Abstract
Introduction
Cutaneous leishmaniasis in Sri Lanka is a newly established parasitic disease caused by the usually visceralizing Leishmania donovani. Skin lesions manifest as non-itchy, non-tender papules, nodules or ulcers. In situ cytokine expression provides clues for immunopathogenesis of this localized form of disease.
Methods
Skin biopsies from 58 patients were analyzed for histological appearance and in situ cytokine expression of T- helper 1 (Th1) and T- helper 2 (Th2) cytokines, namely interferon (IFN)-γ, interleukin (IL)-12A, tumor necrosis factor (TNF)-α, IL-4 and IL-10 by real-time RT- PCR.
Results
Significant up regulation of the Th1 cytokine IFN-γ and down regulation of the Th2 cytokine IL-4 was seen in patients compared to healthy controls. Significantly elevated tissue expression of IFN-γ and TNF-α was seen in lesions that presented later than 6 months from the time of onset, while IL-4 expression was more prominent in lesions that responded poorly to antimony therapy.
Conclusion
A prominent Th1 response appears to support resolving of lesions, whereas a Th2 biased milieu tends to favor poor responsiveness to antimony and delayed lesion healing in L. donovani infections in Sri Lanka.
Keywords: Cell mediated immunity, IFN- γ, IL-4, TNF- α, histopathology, Sri Lanka
Introduction
Leishmaniasis is a vector-borne parasitic disease, caused by the genus Leishmania, which are obligate intracellular parasites of the macrophage – dendritic cell lineage1. It presents in three major forms as cutaneous, mucocutaneous or visceral leishmaniasis (VL). Leishmaniasis is an important global health problem; currently endemic in 98 countries, with an estimated 0.2 to 0.4 million VL and 0.7 to 1.2 million cutaneous leishmaniasis (CL) cases occurring each year 2. It was considered as an exotic disease in Sri Lanka until the first locally-acquired case was described in 1992 3. At present, it is an established parasitic disease with CL as the predominant form of presentation4. Cutaneous Leishmaniasis in Sri Lanka is caused by Leishmania donovani MON-37 5, which is closely related to Leishmania donovani MON-2 that causes VL in the Indian subcontinent 6. This zymodeme has also been recently isolated from cutaneous lesions in a tribal population from Kerala, India 7, but is better known for its ability to cause VL in many countries including India, Kenya, Israel and Cyprus 8.
In addition to CL, several cases of mucosal leishmaniasis 9,10 and VL 11,12 have been recently reported in Sri Lanka. The causative agent of autochthonous VL also has been confirmed as Leishmania donovani MON-37 12. From the current picture, it is clear that Sri Lanka is dealing with a Leishmania donovani strain that manifests mainly as CL, however with visceralizing potential. Hence, it could be assumed that the host genetics and immunological response may undoubtedly have a role in apparently diverse clinical outcome.
Histopathology of CL is influenced by the geographical origin through diverse host or parasite related factors. It shows a two way spectrum: from anergic macrophage to tuberculoid granuloma in one and from digestion of amastigotes within macrophages to necrosis of infected macrophages in the other. Such necrosis may be either diffuse or focalized 13. Histopathological spectrum of CL in Sri Lanka has been described as similar to leprosy and grouped into four using a modified Ridley classification. It ranged from diffuse infiltrate of parasitized macrophages in group I to well-formed epithelioid granulomata in group IV 14.
Experimental mouse models have shown that outcome of Leishmania major infection depend on preferential activation of Th1 or Th2 subsets of CD4+ T cells, with Th1 type response leading to host resistance and Th2 type response causing progressive disease 15. Different mouse strains are recognized as genetically resistant or susceptible to L. major, depending on their ability to produce Th1 or Th2 cytokine profiles respectively. However, this clear dichotomous response is apparently not obvious in human leishmaniasis 16.
Varying cytokine profiles have been associated with different clinical manifestations and infecting species in leishmaniasis. Leishmania braziliensis infection which may manifests as localized cutaneous leishmaniasis (LCL), mucocutaneous leishmaniasis (MCL) or disseminated cutaneous leishmaniasis (DCL), has demonstrated a predominant Th1 response in LCL, a mixed Th1/Th2 response in MCL and a prominent Th2 response in DCL 17,18. Similarly, a predominant Th1 type cytokine profile is seen in localized L. mexicana and L. tropica infections 19,20. In contrast a prominent Th2 response was observed in lesions caused by L. guyanensis 21. Unfavorable clinical evolution in L. donovani-induced post kala-azar dermal leishmaniasis (PKDL) has been attributed to a mixed Th1/Th2 cytokine response22.
Hence, the in situ cytokine profile in leishmaniasis is determined by the causative Leishmania spp. 18,19,21, the parasite strain 23,24, and the host immune response 25. Cytokine expression in dermatropic L. donovani infections however, has not been previously evaluated. The present study is the first to characterize the in situ cytokine gene expression in CL due to Leishmania donovani.
Materials and Methods
Ethical issues
This study received ethical approval from the Ethics Review Committee of the Faculty of Medicine, University of Kelaniya, Sri Lanka and was conducted adhering to the approved protocol and in agreement with the Helsinki Declaration. Patients and controls were recruited on a voluntary basis and informed written consent was obtained prior to sample collection.
Study population
A total of 108 patients with suspected skin lesions attending dermatology clinics in the Sri Lanka Army and District General Hospitals of Polonnaruwa and Hambantota were included. Patients with major co-morbidities such as diabetes mellitus and those already on treatment for CL were excluded from the study.
Sample collection
Two punch biopsies each measuring 2–3 mm were obtained from the lesion under local anesthesia. One was immediately submerged in RNAlater (Qiagen, Hilden, Germany), to stabilize RNA, and stored at −20°C until further analysis. The second biopsy was gently rolled over a microscope slide to prepare an impression smear. The biopsy specimen thereafter was fixed in 10% neutral buffered formalin (NBF) for routine histopathological processing, where tissue sections separately stained with haematoxylin and eosin (H & E) and Giemsa were prepared. Impression smears made on glass slides were air dried, fixed in methanol, stained with Giemsa and examined under a light microscope for the presence of parasites. Fifty eight patient samples with confirmed diagnosis of leishmaniasis, following the demonstration of parasites in impression smears or histological sections were further analyzed for cytokine gene expression. Control skin specimens were obtained from incision sites of 25 patients with no signs or symptoms of leishmaniasis, who underwent minor surgical procedures due to unrelated surgical causes. Patients were treated by the dermatologists in charge of the clinics with intralesional sodium stibogluconate weekly or fortnightly until the lesions healed.
Histopathological analysis
Forty six H and E stained sections were examined under a conference microscope (Olympus BX50, Tokyo, Japan) and categorized according to modified Ridley’s criteria for leishmaniasis as previously described 14. These categories are defined and illustrated in figure 2 and 3. In the group IV pattern where amastigotes were not seen, diagnosis was based on positive impression smear result. Histology was not performed in 12 patients due to inadequacy of specimen or its poor quality. Each specimen was examined by two histopathologists for confirmation of findings.
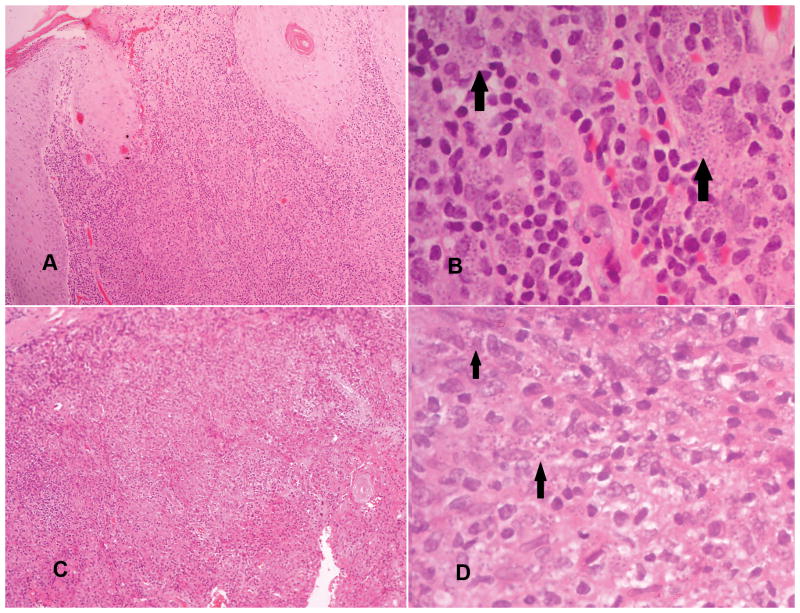
Figure 2.
Histopathological groups I and II.
Group I (pictures A and B): Parasitized macrophages with variable lymphocytes and plasma cells in a diffuse infiltrate (amastigotes are shown by arrows in B).
Group II (pictures C and D): Parasitized macrophages (shown by arrows in D) with lymphocytes, plasma cells and ill formed histiocytic granulomata. (A and C: haematoxylin and eosin 10 x 20, B and D: haematoxylin and eosin 10x40).
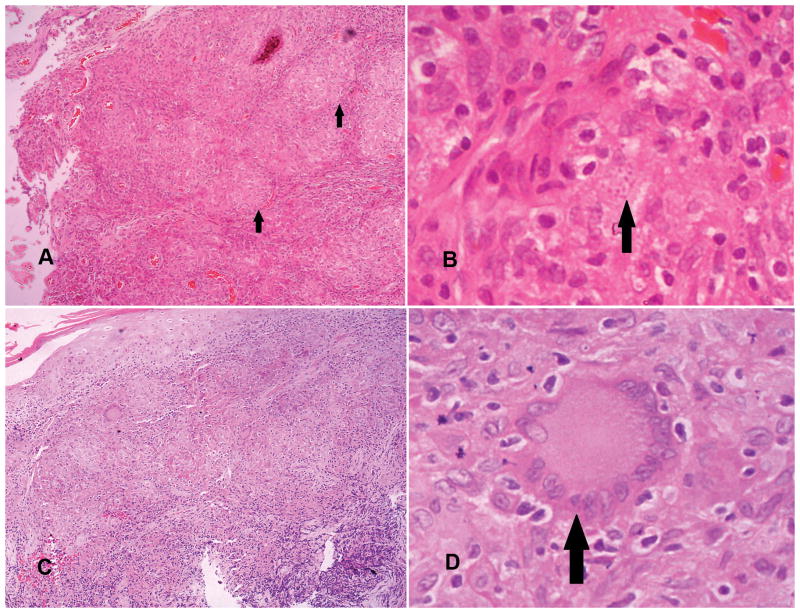
Figure 3.
Histopathological groups III and IV.
Group III (pictures A and B): A mixture of macrophages with or without parasites (amastigotes focally present are indicated by arrows in B), lymphocytes, plasma cells and epithelioid granulomata (shown by arrows in A).
Group IV (pictures C and D): Epithelioid granulomatous response with or without Langhans type multinucleated giant cells (shown with arrow in D), few lymphocytes, plasma cells but no amastigotes (A and C: haematoxylin and eosin 10 x 20, B and D: haematoxylin and eosin 10x40).
Altogether, cytokine gene expression was quantified in 58 patient and 25 control samples and it was correlated with histopathology in 46 patient samples. Forty four patients were followed up to assess the duration of treatment.
Total RNA extraction
Tissue specimens in RNAlater were disrupted and homogenized with RNA lysis buffer containing guanidine isothiocyanate using 1 g of 2.00 mm diameter Zirconia beads in a Mini-Beadbeater (Biospec, Bartlesville, OK, USA). Total RNA was extracted using the RNeasy Plus Universal Mini Kit (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions. RNA purity and concentration measured using the NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Integrity of RNA was established by the presence of 28s and 18s bands on a 1% agarose gel electrophoresis according to established methods 26.
Reverse transcription-quantitative real- time polymerase chain reaction (RT-qPCR) for cytokine gene expression
Gene expression was analyzed for 5 cytokine genes (IL-12A, IFN-γ, TNF-α, IL-4 and IL-10) that represent both the Th1 and Th2 responses in the lesion. Cytokine primers validated for quantitative real-time PCR were selected from the PrimerBank 27 (Table 1). Optimization of RT-qPCR was carried out following the MIQE guidelines 28. Complementary DNA (cDNA) was synthesized using a unique blend of oligo (dT) and random hexamer primers provided by the iScript® cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), according to manufacturer’s instructions. Briefly, 100 ng of RNA was used per 20 μl total reaction volume, which included 4 μl of 5x iScript® reaction mix and 1 μl of iScript® reverse transcriptase. Reactions were incubated for 5 minutes at 25°C, 30 minutes at 42°C and 5 minutes at 85°C. A water control was included for each batch of cDNA synthesized. As recommended by the manufacturer, one-tenth of the cDNA reaction volume (2 μl) was used for qPCR. Real-time PCR reactions were set up with 2 μl of cDNA, 2.5 μl of primer mix with a concentration of 2.5 μM of each forward and reverse primers, 12.5 μl of iQ™SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA) and 8 μl of nuclease free water in a total reaction volume of 25μl in each well on Microseal®96-well skirted PCR plates (Bio-Rad, Hercules, CA, USA). For each set of primers, all samples were run in duplicates. Beta-actin was used as the reference gene and qPCR was performed for 58 patient samples and 25 control samples in a CFX96™ Real-time system (Bio-Rad, Hercules, CA, USA). Thermal cycling protocol consisted of an initial denaturation step at 95°C for 10 min followed by 45 cycles each consisting of 95°C for 15s, 60°C for 30s, 72°C for 30s and 78°C for 5s, and held at 72°C for 10 min. Post-amplification melting curve analysis was done to ensure reaction specificity, starting at 50°C and increasing up to 95°C in increments of 0.5°C for 5s. A negative control was included for each batch of cDNA samples.
Table 1.
Cytokine primer sequences used in this study for mRNA quantification by RT-qPCR.
| Gene | Genbank accession | PrimerBank ID | Primer Sequence (5′-3′) | Amplicon size (bp) | Melting Temperature (°C) |
|---|---|---|---|---|---|
| β-actin | NM_001101 | 4501885a1 | F: CATGTACGTTGCTATCCAGGC R: CTCCTTAATGTCACGCACGAT |
250 | 60.8 60.2 |
| IFN-γ | NM_000619 | 56786137c1 | F: TCGGTAACTGACTTGAATGTCCA R: TCGCTTCCCTGTTTTAGCTGC |
93 | 61.2 62.9 |
| IL-12A | NM_000882 | 325974478c1 | F: CCTTGCACTTCTGAAGAGATTGA R: ACAGGGCCATCATAAAAGAGGT |
181 | 60.2 61.1 |
| TNF-α | NM_000594 | 25952110c1 | F: CCTCTCTCTAATCAGCCCTCTG R: GAGGACCTGGGAGTAGATGAG |
220 | 60.8 60.2 |
| IL-4 | NM_000589 | 4504669a1 | F: CCAACTGCTTCCCCCTCTG R: TCTGTTACGGTCAACTCGGTG |
150 | 62 61.7 |
| IL-10 | NM_000572 | 24430216c1 | F: GACTTTAAGGGTTACCTGGGTTG R: TCACATGCGCCTTGATGTCTG |
112 | 60.5 63 |
Source:29
Abbreviations: bp, base pairs.
Relative quantification of gene expression was done using the equation 2−ΔΔCt = 2−(Ct target gene – Ct β-actin) sample-(Ct target gene-Ct β-actin) control as previously described 29.
Statistical analysis
Data were analyzed by IBM SPSS statistics for Windows, version 21.0 (Armonk, NY, USA). Non parametric tests were used for comparison of cytokine gene expression between patient and control groups as cytokine levels did not show a normal distribution. Mann – Whitney U test was used for comparison between two groups and Kruskal-Wallis test was used for comparison between more than two groups. Spearman’s correlation test was used for analyzing correlation between continuous variables. Significance was estimated at 0.05 level for all above methods.
Results
Patient and control group characteristics
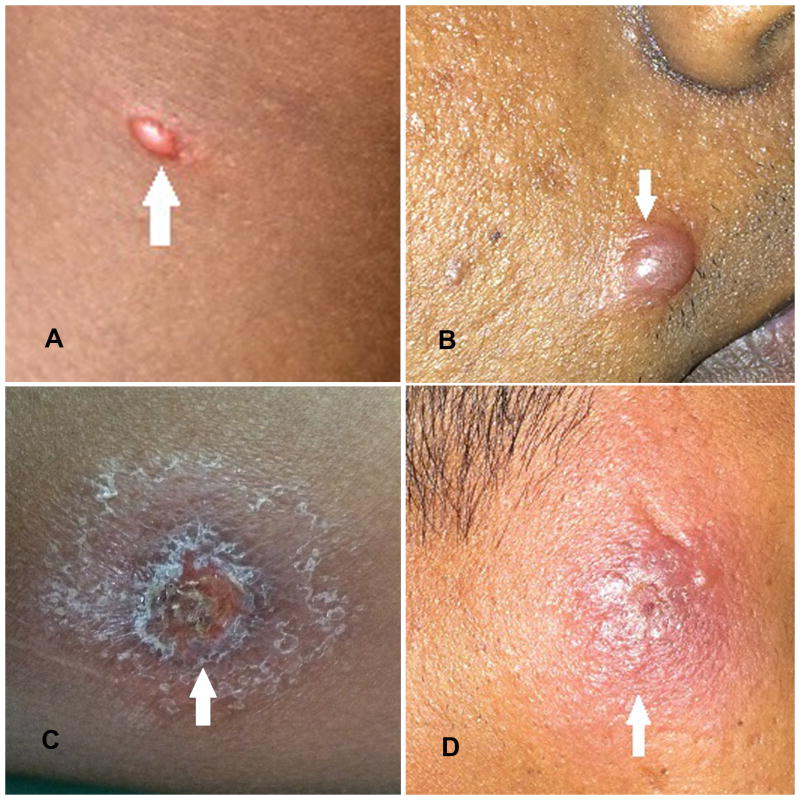
The two groups were comparable with no significant difference in their age (p = 0.19) and sex (p = 0.72) distribution (Table S1). Patients presented with 1–4 lesions and majority (75.9%, 44/58) had only a single lesion. Lesions were mainly on upper limb (51.7%, 30/58) and lower leg (34.5%, 20/58), and lesion type varied from discrete papules and nodules to ulcerated plaques (Figure 1). Majority had nodular lesions (44.8%, 26/58). Lesions had a mean duration of 6.75 ±9.1 months (range: 1–48 months) and a mean size of 176.59 ±185.76 mm2 (range: 12.6 – 908.3 mm2).
Figure 1.
Clinical presentations of cutaneous leishmaniasis due to L. donovani in Sri Lanka. Pictures A, B, C and D demonstrate a papule, nodule, ulcer and an indurated plaque lesion respectively.
Histopathological characteristics
Epidermal changes included hyperkeratosis (91.3%, 42/46), irregular acanthosis (54.3%, 25/46), parakeratosis (34.8%, 16/46), follicular plugging (21.7%, 10/46) and hyperplasia (10.9%, 5/46) (Figure S1). Dermal changes were characterized by marked inflammatory infiltrate composed of macrophages, lymphocytes and plasma cells with or without granuloma formation (Figure 2 and 3). Distribution of sample numbers, lesion types and mean lesion duration among histopathological groups are given in table 2. Necrosis was not seen in any of these specimens. Histological grouping failed to show any obvious association with the lesion type, size or duration.
Table 2.
Distribution of sample numbers and lesion characteristics among histopathological groups
| Group I | Group II | Group III | Group IV | |
|---|---|---|---|---|
| Number of samples | 13 | 17 | 10 | 6 |
| Papules | 0 | 0 | 1 | 0 |
| Nodules | 3 | 10 | 3 | 5 |
| Plaques | 2 | 2 | 1 | 0 |
| Ulcers | 8 | 5 | 5 | 1 |
| Mean lesion duration in months (SD) | 4.6 (2.5) | 8.1 (11.6) | 11.8 (14.4) | 5.7 (4.3) |
In situ cytokine gene expression and association with patient and lesion characteristics
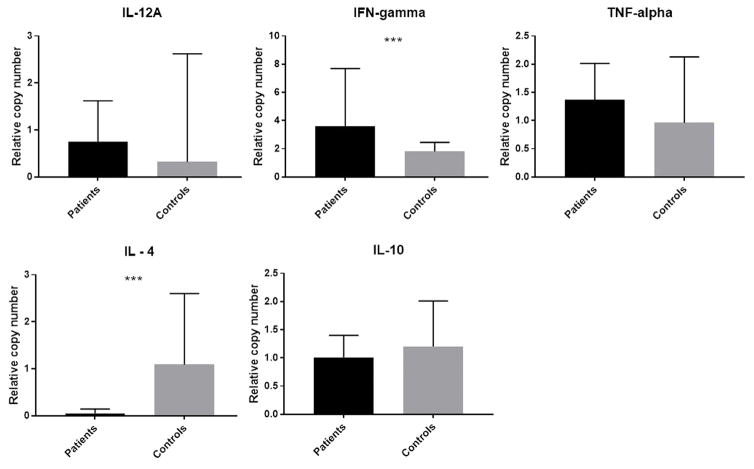
All cytokines tested were expressed at detectable levels in patients’ tissue samples. In the control group all had detectable levels of IL-10, IFN-γ and TNF-α. However, IL-12A and IL-4 were detectable only in 17/25 and 21/25 controls respectively. Significant up regulation of IFN-γ (p < 0.001) and down regulation of IL-4 (p < 0.001) was seen in the patients compared to controls (Figure 4).
Figure 4.
Gene expression for IL-12A, IFN-γ, TNF-α, IL-4 and IL-10 in patient and control groups. Cytokine mRNA in dermal lesions and control skin specimens were quantified by RT-qPCR and expressed as relative mRNA copy numbers by 2ΔΔCt method. Beta actin was used as the reference gene and controls as the calibrators. Graphs represent the median relative mRNA copy numbers and interquartile range. Difference between patient and control groups were significant by Mann-Whitney U test for IFN- γ and IL-4 (***p <0.001).
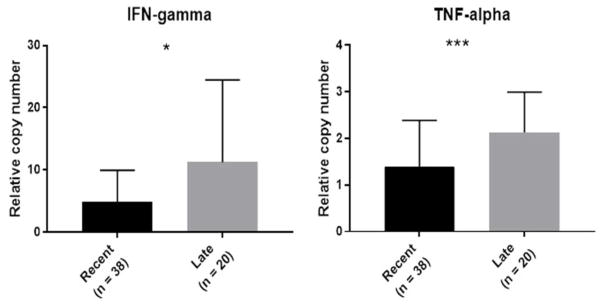
Expression of TNF-α increased significantly with lesion duration (Spearman r =0.294, p=0.025). When lesions were categorized as recent (those with a duration of < 6 months at presentation) and late (duration of 6 months or more), there was significant increase in both IFN-γ (p=0.018) and TNF-α (p<0.001) in late lesions (Figure 5). Females showed higher mean level of Th1 type cytokine IL-12A (p= 0.03). On the other hand, males had higher mean levels of Th2 type cytokines IL-4 and IL-10 as well as Th1 type IFN-γ, but none were statistically significant. Cytokine expression was not significantly associated with the lesion size, lesion type, ulceration or histopathological grouping. Expression of all cytokine genes correlated significantly with each other, with the strongest correlation in the expression of IL-12A with IFN-γ (r =0.786) (Table 3).
Figure 5.
Expression of IFN-gamma and TNF-alpha in recent (< 6 months) and late (≥6 months) lesions. Both genes were expressed significantly more in late lesions compared to recent lesions. Graphs represent median relative mRNA copy numbers and interquartile range. (p values as calculated by Mann Whitney U test: * p <0.05 and ***p <0.001).
Table 3.
Spearman’s rank correlation coefficients for intralesional cytokine gene expressions
| IL-12A | IFN- γ | TNF-α | IL-4 | |
|---|---|---|---|---|
| IFN- γ | 0.786 (p <0.001) | |||
| TNF-α | 0.594 (p <0.001) | 0.679 (p <0.001) | ||
| IL-4 | 0.565 (p <0.001) | 0.615 (p <0.001) | 0.395 (p =0.002) | |
| IL-10 | 0.507 (p <0.001) | 0.597 (p <0.001) | 0.485 (p <0.001) | 0.659 (p <0.001) |
Values indicate correlation coefficients with level of significance in parentheses, determined by the non-parametric Spearman’s rank correlation test to calculate the degree of linear correlation among the relative copy numbers for cytokine mRNAs expressed at the site of lesion. A correlation coefficient close to 1 indicate a linear relationship in the expression of the two cytokines.
Abbreviations: IL, interleukin; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor-alpha.
All lesions followed up achieved complete healing and the treatment duration ranged from 1.5 to 8 months with a mean of 3.0 ± 1.75 months. Time taken to heal showed a significant positive correlation with in situ expression level of IL-4 (Spearman’s r =0.321, p=0.034).
Discussion
Localized immune response at the site of infection plays an important role in pathogenesis and outcome of CL18–20,30,31. To better understand the role of cytokines in the pathogenesis of CL due to dermatropic L. donovani, we investigated and quantified for the first time, the in situ expression of both Th1 and Th2 type cytokine profiles together with local histopathological changes. Different types of cutaneous lesions, as previously described 4 were studied and all appeared to heal completely following treatment with intralesional sodium stibogluconate, although the rate of healing varied between patients. There was a mixed Th1/Th2 cytokine profile at the site of infection, similar to observations made on human CL due to other Leishmania species 17–19. However, it was interesting to note a markedly up-regulated IFN-γ and down-regulated IL-4 expression with varying histopathological characteristics.
The most striking feature in histopathology was the presence of a marked inflammatory cell infiltrate in the dermis, composed of histiocytes, plasma cells and lymphocytes. Its organization ranged from diffuse inflammatory infiltrate with parasitized macrophages to varying degrees of granuloma formation that extended from ill-formed histiocytic to epithelioid granulomata. Similar histological changes have been observed in previous studies done on CL due to L. major 32 and L. donovani in the local setting 14. Varying degrees of necrosis have been described in American tegumentary leishmaniasis 33,34 as well as in CL in the old world 32. However, necrosis was not a prominent feature in this study, and therefore parasite elimination in CL due to L. donovani appears to depend on activation of macrophages to form epithelioid granulomas, rather than through a necrotizing process, as previously suggested 14. Interestingly, up-regulation of IFN-γ seen in this study provide further support for this assumption, as IFN-γ is a potent activator of macrophages 35. Therefore, it can be concluded that macrophage activation plays a key role in parasite elimination in CL due to L. donovani in Sri Lanka. No significant association between histological grouping and cytokine expression was seen however, which may have been due to the inadequate numbers in some of the groups that hindered a proper comparison. Furthermore, failure to measure the cytokines produced also may have been an added limitation.
Localized CL is associated with increased in situ expression of IFN-γ as demonstrated in L. tropica 20 and L. major 31 infections, which are clinically comparable to L. donovani-induced CL in Sri Lanka. Interferon-γ is a potent activator of macrophages 35 and acts synergistically with TNF-α to kill intracellular pathogens by induction of Nitric Oxide Synthase (iNOS) to produce nitric oxide 36. Significant up-regulation of IFN-γ and significant positive correlation between IFN-γ and TNF-α gene expression seen in this study further suggest that, macrophage activation is responsible for parasite elimination. Interleukin-12 plays a major role in the development of a Th1 response at the site of infection 35. Leishmania spp. are known to down-regulate production of IL-12, which enable their establishment within macrophages without activation, and hence facilitate ‘silent entry’ 37. A similar tendency to down-regulate IL-12 was seen in the patient population compared to controls, but the strong positive correlation seen between the expressions of IL-12A and IFN-γ indicates a well regulated expression of these two cytokines in the local milieu.
Although essential for parasite clearance, an exaggerated Th1 response with excessive IFN-γ levels causes damage to host tissues. Subclinical infections have been associated with low levels of IFN-γ production in L. mexicana and L. braziliensis infections 30,38. Furthermore, elevated in situ expression of IFN-γ has been demonstrated in late lesions (> 6 months) in L. major infections 39. In this study, significantly increased expression of IFN-γ was seen in lesions diagnosed late (≥ 6 months) and a tendency to have higher levels of IFN-γ was observed in lesions that healed more slowly. Further enhancement of the pro-inflammatory milieu at the site of lesions as they evolved with time was evident by the significant positive correlation between the expression of TNF-α and lesion duration. This adds to the existing theory of the role of TNF-α in MCL and chronic manifestations in human leishmaniasis 17–19.
Development of an anti-inflammatory milieu at the site of infection favors persistence of parasites and disease progression in mouse models 15. Interleukin - 4 secreted by Th2 subset of lymphocytes plays a major role in down regulating the Th1 response and inhibiting nitric oxide production 1. In general, in situ expression of IL-4 is low in human CL 19,31, but a greater expression has been evident in MCL and DCL 17–19. In human infections with L. major, IL-4 production was associated with severe disease, while patients with mild disease had no IL-4 production 40. A very low and significantly down regulated IL-4 response was observed in patients compared to healthy controls, in the current study. This might provide a clue as to the non-visceralizing nature of the local parasite with relatively more favorable outcome of leishmaniasis in Sri Lanka, despite the virulence and visceralizing potential of the species L. donovani. Furthermore, a significant positive correlation was observed between IL-4 expression and time taken for lesions to heal, which points toward a role of IL-4 in interfering with the healing process. Interleukin-10, which is another cytokine with anti-inflammatory properties, was co-expressed with IL-4 demonstrating a significant positive correlation. Modulatory actions of IL-10 is important to prevent excessive tissue damage in inflammatory reactions by inhibiting pro-inflammatory cytokines 41,42 and hence higher expression has been recorded in MCL than LCL 18. High IL-10 has also been associated with poor response to treatment in infections with L. guyanensis 43, more slowly healing lesions in L. major31, persistence of disease with L. mexicana 44 and Indian PKDL 45, which has led to the suggestion that an optimum balance between macrophage activating IFN-γ and de-activating IL-10 is required for favorable outcome in leishmaniasis 46. Interestingly, we observed a significant positive correlation between expression of IFN-γ and IL-10 in lesions, with suggestive favorable balance between the pro-inflammatory and immune-regulatory responses among CL patients in Sri Lanka, which possibly explains its uncomplicated course in almost all patients.
Male and female sex hormones have a role in modulating the immune responses as evidenced by the pro-inflammatory properties of estrogen or 17β-estradiol and anti-inflammatory properties of testosterone 47. Furthermore, studies have shown that 17β-estradiol can increase nitric oxide production from macrophages in a pro-inflammatory cytokine independent pathway48. However, experimental models have shown different outcomes in male and female mice depending on the Leishmania species 47. Influence of sex was not very marked in the present study though there was an apparent tendency for increased expression of pro-inflammatory cytokine TNF-α and IL-12A in females and IL-4 and IL-10 with anti-inflammatory bias in males. However, the use of large sample numbers may increase the statistical power of such analysis.
Evidence from these cytokine profiles points out a significant correlation between all the genes studied, with strong correlations apparent for IFN-γ with IL-12A and TNF-α. Similar significant correlation for IFN-γ with IL-12 and TNF-α has been found in other studies that involve Leishmania 20,31. Interestingly we also observed a significant positive correlation between the expression of IFN-γ and IL-4 and IL-4 and IL-10, indicating the fine balance between the pro- and anti-inflammatory modulators in local tissues of CL patients.
In conclusion, these observations point towards an existence of a mixed Th1/ Th2 cytokine expression profile at the site of lesion with an obvious bias towards Th1 response, that has positively influenced the outcome of the resultant localized form of leishmaniasis caused by a variant strain of Leishmania donovani among Sri Lankan patients. However, in order to directly implicate cytokines in disease pathogenesis and/or its outcome, more detailed investigations with adequate number of samples in each category of histopathology and proteomic studies would be required, which may be combined with a comprehensive analysis of chemokines and other anti-inflammatory cytokines such as IL-17, TGF-β.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health [grant number R01AI099602 to NDK.]; the University Grants Commission of Sri Lanka [grant number UGC/VC/DRIC/PG/2013/KLN/03 to NHM]; the University of Kelaniya, Sri Lanka [grant number RP/03/04/06/01/2014 to WA]; and the Ohio State University Intramural Grant [to ARS]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any other funding agency.
Authors would like to acknowledge Drs. UADD Munidasa and KKVN Somaratne for permission to collect patient samples; Drs. D.T. Gunasena, N. L. Munasinghe and R. Premathilaka for assistance with collection of control specimens; Dr. Charisma S. Fernando for histopathological analysis of specimens; Prof. A. Pathmeswaran and Dr. E.P.D.S. Ediriweera for guidance on statistical analysis and all the patients for participating and consenting to photograph lesions.
Abbreviations
- CL
cutaneous leishmaniasis
- DCL
disseminated cutaneous leishmaniasis
- LCL
localized cutaneous leishmaniasis
- MCL
mucocutaneous leishmaniasis
- VL
visceral leishmaniasis
- IFN
Interferon
- IL
Interleukin
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- Th
T helper
Footnotes
Author Contributions
NHM designed, performed the experiments, analyzed the data and wrote the manuscript. SO contributed to experimental work by designing cytokine mRNA quantification. Clinical management was done by NP. VCdeS performed the histopathological analysis of specimens. WA contributed to the design and provided supervision. ARS provided essential reagents and laboratory facilities. NDK contributed to design, undertook overall supervision, managed collaborations and edited the manuscript.
Disclosures
The authors have no potential conflict of interest to disclose.
Disclosures: None.
References
- 1.Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112(Pt 18):2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athukorale DN, Seneviratne JK, Ihalamulla RL, Premaratne UN. Locally acquired cutaneous leishmaniasis in Sri Lanka. J Trop Med Hyg. 1992;95(6):432–433. [PubMed] [Google Scholar]
- 4.Karunaweera ND, Rajapaksa US. Is Leishmaniasis in Sri Lanka benign and be ignored? J Vector Borne Dis. 2009;46(1):13–17. [PubMed] [Google Scholar]
- 5.Karunaweera ND, Pratlong F, Siriwardane YD, Ihalamulla RL, Dedet JP. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans R Soc Trop Med Hyg. 2003;97(4):380–381. doi: 10.1016/S0035-9203(03)90061-7. [DOI] [PubMed] [Google Scholar]
- 6.Alam MZ, Haralambous C, Kuhls K, et al. The paraphyletic composition of Leishmania donovani zymodeme MON-37 revealed by multilocus microsatellite typing. Microbes Infect. 2009;11(6–7):707–715. doi: 10.1016/j.micinf.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Kumar NP, Srinivasan R, Anish TS, Nandakumar G, Jambulingam P. Cutaneous leishmaniasis caused by Leishmania donovani in the tribal population of the Agasthyamala Biosphere Reserve forest, Western Ghats, Kerala, India. J Med Microbiol. 2015;64(Pt_2):157–163. doi: 10.1099/jmm.0.076695-0. [DOI] [PubMed] [Google Scholar]
- 8.Karunaweera ND. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: a wolf in sheep’s clothing? Trends Parasitol. 2009;25(10):458–463. doi: 10.1016/j.pt.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Rajapaksa US, Ihalamulla RL, Karunaweera ND. First report of mucosal tissue localisation of leishmaniasis in Sri Lanka. Ceylon Med J. 2005;50(2):90–91. [PubMed] [Google Scholar]
- 10.Rathnayake D, Ranawake RR, Sirimanna G, Siriwardhane Y, Karunaweera N, De Silva R. Co-infection of mucosal leishmaniasis and extra pulmonary tuberculosis in a patient with inherent immune deficiency. Int J Dermatol. 2010;49(5):549–551. doi: 10.1111/j.1365-4632.2010.04376.x. [DOI] [PubMed] [Google Scholar]
- 11.Abeygunasekara P, Costa Y, Seneviratne N, Ratnatunga N, Wijesundera MDS. Locally aquired visceral leishmaniasis in Sri Lanka. Ceylon Med J. 2007;52(1):30–31. doi: 10.4038/cmj.v52i1.1047. [DOI] [PubMed] [Google Scholar]
- 12.Ranasinghe S, Zhang W-W, Wickremasinghe R, et al. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathog Glob Health. 2012;106(7):421–424. doi: 10.1179/2047773212Y.0000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridley DS, Ridley MJ. The evolution of the lesion in cutaneous leishmaniasis. J Pathol. 1983;141:83–96. doi: 10.1002/path.1711410109. [DOI] [PubMed] [Google Scholar]
- 14.Herath CH, Ratnatunga NV, Waduge R, Ratnayake P, Ratnatunga CN, Ramadasa S. A histopathological study of cutaneous leishmaniasis in Sri Lanka. Ceylon Med J. 2010;55:106–111. doi: 10.4038/cmj.v55i4.2626. [DOI] [PubMed] [Google Scholar]
- 15.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander J, Brombacher F. T helper1/T helper2 cells and resistance/susceptibility to Leishmania infection: Is this paradigm still relevant? Front Immunol. 2012;3:80. doi: 10.3389/fimmu.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cáceres-Dittmar G, Tapia FJ, Sánchez MA, et al. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin Exp Immunol. 1993;91(3):500–505. doi: 10.1111/j.1365-2249.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human Leishmaniasis. J Clin Invest. 1993;91(4):1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun. 1994;62(3):837–842. doi: 10.1128/iai.62.3.837-842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R, Bumb RA, Salotra P. Evaluation of localized and systemic immune responses in cutaneous leishmaniasis caused by Leishmania tropica: interleukin-8, monocyte chemotactic protein-1 and nitric oxide are major regulatory factors. Immunology. 2010;130(2):193–201. doi: 10.1111/j.1365-2567.2009.03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourreau E, Prévot G, Pradinaud R, Launois P. Interleukin (IL)-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis lesions and renders specific CD4+ T cells unresponsive to IL-12. J Infect Dis. 2001;183(6):953–959. doi: 10.1086/319249. [DOI] [PubMed] [Google Scholar]
- 22.Ansari NA, Ramesh V, Salotra P. Interferon (IFN)-gamma, tumor necrosis factor-alpha, interleukin-6, and IFN-gamma receptor 1 are the major immunological determinants associated with post-kala azar dermal leishmaniasis. J Infect Dis. 2006;194(7):958–965. doi: 10.1086/506624. [DOI] [PubMed] [Google Scholar]
- 23.Asadpour A, Riazi-Rad F, Khaze V, Ajdary S, Alimohammadian MH. Distinct strains of Leishmania major induce different cytokine mRNA expression in draining lymph node of BALB / c mice. Parasite Immunol. 2013;35(1):42–50. doi: 10.1111/pim.12018. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira MJ, Fernandes JD, Teixeira CR, et al. Distinct Leishmania braziliensis isolates induce different paces of chemokine expression patterns. Infect Immun. 2005;73(2):1191–1195. doi: 10.1128/IAI.73.2.1191-1195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gollob KJ, Viana AG, Dutra WO. Immunoregulation in human American leishmaniasis: balancing pathology and protection. Parasite Immunol. 2014;36(8):367–376. doi: 10.1111/pim.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forlenza M, Kaiser T, Savelkoul HF, Wiegertjes GF. The use of real-time quantitative PCR for the analysis of cytokine mRNA levels. Methods Mol Biol. 2012;820:7–23. doi: 10.1007/978-1-61779-439-1_2. [DOI] [PubMed] [Google Scholar]
- 27.PCR primers for gene expression detection and quantification. [Accessed August 20, 2015];PrimerBank web site. https://pga.mgh.harvard.edu/primerbank/
- 28.Bustin SA, Benes V, Garson JA, et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin Chem. 2009;55(4):1–12. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2^(−ΔΔCT) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Valencia-Pacheco G, Loría-Cervera EN, Sosa-Bibiano EI, et al. In situ cytokines (IL-4, IL-10, IL-12, IFN-γ) and chemokines (MCP-1, MIP-1α) gene expression in human Leishmania (Leishmania) mexicana infection. Cytokine. 2014;69(1):56–61. doi: 10.1016/j.cyto.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Louzir H, Melby PC, Ben Salah A, et al. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J Infect Dis. 1998;177:1687–1695. doi: 10.1086/515297. [DOI] [PubMed] [Google Scholar]
- 32.Gaafar A, el Kadaro AY, Theander TG, et al. The pathology of cutaneous leishmaniasis due to Leishmania major in Sudan. Am J Trop Med Hyg. 1995;52(5):438–442. doi: 10.4269/ajtmh.1995.52.438. [DOI] [PubMed] [Google Scholar]
- 33.Bittencourt AL, Barral A. Evaluation of the histopathological classification of American cutaneous and mucocutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 1991;86(1):51–56. doi: 10.1590/s0074-02761991000100009. [DOI] [PubMed] [Google Scholar]
- 34.Martins ALGP, Barreto JA, Lauris JRP, Martins ACGP. American tegumentary leishmaniasis: Correlations among immunological, histopathological and clinical parameters. An Bras Dermatol. 2014;89(1):52–58. doi: 10.1590/abd1806-4841.20142226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: Paradox rather than paradigm. Immunol Lett. 2005;99(1):17–23. doi: 10.1016/j.imlet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Liew FY, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145(12):4306–4310. [PubMed] [Google Scholar]
- 37.Gupta G, Oghumu S, Satoskar AR. Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol. 2013;82:155–184. doi: 10.1016/B978-0-12-407679-2.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bittar RC, Nogueira RS, Vieira-Gonçalves R, et al. T-cell responses associated with resistance to Leishmania infection in individuals from endemic areas for Leishmania (Viannia) braziliensis. Mem Inst Oswaldo Cruz. 2007;102(5):625–630. doi: 10.1590/s0074-02762007005000069. [DOI] [PubMed] [Google Scholar]
- 39.Hoseini SG, Javanmard SH, Hejazi SH, et al. Comparison of immune regulatory factors in acute and chronic lesions of cutaneous leishmaniasis due to Leishmania major. J Res Med Sci. 2014;19(Suppl 1):S36–40. [PMC free article] [PubMed] [Google Scholar]
- 40.Gaafar A, Kharazmi A, Ismail A, et al. Dichotomy of the T cell response to Leishmania antigens in patients suffering from cutaneous leishmaniasis; absence or scarcity of Th1 activity is associated with severe infections. Clin Exp Immunol. 1995;100(2):239–245. doi: 10.1111/j.1365-2249.1995.tb03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bacchetta R, Gambineri E, Roncarolo M-G. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120(2):227–235. doi: 10.1016/j.jaci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Cummings HE, Tuladhar R, Satoskar AR. Cytokines and their STATs in cutaneous and visceral leishmaniasis. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/294389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourreau E, Prévot G, Gardon J, Pradinaud R, Launois P. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J Infect Dis. 2001;184:1628–1630. doi: 10.1086/324665. [DOI] [PubMed] [Google Scholar]
- 44.Melby PC, Andrade-Narvaez F, Darnell BJ, Valencia-Pacheco G. In situ expression of interleukin- 10 and interleukin- 12 in active human cutaneous leishmaniasis. FEMS Immunol Med Microbiol. 1996;15(2–3):101–107. doi: 10.1111/j.1574-695X.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 45.Ganguly S, Das NK, Panja M, et al. Increased levels of interleukin-10 and IgG3 are hallmarks of Indian post-kala-azar dermal leishmaniasis. J Infect Dis. 2008;197(12):1762–1771. doi: 10.1086/588387. [DOI] [PubMed] [Google Scholar]
- 46.Gomes-Silva A, De Cássia Bittar R, Dos Santos Nogueira R, et al. Can interferon-γ and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007;149(3):440–444. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snider H, Lezama-Dávila CM, Alexander J, Satoskar AR. Sex hormones and modulation of immunity against leishmaniasis. Neuroimmunomodulation. 2009;16(2):106–113. doi: 10.1159/000180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lezama-Dávila CM, Isaac-Márquez AP, Barbi J, Oghumu S, Satoskar AR. 17β-estradiol increases Leishmania mexicana killing in macrophages from DBA/2 mice by enhancing production of nitric oxide but not pro-inflammatory cytokines. Am J Trop Med Hyg. 2007;76(6):1125–1127. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.