Abstract
Several studies have reported declines in adaptive behavior amongst children with fragile X syndrome (FXS) starting in middle childhood. We examined the effects of maternal responsivity on adaptive behavior in 55 children with FXS visited 5–6 times in their homes from early through middle childhood. Our analyses indicated that sustained maternal responsivity had a significant positive impact on the trajectories of communication and to a lesser extent other adaptive behavior domains through middle childhood with many effects remaining significant after controlling for autism symptoms and developmental level. For children who showed declines in adaptive behavior during middle childhood, sustained high levels of maternal responsivity minimized the amount of decline observed in the communication, socialization, and daily living domains.
Keywords: fragile X syndrome, maternal responsivity, adaptive behavior
Fragile X syndrome (FXS) is the most common known inherited cause of intellectual disability (Crawford, Acuna, & Sherman, 2001; Turner, Webb, Wake, & Robinson, 1996). It is caused by a mutation on the X chromosome that disrupts the expression of the FMR1 gene (Verkerk et al., 1991). This leads to atypical brain development (Devys, Lutz, Rouyer, Bellocq, & Mandel, 1993; Tamanini, 1997) and a range of impairment from learning disabilities to severe intellectual disabilities (Loesch, Huggins, & Hagerman, 2004). Because FXS is an X-linked disorder there is a higher incidence rate for males (1 in 4,000) than for females (1 in 6,000) (Hagerman, 2007; CDC, 2011). FXS is also the most common known genetic disorder linked to autism spectrum disorder (ASD) with estimates ranging from 30% to 45% of males and 10% of females having a co-occurring diagnosis of ASD (Bailey, Raspa, Olmstead & Holiday, 2008; Demark, Feldman, & Holden, 2003; Philofsky, Hepburn, Hayes, Hagerman, & Rogers, 2004).
Our research has shown that a group of specific parenting behaviors collectively referred to as “maternal responsivity” have significant early (Warren, Brady, Sterling, Fleming, & Marquis, 2010) and sustained (Brady, Warren, Fleming, Keller, & Sterling, 2014) effects on language and communication development in children with FXS even when controlling for the effects of development, sex, ASD status, and maternal education level. Maternal responsivity has been broadly defined as a healthy, growth-producing relationship characterized by warmth, nurturance, and stability as well as specific behaviors, such as continuing positive responses to child initiations (Shonkoff & Phillips, 2000; Spiker, Boyce, & Boyce, 2002). Forms of maternal responsivity are not mutually exclusive and have been shown across an array of studies of typically and atypically developing children to impact language and communication development as well as emotional, social, and cognitive development (Warren & Brady, 2007). However, in the present study, as in most of our previous studies with this cohort (Brady et al., 2014; Warren et al., 2010), we focused on a set of specific maternal behaviors directed to the child such as commenting on the child’s behavior and/or focus of attention, requesting a verbal response, and recoding and/or expanding a child’s previous response. We also measured and assessed the effects of specific behavior management behaviors used by mothers such as requests for child behavior compliance (e.g., “stop that”, “come over here”).
Although a great deal of emphasis has been placed on the importance of early maternal responsivity, consistent sustained responsivity throughout childhood may provide the optimal environment for promoting continued growth in language, cognition, and other developmental domains (Warren & Brady, 2007). Continuous adjustment of the types and amounts of responsivity provided in response to changing child behaviors is the hallmark of healthy, adaptive parenting (Hauser-Cram, Warfield, Shonkoff, & Krauss, 2001; Legerstee, Vargehese, & van Beek, 2002). Specifically, children who experienced consistently high maternal responsivity throughout their first five years had better outcomes than children whose mothers were inconsistently responsive or consistently low (Landry, Smith, Swank, Assel, & Vellet, 2001). However, it may be difficult for a parent to remain responsive over an extended period of time if the child does not initiate frequently, does not produce clear communication signals, and/or develops new skills at a very slow pace. Thus, it may be especially important to examine the impact of sustained responsivity over time for children with intellectual and developmental disabilities such as FXS and/or ASD.
Our previous findings from a longitudinal study of 53 children with FXS indicated that children whose mothers demonstrated more sustained responsivity behaviors through the age of 9 produced significantly higher numbers of different words and displayed higher rates of growth in vocabulary over time (Brady et al., 2014). Our analyses controlled for early maternal responsivity, autism symptoms, nonverbal IQ, and maternal education level in order to determine the unique contribution of sustained responsivity on child vocabulary development.
Although our previous studies focused on relationships between maternal responsivity and language outcomes, we hypothesize that maternal responsivity is also significantly related to variability in children’s adaptive behavior (i.e., an individual’s level of skills needed to function in their daily environment; Ditterline & Oakland, 2009). There is ample evidence that many children with FXS acquire increasingly sophisticated communication, social, and daily living skills during early and middle childhood (Dykens et al., 1996; Dykens, Hodapp, Ort, & Leckman, 1993; Fisch, Carpenter, Holden, Simensen, et al., 1999; Hahn, Brady, Warren, & Fleming, 2015; Hatton et al., 2003). However, a growing body of research has also documented that a substantial portion of children with FXS begin experiencing declines in adaptive behavior development as measured by the Vineland Adaptive Behavior Scales (VABS; Sparrow, Balla, & Cicchetti, 1984; Sparrow, Cicchetti, & Balla, 2005) as early as middle childhood and extending into adolescence (Fisch et al., 2012; Freund, Peebles, Aylward, & Reiss, 1995; Hahn et al., 2015; Klaiman et al., 2014).
Previous results from our research showed significant linear and quadratic growth for the overall adaptive behavior raw scores on the VABS (i.e., adaptive behavior composite) and within the domains of communication, daily living, and socialization, over time with a slowing in the rate of growth during the middle childhood years (Hahn et al., 2015). Importantly, the quadratic trends were negative, indicating slight declines. Additionally, children in our sample who had higher nonverbal cognitive abilities and less autistic behaviors had higher adaptive behavior trajectories.
Closer examination of the individual trajectories of adaptive behavior revealed that while 44% of children in our study showed advances in adaptive behavior from toddlerhood through middle childhood (similar to past research findings; i.e., Hatton et al., 2003), the other 56% of children showed declines in adaptive behavior at or before the age of 10 (average age for the beginning of decline = 7 years; Hahn et al, 2015). The timing of this decline is also consistent with other studies that have indicated declines in adaptive behavior starting in middle childhood (Dykens et al., 1996, 1993). As in Hahn et al. (2015), in the present study we defined a decline as a decrease of at least 5 points for one or more of the VABS subscales (i.e. communication, socialization, daily living) over at least two time points that were separated by approximately 18 months.
These findings of raw score declines in the overall VABS composite and within the different domains of the VABS suggest that middle childhood may be a pivotal age period for adaptive behavior development in children with FXS (Hahn et al., 2015). Furthermore, our use of raw scores for this analysis differs from past studies that have reported declines or plateaus in adaptive behavior in childhood based on either standard or age-equivalent scores (Dykens et al., 1996, 1993; Fisch et al., 2012, 2002; Fisch, Carpenter, Holden, Howard-Peebles, et al., 1999; Fisch, Carpenter, Holden, Simensen, et al., 1999; Freund et al., 1995). The use of these types of scores make it difficult to discern whether children with FXS are truly showing declines in adaptive behavior or if they are simply showing slower growth in these skills relative to the normative sample. By using raw scores in a true longitudinal design, a decline means that these children, according to parent reports on the VABS, had actually lost some of these important skills over time suggesting a true regression (Hahn et al., 2015). Additionally, this approach allowed for a more nuanced examination of changes in adaptive behavior over time. In summary, our previous research, and the previously cited studies, indicate that a substantial proportion of children with FXS lose adaptive behavior skills in middle and/or late childhood both in relation to their peers and, as our previous study showed, in absolute terms.
The encouraging results of our previous analyses of maternal responsivity on the language development of children with FXS (Brady et al., 2014; Warren et al., 2010) in combination with our disconcerting discovery that a substantial portion of children in this same cohort showed true raw scores declines in one or more domain of adaptive behavior in middle childhood (Hahn et al., 2015) lead us to an obvious question: What is the relationship, if any, of parenting to the adaptive behavior development of children with FXS in middle childhood?
Our interest in conducting the present analysis was further peaked by three aspects of our sample. First, observing actual regression in children’s skills as they develop might suggest that the children who regress are simply functioning at a lower developmental level than children who show no declines. However, we found no statistically significant differences between the children in our study who did and or did not show declines on adaptive behavior on our initial measures of nonverbal cognition or autism symptoms (Hahn et al., 2015). Second, it has been reported in other studies of adaptive behavior in individuals with FXS that declines are related to sex (they are more common in males, which makes logical sense given the X-linked nature of FXS) and co-morbid ASD status. We have observed these same relationships in our sample (Hahn, et al., 2015). But adaptive behavior declines have been reported across all FXS sub phenotypes (Fisch, Carpenter, Holden, Howard-Peebles, et al., 1999; Fisch, Carpenter, Holden, Simensen, et al., 1999; Klaiman et al., 2014). Furthermore, we observed a spread of declines in our sample by middle childhood across all FXS sub phenotypes as well. Specifically, 4 of 11 females (36%) showed adaptive behavior declines of 5 or more points in one or more domains, 10 of 14 males with FXS and ASD (71%) declined, and 16 of 28 males with “FXS only” (57%) declined (Hahn et al., 2015). So while “being female” or “being male without ASD” may have afforded some protection from declines in adaptive behavior, there was nevertheless a substantial portion of those sub-groups who experienced raw score declines in one or more domain in middle childhood.
Our third observation was the considerable amount of variability in our sample in terms of maternal responsivity that spanned early through middle childhood. Specifically, 15 of 53 mothers for whom we had data across early and middle childhood, had relatively low-responsivity across 5 to 6 time points; 9 had high responsivity at all-time points; 22 started low but steadily increased their responsivity across time points; and 7 were variable, fluctuating from high to low responsivity and back (Brady et al., 2014). This variability did not correlate with maternal educational status. However, it may be relevant that the biological mothers of children with FXS are themselves premutation carriers of FXS, and three mothers in our sample had the full mutation. Indeed, research suggests that all carrier mothers of children with FXS may be at risk for a range of subtle to severe cognitive and/or emotional problems that could hinder their interactions with their children especially as they age (Mailick et al., 2014). These include an increasing risk of cognitive deficits in attention, verbal memory, and executive function as the mother’s age (Freund, Reiss, & Abrams, 1993; Sobesky, Hull, & Hagerman, 1994). They are also more prone to depression and social anxiety, and may be more affectively labile (Roberts et al, 2016; Hagerman & Hagerman, 2002; Mazzocco, 2000; Sobesky et al., 1994; Thompson, Rogeness, McClure, Clayton, & Johnson, 1996). These risk factors have been previously associated with lower maternal responsivity in other non-FXS samples (Goldsmith & Rogoff, 1995; Osofsky, Thompson, Shonkoff, & Meisels, 2000).
In summary, the degree of variability in maternal responsivity we observed and the variability of declines in adaptive behavior, led us to an obvious question: Is there a relationship between parenting and the development and/or decline of adaptive behaviors as measured by the VABS? To answer this question, we chose to examine the role of specific, contingent parenting behaviors that make up the constructs of maternal responsivity and behavior management on the adaptive behavior trajectories of the same cohort of children described in our previous study (see Hahn et al., 2015).
Methods
Participants
The participants in our ongoing longitudinal study are 55 children with FXS and their mothers. Fifty-three of the dyads were part of our longitudinal study into middle childhood. The other two participated only during the early childhood period. All participants represented a sample of convenience that were recruited from across the United States through advertisements at national conventions, use of a national research registry, and networking with the community of families who have a child with FXS. The present sample represents a sample of convenience because FXS is a rare disorder and, thus, it was not feasible to recruit a representative sample of all young children with this disorder either locally or nationally.
Maternal characteristics
The mean age of mothers was 33.5 years at the first observation, ranging between 20.5 and 41.75 years. At the final observation the mean age for mothers was 40.0 years with a range of 27.1 to 48.4 years. Three of the mothers had full mutation FXS, and 52 were premutation carriers. The FMR1 gene is made up of CGG trinucleotide repeats, and elevated repeats beyond 55 signify either the premutation (55–200 repeats) or the full mutation (> 200 repeats; Loesch et al., 2004). At the beginning of the study, each mother completed the Wechsler Adult Intelligence Scale – Third edition (Wechsler, 1997). The mothers’ IQs ranged widely from 55 to 130, with the mean score being 107. The IQs of the three mothers with full mutation FXS were 55, 89, and 103 (see Table 1 for additional demographic information).
Table 1.
Child Developmental Information
| Characteristic | M/% | SD | Range |
|---|---|---|---|
| Child | |||
| Age at First Observation (in months) | 34.11 | 5.58 | 24–55 |
| Age at Last Observation (in months) | 112.58 | 7.44 | 67–121 |
| MSEL Early Learning Composite at First Observation | 55.89 | 11.50 | 49–101 |
| MSEL Nonverbal Raw Score at First Observation | 44.31 | 8.95 | 29–73 |
| CARS at First Observation | 26.07 | 5.78 | 15.5–42 |
| Ethnicity (%) | |||
| Caucasian | 87.3 | ||
| Hispanic | 3.6 | ||
| Biracial | 7.3 | ||
| Mother | |||
| Education level in years at Last Observation | 15.5 | 2.33 | 9–20 |
| % Graduate from college | 61 | ||
| % Married | 72 | ||
| % Working Outside the Home (Part or Full Time) | 61 | ||
| Household Income (%) | |||
| > $30,000 | 11 | ||
| $31,000–$99,999 | 56 | ||
| > $100,000 | 33 | ||
| Ethnicity (%) | |||
| Caucasian | 91 | ||
| African American | 3.6 | ||
| Pacific Islander | 1.8 | ||
| Biracial | 1.8 | ||
| Choose not to respond | 1.8 |
Note. MSEL = Mullen Scales of Early Learning; CARS = Childhood Autism Rating Scale.
14 of the participants had CARS scores that exceeded 30 at the first observation. All presented means are prior to grand mean centering
Child characteristics
Forty-four boys and 11 girls with full mutation FXS participated in the study beginning in early childhood (see Table 1 for developmental information). The age of children at recruitment and first observation varied between 11 months and 4 years with a mean age of 28.6 months. The current analyses include all data points beginning when children were 2 years old or older (median age of first data point was 2.9 years). All data collected for each child between the ages of 2 and 10 years were included in the growth curve analyses.
Eighteen children (16 males and 2 females) had a stable diagnosis of autism throughout the early and middle childhood period, according to maternal report of diagnostics conducted by professionals outside our research team. Of the 18 children with comorbid autism, 11 were diagnosed by a pediatrician, 1 was diagnosed by a developmental pediatric neurologist, and 6 were diagnosed by a psychologist. We also measured each child’s level of autism symptoms displayed during each data collection visit using the Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1986).
Measures
Maternal parenting measures
Specific maternal responsivity and behavior management behaviors were based on mother-child interactions measured cumulatively over the early and middle childhood periods (described below). We coded mothers’ communication toward their child on a behavior-by-behavior basis using Noldus Observer XT software (Noldus Information Technology, 2008). The coding system was described in previous studies (Warren et al., 2010; Brady et al., 2014).
All maternal communication directed toward the child during the observation was coded. When mothers’ communication included several utterances in succession, the last utterance spoken to the child was coded based on the assumption that the child’s response would typically be anchored to the mother’s final utterance. Using a three-tiered coding system, maternal communication acts were coded according to both their level of attention (tier 1: maintain the child’s attention, introduce a new topic, or redirect child attention) and the function of the specific communication act (tier 2: request for verbal response from the child, request for behavioral compliance by the child, or comment to the child). Within these two tiers, these codes were mutually exclusive, and in combination across tiers they were exhaustive. For example, if a mother said, “come over here” while the child was trying to leave the room, it would be coded as a mother redirect-request for behavioral comply from the child. Or, if mother said “that’s a big bear” while looking at a book with the child, it would be coded as mother maintain- and comment. In addition, the following codes were supplemental (tier-three) in that they could be added to any maternal behavior if observed: maternal gesture, maternal recode (e.g., an expansion of a child communication act), maternal communication breakdown (e.g., requesting clarification from the child), or zap (e.g., admonishment of the child).
Principal Components Analysis (PCA; Gorsuch, 2003) was used to determine which of the behaviors could be combined as part of data reduction (see Brady et al., 2014 for complete description of the principal components analysis). Using this approach, we identified a maternal responsivity composite that was comprised of maintain-comment, maintain-request-for-verbal-comply, and recodes. A similar process was followed to create the behavior management variable, which was comprised of two maternal behaviors–redirect-request for behavioral complies and zaps/admonishments of child behavior. The average scores from these two constructs over all available observations was grand mean centered and used as predictors in the growth models described in our analysis section. Definitions and examples of the behaviors that made up these two empirically determined composites are shown in Table 2.
Table 2.
Definitions of Maternal Behaviors
| Behavior | Definition | Example |
|---|---|---|
| Maternal Responsivity Construct | ||
| Maintain comments | Behaviors that maintained the child’s focused and verbally commented | Child is making a sandwich and Mom says, “that looks good” |
| Maintain Request verbal complies | Questions or statements intended to elicit a verbal response, while maintaining child focus | Mom says, “Do you want help with that sandwich?” |
| Recode | Verbal interpretation of child’s communication act | Child says “ba” and mom says, “Do you want your ball?” |
| Behavior Management Construct | ||
| Redirect request for behavioral complies | Mom requests or directs child to complete a behavior that is outside of the child’s current focus of attention | Child is playing with toy truck and Mom says “tie your shoe” |
| Zap/Admonishment | Verbal or non-verbal restriction of child’s behavior | Mom says “Don’t do that” and shakes her finger at the child |
Coding and reliability
Research assistants completed the primary and reliability coding. Each coder was initially trained to be at least 80% reliable with a master coder on a set of videos that were not part of the current analysis. Following this training, two coders independently coded child and maternal behaviors for each taped observation. After completing independent coding, the two coders compared transcripts, and if agreement was below 80% the coders resolved differences by consensus. This process was implemented to ensure consistency across coders and over time. Approximately 68% files were consensus coded. To determine the inter-judge reliability for each behavior that made up the maternal responsivity composite (Maintain-Comments, Maintain-Request for Verbal Complies, and Recodes), and maternal behavior management (Redirect-Request for Verbal Complies, Zap/Admonishment of Child Behavior) we calculated intra-class correlation coefficients (ICCs), using the absolute agreement and single measure values for each score (Shrout & Fleiss, 1979). ICCs were calculated between the primary and reliability scores, as well as between primary scores and those arrived at by consensus codes. ICCs for maternal behaviors were also high, ranging from .73 and .96 between primary and reliability coders. The ICC of .73 was for maternal recodes, which did not occur as often as other behaviors. ICCs between primary and consensus coders were .99 for all maternal behaviors.
Vineland Adaptive Behavior Scales – Interview Edition, Survey Form (Sparrow et al., 1984, 2005)
The VABS is a widely used standardized semi-structured parent interview that assesses personal and social functioning of individuals from birth through adulthood in four domains: communication, socialization, daily living skills, and motor skills (Sparrow et al., 1984; 2005). The motor skills domain is usually only administered to children under the age of 6, so we did not include it in this analysis. The survey form contains 297 items that are scored using a 3-point Likert scale (never, sometimes/partially, or usually). Items may also be scored as parent does not know if the child can perform the activity or as the child has not had the opportunity to perform the activity. For the domains of the VABS, inter-rater reliability coefficients and the test-rest reliability coefficients are in the good to excellent range with split-half reliability coefficients ranging from .70s to .90s. The VABS has established construct, criterion, and content validity and has satisfactory levels of internal consistency (Sparrow et al., 1984).
The Mullen Scales of Early Learning (Mullen, 1995)
The Mullen Scales of Early Learning (MSEL) is a standardized developmental assessment for children from birth to 68 months. The MSEL measures five domains of development (i.e., gross motor, fine motor, visual reception, expressive language, and receptive language) that are combined to create an overall standard score—Early Learning Composite—that represents an estimate of overall developmental functioning. The MSEL has established content, construct, and predictive validity and strong concurrent validity with other well-known developmental assessments for young children (e.g., Bayley Scales of Infant Development [Bayley, 1993]). In addition, strong test-retest reliability (.82–.85), inter-rater reliability (.91–.99), and internal consistency coefficients have been established for the MSEL (.83–.95; Mullen, 1995).
In the present study we used nonverbal cognitive abilities score, as opposed to the Early Learning Composite, to help control for the potential confounding influence of language abilities on this overall score. This score can be calculated by combining the scores from the Visual Reception and Fine Motor domain (Mullen, 1995). Additionally, raw scores were used in the present study because there was little variability in standard scores and many of the children had the minimum standard score (i.e., floor effects). The nonverbal raw score, obtained at the first observation after age 24 months was used as a covariate in our analyses because this time point is when all 55 participants were observed (mean MSEL nonverbal raw score at the first observation was 44.31 [SD = 8.95]).
The Childhood Autism Rating Scale (Schopler et al., 1986)
The Childhood Autism Rating Scale (CARS) is a 15-item general rating scale of observed autism symptoms. Each item is scored on a 4-point Likert scale, where 1 is within normal limits for age and 4 is severely abnormal for age or developmental level. An overall score of autistic behavior is calculated based on these ratings, such that a Total CARS score of 30 to 37 indicates the presence of mild to moderate autism behavior symptoms and scores above 37 indicate more severe symptoms (Schopler et al., 1988). We did not use the CARS as a diagnostic measure but instead used its scores from each child’s first observation as a predictor variable to describe each child’s level of autism symptoms concurrent with our observations. The mean CARS score at the first observation at 24 months or later was 26.07 (SD = 5.78). Although, this score is elevated compared to typically developing children of a similar age (Mayes et al., 2009), it is similar to the mean CARS scores reported by other studies of children with FXS (e.g., Bailey, Hatton, et al., 2001; Bailey, Mesibov et al., 2001; Hatton et al., 2006). The CARS score added as a predictor to the models was grand mean centered to facilitate interpretation.
Measurement schedule
Fifty-three of the 55 participants contributed data from 5 observations across early and middle childhood. The other two participants only contributed data during early childhood phase. The first three home visits were approximately 16 to 18 months apart. The fourth home visits occurred between 30 and 31 months after the third home visit, due to a change in project funding. The time between the fourth and fifth home visit was 16 to 18 months. All of the available VABS and maternal parenting data collected between the ages of 2 and 10 years were used in the growth curve analysis described below, which allowed for the modeling of trajectories with different numbers of observations and different assessment intervals (Signer & Willet, 2003). Therefore, no form of data imputation was used to account for missing data in this study.
Results
Modeling Approach
Because our research questions focused on the relationship between parenting and the development of/or decline in adaptive behavior and because some subscales showed more decline than others, we chose to analyze the subscales of the VABS individually. Given the observed trajectories for each of the VABS outcomes of interest—communication, socialization, daily living—fixed effects for linear, quadratic, and cubic growth terms were evaluated initially in all growth models using SAS PROC MIXED. This is the same approach we took in the Hahn et al study (2015). Age was centered at 30 months, a point early in the trajectory around which most participants were observed. Random intercepts, linear growth terms, and covariance between random intercepts and slopes were also included in the models. Model comparisons were made using two types of indices: the deviance statistic (change in the −2 log likelihood) and the Bayesian Information Criterion (BIC). Deviance statistics are distributed asymptotically as a Chi Square with degrees of freedom equal to the difference in the number of parameters estimated by the two models. Smaller BIC values indicate better fit. For each of the outcomes of interest, we first ran an intercept-only model in order to examine within and between person variance and calculate intra-class correlation coefficients. Next we ran a growth model with no predictors, followed by Model 3, which added parenting predictors (either maternal responsivity or behavior management), and finally Model 4 added child predictors for autism symptoms as measured during home visits using the CARS, and nonverbal ability as measured by the Mullen from the first observation.
Because the growth models indicated that there was a significant decline in skill for some participants at the later observation periods, we ran additional analyses for the subgroup of participants who experienced a decline to determine how maternal responsivity influenced decline. For these analyses, we centered time at 110 months, the point in middle childhood when declines started to be observed, and specifically examined maternal responsivity as a predictor of intercepts and slopes at this middle childhood period. The results are presented first for the analyses completed with the entire group of participants, followed by the analyses for just those who showed declines.
Preliminary Analyses
Preliminary analyses were conducted to examine early responsivity as a predictor of adaptive behavior. Early responsivity was only significantly related to intercepts at 30 months for the communication subscale outcome whereas sustained responsivity (which included early and later responsivity) was related to both intercepts and slopes for outcomes as described below.
Vineland Communication Domain
Figure 1 shows a pattern of generally increasing growth on the VABS communication scale by chronological age. As expected, females with “FXS only” tend to have the steepest increases across age and males and females with autism tend to have the flattest trajectories with males (no autism) in the middle. At 30 months, the average VABS communication score was just over 29 and scores increased by .87 each month on average with a significant negative quadratic parameter estimate of −.004. See Table 3, Model 2 for a complete description. As a point of comparison, an average typically developing child has a raw score of 54 at 30 months on the VABS communication subscale.
Figure 1.
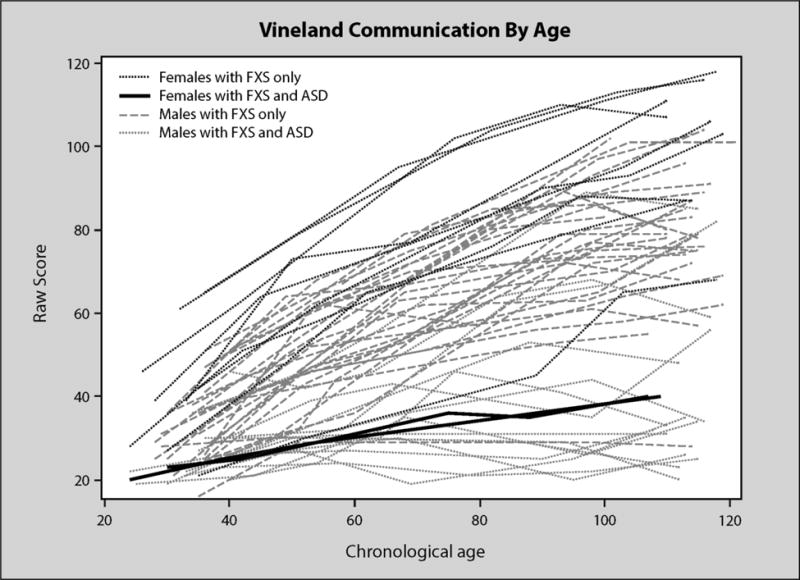
VABS communication by age in months
Table 3.
Fixed Effect Estimates (Top) and Variance-Covariance Estimates (Bottom) for VABS Communication Scores with Responsivity and Child Predictors
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||
| Intercept | 54.32*** | 2.52 | 29.49*** | 1.62 | 29.35*** | 1.38 | 29.56*** | 1.17 |
| Level 1 | ||||||||
| Linear | .87*** | .06 | .87*** | .05 | .86*** | .05 | ||
| Quadratic | −.004*** | .0005 | −.005*** | .0005 | −.004*** | .0005 | ||
| Level 2 Predictors | ||||||||
| Mat. Resp. on Inter. | 2.32** | .68 | .83 | .64 | ||||
| Mat. Resp. on Linear | .13*** | .02 | .12*** | .02 | ||||
| Mat. Resp. on Quad | −.0007** | .0002 | −.0007** | .0002 | ||||
| CARS on Intercept | −.44* | .22 | ||||||
| CARS on Linear | −.01** | .005 | ||||||
| Mullen on Intercept | .51** | .16 | ||||||
| Random Parameters | ||||||||
| Intercept | 278.28*** | 67.14 | 111.50*** | 24.66 | 73.83*** | 17.21 | 43.52*** | 11.59 |
| Slope | .06*** | .01 | .04*** | .009 | .03*** | .008 | ||
| Covariance | 1.31** | .43 | .35 | .27 | .04 | .21 | ||
| Residual | 348.72*** | 33.79 | 26.27*** | 2.95 | 24.94*** | 2.80 | 24.95*** | 2.80 |
| Model Fit | ||||||||
| −2 log likelihood | 2416.6 | 1934.0 | 1890.9 | 1860.8 | ||||
| BIC | 2428.6 | 1962.1 | 1931.0 | 1912.9 | ||||
p < .05,
p < .01,
p < .001
Sustained maternal responsivity was significantly related to intercept, linear growth, and quadratic change for VABS communication as can be seen in Table 3. Variance in intercepts and slopes for the communication subscale were reduced by 34% and 33%, respectively, with the addition of maternal responsivity to the model. The explained variance can be interpreted as a pseudo-R-square. Accordingly, we applied Cohen’s (1988) criteria for R-square change formula, which indicated the observed effects were “large”. Children with highly responsive mothers had higher intercepts at 30 months and larger increases in communication scores over time. Overall maternal responsivity influenced the intercept such that communication scores were 2.32 points higher at 30 months for every 1-point increase in maternal responsivity. It also influenced the slope, increasing by .13 points for every 1-point increase in maternal responsivity. Maternal responsivity influenced the quadratic term increasing the rate of growth by .0007 for every 1-point increase in maternal responsivity. Thus, the change in slope as the children aged was more positive for children whose mothers were highly responsive. Specifically, children who were declining, declined less with highly responsive moms. The positive influence of maternal responsivity on the slope and quadratic terms was maintained even after child autism symptoms and nonverbal skills were added as predictors, as can be seen in Table 3 under Model 4. ASD symptoms and nonverbal scores were significant predictors of intercept. Maternal responsivity was no longer a significant predictor of the intercept when these child predictors were added to the model but maternal responsivity was still significantly related to linear and quadratic growth trajectory.
Figure 2 shows an interaction between maternal responsivity and CARS scores such that children with highly responsive mothers and low CARS scores show the steepest communication growth trajectory across age. Children with high CARS scores and high maternal responsivity had a somewhat lower growth trajectory but outperformed children with low CARS score and low responsive mothers. Children with the lowest trajectory had low responsive mothers and high CARS scores. Communication scores were .44 lower at 30 months for every 1-point increase above the mean CARS score. For example, if the CARS score observed for a child was 5 points above the mean CARS score for the sample, then their communication score was 2.20 points lower on average. Mullen Nonverbal raw scores (not shown in Figure 2) also influenced the intercept such that communication scores at 30 months were .51 higher for every 1-point increase in the Mullen Nonverbal raw score.
Figure 2.
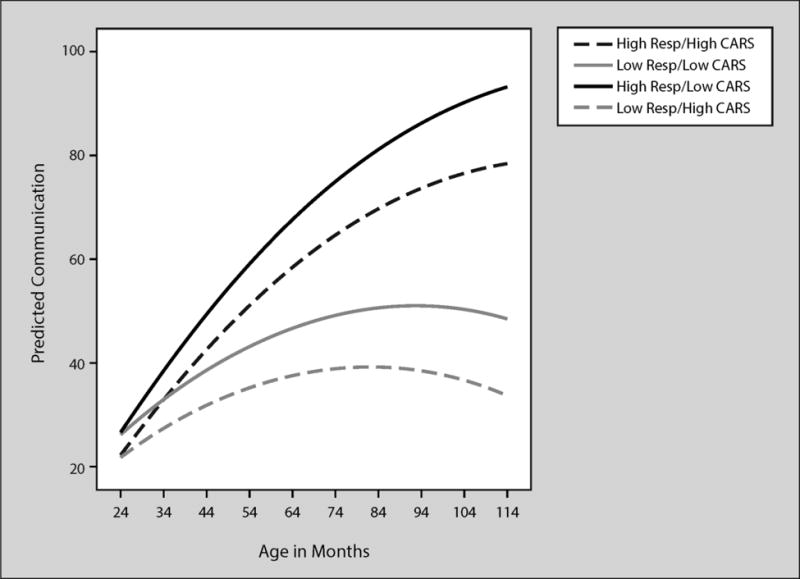
Predicted VABS communication by age in months
Table 4 provides the parameter estimates for the model of communication domain scores when behavior management was the parenting predictor of interest. Higher rates of behavior management were significantly related to slower increases in communication scores over time with slopes decreasing by .14 for every 1-point increase in the rate of behavior management. However, behavior management was not a significant predictor of communication parameters once autism symptoms and nonverbal skills were added to the model.
Table 4.
Fixed Effect Estimates (Top) and Variance-Covariance Estimates (Bottom) for VABS Communication Scores with Behavior Management and Child Predictors
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||
| Intercept | 54.32*** | 2.52 | 29.49*** | 1.62 | 29.51*** | 1.60 | 29.85*** | 1.23 |
| Level 1 | ||||||||
| Linear | .87*** | .06 | .87*** | .05 | .85*** | .05 | ||
| Quadratic | −.004*** | .0005 | −.004*** | . 0005 | −.004*** | .0005 | ||
| Level 2 Predictors | ||||||||
| BehMan. Inter. | −2.70 | 2.53 | .70 | 1.93 | ||||
| BehMan Linear | −.14* | .06 | −.10 | .05 | ||||
| CARS on Intercept | −.46 | .24 | ||||||
| CARS on Linear | −.02** | .006 | ||||||
| Mullen on Intercept | .68*** | .15 | ||||||
| Random Parameters | ||||||||
| Intercept | 278.28*** | 67.14 | 111.50*** | 24.66 | 108.82*** | 24.14 | 50.21*** | 13.15 |
| Slope | .06*** | . 01 | .06*** | . 01 | .05*** | .01 | ||
| Covariance | 1.31** | .43 | 1.17** | .40 | .39 | .27 | ||
| Residual | 348.72*** | 33.79 | 26.27*** | 2.95 | 26.27*** | 2.95 | 26.29*** | 2.96 |
| Model Fit | ||||||||
| −2 log likelihood | 2416.6 | 1934.0 | 1927.9 | 1891.7 | ||||
| BIC | 2428.6 | 1962.1 | 1964.0 | 1939.8 | ||||
p < .05,
p < .01,
p < .001
Vineland Socialization Domain
Figure 3 shows a pattern of generally increasing growth on the VABS socialization scores as children with FXS aged. Similar to Figure 1, growth was steepest for females and males without autism. Males with ASD and one of the two females with ASD tended to have the lowest trajectories. However, the overall pattern of growth generally increased with a significant negative quadratic term as individual’s rate of growth in socialization scores slowed as they aged. As is apparent from the figure, many children actually declined in socialization scores at the latter time points.
Figure 3.
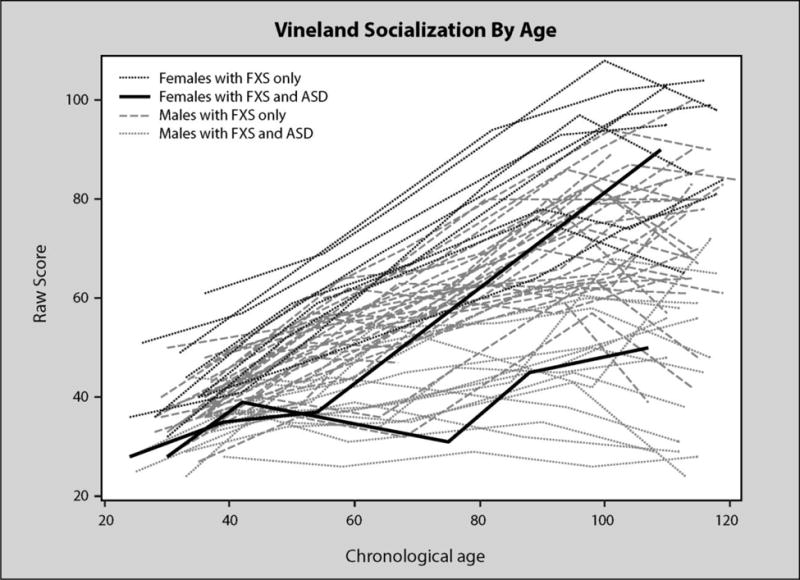
VABS socialization by age in months
Maternal responsivity was a significant predictor of both intercept and slope of socialization scores, with socialization scores 2.00 points higher at 30 months and slopes .03 higher for every 1-point increase in maternal responsivity as can be seen in Table 5, Model 3. Variance in intercepts and slopes for the socialization subscale were reduced by 61% (a large effect) and 25% (a medium to large effect), respectively with the addition of maternal responsivity to the model. Maternal responsivity was a significant predictor of intercept even with child predictors added to the model, as can be seen in Table 5 Model 4. CARS scores were significantly related to slopes when all of the predictors were in the model. Behavior management was not a significant predictor of socialization scores and thus no table is provided.
Table 5.
Fixed Effect Estimates (Top) and Variance-Covariance Estimates (Bottom) for VABS Socialization Scores with Maternal Responsivity and Child Predictors
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||
| Intercept | 54.82*** | 1.70 | 34.79*** | 1.15 | 35.08*** | 1.00 | 35.45*** | .93 |
| Level 1 | ||||||||
| Linear | .67*** | .06 | .65*** | .06 | .63*** | .06 | ||
| Quadratic | −.003*** | .0006 | −.003*** | .0006 | −.003*** | .0006 | ||
| Level 2 Predictors | ||||||||
| Mat. Resp. Inter. | 2.00*** | .39 | 1.12** | .39 | ||||
| Mat. Resp. Linear | .03* | .01 | .02 | .01 | ||||
| CARS on Inter. | −.28 | . 14 | ||||||
| CARS on Linear | −.01* | .005 | ||||||
| Mullen on Intercept | .28** | .10 | ||||||
| Random Parameters | ||||||||
| Intercept | 105.31*** | 30.73 | 26.92** | 10.17 | 10.62 | 6.97 | 2.23 | 4.74 |
| Slope | .04*** | .01 | .03*** | .009 | .03*** | .007 | ||
| Covariance | .56** | .22 | .30 | .17 | ||||
| Residual | 260.57*** | 25.24 | 38.51*** | 4.30 | 38.49*** | 4.29 | 37.78*** | 4.02 |
| Model Fit | ||||||||
| −2 log likelihood | 2311.1 | 1932.4 | 1905.8 | 1877.5 | ||||
| BIC | 2323.2 | 1960.5 | 1941.9 | 1921.6 | ||||
p < .05,
p < .01,
p < .001
Vineland Daily Living
Figure 5 shows a pattern of generally increasing growth in daily living that slows over time. A few individuals show clear declines at the upper age levels. As was the case with VABS communication and socialization scores, females tended to have the highest daily living trajectories. A few male trajectories overlapped with these females. Males with ASD showed low growth trajectories across age. Daily living scores averaged 21.72 at 30 months with an increase of 1.05 for every month of age and a decrease in the rate of growth over time indicated by a significant negative quadratic term.
Figure 5.
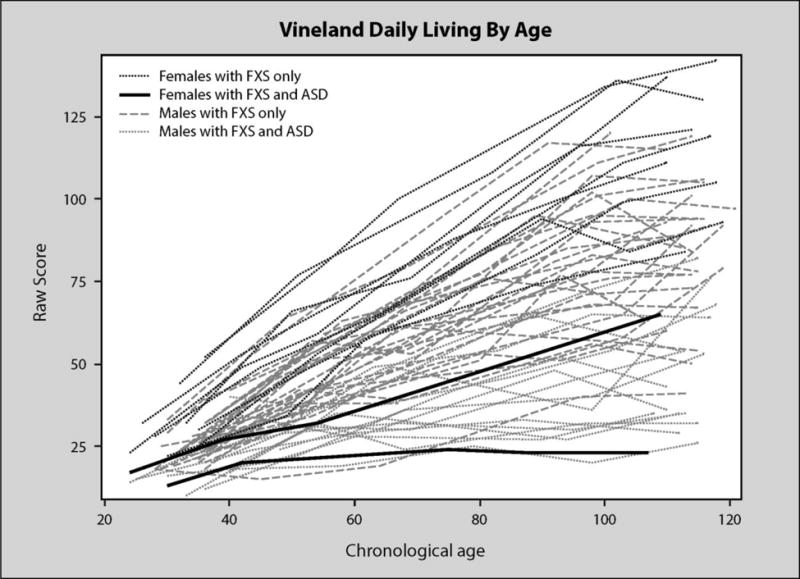
VABS daily living by age in months
Scores at 30 months were 1.47 higher and slopes of daily living Scores increased by .08 for every 1point increase in maternal responsivity as can be seen in Table 6, Model 3. Variance in intercepts and slopes for the daily living subscale were reduced by 19% and 22% (medium size effects), respectively with the addition of maternal responsivity to the model. Maternal responsivity significantly influenced slope even when child predictors were added to the model. CARS scores influenced intercept such that daily living scores were .45 lower at 30 months for every 1-point increase in CARS scores. Slope for daily living scores were .03 lower for every 1-point increase in CARS scores. Behavior management was not significantly related to daily living slopes or intercepts. As the predicated trajectories indicate, children of responsive mothers and few autism symptoms tended to have the highest daily living scores. In contrast, children with low responsive mothers and many autism symptoms had the lowest daily living scores. The other two groups (high responsivity/high CARS and low responsivity/low CARS) had very similar scores and trajectories.
Table 6.
Fixed Effect Estimates (Top) and Variance-Covariance Estimates (Bottom) for VABS Daily Living Scores with Maternal Responsivity and Child Predictors
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||
| Intercept | 54.45*** | 2.61 | 21.72*** | 1.33 | 21.72*** | 1.27 | 22.09*** | 1.12 |
| Level 1 | ||||||||
| Linear | 1.05*** | .07 | 1.05*** | .07 | 1.03*** | .06 | ||
| Quadratic | −.004*** | .0006 | −.004*** | .0006 | −.004*** | .0006 | ||
| Level 2 Predictors | ||||||||
| Mat. Resp. Inter. | 1.47** | .53 | .28 | .51 | ||||
| Mat. Resp. Linear | .08*** | .02 | .06** | .02 | ||||
| CARS on Inter. | −.45* | . 19 | ||||||
| CARS on Linear | −.03*** | .006 | ||||||
| Mullen Intercept | .37** | .14 | ||||||
| Random Parameters | ||||||||
| Intercept | 243.37*** | 72.27 | 45.89*** | 14.65 | 36.96** | 12.85 | 15.39 | 9.38 |
| Slope | .09*** | .02 | .07*** | .02 | .05*** | .01 | ||
| Covariance | 1.58*** | .41 | 1.13*** | .33 | .48* | .23 | ||
| Residual | 631.64*** | 61.18 | 44.19*** | 5.06 | 44.23*** | 5.07 | 44.52*** | 5.13 |
| Model Fit | ||||||||
| −2 log likelihood | 2546.7 | 2005.8 | 1989.6 | 1956.3 | ||||
| BIC | 2558.7 | 2033.8 | 2025.6 | 2004.4 | ||||
p < .05,
p < .01,
p < .001
Declines in Domains
A subgroup of 30 individuals representing 56% of our sample, previously discussed in Hahn et al. (2015), demonstrated a decline of at least 5 points in the raw score on at least one of the Vineland subscales (communication, socialization, daily Living) over two or more longitudinal data points. This represented a change, for example, from a score of 2 “always does” to a score of 1 “sometimes does” on at least 5 items within a single domain, or a change from 2 “always does” to 0 “never does” on at least 3 items. We examined whether sustained responsivity was specifically related to the intercepts and slopes for these 30 participants. As previously stated, for this analysis we centered age at 110 months, the age point at which, on average, we started to observe declines in middle childhood.
The average communication score for participants showing declines at 110 months was 66.17(SE = 3.54). The average slope of communication was not significantly different from 0, .01(SE=.06), at 110 months and there was a significant, negative quadratic term −.006(SE=.00066) indicating a less positive slope over time. Sustained responsivity was positively related to intercepts 7.96(SE=1.40) and slopes .06(SE=.01). Children whose mothers were highly responsive had higher scores at 110 months and more positive slopes.
The average socialization score for these 30 participants at 110 months was 63.83(SE=3.12). The average slope of socialization was not significantly different from 0, −.05(SE=.07) and there was a significant, negative quadratic term −.005(SE=.0008) indicating a less positive slope over time. Sustained responsivity was positively related to both intercepts 4.68 (SE=1.23) and slopes .03(SE=.01). That is, participants in this group whose mothers were more responsive showed relatively more growth.
The average daily living score for participants in this group at 110 months was 75.08(SE=4.75). The average slope was positive, .16(SE=.07) at 110 months and there was a significant, negative quadratic term −006(SE=.0008). Sustained responsivity was positively related to both intercepts 6.92(SE=1.88) and slopes .06(SE=.02). The significant relationship between slopes and sustained responsivity further indicates that even within this group of children who showed declines, relatively more growth was associated with responsive parenting.
Discussion
Our findings suggest that middle childhood may be an especially pivotal time for the development of adaptive behaviors in individuals with FXS. Our prior study of adaptive behavior declines indicated that 56% of our sample had lost important adaptive behavior skills and regressed to some extent in middle childhood (Hahn et al., 2015). Raw score declines were documented in 3 of 9 females with FXS only, 10 males with FXS and co-morbid autism, and 17 males with FXS only.
The underlying reasons for declines in adaptive behavior in children with FXS as reported in several other studies have been a mystery beyond having some relationship to autism symptoms and cognition. However, our findings suggest that parenting may play an important role in this developmental process. In our analysis we found that maternal responsivity significantly predicted quadratic change, slope, and intercept for all children on the VABS communication domain, as well as slope and intercept for those that declined on the socialization and daily living domains. These effects were highly significant statistically and generated large to moderate effects sizes depending on specific VABS domain. Many, but not all, of these effects were maintained after controlling for autism symptoms and nonverbal cognitive development. Thus, our data suggest that highly responsive parenting practices act to increase positive trajectories and decrease the amount of decline in some domains. The strongest effects were apparent for the communication domain. Whether these effects continue into adolescence and beyond remains to be seen.
Perhaps these data should not be viewed as surprising. We previously reported on the early and sustained impact of maternal responsivity on language development in this same sample of children (Warren et al., 2010; Brady et al., 2014). In this respect our discovery of a substantial relationship between maternal responsivity and the VABS communication domain is further verification of our earlier findings. More generally, maternal responsivity has been repeatedly shown to be among the most potent environmental influences on a variety of child development measures across both typically and atypically developing children (Landry et al., 2006, 2001; Spiker et al., 2002; Warren & Brady, 2007; Shire et al, 2016). Furthermore, our findings regarding the influence of contingent maternal responsivity on child adaptive behavior underscores the fact that the manifestation of FXS is not just the product of innate biology, but is attributable to the dynamic and cumulative interaction of biology, behavior, and environment over time.
In contrast to maternal responsivity, the effects of maternal behavior management on adaptive behavior appeared to be minimal in terms of the outcomes we measured. One might have expected behavior management to positively or even negatively impact adaptive behavior. However, our data only revealed one statistically significant relationship, a relatively weak negative one between behavior management on the growth of the communication domain. This finding fits with our casual observations of the mothers in our study during the home visits. That is, mother’s parenting approaches ranged from mostly positive to neutral and their use of behavior management techniques were generally appropriate to the situation at the moment. Of course there might have been some effect of an observer being present in terms of the behavior they displayed toward their child. Additional research with other samples and data collection approaches might reveal a different picture.
Importantly, to our knowledge no other published research has examined the role of parenting on child adaptive behavior advances and declines associated with FXS. This suggests the need for extra caution in interpreting these results. It is also possible that the declines of one or more adaptive skills that we found for many of these children may be transitory and that these skills might increase again during adolescence. This may be especially true given that we analyzed raw scores. Thus, we might observe increases in raw scores among a broad group of our sample during adolescence even though their standardized/age equivalent scores could still show further declines. Fortunately, we will be collecting further data on this sample during adolescence.
The definition of maternal responsivity that we used in this study—and most of our other work on parenting and FXS—is different from many studies on maternal responsivity that focus more on the affective qualities (e.g. warmth, flexibility, positive affect, harsh vocal tone, etc.) of parent behavior (e.g. Landry et al 2006). Instead our focus was on the cumulative effects of specific maternal behaviors in response to specific child behaviors (see Table 2 for examples). These maternal behaviors may function as in-vivo “incidental teaching procedures” to help children build specific adaptive skills in communication, socialization, and daily living (Hart & Risley, 1999). However, they may or may not have a strong correlational relationship with measures of maternal affect such as “warmth” or “flexibility”. This issue is obviously worthy of further study. Our point is that how maternal responsivity is specifically defined and measured is an important topic in and of itself and may have played an important role in the results of this study.
In our view this study has several important strengths. First, it is based on an intact longitudinal sample spanning the period of early to middle childhood, not a cross-sectional sample like most of the extant literature on this topic. Second, we directly measured mother-child interaction across varied activities in the child’s natural environment (home). Third, our definition of “decline” was conservative (raw declines over two or more longitudinal data points 16–18 months apart. Finally, the definitions of “responsive parent behaviors” are ones that conceptually relate to contingently teaching and supporting new and emerging communication, social, and daily living skills in children with and without FXS. Our study also has limitations. Most obvious are that it’s based on a sample of convenience drawn from 28 states and on what are essentially correlational data.
In conclusion, the importance of this study lies in the obvious seriousness of raw score declines in adaptive behavior development and our discovery that growth in adaptive behaviors are more positive when parents engage in higher rates of the type of maternal responsivity measured in our study. Correspondingly, the amount of decline observed in some participants was lessened for those with highly responsive mothers. These results may have important clinical and educational implications for children with FXS. Nevertheless, since to our knowledge we are the first to report a possible connection to parenting style to adaptive behavior declines, it would be prudent to consider these results with caution until they have been replicated by other investigators. In the meantime, we see no downside and potentially a considerable upside in training efforts aimed at enhancing and supporting sustained highly responsive parenting for children with FXS during both early and middle childhood.
Ethics Approval: All procedures performed in this study involved human participants and were in accordance with the ethical standards of the institutional research committee at the University of Kansas and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Figure 4.
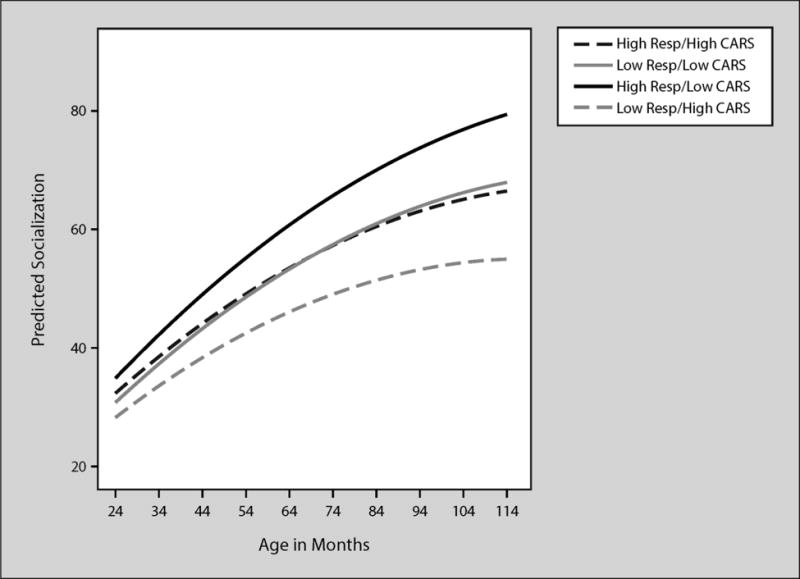
Predicted VABS socialization by age in months
Figure 6.
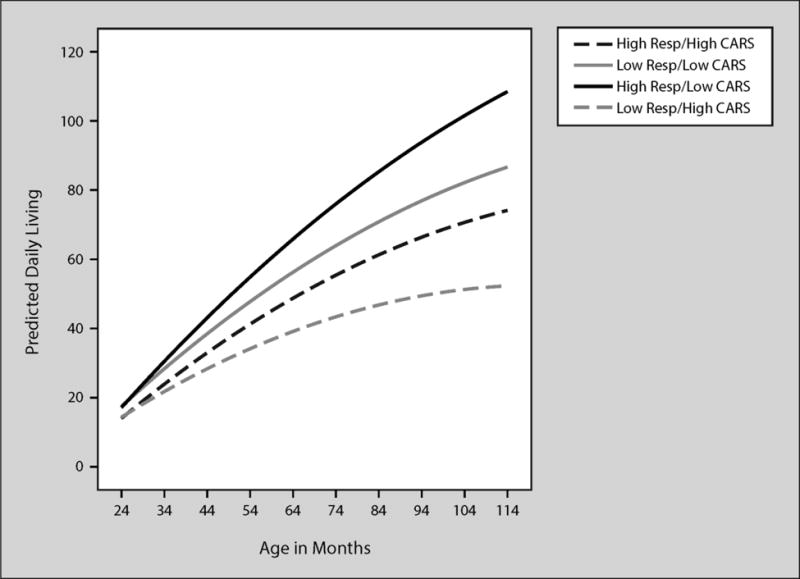
Predicted VABS daily living by age in months
Acknowledgments
This research was supported by National Institute of Child Health and Human Development grants P30 HD003110, P30 HD002528, and RO1-HD84663. We wish to express our profound gratitude to the 55 families from across the United States that participated in this research.
Footnotes
Conflict of Interest: The authors declare they have no conflict of interest.
Contributor Information
Steven F. Warren, Department of Speech-Language-Hearing: Sciences and Disorders, The University of Kansas, 1000 Sunnyside Avenue, #3045, University of Kansas, Lawrence, KS, 66045-7555
Nancy Brady, Department, Department of Speech-Language-Hearing: Sciences and Disorders, The University of Kansas, 1000 Sunnyside Avenue, The University of Kansas, Lawrence, KS, 66045-7555.
Kandace K. Fleming, Institute for Life Spans Studies, 1000 Sunnyside Avenue, The University of Kansas, Lawrence, KS, 66045-7555
Laura J. Hahn, Department of Speech and Hearing Science, University of Illinois, 901 S. Sixth St. Champaign, IL 61820
References
- Bailey DB, Raspa M, Olmstead M, Holiday DB. Co-occuring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics. 2008;146A(16):2060–9. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2nd. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Bornstein MH, Tamis-LeMonda CS, Hahn CS, Haynes OM. Maternal responsiveness to young children at three ages: longitudinal analysis of a multidimensional, modular, and specific parenting construct. Developmental Psychology. 2008;44(3):867. doi: 10.1037/0012-1649.44.3.867. [DOI] [PubMed] [Google Scholar]
- Brady NC, Warren SF, Fleming K, Keller J, Sterling A. Effect of sustained maternal responsivity on later vocabulary development in children with fragile X syndrome. American Journal of Speech, Language, and Hearing Research. 2014;57:212–226. doi: 10.1044/1092-4388(2013/12-0341)212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady N, Fleming K, Thiemann-Bourque K, Olswang L, Dowden P, Saunders M, Marquis J. Development of the Communication Complexity Scale. American Journal of Speech-Language Pathology. 2012;21(1):16–2. doi: 10.1044/1058-0360(2011/10-0099). [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Center for Disease Control. FMR1 and the fragile X syndrome. 2011 http://doi.org/Retrieved January 31, 2013 from http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/fragile_x.pdf.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark JL, Feldman MA, Holden JJA. Behavioral relationship between autism and fragile X syndrome. American Journal on Mental Retardation. 2003;108(5):314–326. doi: 10.1352/0895-8017(2003)108<314:BRBAAF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nature Genetics. 1993;4(4):335–40. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Ditterline J, Oakland T. Relationships between adaptive behavior and impairment. In: Naglieri J, Goldstein S, editors. Assessing Impairment: From Theory to Practice. New York, NY: Springer Science + Business Media, LLC; 2009. pp. 31–48. [DOI] [Google Scholar]
- Dix T. The affective organization of parenting: Adaptive and maladaptative processes. Psychological Bulletin. 1991;110(1):3–25. doi: 10.1037/0033-2909.110.1.3. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Hodapp R, Ort S, Leckman J. Trajectories and profiles of adaptive behavior in males with fragile X syndrome. Jounral of Autism and Developmental Disorders. 1993;23(1):135–145. doi: 10.1007/BF01066423. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Ort S, Cohen I, Finucane B, Spiridigliozzi G, Lachiewicz A, O’Conner R. Trajectories and profiles of adaptive behavior in males with fragile X syndrome. Journal of Autism & Developmental Disorders. 1996;(3):287–301. doi: 10.1007/BF02172475. [DOI] [PubMed] [Google Scholar]
- Fisch G, Carpenter N, Holden JJ, Howard-Peebles PN, Maddalena A, Borghgraef M, Fryns JP. Longitudinal changes in cognitive and adaptive behavior in fragile X females: a prospective multicenter analysis. American Journal of Medical Genetics. 1999;83(4):308–12. [PubMed] [Google Scholar]
- Fisch G, Carpenter N, Howard-Peebles PN, Holden JJA, Tarleton J, Simensen R, Battaglia A. Developmental trajectories in syndromes with intellectual disability, with a focus on Wolf-Hirschhorn and its cognitive-behavioral profile. American Journal on Intellectual and Developmental Disabilities. 2012;117(2):167–79. doi: 10.1352/1944-7558-117.2.167. [DOI] [PubMed] [Google Scholar]
- Fisch G, Carpenter NJ, Holden JJ, Simensen R, Howard-Peebles PN, Maddalena A, Nance W. Longitudinal assessment of adaptive and maladaptive behaviors in fragile X males: growth, development, and profiles. American Journal of Medical Genetics. 1999;83(4):257–63. doi: 10.1002/(sici)1096-8628(19990402)83:4<257::aid-ajmg5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fisch G, Simensen R, Schroer R. Longitudinal changes in cognitive and adaptive behavior scores in children and adolescents with the fragile X mutation or autism. Journal of Autism and Developmental Disorders. 2002;32(2):107–114. doi: 10.1023/A:1014888505185. [DOI] [PubMed] [Google Scholar]
- Freund LS, Peebles C, Aylward E, Reiss AL. Preliminary report on cognitive and adaptive behaviors in preschool-ages males with fragile X. Developmental Brain Dysfunction. 1995;8:242–251. [Google Scholar]
- Freund LS, Reiss AL, Abrams MT. Psychiatric Disorders Associated With Fragile X in the Young Female. Pediatrics. 1993;91(2):321–329. [PubMed] [Google Scholar]
- Goldsmith DF, Rogoff B. Sensitivity and teaching by dysphoric and nondysphoric women in structured versus unstructured situations. Developmental Psychology. 1995;31(3):388. [Google Scholar]
- Gorsuch RL. Factor analysis. In: Schinka JA, Velicer WF, editors. Handbook of psychology: Research methods in psychology. Vol. 2. Hoboken, NJ: Wiley; 2003. pp. 143–146. [Google Scholar]
- Hagerman RJ. Etiology, diagnosis, and development in fragile X syndrome. In: Roberts JE, Chapman RS, Warren SF, editors. Speech and language development and intervention in Down syndrome and fragile X syndrome. Baltimore, MD: Brookes; 2007. pp. 27–42. [Google Scholar]
- Hagerman RJ, Hagerman PJ. The fragile X premutation: into the phenotypic fold. Current Opinion in Genetics & Development. 2002;12(3):278–283. doi: 10.1016/s0959-437x(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Rivera SM, Hagerman PJ. The Fragile X Family of Disorders: A Model for Autism and Targeted Treatments. Current Pediatric Reviews. 2008;4(1):13. http://dx.doi.org/10.2174/157339608783565770. [Google Scholar]
- Hahn LJ, Brady NC, Warren SF, Fleming KK. Do children with fragile X syndrome show declines or plateaus in adaptive behavior? American Journal on Intellectual and Developmental Disabilities. 2015;120(5):412–432. doi: 10.1352/1944-7558-120.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley TR. The social world of learning to talk. Baltimore: Paul Brookes Publishing Co; 1999. [Google Scholar]
- Hatton DD, Wheeler AC, Skinner ML, Bailey DB, Jr, Sullivan K, Roberts J, Clark R. Adaptive behavior in children with fragile X syndrome. American Journal on Mental Retardation. 2003;108(6):373–390. doi: 10.1352/0895-8017(2003)108%3C373:ABICWF%3E2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hauser-Cram P, Warfield M, Shonkoff JP, Krauss M. Children with disabilities: A longitudinal study of child development and parent well-being. Monographs of the Society for Research in Child Development. 2001;66(3):1–5. doi: 10.1111/1540-5834.00151. [DOI] [PubMed] [Google Scholar]
- Klaiman C, Quintin EM, Jo B, Lightbody AA, Hazlett HC, Piven J, Reiss AL. Longitudinal profiles of adaptive behavior in fragile X Syndrome. Pediatrics. 2014;134(2):315–324. doi: 10.1542/peds.2013-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry S, Smith KE, Swank PR. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Developmental Psychology. 2006;42(4):627–42. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- Landry S, Smith K, Swank PR, Assel MA, Vellet S. Does early responsive parenting have a special importance for children’s development or is consistency across early childhood necessary? Developmental Psychology. 2001;37(3):387–403. doi: 10.1037//0012-1649.37.3.387. [DOI] [PubMed] [Google Scholar]
- Legerstee M, Vargehese J, van Beek Y. Effects of maintaining and redirecting infant attention on the production of referential communication in infants with and without Down syndrome. Journal of Child Language. 2002;29(01):23–48. doi: 10.1017/S0305000901004895. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Mailick M, Greenberg JS, Smith LE, Sterling A, Brady N, Warren SF, Hong J. Fragile X–associated disorders: How the family environment and genotype interact. In: Burack JA, Schmidt LA, editors. Cultural and Contextual Perspectives on Developmental Risk and Well-Being. New York, NY: Cambridge University Press; 2014. pp. 221–253. [Google Scholar]
- Mazzocco MMM. Advances in research on the fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(2):96–106. doi: 10.1002/1098-2779(2000)6:2<96::AID-MRDD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Noldus Information Technology. The Observer XT (Version 8.0) [Computer software] Wageningen the Netherlands: Author; 2008. [Google Scholar]
- Osofsky JD, Thompson MD, Shonkoff JP, Meisels SJ. Adaptive and maladaptive parenting: Perspectives on risk and protective factors. Handbook of Early Childhood Intervention. 2000;2:54–75. [Google Scholar]
- Philofsky A, Hepburn SL, Hayes A, Hagerman R, Rogers SJ. Linguistic and cognitive functioning and autism symptoms in young children with fragile X syndrome. American Journal on Mental Retardation. 2004;109(3):208–218. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Tonnesen BL, Lindsay MM, Ford AL, Golden RN, Bailey DB. Trajectory and predictors of depression and anxiety disorders in mothers with the FMR1 premutation. Biological Psychiatry. 2016;79:850–857. doi: 10.1016/j.biopsych.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS): For diagnostic screening and classification of autism. Irvington; New York: 1986. [Google Scholar]
- Shire S, Gulsrud A, Kasari C. Increasing responsive parent-child interactions and joint engagement: Comparing the influence of parent-mediated intervention and parent psychoeducation. Journal of Autism and Developmental Disorders. 2016;46:1737–1747. doi: 10.1007/s10803-016-2702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: The science of early childhood development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- Sobesky WE, Hull CE, Hagerman RJ. Symptoms of schizotypal personality disorder in fragile X women. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33(2):247–255. doi: 10.1097/00004583-199402000-00014. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti D. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow SS, Cicchetti D, Balla DA. Vineland-II: Vineland Adaptive Behavior Scales. Second. Minneapolis, MN: Pearson; 2005. [Google Scholar]
- Spiker D, Boyce GC, Boyce LK. Parent-child interactions when young children have disabilities. International Review of Research in Mental Retardation. 2002;25:35–70. [Google Scholar]
- Tamanini F. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Human Molecular Genetics. 1997;6(8):1315–1322. doi: 10.1093/hmg/6.8.1315. [DOI] [PubMed] [Google Scholar]
- Thompson NM, Rogeness GA, McClure E, Clayton R, Johnson C. Influence of depression on cognitive functioning in fragile X females. Psychiatry Research. 1996;64(2):97–104. doi: 10.1016/0165-1781(96)02785-0. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64(1):196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DPA, Pizzuti A, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-H. [DOI] [PubMed] [Google Scholar]
- Warren SF, Brady NC. The role of maternal responsivity in the development of children with intellectual disabilities. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:330–338. doi: 10.100/mrdd.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SF, Brady NC, Sterling A, Fleming K, Marquis J. Maternal responsivity predicts language development in young children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2010;115(1):54–75. doi: 10.1352/1944-7558-115.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale Administration and Scoring Manual, Third Edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]


