Abstract
Background
Despite a recent upsurge of HIV/AIDS epidemic among young people in China, youth-specific HIV data were limited.
Methods
Altogether 56621 HIV/AIDS cases, aged 15–24, registered in the Case Reporting System of China between 2005 and 2012 and having complete spatial information were included in the present analysis. Spatial autocorrelation (general and local) and space-time scanning were performed using ArcGIS10.2 and SaTScan 9.3 software, respectively.
Results
During 2005–2012, the number of reported HIV/AIDS cases and proportion of HIV cases increased while proportion of AIDS cases decreased. Sexual contact evolved as the pre-dominant route of transmission in later years. Spatial analysis showed marked geographic variations of HIV infection among young people throughout China during 2005–2012. The number of new hotspots increased over years. They were mainly localized in southeast coastal areas and southwest bordering provinces or autonomous regions of Guangxi, Yunnan and Sichuan and Beijing municipality. Later these hotspots shifted towards northeastern part. Significant clusters of HIV positive cases were identified in three different time-periods, which indicated high HIV transmission among young Chinese in the recent past. The risk of HIV was highest in the first cluster (2009–2012, largest in size) covering Guizhou and Yunnan province, Chongqing municipality, Guangxi and Sichuan province. The second cluster (2010–2012) was mostly located in Shanghai, South Jiangsu, Zhejiang and South Anhui area while the third cluster (2010–2012) was in Beijing and Tianjin.
Conclusions
Target-specific comprehensive behavioral interventions were urgently needed to contain further spread of HIV epidemic among young people.
Keywords: HIV, young people, China, spatial analysis, cluster, hot points
Introduction
Globally, HIV is disproportionately afflicting young people worldwide, more so in the developing countries [1–4]. Limited access to comprehensive sex education and subsequent lack of awareness, adolescents’ risk-taking behaviors, concerns about rights and confidentiality, sexual violence against young girls and non-availability of youth-friendly quality reproductive health services could be the main reasons for this scenario [1–4]. Currently, about 6% of all people living with HIV and 12% of new adult HIV infections are attributed to adolescents [5]. Overall, an estimated of 5 million young people aged 15–24 were living with HIV in 2009 and accounted for 41% (about 890,000 cases) of new HIV infections globally [6 7]. Perhaps most alarming of all was that adolescent was the only age group where AIDS-related deaths increased by 50% between 2005 and 2012 and showed no decline thereafter [1 5 8]. Despite global commitments and continuous preventive efforts, young subjects continued to demonstrate a persistently high risk for HIV (an estimated 670,000 individuals between 15–24 became newly HIV infected in 2013) [5 8].
Historically driven by plasma-donors, dynamics of HIV transmission changed over the past few decades in China [9–11]. Moreover, HIV epidemics showed marked variation among the different risk groups in this country. Estimated HIV-positive rates remained low in the general population (0.1%) and female sex workers (0.2%), decreased among injecting drug users (IDU, 6.3%), while it increased among men who have men with men (MSM, 7.3%)and older people aged 50 years or more [9 12 13]. The number of HIV-positive cases also increased among young people in the recent years and sexual transmission (especially pre-marital sex) accounted for more than 90% of these new cases. Another point of huge concern was the potential for missing out high-risk young people like school drop-outs, young migrants, those in juvenile centers and residents of high-prevalent zone, from the National HIV prevention initiatives [14–16].
Although the vulnerabilities among young people became increasingly evident, there had been little efforts towards identification of their social networks and their role in potential HIV transmission. Limited data on geographical clustering of HIV-positive young persons in China further called for a detailed investigation. In 2014, we conducted a study on evaluating the HIV/AIDS epidemic among older adults in China through spatial analysis [17], and this study became a landmark for program planning and intervention designing targeting older adults in China. We believe that conduct a similar study among young people will have similar impact on program planning and intervention designing targeting young people in China.
The objectives of the current research were to describe the epidemiological and spatial distributions of HIV among young people between 2005 and 2012 in China so that policy makers could identify and address their specific needs for HIV prevention, care and treatment.
MATERIALS AND METHODS
Data Collection
HIV/AIDS case reporting system (CRS) in China was established in 1985 and became online in 2005. In this web-based reporting system, every new case identified at local hospitals, clinics run by Centers for Disease Control and Prevention (CDCs) and blood banks is reported at regular intervals [18]. Based on the principles of National HV/AIDS testing protocol, laboratory technology regulation and diagnostic criteria under CDC, blood samples were initially screened for HIV using Enzyme-linked immunosorbent-assay (ELISA): Chemiluminescence or Imunofluorescence and rapid testing. Samples positive at screening were confirmed by Western blot (WB), Radioimmunoprecipitation assay (RIPA) and Immunofluorescence test (IFA). Samples positive in the confirmatory tests were considered as HIV sero-positive [19]. In addition, as per the protocol and using a standardized case-report form, a face-to-face interview was conducted in a private room to gather information on socio-demographic characteristics [age, gender (male/female), occupation (student/other occupation), education (illiteracy/primary school/junior high school/senior high school/college or above/unknown), disease status (HIV/AIDS)], routes of transmission (heterosexual/homosexual/blood or plasma donor/injecting drugs/unknown or others) and risk behaviors from each eligible subject. For ensuring data quality through multiple logic checks, all completed case report forms were dispatched to the National Center for AIDS/STD Control and Prevention (NCAIDS) after ascertainment of new cases. To avoid or minimize the likelihood of duplication of reported cases, local CDC staffs rechecked ID numbers, names, addresses and other relevant information at the field-level. All information were also verified and evaluated at the national level by NCAIDS personnel to identify residual mistakes, if any. If any duplicate record was detected, single record was retained for each duplicated subject. Thus, this extensive and robust CRS procedure helped us to obtain detailed information on new cases as well as geographic distribution throughout the country.
In our study, “HIV”, “AIDS” and “HIV/AIDS” respectively referred to the presence of HIV infection at the time of reporting, development of AIDS and having either HIV infection or AIDS. Considering the probable age of becoming sexually active and inclination for risky behaviors as evidenced from prior literature, in the present study we defined young people as persons aged between 15–24 years consistent with the definition outlined by Joint United Nations Program on HIV/AIDS (UNAIDS) [4].
Data management
All young HIV/AIDS cases aged 15–24, identified in CRS during 2005–2012 were considered for the analysis. Subjects with inconsistent address were excluded from the study. To protect confidentiality of subjects, all personal identification information was removed from the database before starting data analysis. All reported case records were geocoded to the street address at provincial and city levels to obtain a valid geographical coordinate location (latitude and longitude)of the current place of residence.
General Spatial autocorrelation
Assuming consistent spatial distribution of HIV infection across different provinces in China, Moran’s Index (I) was used to measure the patterns (cluster/disperse/random) of distribution of HIV/AIDS-cases among young people throughout the country. Values for Moran’s I ranged from −1 to +1. The pattern of distribution was considered to be clustered if the value of Moran’s I was >0 and Z-value was >1.96 or the Moran’s I was<0 and Z-value was <−1.96. Otherwise the pattern was considered to be random or diverse [20].
Local Spatial autocorrelation
Getisstatistic was used to identify clusters at local levels or so called hotspots which helped us to compare the local pattern with the global pattern (that might be either driving the overall clustering pattern or reflect heterogeneities within the global pattern). The local clusters with a Z-value>1.96, were identified as hotspots, indicating elevated rates of HIV infection and with greatest public health needs. Local autocorrelation was conducted at city levels to detect hotspots among young people in China. Spatial analysis was conducted using ArcGIS 10.2 software (ESRI Inc., Redlands, CA, USA)
Space-time scanning
SaTScan 9.3 software was used to detect the distribution of HIV clusters over space, time or both under the assumption of space-time permutation probability (Poisson) model, which scanned areas of high HIV incidence, time allocation of 1 year, a maximum size of spatial cluster equal to 50% of the at-risk population, maximum size of temporal cluster equal to 50% of the study period and a maximum of 999 Monte Carlo simulations. The principle component of spatial scan statistic was based on the geometry of the scanning window. Usually, a cylindrical window was moved over each defined region where the base of the cylinder represented a specific geographic area and height of the cylinder represented a specific time period. A log likelihood ratio (LLR), and relative risk (RR=observed number of cases/expected number of cases) were calculated. A cluster was considered statistically significant if p value was <0.05. The formula used for LLR was:
where C was total cases in the data set, c was observed cases within the scanning window and μ was expected number of cases in that window. Scanning window with the maximum LLR value was defined as cluster 1, windows with lower LLR values were defined cluster 2, cluster 3 and so on.
Results
Between 2005 and 2012, 56,667 young HIV/AIDS cases aged 15–24 years were identified and reported to CRS. Of them, 46 cases (0.1%) were excluded due to inconsistencies in the addresses at the city level. Thus, 56, 621 (99.9%) cases were finally included in the analysis.
While the number of reported young HIV/AIDS cases increased during the study period (from 5186 in 2005 to 10195 in 2012) along with overall HIV cases, the results from trend analysis indicated that the proportions of young cases among all reported cases of HIV/AIDS in each year decreased significantly, from 13.9% to 12.6% in 2012, with P<0.001). Proportion of individuals acquiring infection through sexual route increased over the years. In 2012, more than 90% of reported HIV/AIDS cases acquired the virus through sexual contact. Proportions of individuals infected through homosexual transmission increased over the study period (Increased from 1.4% at 2005 to 42.2% at 2012), while the proportion infected through injecting drug declined over time (Decreased from 39.2% in 2005 to 7.5% in 2012). The proportion of reported HIV cases increased slightly during the study period (from 76.7% to 84.7%) while proportion of AIDS cases declined over the same time period among young people. Compared to females, the number of reported HIV/AIDS cases among males showed a marked increase after 2007 onwards. Number of HIV/AIDS cases increased among students over years (1.9% in 2005 to 13.0% in 2012). Overall, the distribution of age (approximately more than 80% were 20–24 years) was similar across years. Distribution of educational attainment revealed that most of them received education up to junior high school level (about 35–40%) followed by primary level (about 15–25%) in each year (Table 1).
Table 1.
Distribution of young HIV/AIDS cases in China, 2005–2012
| 2005 (%) | 2006 (%) | 2007 (%) | 2008 (%) | 2009 (%) | 2010 (%) | 2011 (%) | 2012 (%) | |
|---|---|---|---|---|---|---|---|---|
| Reported cases | 5186 | 4872 | 5524 | 6628 | 7416 | 7875 | 8925 | 10195 |
| Transmission route | ||||||||
| Heterosexual | 1370 (26.4) | 1877 (38.5) | 2553 (46.2) | 3479 (52.5) | 3875 (52.3) | 4157 (52.8) | 4598 (51.5) | 4998 (49.0) |
| Homosexual | 72 (1.4) | 158 (3.2) | 395 (7.2) | 964 (14.5) | 1619 (21.8) | 2149 (27.3) | 3043 (34.1) | 4305 (42.2) |
| Blood/Plasma | 120 (2.3) | 108 (2.2) | 101 (1.8) | 134 (2.0) | 122 (1.6) | 125 (1.6) | 111 (1.2) | 73 (0.7) |
| Injecting drugs | 2033(39.2) | 1578 (32.4) | 1703 (30.8) | 1648 (24.9) | 1482 (20.0) | 1254 (15.9) | 1060 (11.9) | 768 (7.5) |
| Unknown/others | 1591 (30.7) | 1151 (23.6) | 772 (14.0) | 403 (6.1) | 318 (4.3) | 190 (2.4) | 113 (1.3) | 51 (0.5) |
| Gender | ||||||||
| Male | 3059 (59.0) | 2642 (54.2) | 2990 (54.1) | 3756 (56.7) | 4343 (58.6) | 4896 (62.2) | 5994 (67.2) | 7298 (71.6) |
| Female | 2127 (41.0) | 2230 (45.8) | 2534 (45.9) | 2872 (43.3) | 3073 (41.4) | 2979 (37.8) | 2931 (32.8) | 2897 (28.4) |
| Occupation | ||||||||
| Student | 97 (1.9) | 142 (2.9) | 176 (3.2) | 384 (5.8) | 560 (7.6) | 691 (8.8) | 984 (11.0) | 1326 (13.0) |
| Other occupation | 5089 (98.1) | 4730 (97.1) | 5348 (96.8) | 6244 (94.2) | 6856 (92.4) | 7184 (91.2) | 7941 (89.0) | 8869 (87.0) |
| Age | ||||||||
| 15–19 | 851 (16.4) | 760 (15.6) | 858 (15.5) | 1044 (15.8) | 1115 (15.0) | 1144 (14.5) | 1410 (15.8) | 1752 (17.2) |
| 20–24 | 4335 (83.6) | 4112 (84.4) | 4666 (84.5) | 5584 (84.2) | 6301 (85.0) | 6731 (85.5) | 7515 (84.2) | 8443 (82.8) |
| Education | ||||||||
| Illiteracy | 284 (5.5%) | 391 (8.0) | 613 (11.1) | 950 (14.3) | 750 (10.1) | 681 (8.6) | 551 (6.2) | 450 (4.4) |
| Primary school | 1185 (22.8) | 1201 (24.7) | 1344 (24.3) | 1532 (23.1) | 1587 (21.4) | 1336 (17.0) | 1352 (15.1) | 1230 (12.1) |
| Junior high school | 1736 (33.5) | 1719 (35.3) | 2141 (38.8) | 2637 (39.8) | 2874 (38.8) | 3055 (38.8) | 3280 (36.8) | 3435 (33.7) |
| Senior High school | 327 (6.3) | 346 (7.1) | 465 (8.4) | 911 (13.7) | 1209 (16.3) | 1479 (18.8) | 1830 (20.5) | 2414 (23.7) |
| College or above | 93 (1.8) | 161 (3.3) | 260 (4.7) | 597 (9.0) | 996 (13.4) | 1324 (16.8) | 1912 (21.4) | 2666 (26.2) |
| Unknown | 1561 (30.1) | 1054 (21.6) | 701 (12.7) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Disease Status when identified | ||||||||
| HIV | 3980 (76.7) | 3622 (74.3) | 3921 (71.0) | 4783 (72.2) | 5507 (74.3) | 6010 (76.3) | 7102 (79.6) | 8632 (84.7) |
| AIDS | 1206 (23.3) | 1250 (25.7) | 1603 (29.0) | 1845 (27.8) | 1909 (25.7) | 1865 (23.7) | 1823 (20.4) | 1563 (15.3) |
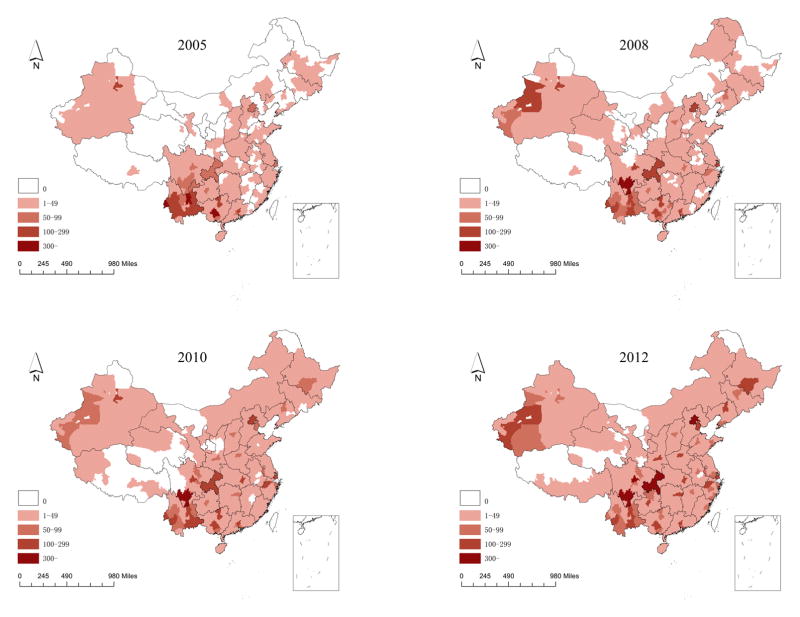
Majority of young HIV/AIDS cases were reported from 12 cities namely Tianjin, Shenyang, Harbin, Hangzhou, Zhengzhou, Wuhan, Changsha, Chengdu, Liangshan, Xi’an, Aksu, Kashgar which increased 5-folds between 2005 and 2012 (Figure 1)
Figure 1.
Geographical distribution of the total number of reported young HIV/AIDS cases at city level in the years 2005, 2008, 2010 and 2012
Spatial analysis
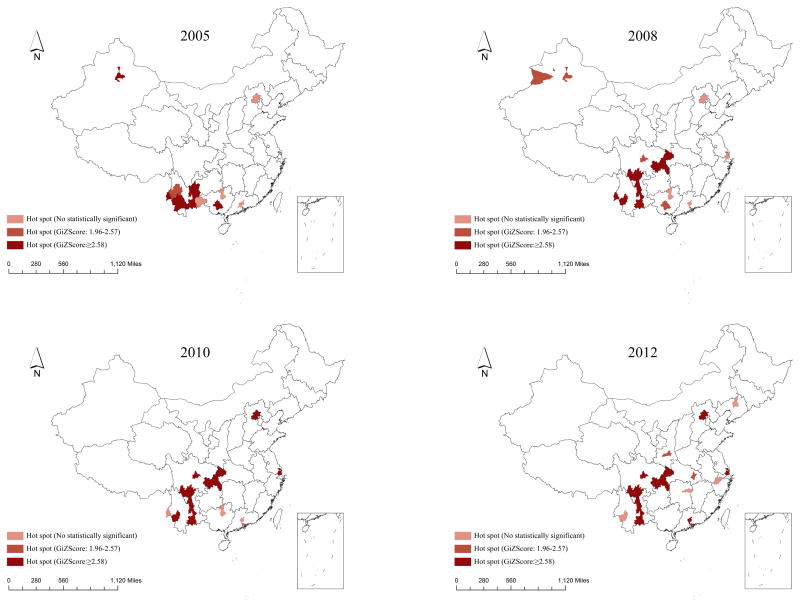
General spatial autocorrelation tests revealed significant clustering of PLWHA (aged 15–24) throughout the country during each year of the study period. Local spatial correlation using the same matrix was implemented to determine the year-wise distribution of hot spots at city levels throughout China (Figure 2). The hotspots were mainly located in south-east coastal areas and south-west bordering provinces or autonomous regions of Guangxi, Yunnan and Sichuan provinces and Beijing municipality. The number of hotspots significantly increased between 2005 and 2012. Further, hot spots were mainly clustered in southwestern part of China during the early years of our study, but gradually spread to central and northeastern part after 2008 in Xi’an, Wuhan, Changsha, Hangzhou and Shanghai (Figure 2).
Figure 2.
Hot spots of young HIV/AIDS cases at city level by year Space-time scanning
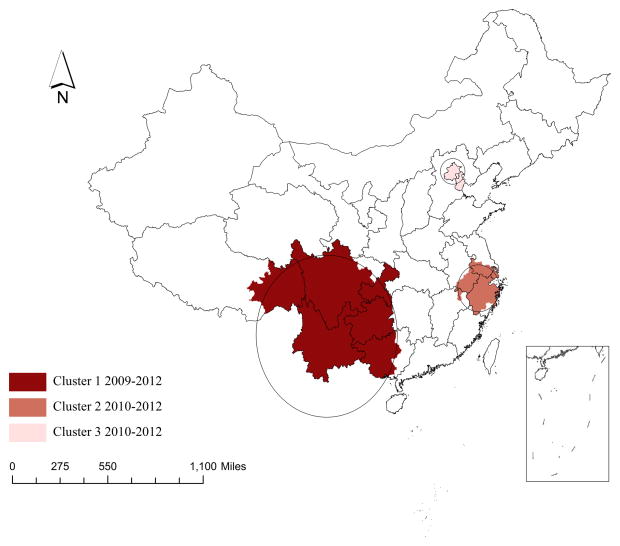
The space-time permutation scan statistics identified three significant clusters over different time intervals and different spaces (Table 2). The first cluster (largest in size) was detected between 2009 and 2012 with LLR=13415.26, radius=835.11 km and RR=6.28, covering 54 cities in Guizhou and Yunnan provinces, Chongqing municipality, some western cities in Guangxi Zhuang autonomous region and some central and southern cities in Sichuan province (Figure 3). Compared to neighboring cities, those identified in this cluster had 6.28 times higher HIV risk among young people. The second cluster was identified between 2010 and 2012 with LLR=1124.26, radius=237.18 and RR=3.04 and spread over 22 cities, mostly involving Shanghai, South Jiangsu, Zhejiang and South Anhui regions. The third cluster (smallest in size) was detected between 2010 and 2012 with LLR=1006.57, radius=122.68 and RR=5.16 in Beijing and Tianjin.
Table 2.
Spatial and temporal cluster of young HIV/AIDS cases in China, 2005–2012
| Potential clusters | Latitude | Longitude | Radius (km) | Time interval | Total locations | Log likelihood ratio | Relative risk | P value |
|---|---|---|---|---|---|---|---|---|
| Cluster 1 | 25.36 | 101.60 | 835.11 | 2009–2012 | 54 | 13415.29 | 6.28 | <0.001 |
| Cluster 2 | 29.88 | 119.51 | 237.18 | 2010–2012 | 22 | 1124.26 | 3.04 | <0.001 |
| Cluster 3 | 40.25 | 116.46 | 122.68 | 2010–2012 | 2 | 1006.57 | 5.16 | <0.001 |
Figure 3.
Case clusters among young people through space-time analysis, 2005–2012, China
Discussion
The present study clearly indicated that young residents in China accounted for a substantial number of HIV cases during 2005–2012. The number of PLWHA aged 15–24 and proportion of sexual (especially homosexual) transmission increased over time. Spatial analysis showed significant clustering of PLWHA throughout China during each year of the study period. Further, the number of hotspots increased over years and was mainly located in southeast coastal areas and southwest bordering provinces or autonomous regions of Guangxi, Yunnan and Sichuan and Beijing municipality.
Similar upsurge of HIV epidemic among young people was also reported from other countries in the world. Of estimated 35.3 million people living with HIV (PLWH) globally during 2012, 2.1 million were adolescents. Highest number adolescents living with HIV were reported from sub-Saharan Africa (1.7 million, about 85% of the total burden) followed by South Asia (130,000, about 6%), East Asia and Pacific (110,000), Latin America and the Caribbean (81,000), Eastern Europe and Central Asia (22,000) and Middle East and North Africa (17,000). Approximately 300,000 individuals between 15–19 years became newly HIV infected in 2012. [8]
There were a myriad of factors that could have resulted in higher number of HIV positives in this group. Societal shifts and behavioral patterns coupled with unique development changes were known to increase vulnerability to HIV infection faced by young people [21]. Previous studies in China revealed that about 11–26% college students had sexual experience and were less likely to use condom [22 23]. Inconsistent condom use and several risk-taking behaviors were also reported among young people in United States (African-Americans aged 18–21) and Uganda [24 25], Poor HIV-related knowledge and low self-perceived risk might be associated with this rising burden of HIV among young people [8 26 27]. Basic understanding of HIV was also found to be appallingly low among Chinese young people, as more than 85% of them had no knowledge regarding HIV prevention [14 28 29]. Apart from individual risk-taking behaviors, improved detection of PLWHA through robust CRS system in China might be another contributing factor to this rise in number of HIV cases in recent years [15 18]. In addition, the proportion of people that already progressed to AIDS was decreased during the study period, which might partially support a rising awareness to get HIV testing among young people. Thus, in light of above concerns, it seemed that improved access to youth-friendly counseling centers and enhanced coverage of sustained prevention and support services specific for adolescents might be effective in bringing positive behavior change with subsequent reduction in high-risk activities among this socially vulnerable group.
Spatial analysis in our study demonstrated significant clustering of PLWHA (15–24 years) throughout China with a gradual rise in number of hot spots over time. Further, several new hotspots were identified in Guangxi, Yunnan, Sichuan and Xinjiang provinces between 2005 and 2012. In recent years, HIV epidemic remained concentrated among high-risk groups and in some areas with a marked variation in the severity and other epidemiological characteristics across counties (districts) in 31 provinces of China. The highest number of HIV cases was mostly reported from Yunnan, Guangxi, Xinjiang and Sichuan in 2013 [9 30]. Evidences from earlier studies revealed that overland heroin export routes in Burma (largest producer of world’s heroin, about 60%) and Laos (third largest producer) lead to dual epidemic of IDU and new HIV infection in Yunnan, Xinjiang and Guangxi provinces [11 31 32]. Although the HIV epidemic in southern areas was initially driven by IDUs, heterosexual transmission became predominant in later phase. Henan was the only province, which showed significant clusters of blood/plasma donors. However, throughout the country the HIV burden remained high among MSM[30]. Hotspots were also detected in densely populous cities like Beijing, Shanghai and Guangzhou since 2010 in the present analysis. In addition, new clusters were identified in provincial capital cities like Xi’an, Wuhan, Changsha, Hangzhou and Shenyang in 2012. Though not confirmed, still it might be reasonable to speculate that, as most of the universities are located in these cities, where a huge number of students were enrolled each year. College atmosphere along with current sexual revolution in historically conservative Chinese society, particularly acceptance of pre-marital sex among young people, might create conducive environment where young people constellate for high-risk behaviors [26 33]. Thus, promotion and acceptance of comprehensive sex education incorporating accurate and age-appropriate information on HIV in academic institutes seemed to be the need of the hour in order to interrupt HIV transmission among students.
Although clusters of PLWHA mostly overlapped with the locations of hotspots, there were few non-overlapping zones. This difference might be attributed to the use of different principles in local spacing autocorrelation (ArcGIS software) and space-time analysis (SaTScan software), Thus, findings from each analysis should be considered in tandem rather than individually [34–36]. If the current pattern of clustering continues, it is likely that many young people would be infected over the next few years. These findings clearly supported the need to roll out target-specific socio-behavioral programs among Chinese young people, ensuing sufficient access, frequent use and quality services.
There were several limitations in our study. Since this was a record-based study, number of reported HIV/AIDS cases among young people might be affected by availability and uptake of HIV-antibody testing as well as possible impact of prevention efforts. Moreover, unidentified cases (either not detected or reported) in CRS might have influenced the determination of hotspots in our study. Although we could not control these factors in our analyses, which might have introduced some biases in the present study, but we think the probability of such biases would be minimal because of an extensive ongoing HIV/AIDS surveillance and monitoring system across the country. In addition, individuals with non-specific spatial information were dropped from our study. But this percentage was very small and thus unlikely to introduce any bias. The data that we used unfortunately did not have information on migration and health-care access (including diagnostic facilities) as by design it was record-based, hence it was not also possible to look in to these factors in our study. Based on the design it was also not possible for us to identify the changes in the reporting fraction, hence we could not measure what fraction of the observed change in hotspots were attributable to the increase in recognition and reporting of HIV cases. Further the newly reported cases should not be taken as incidence; rather it should be interpreted as newly observed burden. Despite of study limitations and scarcity of information on spatial distribution of HIV cases among Chinese youth, by virtue of large sample size, cost-effective robust methodology, efficient detection of significant clusters and hot spots through advanced technologies and strength of data analyses, we believe this study generated important insights into dynamics of HIV transmission among young people.
Conclusion
An increasing number of young people were infected with HIV in China between 2005 and 2012. According to the spatial analysis, marked geographic variation of HIV infection existed at regional level. Several new hotspots and significant HIV clusters were initially detected towards the southeast coastal areas and southwest bordering provinces with gradual shift to northeastern part of China afterwards. Thus, HIV among young people should be viewed more contextually and preventive efforts should be targeted towards changing risky environments as well as risky individual behaviors. In addition for designing effective behavioral change interventions, future research into spatial analysis of behavioral risk factors associated with HIV among young people seemed pertinent for controlling further spread of the epidemic.
Key message: Summary.
Number of people living with HIV/AIDS, aged 15–24 years increased in China during 2005–2012;
Several new hotspots and significant HIV clusters were detected towards the southeast and southwest provinces with gradual shift to northeastern and central part of China;
Target-specific comprehensive behavioral interventions were urgently needed to contain further spread of HIV epidemic among young people.
Acknowledgments
Funding
This work was supported by the mega-projects of national science research under the 12th Five-Year Plan of China (2012ZX10001001), National Institutes of Health (NIAID 1R01AI114310, 1D43TW009532) and NIH Fogarty International Center (5R25TW009340).
We would like to acknowledge all staff of HIV/AIDS case reporting system in China and all staff from Division of Epidemiology, National Center for AIDS/STD Control and Prevention, National Center for Disease Control and Prevention.
Footnotes
Conflicts of interest: None
Ethics Statements
This study was approved by Institutional Review Board of National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention (X120331209).
Consent
Our study was secondary analysis based on reported data and all individual information was removed before analysis. We didn’t design informed consent in this study.
Author Contributions
XZ, WT and YL wrote this manuscript. TM, YF, ML, FC and PL collected and cleaned data. JX, SQ and LG conducted statistical analysis. LW and NW contributed to designing this study. SM and TM revised this manuscript before submission.
References
- 1.Tang W, Babu GR, Li J, et al. The difference between HIV and syphilis prevalence and incidence cases: results from a cohort study in Nanjing, China, 2008–2010. International journal of STD & AIDS. 2014 doi: 10.1177/0956462414550170. 0956462414550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binagwaho A, Fuller A, Kerry V, et al. Adolescents and the right to health: eliminating age-related barriers to HIV/AIDS services in Rwanda. AIDS Care. 2012;24(7):936–42. doi: 10.1080/09540121.2011.648159. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Pettifor A, Bekker LG, Hosek S, et al. Preventing HIV among young people: research priorities for the future. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S155–60. doi: 10.1097/QAI.0b013e31829871fb. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis B, Taboada F, Andiorio E, et al. Demonstration of Improved Sensitivity and Specificity in Blood-Donors by a Prototype Hcv-3. 0 Elisa. Transfusion. 1993;33(9):S32–S32. [Google Scholar]
- 5.Riezuboj JI, Parker D, Civeira MP, et al. Detection of Hepatitis-C Virus-Antibodies with New Recombinant Antigens - Assessment in Chronic Liver-Diseases. Journal of Hepatology. 1992;15(3):309–13. doi: 10.1016/0168-8278(92)90061-s. [DOI] [PubMed] [Google Scholar]
- 6.Feng L, Li P, Wang X, et al. Distribution and Determinants of Non Communicable Diseases among Elderly Uyghur Ethnic Group in Xinjiang, China. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W, Wang L, Han N, et al. Pre-exposure prophylaxis of HIV: A right way to go or a long way to go? Artificial cells, nanomedicine, and biotechnology. 2014;(0):1–8. doi: 10.3109/21691401.2014.934458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S144–53. doi: 10.1097/qai.0000000000000176. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 9.2014 China AIDS Response Progress Report. National Health and Family Planning Commission of the People’s Republic of China. www.unaids.org/sites/default/files/.../CHN_narrative_report_2014.pdf. Secondary 2014 China AIDS Response Progress Report, National Health and Family Planning Commission of the People’s Republic of China, www.unaids.org/sites/default/files/.../CHN_narrative_report_2014.pdf.
- 10.Wang LD. Overview of the HIV/AIDS epidemic, scientific research and government responses in China. Aids. 2007;21:S3–S7. doi: 10.1097/01.aids.0000304690.24390.c2. [DOI] [PubMed] [Google Scholar]
- 11.Wu ZY, Rou K, Cui HX. The HIV/AIDS epidemic in China: history, current strategies and future challenges. AIDS Educ Prev. 2004;16(Supplement A):7–17. doi: 10.1521/aeap.16.3.5.7.35521. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Chow EP, Jing J, et al. HIV prevalence in China: integration of surveillance data and a systematic review. Lancet Infect Dis. 2013;13(11):955–63. doi: 10.1016/s1473-3099(13)70245-7. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Xing J, Li YG, Tang W, et al. HIV/AIDS epidemic among older adults in China during 2005–2012: results from trend and spatial analysis. Clin Infect Dis. 2014;59(2):e53–60. doi: 10.1093/cid/ciu214. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Emshaty WM, Raafat D, Elghannam DM, et al. Diagnostic Performance of an Immunoassay for Simultaneous Detection of Hcv Core Antigen and Antibodies among Haemodialysis Patients. Brazilian Journal of Microbiology. 2011;42(1):303–09. doi: 10.1590/S1517-83822011000100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Ding ZW, Yan RX, et al. HIV/AIDS epidemic situation and data analysis among young students from 2006–2009 in China. Chinese Journal of Epidemiology. 2010;31(9):1017–21. [PubMed] [Google Scholar]
- 16.Chen L, Mahapatra T, Fu G, et al. Male Clients of Male Sex Workers in China: An Ignored High Risk Population. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016 doi: 10.1097/QAI.0000000000000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing J, Li Y-g, Tang W, et al. HIV/AIDS epidemic among older adults in China during 2005–2012: Results from trend and spatial analysis. Clinical Infectious Diseases. 2014;59(2):e53–e60. doi: 10.1093/cid/ciu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun LN, Fu SG, Wang L. The history and situation of HIV/AIDS case reporting. Modern Preventive Medicine. 2011;4:070. [Google Scholar]
- 19.Constantine NT, Callahan JD, Bansal J, et al. Concordance of Tests to Detect Antibodies to Hepatitis-C Virus. Laboratory Medicine. 1992;23(12):807–10. [Google Scholar]
- 20.Peng ZH, Cheng YJ, Reilly KH, et al. Spatial distribution of HIV/AIDS in Yunnan province, People’s Republic of China. Geospat Health. 2011;5(2):177–82. doi: 10.4081/gh.2011.169. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 21.Bearinger LH, Sieving RE, Ferguson J, et al. Global perspectives on the sexual and reproductive health of adolescents: patterns, prevention, and potential. Lancet. 2007;369(9568):1220–31. doi: 10.1016/s0140-6736(07)60367-5. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Ji CY. Sexual intercourse and high-risk sexual behaviours among a national sample of urban adolescents in China. J Public Health. 2010;32(3):312–21. doi: 10.1093/pubmed/fdp123. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Z, Palmgreen P, Zimmerman R, et al. Adapting and applying a multiple domain model of condom use to Chinese college students. AIDS Care. 2010;22(3):332–38. doi: 10.1080/09540120903193609. [DOI] [PubMed] [Google Scholar]
- 24.Kogan SM, Brody GH, Chen YF, et al. Risk and protective factors for unprotected intercourse among rural African American young adults. Public Health Rep. 2010;125(5):709. doi: 10.1177/003335491012500513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chimoyi LA, Musenge E. Spatial analysis of factors associated with HIV infection among young people in Uganda, 2011. BMC public health. 2014;14(1):555. doi: 10.1186/1471-2458-14-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adefuye AS, Abiona TC, Balogun JA, et al. HIV sexual risk behaviors and perception of risk among college students: implications for planning interventions. BMC Public Health. 2009;9:281. doi: 10.1186/1471-2458-9-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nogues D, David A, Falcou Briatte R. Comparative studies of the access HCV Ab plus assay. Biochimica Clinica. 2013;37:S458. [Google Scholar]
- 28.Cai Y, Shi R, Li S, et al. Study of HIV/AIDS-related knowledge among junior high-school students in Shanghai, China. Int J STD AIDS. 2012;23(3):e9–e12. doi: 10.1258/ijsa.2009.009065. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Li W. Knowledge, attitude and behaviors about HIV/AIDS among middle school students in Dalian city. Chinese Journal of Public Health. 2013;5:036. [Google Scholar]
- 30.Wang L, Tang W, Wang L, et al. The HIV, syphilis, and HCV epidemics among female sex workers in China: Results from a serial cross-sectional study between 2008 and 2012. Clinical Infectious Diseases. 2014;59(1):e1–e9. doi: 10.1093/cid/ciu245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyrer C, Razak MH, Lisam K, et al. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 2000;14(1):75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- 32.Qian HZ, Schumacher JE, Chen HT, et al. Injection drug use and HIV/AIDS in China: review of current situation, prevention and policy implications. Harm reduction journal. 2006;3(1):4. doi: 10.1186/1477-7517-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Z, Mehrotra P, Zimmerman R. Sexual revolution in China: implications for Chinese women and society. AIDS Care. 2011;23(Suppl 1):105–12. doi: 10.1080/09540121.2010.532537. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 34.Anselin L. Local indicators of spatial association—LISA. Geographical analysis. 1995;27(2):93–115. [Google Scholar]
- 35.Hanson CE, Wieczorek WF. Alcohol mortality: a comparison of spatial clustering methods. Soc Sci Med. 2002;55(5):791–802. doi: 10.1016/s0277-9536(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 36.Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Statistics in medicine. 1995;14(8):799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]