with inactivating mutations in the ATRX and DAXX genes, which encode chromatin remodeling proteins required for incorporation of histone H3.3 at the telomeres (14, 15, 21).
Overall, in this Korean population, ALT activation was identified in 20.8% of primary PanNETs. Interestingly, this prevalence is considerably lower than that of the previous studies in Western populations (48–61%) (15, 21, 24) and in a Chinese population (54.1%) (32). However, the prevalence of ALT in PanNETs in the present study was similar to that of the previous Korean study assessing a smaller number of PanNETs (15%, 7 of 47 cases) (23). Combining the results of the present study with those from the previous reports, we hypothesize that differences in ALT prevalence exist among Western, Chinese, and Korean populations.
In the present study, ALT-positive primary PanNETs were associated with larger tumor size and higher pT classification, and ALT was not observed in any of the 19 neuroendocrine microadenomas. Strikingly, the prevalence of ALT dramatically increased with tumor size, suggesting that ALT activation occurs as a late event in PanNET tumorigenesis. These results are in concordance with those from de Wilde et al. who assessed neuroendocrine microadenomas and PanNETs from MEN-1 syndrome patients. The authors reported intact nuclear ATRX and DAXX expression and no ALT-positivity in all 47 neuroendocrine microadenomas; however, loss of nuclear ATRX or DAXX expression was only observed in a subset of large (>3 cm) PanNETs (31). Similarly, Marinoni et al. reported only 14.2% of small tumors (<2 cm) with loss of ATRX or DAXX expression were ALT-positive, whereas larger tumors (>2 cm) or metastatic PanNETs showed an almost perfect correlation between loss of ATRX or DAXX expression and ALT-positivity (21). In addition to these findings, we also observed that 6 ALT-positive PanNETs retained nuclear ATRX and DAXX expression. These findings may result from genetic alterations in ATRX or DAXX that confer a loss-of-function, but not loss of nuclear protein expression. Conversely, it is possible that additional drivers of ALT in PanNETs have not yet been identified. Taken together, we conclude that loss of ATRX or DAXX expression and activation of ALT occur as a relatively late event in PanNET tumorigenesis.
We also observed that ALT-positive PanNETs displayed aggressive behavior, such as higher grade, presence of lymphovascular and perineural invasion, and lymph node and distant metastasis. Previously, PanNET patients with insulin, GLP-1, and multiple hormone expression were reported to have a better survival than those without insulin, GLP-1, and multiple hormonal expression (29). In the present study, ALT-positive PanNETs correlated with a lack of insulin, GLP-1, and multiple hormone expression, which were previously associated as poor prognostic factors in PanNET patients.
Previous studies evaluating the prognostic effect of ALT and ATRX or DAXX alterations in PanNETs are contradictory. Jiao et al. and Dogeas et al. both reported that PanNET patients with ALT-positive or ATRX or DAXX altered tumors had better clinical outcomes (14, 24). In contrast, in a larger study, Marinoni et al. concluded that PanNET patients with ALT activation and loss of ATRX or DAXX displayed a worse prognosis (21). The discrepancy between previous studies could be explained by different study populations, as was indicated in Marinoni’s study (21). In the previous studies of Jiao and Dogeas, all patients were metastatic and most of them had higher pT classification (pT3 or pT4) (14, 24). In contrast, most of the patients included in Marinoni’s study were pT1 and pT2 and non-metastatic primary PanNETs (21). Our observations herein may help explain these apparent discrepancies. First, we observed a worse recurrence-free survival in patients with ALT-positive primary PanNETs which confirms the findings by Marioni and colleagues. Second, we also observed that ALT activation in primary PanNETs was significantly correlated with better overall survival when patients developed metastasis. This result is in agreement with those described by Jiao and colleagues and Dogeas and colleagues, who both observed that PanNET patients with ALT-positive or ATRX or DAXX mutated liver metastases had a longer survival time (14, 24). Our observations confirm the prognostic significance of ALT and ATRX or DAXX status with those of the previous studies. The presence of ALT was independently predictive of a worse prognosis in primary PanNETs and of a better prognosis in metastatic PanNETs. Thus, these results suggest that ALT may be used as a potential prognostic marker depending on the context of the disease. Possible explanations for this observation include potential differences in treatment response or that once ALT-positive clones establish as distant metastatic foci, these clones may grow more slowly in the new microenvironment. However, the exact molecular mechanism underlying ALT activation has not been elucidated and warrants further study.
Given the relatively large proportion of PanNETs that display the ALT phenotype and have ATRX or DAXX loss, the identification of therapies that target ALT-positive (or ATRX/DAXX-null) cancers would be tremendous for the field. Previous in vitro work has indicated that ALT-positive cancer cells are sensitive to inhibition of the DNA-damage mediator, ATR (25), while recent data suggests that ATRX loss sensitizes mouse-derived glioma cells to radiation and topoisomerase inhibitors (26). The data presented herein indicate that both ATRX or DAXX loss and ALT are effective prognostic biomarkers. Further study will elucidate whether ATRX or DAXX loss and/or the presence of ALT can predict response to therapy in PanNETs.
The present study has several strengths. We were able to analyze a large, well-characterized cohort of primary PanNET patients with detailed clinical and pathological information. Recurrence-free survival was calculated for PanNETs without metastasis, thus making a more precise estimation of the effect of ALT on PanNET patients without metastatic disease. Additionally, the telomere-specific FISH assay used to detect ALT was performed with the same methodology as some of the previous studies, thus facilitating direct comparison of the prevalence of ALT between different populations. However, this study also has some limitations. We report the ALT status in primary PanNET tissues only; clinical data provided the status of synchronous or metachronous metastases. We did not directly compare matched primary and metastatic PanNETs tissues. In addition, the median follow-up period of 28 months and the limited number of G3 tumors may not be adequate for complete evaluation of the biology of patients with PanNETs; thus, it would be informative to investigate these observations in cohorts with more extended follow-up information. Further, the use of tissue microarrays for our studies limits our ability to assess potential intra-tumoral heterogeneity. Finally, our cohort represents a population from a single center with a homogeneous ethnic background.
In conclusion, ALT activation and loss of ATRX or DAXX expression occurs at a relatively late stage of tumor progression. In our cohort, we have demonstrated that ALT-positive primary PanNETs display aggressive clinicopathologic behavior and have poor recurrence-free survival compared to their ALT-negative counterparts. In contrast, ALT activation is associated with better survival in patients with metastatic disease. In summary, ALT or loss of ATRX or DAXX expression may be used as potential prognostic markers depending on the context of the disease and may be eventually used to identify patients who may benefit from targeted therapies.
Supplementary Material
TRANSLATIONAL RELEVANCE.
A better understanding of the molecular mechanisms driving pancreatic neuroendocrine tumors (PanNETs) can help identify patients who will benefit from targeted therapies. We performed telomere-specific fluorescent in situ hybridization to determine the alternative lengthening of telomeres (ALT) status of 269 primary PanNETs and compared ALT status with nuclear ATRX and DAXX protein expression and clinicopathologic factors. In primary PanNETs, the ALT-positive tumors behaved aggressively, and these patients had a shorter recurrence-free survival. Conversely, when limited to patients with distant metastases, ALT-positivity conferred significantly better survival. One possible explanation for this observation is that, although ALT-positive primary PanNET clones seem to progress and metastasize readily to other organ sites, these metastases may grow more slowly in the new microenvironment.
Acknowledgments
Grant Support: This work was supported by a grant (2015-554, SMH) from the Asan Institute for Life Sciences, Seoul, Korea, by NRF-2016R1A2B4009381 (SMH) from the National Research Foundation of Korea, and by R01CA172380 (AKM) from the National Cancer Institute.
Footnotes
Disclosure of potential conflicts of interest: No potential conflicts of interest were disclosed.
This work was presented, in part, at the 2016 annual meeting of the United States and Canadian Academy of Pathology, Seattle.
REFERENCES
- 1.Fischer L, Kleeff J, Esposito I, Hinz U, Zimmermann A, Friess H, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95:627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 2.Tsutsumi K, Ohtsuka T, Mori Y, Fujino M, Yasui T, Aishima S, et al. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47:678–685. doi: 10.1007/s00535-012-0540-0. [DOI] [PubMed] [Google Scholar]
- 3.Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 4.Jarufe NP, Coldham C, Orug T, Mayer AD, Mirza DF, Buckels JA, et al. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg. 2005;22:157–162. doi: 10.1159/000087148. [DOI] [PubMed] [Google Scholar]
- 5.Norton JA. Surgery for primary pancreatic neuroendocrine tumors. J Gastrointest Surg. 2006;10:327–331. doi: 10.1016/j.gassur.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 7.Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409–427. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredrich M, Reisch A, Illing RB. Neuronal subtype identity in the rat auditory brainstem as defined by molecular profile and axonal projection. Exp Brain Res. 2009;195:241–260. doi: 10.1007/s00221-009-1776-7. [DOI] [PubMed] [Google Scholar]
- 9.Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–7803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 10.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Shi C, Klimstra DS. Pancreatic neuroendocrine tumors: pathologic and molecular characteristics. Semin Diagn Pathol. 2014;31:498–511. doi: 10.1053/j.semdp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Kulke MH, Shah MH, Benson AB, 3rd, Bergsland E, Berlin JD, Blaszkowsky LS, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 13.Amin S, Kim MK. Islet Cell Tumors of the Pancreas. Gastroenterol Clin North Am. 2016;45:83–100. doi: 10.1016/j.gtc.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 17.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henson JD, Hannay JA, McCarthy SW, Royds JA, Yeager TR, Robinson RA, et al. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin Cancer Res. 2005;11:217–225. [PubMed] [Google Scholar]
- 19.Henson JD, Reddel RR. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett. 2010;584:3800–3811. doi: 10.1016/j.febslet.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Pickett HA, Reddel RR. Molecular mechanisms of activity and derepression of alternative lengthening of telomeres. Nat Struct Mol Biol. 2015;22:875–880. doi: 10.1038/nsmb.3106. [DOI] [PubMed] [Google Scholar]
- 21.Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146:453–460. e5. doi: 10.1053/j.gastro.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 22.O'Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HS, Lee HS, Nam KH, Choi J, Kim WH. Telomere length abnormalities and telomerase RNA component expression in gastroenteropancreatic neuroendocrine tumors. Anticancer Res. 2015;35:3501–3510. [PubMed] [Google Scholar]
- 24.Dogeas E, Karagkounis G, Heaphy CM, Hirose K, Pawlik TM, Wolfgang CL, et al. Alternative lengthening of telomeres predicts site of origin in neuroendocrine tumor liver metastases. J Am Coll Surg. 2014;218:628–635. doi: 10.1016/j.jamcollsurg.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science. 2015;347:273–277. doi: 10.1126/science.1257216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koschmann C, Calinescu AA, Nunez FJ, Mackay A, Fazal-Salom J, Thomas D, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med. 2016;8:328ra28. doi: 10.1126/scitranslmed.aac8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas. Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 28.Bosman FTCF, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 29.Kim JY, Kim MS, Kim KS, Song KB, Lee SH, Hwang DW, et al. Clinicopathologic and prognostic significance of multiple hormone expression in pancreatic neuroendocrine tumors. Am J Surg Pathol. 2015;39:592–601. doi: 10.1097/PAS.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 30.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AI. AJCC Cancer Staging Manual. 7th. Springer; 2010. [Google Scholar]
- 31.de Wilde RF, Heaphy CM, Maitra A, Meeker AK, Edil BH, Wolfgang CL, et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod Pathol. 2012;25:1033–1039. doi: 10.1038/modpathol.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan F, Shi M, Ji J, Shi H, Zhou C, Yu Y, et al. KRAS and DAXX/ATRX gene mutations are correlated with the clinicopathological features, advanced diseases, and poor prognosis in Chinese patients with pancreatic neuroendocrine tumors. Int J Biol Sci. 2014;10:957–965. doi: 10.7150/ijbs.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.