Abstract
Research in non-human animals reveals threat-sensitive generalization of defensive behavior that favors widespread generalization when threat intensity is high and limited generalization (i.e., specificity) when threat intensity is low. Here, we used Pavlovian fear-conditioning to systematically investigate whether threat intensity widens behavioral generalization gradients to stimuli that decreasingly resemble a learned threat cue. Using a between-subjects design, volunteers underwent fear-conditioning with a tone paired with either a high-intensity or low-intensity aversive stimulus prior to a test of fear-generalization to novel tones. Results showed no effect of threat intensity on initial acquisition of conditioned fear. However, volunteers who underwent fear-conditioning with a high-intensity aversive stimulus exhibited widespread generalization of autonomic arousal (skin conductance responses) as compared to volunteers who received a low-intensity aversive stimulus. These results show a transition from normal (selective) to overgeneralized fear as threat intensity increases, and have implications for understanding overgeneralization characteristic of trauma- and stress-related disorders.
Keywords: Pavlovian fear-conditioning, anxiety, arousal, shocks, generalization, pattern separation
Detection and quick reactions to signals of threat in the environment helps ensure survival when threat is imminent. However, cues in the environment associated with threat (e.g., the sound of rustling leaves in the forest) do not always portend impending danger (e.g., a harmless scurrying animal and not a predator). Excessively responding to any sign of threat constitutes a waste of time and energy, but a failure to respond appropriately could be disastrous (e.g., ignoring the signs of a stalking predator). Accordingly, research shows that animals balance the trade-off between generalization and specificity by factoring the intensity of a potential threat, sometimes referred to as ‘threat-sensitive generalization’ (Ferrari, Messier, & Chivers, 2008). If threat intensity is low, defensive behavior is limited to cues strongly and directly associated with threat; if threat intensity is high, animals generalize defensive behavior broadly to a wide range of cues that might portend danger but that have not been directly associated with threat (‘better-safe-than-sorry’). Put simply, the higher the risk of serious harm, the more widespread the fear. This relationship between threat intensity and generalization of defensive behavior emulates symptoms of psychiatric conditions characterized by excessive fear and anxiety to harmless cues following extremely intense emotional events, best exemplified by posttraumatic stress disorder. Here, we investigated whether threat-sensitive fear generalization is an empirical phenomenon in humans by varying threat intensity and measuring gradients of threat-related responses from a known threat to similar but harmless stimuli.
The importance of outcome intensity to Pavlovian learning is formalized in a number of influential learning models (Pearce & Hall, 1980; Rescorla & Wagner, 1972; Wagner, 1981), and has been empirically validated in fear-conditioning studies that vary foot-shock intensity in rodents (Ader, Weijnen, & Moleman, 1972; Annau & Kamin, 1961; Baldi, Lorenzini, & Bucherelli, 2004; Cordero, Merino, & Sandi, 1998; Davis & Astrachan, 1978). In fear-conditioning, the subject learns the association between a conditioned stimulus (CS) and an aversive unconditioned stimulus (US). The CS-US association establishes the CS as a reliable indicator of impending threat, thereby initiating increases in autonomic activity, endocrine changes, and defensive behaviors. At weak shock intensities, rodents show little or no defensive behavior (Baldi et al., 2004; Phillips & LeDoux, 1992). At moderate shock intensities, defensive behavior emerges selectively to the cue or context predictive of shock (i.e., CS+). At high shock intensities, rodents not only respond to the CS+ but also generalize to unpaired stimuli or contexts (i.e., CS−) (Baldi et al., 2004; Laxmi, Stork, & Pape, 2003), a process subserved by a loss of cue-specificity in lateral amygdala neurons (Ghosh & Chattarji, 2015).
Although the role of US intensity in animal learning is acknowledged, the question remains whether fear-conditioning with a high-intensity US leads to stronger and more generalized fear in humans. Additionally, in the existing laboratory animal research on effects of shock intensity fear-generalization is defined as increased responding to a single unpaired cue or context; it is therefore unclear if high-intensity outcomes lead to widespread generalization to graded stimuli that vary in similarity to the CS+, or if generalization gradually weakens as similarity to the CS+ diminishes.
In the present study, volunteers first underwent Pavlovian fear-conditioning to a tone CS+ paired with either a low-intensity or high-intensity aversive event (shown in Figure 1A and described in detail in the Methods) and an unpaired tone (CS−). Volunteers were then tested for generalization to a range of novel tones spanning a frequency continuum. Incorporating a wide range of test stimuli provides a comprehensive estimate of intradimensional discrimination training — learning both what is a threat (CS+) and what is safe (CS−) — on similarity-based generalization gradients (Ghirlanda & Enquist, 2003; Hanson, 1959; Kahnt & Tobler, 2016; Spence, 1937; Thomas, 1993). Half of the novel tones were intermediate along the frequency continuum between the CS− and CS+, and half extended beyond the CS+ in a direction opposite the CS−. We predicted that a high-intensity threat would induce widespread behavioral generalization to intermediate tones and a shift in maximal behavioral generalization toward tones more unlike the learned safety signal. In contrast, we predicted that a low-intensity threat would promote specificity, as reflected by a sharp gradient centered on the CS+ and weak generalization to surrounding novel tones.
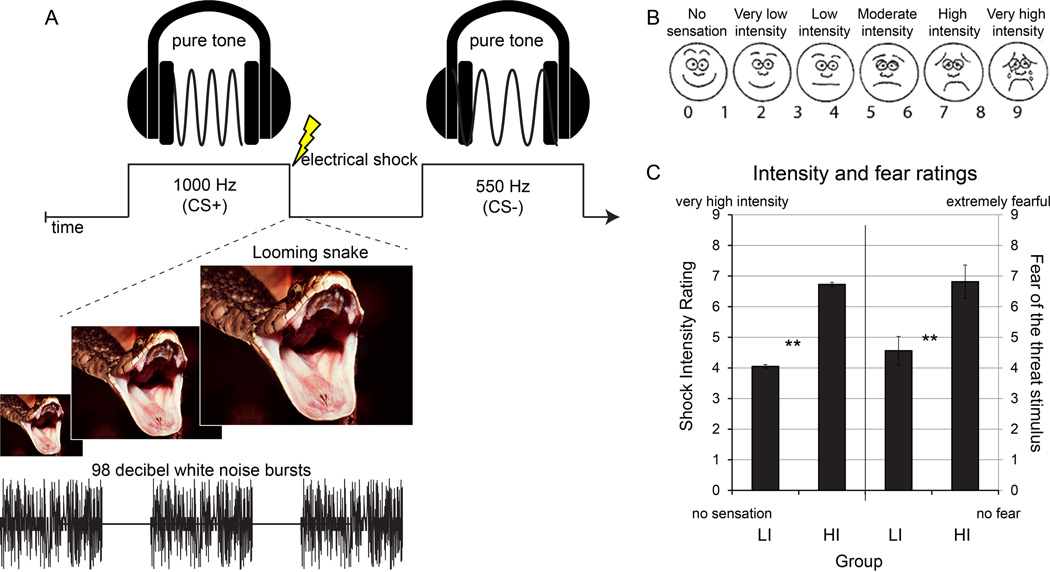
Figure 1. Fear conditioning design and subjective intensity and fear ratings for Experiment 1.
(A) Discriminative fear conditioning included pure tone conditioned stimuli paired (CS+, 1000 Hz) or unpaired (CS−, 550 Hz) with an aversive unconditioned stimulus (US). One group (not depicted) underwent low-intensity fear conditioning that included a low-intensity electrical shock to the right wrist on 40% of CS+ trials. The other group (depicted) underwent high-intensity fear conditioning that included a multimodal US composed of a high-intensity electrical shock, static white noise bursts (~98 decibels), and a looming image of a snake, presented simultaneously on 40% of CS+ trials. A different picture of a snake was used on each trial. (B) A shock work-up procedure was used to calibrate shock to either a low or high level of subjective intensity, between groups. In the low-intensity group, the shock work-up procedure ceased when the volunteer indicated the shock was “very low” to “low.” For the high-intensity group, the calibration ceased when the volunteer indicated the shock was at least “high-intensity.” (C) Post-experimental subjective ratings of intensity of the electrical shock and ratings of fear of the aversive US were greater in the high-intensity (HI) versus low-intensity (LI) group. Error bars reflect standard error of the mean. ** P < .01.
Materials and Method
Participants
Forty-three healthy volunteers gave written informed consent approved by the University Committee on Activities Involving Human Subjects at New York University. One volunteer was excluded from the final analysis due to normalized mean SCRs that were >3 SD from the group mean. The final sample included 20 volunteers (12 females; Mean Age ± SD: 23.25 ± 4.30) assigned to the Low-Intensity (LI) Group and 22 volunteers (13 females; 23.59 ± 3.97) assigned to the High-Intensity (HI) Group.
Procedures
Low and High-intensity US Calibration and Design
The electrical shock was a 200 ms pulse delivered to the right wrist using disposable pre-gelled electrodes connected to a Grass Medical Instruments stimulator (Warwick, RI). Shocks were calibrated using an ascending staircase procedure starting with a low voltage setting near a perceptible threshold. After each calibration pulse, volunteers provided an assessment of subjective intensity using a modified Pain Intensity scale (Figure 1b and 1c) anchored from “no sensation” to “very high intensity.” For the LI group, the shock calibration ceased when the volunteer indicated the shock was “very low” to “low” in how subjectively intense the shock felt. For the HI group, the calibration ceased when the volunteer assessed the shock as feeling “high-intensity” or stronger.
Importantly, there are unique considerations to study the effects of threat-intensity in humans that go beyond increasing the voltage of the electrical shock. One practical and ethical concern is that the physical (objective) intensity of the shock is limited out of concern of subject safety. Thus, there is a restricted range by which the experimenter can vary voltage levels. Further, unlike laboratory animal research, human volunteers have control in determining the subjective intensity of the shock during the initial shock calibration procedure, have the ability to remove shock electrodes, and know that they can choose to terminate an experiment at any time. Accordingly, there are unique practical considerations to a systematic study of US intensity in humans that are less germane to this type of investigation in laboratory animals (see for example Boren, Sidman, & Herrnstein, 1959). A further methodological challenge to varying shock intensities between subjects is that, due to individual differences in the electrode-skin circuit impedance, there is not a one-to-one correspondence between voltage and subjective intensity (Tursky & Watson, 1964). As a consequence, it is not possible to maintain the identical shock intensity level using constant voltage stimulators; a low shock voltage setting for one volunteer may be considered extremely painful in another volunteer, and vice versa. Shock intensity can also vary within the same individual over the course of a shock study due to increases or decreases in sweating, which will affect the conductance of the skin (and therefore the intensity of the shock) considerably. Therefore, due to safety concerns, variability in the electrode-skin circuit impedance, and variability in subjective pain tolerance thresholds, a threat that consists merely of an electrical shock calibrated to subjective level of “high-intensity” is unsatisfactory for the goal of the present study.
To safely augment the aversive US in the HI group, the electrical shock to the wrist (calibrated to a high subjective intensity as described above) was combined with a simultaneous aversive static burst of white noise (~98 decibels) and looming images of a snake (Figure 1a). For the HI group, each component of the multimodal US was presented simultaneously at the offset of the tone with no lag between tone offset and US. For the LI group, the US consisted of only the low-intensity shock. Note that while the quality of the US was different between groups (a multimodal US versus only a shock) the stated goal of this study was to investigate the effects of high versus low-intensity events on learning and generalization; that the objective components of the US differ between groups is in fact the essence of the manipulation. Further, as multisensory aversive events are the types of experiences encountered in naturalistic environments—and implicated in trauma and stressor related disorders—this US can be considered more ecologically valid and provides a closer approximation of real-world fearful experiences. Finally, we note that despite the use of different USs between groups, the reported dependent measures were anticipatory responses (evoked prior to when the US would occur) for tones that were never paired with the US or CS+ trials unpaired with the US.
Because the high-intensity US consisted of an image of a snake, volunteers in the HI group completed the Snake Phobic Questionnaire (Klorman, Weerts, Hastings, Melamed, & Lang, 1974) at the completion of the study to assess snake phobia (scores can range from 0 to 31). Mean SNAQ Scores were 10.09 (SD = 6.37)—comparable to healthy populations—and volunteers all fell below the criterion of phobia scores set at within 1 SD from the mean of patients with a specific phobia of snakes (Fredrikson, 1983).
Fear conditioning
The experiment occurred across two phases, discriminative fear-conditioning and generalization, separated by a 5 minute break during which time volunteers passively viewed a silent video of a train traveling through British Columbia (see also Dunsmoor, Mitroff, & LaBar, 2009). Stimuli consisted of pure tone sine waves presented binaurally at a moderate volume (< 60 decibels) through headphones (Sennheiser HD-280 PRO) for 2.5 s each and separated by a 7–8 s inter-trial interval. Stimulus presentation was controlled using E-Prime 2.0 (Psychology Software Tools, Sharpburg, PA). CSs were a 1000 Hz and 550 Hz tone that signaled the presence (CS+) or absence (CS−) of the US, respectively. Fear-conditioning included 12 presentations each of unpaired CS+ and CS−. An additional 8 CS+ trials were paired with the US (40% reinforcement rate). Because CS duration was short, all CS+ trials paired with shock were excluded from analysis to mitigate potential confounds introduced by the US.
After fear-conditioning, volunteers were presented with 6 novel tones of increasing frequency ranging between the CS− and CS+ (650, 800, and 900) and extending beyond the CS+ (1100, 1200, and 1350) in a direction opposite the CS−. During the generalization test, tones (including unpaired CS+ and CS−) were presented 7 times each. An additional 5 CS+ trials paired with shock were included during the generalization test to prevent extinction and habituation over the course of the lengthy generalization test (steady-state generalization testing; see also Blough, 1975; Dunsmoor et al., 2009; Lissek et al., 2008).
In all phases, volunteers rated shock expectancy on a three alternative-forced-choice scale corresponding to ‘no risk,’ ‘moderate risk,’ and ‘high risk’ for receiving the US, based on prior fear-conditioning studies (Coelho, Dunsmoor, & Phelps, 2015; Lissek et al., 2008). Volunteers were instructed that their button presses did not affect the outcome on a trial in order to mitigate the potential for volunteers to attribute the outcome to their choice or reaction times (i.e. to prevent an illusory correlation). Volunteers were told to pay attention and try to learn the association between the tones and the shock, but no explicit information was given regarding the CS-US contingencies. Presentation was pseudo-randomized so that no more than 3 presentations of the same tone occurred in a row. After generalization testing, volunteers underwent a hearing test, which validated that all volunteers had normal hearing and the capacity to discriminate between each tone frequency used in the experiment.
Psychophysiology Collection and Data Analysis
SCRs were acquired from the hypothenar eminence of the left palmar surface using disposable pre-gelled snap electrodes connected to the MP-100 BIOPAC System (BIOPAC Systems). Analysis of SCRs used procedures previously described (Dunsmoor, Campese, Ceceli, LeDoux, & Phelps, 2015). In brief, an SCR was considered related to CS presentation if the trough-to-peak deflection occurred 0.5–3 seconds following CS onset, lasted between 0.5 and 5.0 s, and was greater than 0.02 microsiemens (µS). Responses that did not fit these criteria were scored as zero. SCR values were obtained using a custom Matlab (The Mathworks Inc., Natick, MA) script that extracts SCRs for each trial using the above criteria (Green, Kragel, Fecteau, & LaBar, 2013), and subsequently inspected by an independent blinded rater. CS+ trials paired with the US were excluded from all analyses. Raw SCR scores were square root transformed prior to statistical analysis to normalize the distribution (Lykken & Venables, 1971). Results were analyzed separately for fear-conditioning and the generalization test by repeated measures ANOVA incorporating Stimulus as a within-subjects factor and Group as a between-subjects factor, and followed where necessary by paired-samples t-tests or independent-samples t-tests. We focused further analysis on SCRs to tones immediately adjacent to the CS+ in both groups, as these stimuli are the most confusable for the CS+ and therefore the likeliest to elicit a generalized response. A similar approach was used by Lissek and colleagues (2014; 2010) to investigate the change in generalization from the CS+ to the most similar generalized stimulus, and in Dunsmoor & LaBar (2013) to investigate generalization to tones immediately above and below the CS+ frequency. Thus, a planned ANOVA was conducted using the CS+ (1000 Hz), 900, and 1100 Hz tones including Group as a between-subjects factor. Greenhouse-Geisser correction was used when assumption of sphericity was not met. All analyses were considered significant at α < .05, two-tailed.
Results
Shock Intensity and Fear Ratings
Subjective shock intensity prior to fear-conditioning was higher in the HI (Mean±SD: 7.18±0.33) than the LI (4.05±0.32) group (t40 = 31.198, P<0.001). Post-experimental ratings, shown in Figure 1c, remained higher in the HI than the LI group (t40 = 4.64, P<0.001. Volunteers were also asked at the conclusion of the study how much they had feared the US (note: data from this question was not recorded for four subjects); self-reported fear was stronger in the HI than the LI group (t36 = 2.90, P=0.006).
Fear-conditioning
SCRs
Analysis of SCRs during acquisition (Figure 2a) revealed a main effect of Stimulus (CS+, CS−: F1,40 =34.16, P<0.001, η2=0.461), but no effect of Group (P=0.26) and no Stimulus by Group interaction (P=0.39). Planned t-tests between CS+ and CS− confirmed successful fear acquisition in the LI (t19 = 3.27, P=0.004) and the HI (t21 = 5.10, P<0.001) groups. Mean SCRs on CS+ trials were not different between LI (Mean±SEM: 0.58±0.10) and HI (0.73±0.07) groups (P =.24).
Figure 2. Skin conductance responses.
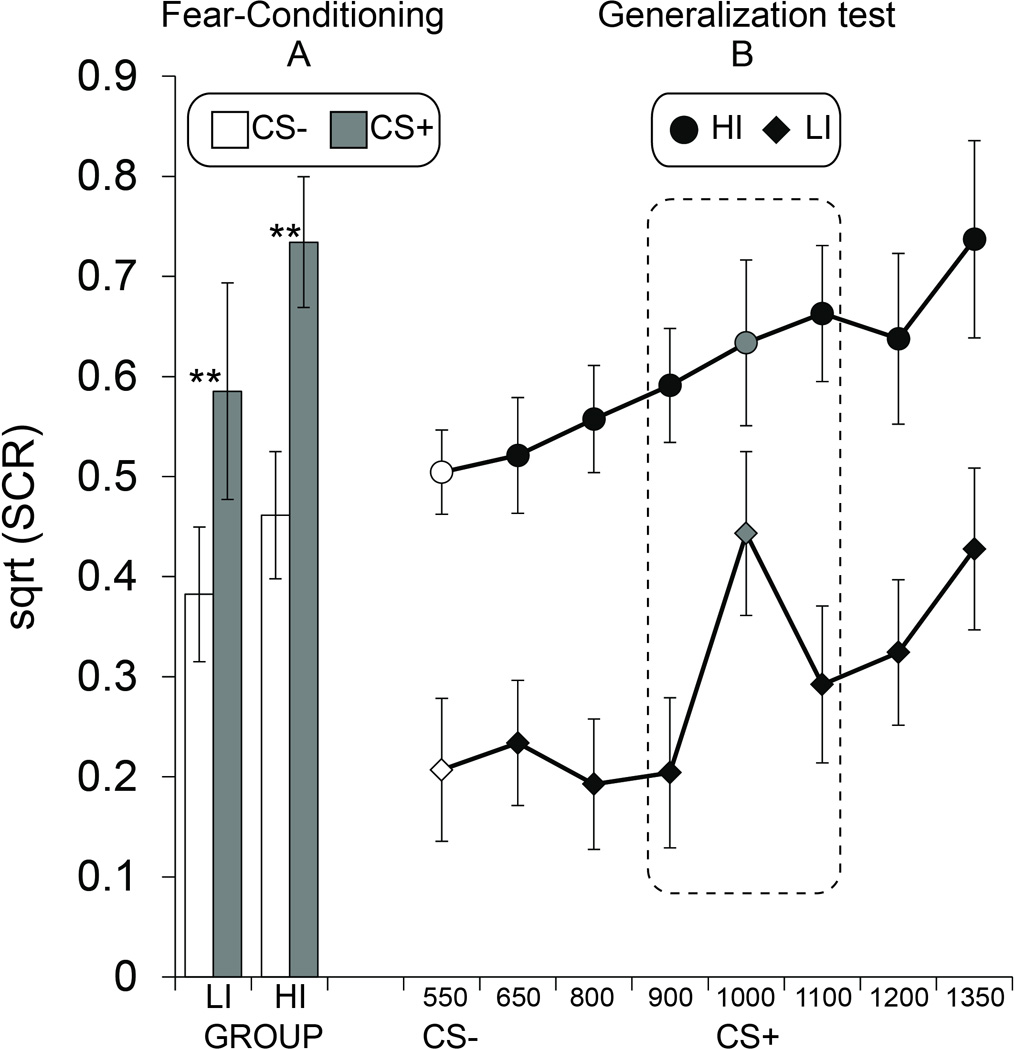
(A) Fear-conditioning involving either a low-intensity (LI) unconditioned stimulus or a high-intensity (HI) US both lead to succesful fear acquisition as defined by greater SCRs on conditioned stimulus trials intermittently paired (CS+) versus unpaired (CS−) with the US. (B) The group who underwent fear-conditioning to a CS+ paired with a low-intensity US (square) produced effectively no generalization of SCRs to novel tones between the CS+ and CS− frequency. In contrast, the group who underwent fear-conditioning to a CS+ paired with a high-intensity US (circles) showed widespread generalization of SCRs. Dashed rectangle indicates a priori analysis of threat-sensitive generalization for stimuli adjacent to the CS+. Data from CS+ trials included trials unpaired with the US only. Grey and White fill denote CS+ and CS−, respectively. Error bars reflect standard error of the mean. ** P < .01.
Expectancy Ratings
Shock expectancy ratings during acquisition (Figure 3a) were likewise characterized by a main effect of Stimulus (F1,40=132.72, P<0.001, η2=0.768), with no effect of Group (P=0.79) and no Stimulus by Group interaction (P=0.3).
Figure 3. Expectancy ratings.
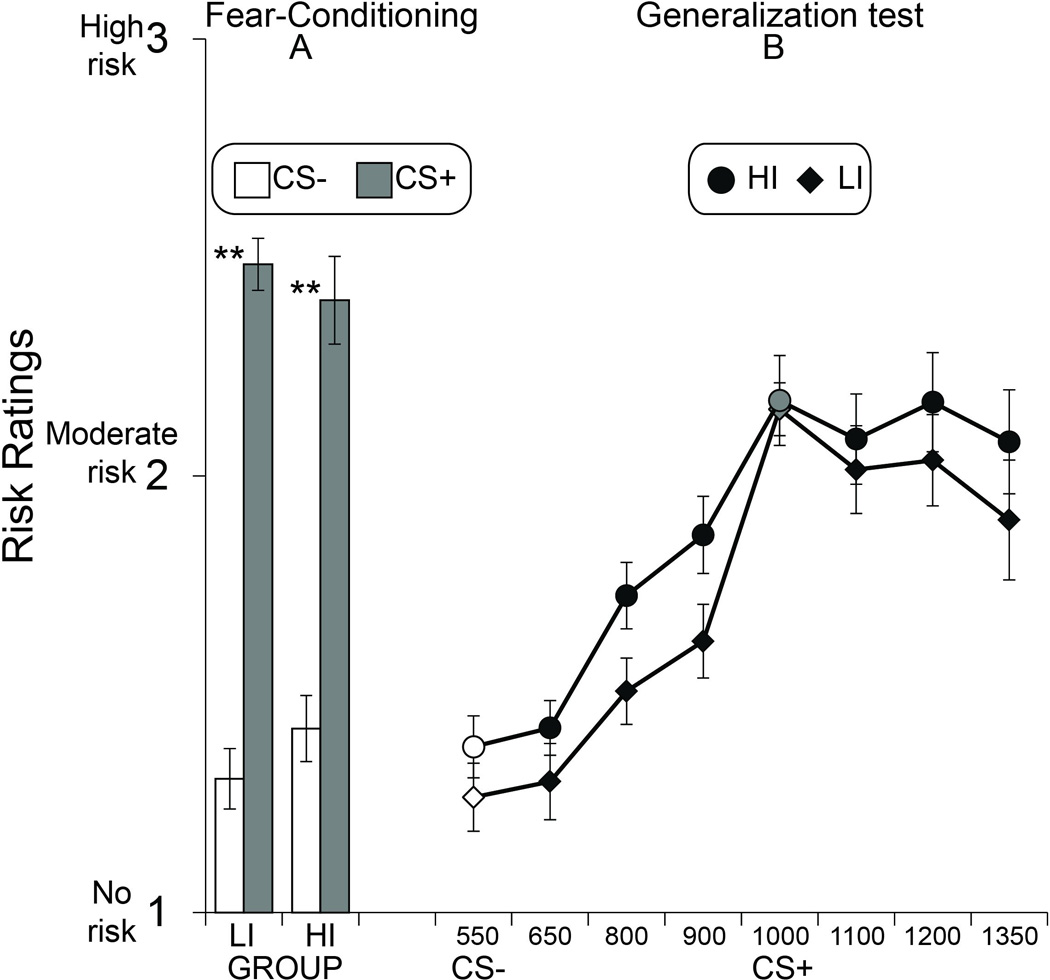
(A) Fear-conditioning involving either a low-intensity (LI) unconditioned stimulus or a high-intensity (HI) US both lead to succesful fear acquisition as defined by greater shock expectancy on conditioned stimulus trials intermittently paired (CS+) versus unpaired (CS−) with the US. (B) Both groups exhibited a decremented gradient of expectancy between the CS+ and CS− that decreased as similarity to the CS+ diminished, with no difference between groups. Expectancy in both groups was skewed to the right of the CS+, reflecting a shift in expectancy in a direction oposite the learned safety signal, CS−. Data from CS+ trials only included trials not paired with the US. Grey and White fill denote CS+ and CS−, respectively. Error bars reflect standard error of the mean. ** P < .01.
Generalization Test
SCRs
Analysis of the entire generalization gradient revealed main effects of Stimulus (F4.955, 198.182=10.01, P<0.001, η2=0.2) and Group (F1, 40=12.03, P=0.001, η2=0.231), but no interaction (P=.21) (Figure 2b). A significant linear trend (F1, 40=27.08, P<.001) was suggestive of an asymmetrical gradient skewed towards stimuli opposite the CS+ most unlike the CS−, i.e., an area-shift (Dunsmoor & LaBar, 2013). As evidence of an area-shift, SCRs to stimuli opposite the CS− (mean of 1100, 1200, 1350 Hz) were heightened relative to stimuli between the CS+ and CS− (mean of 650, 800, 900 Hz) in both the HI (t21 = 3.45, P=.002) and LI (t19 = 3.16, P=.005) groups.
Notably, while both groups exhibited somewhat linear gradients of SCRs along the frequency continuum, there were noticeable between-group differences in the amount of generalization to stimuli surrounding the CS+. In line with our a priori hypothesis, the LI group exhibited an abrupt and pronounced decrease in SCRs to generalization stimuli surrounding the CS+, whereas generalization was markedly elevated to these same stimuli in the HI group (cf. Lissek et al., 2014). Given our a priori hypothesis regarding threat-sensitive generalization to stimuli surrounding the learned threat cue, we focused the ANOVA on the CS+ and stimuli most similar to the CS+, 900 and 1100 Hz (Figure 2b dashed rectangle). This revealed a significant effect of Stimulus (F1.682, 67.275= 5.80, P=0.007, η2=0.127), Group (F1, 40= 10.9, P=0.002, η2=0.214), and a Stimulus by Group interaction (F1.682, 67.275= 3.46, P=0.036, η2=0.08). Follow-up t-tests revealed a significant decrease in SCRs in the LI group between the CS+ and 900 Hz (t19 = 3.16, P=.005) and 1100 (t19 = 2.58, P=.003) Hz tones, while the same comparison in the HI group showed no differences (Ps>.5). Finally, while SCRs to the CS+ were not different between groups (P=.11), mean SCRs to the 900 (t40 = 4.02, P<.001) and the 1100 (t40 = 3.51, P=.001) Hz tones were greater in the HI group as compared to the LI group.
We also examined whether discriminatory (CS+ vs. CS−) fear-conditioning induced a shift in the maximal SCR to a stimulus along the frequency continuum most unlike the learned safety signal—the 1350 Hz tone. Referred to as a peak-shift (Honig & Urcuioli, 1981), this effect can be defined as a significant shift in the maximal response to a stimulus other than the CS+. We detected a moderate but significant peak-shift in the HI group, such that mean SCRs to the 1350 Hz tone were heightened relative to the CS+ (t21 = 2.08, P = .049, d = .44). Importantly, while the LI group did show an area-shift as detailed above (i.e., mean SCRs to stimuli opposite the CS− heightened relative to stimuli between the CS+ and CS−), there was no peak-shift from the CS+ to the 1350 Hz tone (P = .81).
Expectancy Ratings
Shock expectancy ratings (Figure 3b) were characterized by a main effect of Stimulus (F3.672, 146.889= 40.08, P<0.001, η2=0.501) with a linear trend (F1, 40=70.05, P< .001), but no effect of Group (P=0.13) and no Stimulus by Group interaction (P=0.83). In line with the SCR gradient, expectancy to stimuli opposite the CS− (mean of 1100, 1200, 1350 Hz) were heightened relative to stimuli between the CS+ and CS− (mean of 650, 800, 900 Hz) in the HI (t21 = 5.19, P<.001) and LI (t19 = 5.39, P<.001) groups. Unlike the SCR gradient, however, analysis of shock expectancy to the CS+ and tones immediately adjacent to the CS+ (900 and 1100 Hz) did not reveal a significant Stimulus by Group interaction (P=0.2).
Discussion
That more intense outcomes lead to stronger conditioning is a basic tenet of nearly all models of classical conditioning (e.g., Rescorla & Wagner, 1972), and is a core assumption of learning theory accounts of psychopathology (e.g., Foa, Steketee, & Rothbaum, 1989). While the present results did not show stronger fear-conditioning with a high-intensity US, an intense outcome did promote widespread generalization of conditioned fear to harmless stimuli that resembled a learned threat following fear-conditioning. These results provide new empirical support for threat-sensitive generalization (Ferrari et al., 2008) in humans and extends recent findings in laboratory animals (Baldi et al., 2004; Ghosh & Chattarji, 2015) by showing that threat-sensitive fear generalization is widespread and extends beyond a single unpaired stimulus.
Threat-sensitive fear generalization accords with the view that generalization is adaptive when a potential threat is intense (Åhs, Miller, Gordon, & Lundström, 2013; Laufer, Israeli, & Paz, 2016; Resnik & Paz, 2014; Resnik, Sobel, & Paz, 2011). Put another way, when a potential threat presents minimal harm, it may be advantageous to discriminate between those cues that are known to signal threat from similar cues that only might signal threat in order to avoid unnecessarily wasting time and energy. In contrast, when a potential threat poses the risk of intense harm, it may be advantageous to disregard stimulus differences and respond to a range of cues, even to cues that are clearly dissimilar from those experienced in the past, because failing to react could be catastrophic (Dunsmoor & Murphy, 2015; Dunsmoor, Prince, Murty, Kragel, & LaBar, 2011; Laufer et al., 2016; Likhtik & Paz, 2015). Thus, in a dynamic environment where stimuli assume multiple forms from one experience to the next, the balance between discrimination and generalization may shift in accordance with the consequences of ignoring signals of threat.
It is also notable that a high-intensity US did not promote stronger fear acquisition. This finding was surprising insofar as it is widely recognized that moderate to strong foot-shocks produce stronger fear acquisition than weak foot-shocks in rodents (Baldi et al., 2004; Phillips & LeDoux, 1992). It also shows that a shock intensity even somewhat lower than in most human fear-conditioning research (in which the subjective threshold is typically “aversive but tolerable”) is sufficient to generate differential CRs. What can explain equivalent conditioned SCRs and estimates of risk between the low and high-intensity groups? One possibility is that, although the low-intensity US was subjectively weak, its mere presence was sufficient to induce anticipatory anxiety on CS+ trials. This idea fits with prior research showing that human research subjects would rather take a strong shock immediately than a weak shock that is delayed by up to several seconds, presumably because the anticipation or dread of waiting for a weak shock is more unpleasant than the shock itself (Berns et al., 2006). In the context of the present findings, the anticipation of shock—even a weak shock—may be the force driving autonomic responses (“the waiting is the worst part”). In a similar way, the mere instruction that a stimulus will be paired with shock is enough to induce increases in sympathetic arousal and amygdala activity (Olsson & Phelps, 2007). This explanation is also in line with clinical literature showing that the subjective perception of the level of harm posed by a threat mediates the development of PTSD (King, King, Vogt, Knight, & Samper, 2006; Rubin, Berntsen, & Bohni, 2008; Solomon, Mikulincer, & Benbenishty, 1989; van Wingen, Geuze, Vermetten, & Fernández, 2011), and drives emotion-related brain activations in PTSD (Morey et al., 2015; van Wingen et al., 2011) more than the actual level of harm.
Another possibility to explain equivalent fear acquisition is that the high-intensity US was not sufficiently intense. The question is raised, would an extremely intense or painful shock have produced more robust conditioned SCRs to the CS+ than we observed here? Here, we used an augmented multimodal US rather than simply increasing voltage levels between subjects for reasons elaborated in the Methods (e.g., the ceiling on “intense” electrical shock intensity for human volunteers is likely lower than studies that have explicitly investigated the result of shock intensity on fear generalization in laboratory animals). Although subjective ratings for this novel multimodal US—consisting of simultaneous presentations of a physical shock, aversive noise, and a “biologically prepared” stimulus (pictures of a looming snake)—confirmed that subjects found it fearful, it does remain a laboratory approximation of a real-world intense threat event. Yet, it is not clear that an extremely intense or painful outcome would drastically alter the fear-conditioning results, per se; animal research tends to show the largest increase in CRs as foot-shocks increase from low to moderate, with little additional behavioral effect (Baldi et al., 2004; Boren et al., 1959; Phillips & LeDoux, 1992)—or even decrements (Davis & Astrachan, 1978; Leaton & Borszcz, 1985; Witnauer & Miller, 2013)—at extreme shock levels.
The present results extend non-human animal research by testing generalization to a range of stimuli, and not just to a single unreinforced CS−. Testing intradimensional generalization to stimuli above and below the CS+ along a sensory dimension allowed us to examine how broadly conditioned behavior generalizes from a learned threat as a function of similarity. This also revealed an unexpected finding; specifically, despite overall lower generalized SCRs to unreinforced tones, the low-intensity group did show an uptick in SCRs to a tone that was highly dissimilar from the CS+, 1350 Hz. One post-hoc explanation of this uptick in arousal to a highly dissimilar tone is that subjects learned a relational rule during discriminatory fear-conditioning that high pitched tones are more dangerous than low pitched tone. This could have led to transposition (Kohler, 1939; Spence, 1937) of threat value to the most “extreme” generalized stimulus (in terms of pitch), even in subjects for whom generalized arousal was overall reduced.
One possible neurophysiological explanation for broad fear generalization is that high-intensity fear-conditioning promotes short-latency plasticity in the lateral amygdala, whereby neurons switch from specific (CS+ only) to generalized as shock intensity increases (Ghosh & Chattarji, 2015). Neurons in the auditory thalamus have also been implicated in fear generalization; increasing transcription factors in the auditory thalamus enhances fear generalization following low-intensity fear-conditioning to resemble fear generalization seen with high-intensity training (Han et al., 2008). Fear-conditioning induced receptive field plasticity in primary auditory cortex may also play a role in behavioral generalization(Weinberger, 2007). However, Ghosh and Chattarji (2015) found that reversible inactivation of the auditory cortex following high-intensity fear-conditioning did not prevent behavioral generalization, suggesting that the amygdala but not the auditory cortex is a critical site of overgeneralization (see also Armony, Servan-Schreiber, Romanski, Cohen, & LeDoux, 1997).
Although primary predictions centered on the effects of US intensity on generalized physiological arousal, it is important to note the lack of between-group differences on explicit ratings of shock expectancy. Instead, both groups displayed similar gradients of expectancy that diminished linearly between the CS− and CS+ but skewed away from the CS−. Thus, we cannot conclude that US intensity has any effect on explicit measures of shock expectancy. We note however that the design was not optimized to detect subtle differences in shock expectancy, as we used a restricted three-alternative forced choice scale. We also note a previous fear-generalization study using the same ratings scale that detected between group differences in measures of SCR unaccompanied by any differences in explicit shock expectancy (Dunsmoor & Murphy, 2014). A more sensitive measure of explicit risk may be needed to detect possible differences in generalization following high versus low intensity threat conditioning.
In sum, the current study provides new empirical evidence of threat-sensitive generalization in humans, providing new insight into when normal fear transitions to overgeneralized fear. While generalization of threat learning is adaptive to survival, broad overgeneralization of fear and anxiety is characteristic of a host of psychiatric conditions. Indeed, emerging research has begun to characterize broad fear generalization in PTSD (Morey et al., 2015), generalized anxiety disorder (Cha et al., 2014; Laufer et al., 2016), and Panic Disorder (Lissek et al., 2010) relative to psychologically healthy comparison subjects (see Dunsmoor & Paz, 2015 for review). Further research is needed to translate neurophysiological findings from laboratory animals to humans and to determine how high-intensity threat affects the neurocircuitry implicated in the acquisition, expression, and regulation of conditioned fear.
Acknowledgments
This study was supported by NIH RO1 MH097085 to EAP, H2020 Marie Sklodowska-Curie Fellowship and Society in Science-Branco Weiss Fellowship to MCWK, and NIMH K99 MH106719 to JED.
References
- Ader R, Weijnen J, Moleman P. Retention of a passive avoidance response as a function of the intensity and duration of electric shock. Psychonomic Science. 1972;26:125–128. [Google Scholar]
- Åhs F, Miller SS, Gordon AR, Lundström JN. Aversive learning increases sensory detection sensitivity. Biological Psychology. 2013;92:135–141. doi: 10.1016/j.biopsycho.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. Journal of Comparative and Physiological Psychology. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: Effects of auditory cortex lesions in a computational model and in rats. Cerebral Cortex. 1997;7:157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiology of Learning and Memory. 2004;81:162–166. doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berns GS, Chappelow J, Cekic M, Zink CF, Pagnoni G, Martin-Skurski ME. Neurobiological substrates of dread. Science. 2006;312:754–758. doi: 10.1126/science.1123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough DS. Steady-state data and a quantitative model of operant generalization and discrimination. Journal of Experimental Psychology. 1975;104:3–21. [Google Scholar]
- Boren JJ, Sidman M, Herrnstein R. Avoidance, escape, and extinction as functions of shock intensity. Journal of Comparative and Physiological Psychology. 1959;52:420. doi: 10.1037/h0042727. [DOI] [PubMed] [Google Scholar]
- Cha J, Greenberg T, Carlson JM, Dedora DJ, Hajcak G, Mujica-Parodi LR. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. Journal of Neuroscience. 2014;34:4043–4053. doi: 10.1523/JNEUROSCI.3372-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CA, Dunsmoor JE, Phelps EA. Compound stimulus extinction reduces spontaneous recovery in humans. Learning & Memory. 2015;22:589–593. doi: 10.1101/lm.039479.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behavioral neuroscience. 1998;112:885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- Davis M, Astrachan DI. Conditioned fear and startle magnitude: Effects of different footshock or backshock intensities used in training. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Campese VD, Ceceli AO, LeDoux JE, Phelps EA. Novelty-facilitated extinction: providing a novel outcome in place of an expected threat diminishes recovery of defensive responses. Biological Psychiatry. 2015;78:203–209. doi: 10.1016/j.biopsych.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, LaBar KS. Effects of Discrimination Training on Fear Generalization Gradients and Perceptual Classification in Humans. Behavioral Neuroscience. 2013;127:350–356. doi: 10.1037/a0031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory. 2009;16:460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Murphy GL. Stimulus Typicality Determines How Broadly Fear Is Generalized. Psychological Science. 2014;25 doi: 10.1177/0956797614535401. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Murphy GL. Categories, concepts, and conditioning: how humans generalize fear. Trends in Cognitive Sciences. 2015;19:73–77. doi: 10.1016/j.tics.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Paz R. Fear generalization and anxiety: behavioral and neural mechanisms. Biological Psychiatry. 2015;78:336–343. doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS. Neurobehavioral mechanisms of human fear generalization. Neuroimage. 2011;55:1878–1888. doi: 10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MC, Messier F, Chivers DP. Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proceedings of the Royal Society of London B. Biological Sciences. 2008;275:1811–1816. doi: 10.1098/rspb.2008.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Steketee G, Rothbaum BO. Behavioral cognitive conceptualizations of post-traumatic stress disorder. Behavior Therapy. 1989;20:155–176. [Google Scholar]
- Fredrikson M. Reliability and validity of some specific fear questionnaires. Scandinavian Journal of Psychology. 1983;24:331–334. doi: 10.1111/j.1467-9450.1983.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Ghirlanda S, Enquist M. A century of generalization. Animal Behaviour. 2003;66:15–36. [Google Scholar]
- Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nature Neuroscience. 2015;18:112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- Green SR, Kragel PA, Fecteau ME, LaBar KS. Development and validation of an unsupervised scoring system (Autonomate) for skin conductance response analysis. International Journal of Psychophysiology. 2013;91 doi: 10.1016/j.ijpsycho.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Yiu AP, Cole CJ, Hsiang HL, Neve RL, Josselyn SA. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learning & Memory. 2008;15:443–453. doi: 10.1101/lm.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson HM. Effects of discrimination-training on stimulus-generalization. Journal of Experimental Psychology. 1959;58:321–334. doi: 10.1037/h0042606. [DOI] [PubMed] [Google Scholar]
- Honig WK, Urcuioli PJ. The legacy of Guttman and Kalish (1956) - 25 years of research on stimulus-generalization. Journal of the Experimental Analysis of Behavior. 1981;36:405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Tobler PN. Dopamine regulates stimulus generalization in the human hippocampus. eLife. 2016;5 doi: 10.7554/eLife.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LA, King DW, Vogt DS, Knight J, Samper RE. Deployment Risk and Resilience Inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology. 2006;18:89. [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. Psychometric description of some specifici-fear questionnaires. Behavior Therapy. 1974;5:401–409. [Google Scholar]
- Kohler W. Simple structural functions in the chimpanzee and in the chicken. New York: Harcourt, Brace; 1939. [Google Scholar]
- Laufer O, Israeli D, Paz R. Behavioral and Neural Mechanisms of Overgeneralization in Anxiety. Current Biology. 2016;26 doi: 10.1016/j.cub.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Laxmi TR, Stork O, Pape H-C. Generalisation of conditioned fear and its behavioural expression in mice. Behavioural Brain Research. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Leaton RN, Borszcz GS. Potentiated startle: Its relation to freezing and shock intensity in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:421. [Google Scholar]
- Likhtik E, Paz R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends in neurosciences. 2015;38 doi: 10.1016/j.tins.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry. 2014;75:909–915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of Conditioned Fear as a Pathogenic Marker of Panic Disorder. American Journal of Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Morey R, Dunsmoor J, Haswell C, Brown V, Vora A, Weiner J, Marx CE. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Translational Psychiatry. 2015;5:e700. doi: 10.1038/tp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nature Neuroscience. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Appleton-Century-Crofts; 1972. [Google Scholar]
- Resnik J, Paz R. Fear generalization in the primate amygdala. Nature neuroscience. 2014;18 doi: 10.1038/nn.3900. [DOI] [PubMed] [Google Scholar]
- Resnik J, Sobel N, Paz R. Auditory aversive learning increases discrimination thresholds. Nature Neuroscience. 2011;14:791–796. doi: 10.1038/nn.2802. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Berntsen D, Bohni MK. Memory-Based Model of Posttraumatic Stress Disorder: Evaluating Basic Assumptions Underlying the PTSD Diagnosis. Psychological Review. 2008;115:985–1011. doi: 10.1037/a0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon Z, Mikulincer M, Benbenishty R. Locus of control and combat-related post-traumatic stress disorder: The intervening role of battle intensity, threat appraisal and coping. British Journal of Clinical Psychology. 1989;28:131–144. doi: 10.1111/j.2044-8260.1989.tb00823.x. [DOI] [PubMed] [Google Scholar]
- Spence KW. The differential response in animals to stimuli varying within a single dimension. Psychological Review. 1937;44:430–444. [Google Scholar]
- Thomas DR. A model for adaptation-level effects on stimulus-generalization. Psychological Review. 1993;100:658–673. [Google Scholar]
- Tursky B, Watson PD. Controlled physical and subjective intensities of electric shock. Psychophysiology. 1964;1:151–162. doi: 10.1111/j.1469-8986.1964.tb03230.x. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernández G. Perceived threat predicts the neural sequelae of combat stress. Molecular psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning & Memory. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witnauer JE, Miller RR. Conditioned suppression is an inverted-U function of footshock intensity. Learning & Behavior. 2013;41:94–106. doi: 10.3758/s13420-012-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]