Abstract
Background
Early-life exposure to older siblings is associated with a lower risk of asthma. To date, no study has addressed the impact of having siblings on both the airway and fecal microbiota during infancy. We aimed to profile the nasal airway and fecal microbiota in infants, and to examine the association between having siblings and microbiota profiles.
Methods
We conducted a cross-sectional study of 105 healthy infants (aged <1 year). By applying 16S rRNA gene sequencing and an unbiased clustering approach to the nasal airway and fecal samples, we identified microbiota profiles and then determined the association between having siblings and microbiome profiles.
Results
Overall, the median age was 3.4 months (IQR, 2.0–4.7 months); 43% had siblings in the household. Unbiased clustering of nasal airway microbiota identified three profiles: Moraxella-dominant (43%), Corynebacterium/Dolosigranulum-dominant (36%), and mixed (21%). Infants with siblings were more likely to have Moraxella-dominant profile than Corynebacterium/Dolosigranulum-dominant profile (76% vs. 18%) while those without siblings had the opposite pattern (18% vs. 50%) (multivariable-adjusted P<0.001). Fecal microbiota consisted of three profiles: Bifidobacterium-dominant (39%), Escherichia-dominant (31%), and Enterobacter-dominant (30%). Infants with siblings were more likely to have Bifidobacterium-dominant profile than Escherichia-dominant profile (49% vs. 24%) while those without siblings had the opposite pattern (32% vs. 37%) (multivariable-adjusted P=0.04).
Conclusions
In this cross-sectional study, we found that infants with siblings were more likely to have Moraxella-dominant nasal microbiota profile and Bifidobacterium-dominant fecal microbiota profile. Our findings should facilitate further investigation of the interplay between early-life environmental exposures, the microbiome, and childhood asthma.
Keywords: airway microbiota, fecal microbiota, sibling, infants, asthma
BACKGROUND
In recent decades, epidemiologic studies have reported an inverse relationship between exposure to microbially-rich environments in infancy and risk of developing allergic diseases.1–4 For example, the literature has documented that early-life exposure to older siblings and farm animals is protective against the development of childhood asthma.1,2,5,6 This finding was furthered by culture-independent high-throughput sequencing approaches revealing that the human microbiota – highly functional bacterial communities that inhabit humans – influences the rate and pattern of maturation of immune function in early life.7,8 These data suggest that the microbiota may mediate the link between environmental factors and development of childhood asthma.7
To date, most microbiome research has focused on the gut, and, with regard to above, the possible link between the presence of older siblings and specific fecal microbiota.9–11 However, the airway is a major portal for microbial exposure and studies have revealed that the airway microbiota influences immune responses in patients with asthma.7 Further, the airway microbiota may be modulated by host-microbe interactions in the gut and systemic dissemination of metabolites produced by the gut microbiota.12 To the best of our knowledge, no study has addressed the impact of household siblings on both airway and fecal microbiota simultaneously in infants. To address this knowledge gap, we profiled the nasal airway and fecal microbiota in infants, and examined the association between having siblings in the household and microbiota profiles.
METHODS
Design, Setting, and Participants
We conducted a cross-sectional study to examine the nasal and fecal microbiota of healthy infants. The setting and participants have been reported previously.13 Briefly, we enrolled 120 infants from a primary care group practice at Massachusetts General Hospital (Boston, MA) from November 2013 through May 2014. Inclusion criteria were infants aged <1 year and gestational age >32 weeks. Exclusion criteria included comorbidities (i.e., heart-lung disease, immunodeficiency, immunosuppression, or chronic gastrointestinal disorder), previous lower respiratory infection, diarrheal illness in the past week, treatment with antibiotics in the past week, and current acute respiratory infection or gastrointestinal illness.14 The local institutional review board approved the study. Informed consent was obtained from the parents or guardians.
Data Collection
We conducted a structured interview and chart review that assessed participants’ demographic characteristics, family history, prenatal and past medical history, and home environmental characteristic including presence of siblings in the household. Trained investigators collected nasal swabs from the anterior nares (i.e., not from nasal turbinates or nasopharynx), using a standardized protocol,15 during the clinic visit. Both anterior nares were swabbed with a single nylon, pediatric FLOQSwab (Copan, Brescia, Italy). The swab was then added to 2 mL of viral transport medium and frozen at −80°C. Fecal samples were collected, using a standardized protocol,16 within 24 hours of the clinic visit. Diapers containing feces were refrigerated or stored in a cooler immediately after collection. The fecal samples were then added to sterile feces collection containers (Sarstedt, Nümbrecht, Germany) and immediately stored at −80°C. Nasal and fecal samples were shipped on dry ice to the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine, where we characterized the microbiota using 16S rRNA gene sequencing.
16s rRNA Gene Sequencing
16S rRNA gene sequencing methods were adapted from the methods developed for the NIH Human Microbiome Project.17,18 Briefly, bacterial genomic DNA was extracted using MO BIO PowerMag DNA Isolation Kit (Mo Bio Laboratories; Carlsbad, CA). The 16S rDNA V4 region was amplified by PCR and sequenced in the MiSeq platform (Illumina; SanDiego, CA) using the 2x250 bp paired-end protocol yielding pair-end reads that overlap almost completely. The primers used for amplification contain adapters for MiSeq sequencing and single-end barcodes allowing pooling and direct sequencing of PCR products.19
Sequencing read pairs were demultiplexed based on the unique molecular barcodes, and reads were merged using USEARCH v7.0.1090,20 allowing zero mismatches and a minimum overlap of 50 bases. Merged reads were trimmed at the first base with a Q5 quality score. Additionally, a quality filter was applied to the resulting merged reads and reads containing above 0.05 expected errors were discarded. Rarefaction curves of bacterial operational taxonomic units (OTUs) were constructed using sequence data for each sample to ensure coverage of the bacterial diversity present. Samples with suboptimal amounts of sequencings reads (<80% of the taxa are represented) were re-sequenced to ensure that the majority of bacterial taxa were encompassed in our analyses.
16S rRNA gene sequences were clustered into OTUs at a similarity cutoff value of 97% using the UPARSE algorithm.21 OTUs were mapped to the SILVA Database22 containing only the 16S V4 region to determine taxonomies. Abundances were recovered by mapping the demultiplexed reads to the UPARSE OTUs. A custom script constructed a rarefied OTU table from the output files generated in the previous two steps for downstream analyses of alpha-diversity (e.g., Shannon index) and beta-diversity (e.g., Bray-Curtis distance).
Quality Control
The processes involving microbial DNA extraction, 16S rRNA gene amplification, and amplicon sequencing included a set of controls that enabled us to evaluate the potential introduction of contamination or off-target amplification. Non-template controls (extraction chemistries) were included in the microbial DNA extraction process and the resulting material was subsequently used for PCR amplification. Additionally, at the step of amplification, another set of non-template controls (PCR-mix) was included to evaluate the potential introduction of contamination at this step. Similarly, a positive control comprised of known and previously characterized microbial DNA was included at this step to evaluate the efficiency of the amplification process. Before samples (unknowns) were pooled together, sequencing controls were evaluated and the rejection criteria were the presence of amplicons in any of the non-template controls or the absence of amplicons in the positive control. In the present study, no amplicons were observed in the non-template controls and a negligible amount of raw reads were recovered after sequencing.
Statistical Analyses
We calculated the relative abundance of each OTU for each nasal airway sample and fecal sample. As each genus was dominated by one OTU, we collapsed all OTUs assigned to the same genus into a single group for reporting. To identify nasal airway and fecal microbiota profiles, we performed unbiased clustering by the partitioning around medoids method 23 with the use of Bray-Curtis distances. Each cluster was defined by a point designated as the center (the “medoid”) and minimizes the distance between samples in a cluster. The number of clusters to choose for the data was determined by using the gap statistic.24 We also examined the within-subject association between nasal airway microbiota profile and fecal microbiota profile (i.e., the marginal frequencies of two nominal outcomes) by marginal homogeneity test.
To examine the association between the presence of siblings and the microbiota profiles, we constructed multinomial regression model for nasal and fecal microbiota, separately. We adjusted for up to six potential confounders (age, sex, maternal antibiotic use during pregnancy, maternal smoking, breast-feeding status, and history of systemic [i.e., oral, intravenous, intramuscular] antibiotic use in infancy) while being mindful of the relatively small number of subjects within microbiota profiles. These variables were chosen based on clinical plausibility and a priori knowledge.9–11 In the sensitivity analysis, we repeated the multivariable model with the number of siblings as ordinal variable. We also performed unadjusted analyses stratified by mode of delivery in all study participants as well as stratified analyses by feeding status in infants aged <6 months. Analyses used R version 3.2 with the phyloseq package.25
RESULTS
The analytic cohort comprised 105 infants with both nasal and fecal samples. Overall, the median age was 3.4 months (IQR, 2.0–4.7 months), 56% were male, and 52% were non-Hispanic white. With regard to the primary exposure, 43% of infants had siblings in the household (Table 1).
Table 1.
Infant and Microbiota Characteristics, According to the Presence of Siblings in the Household
| Characteristics | Infants with siblings n=45 (43%) | Infants without siblings n=60 (57%) | P-value |
|---|---|---|---|
| Subject characteristics | |||
| Demographics | |||
| Age (mo) | 0.45 | ||
| <2 | 8 (18) | 17 (28) | |
| 2–5.9 | 27 (60) | 31 (52) | |
| 6–12 | 10 (22) | 12 (20) | |
| Male sex | 31 (69) | 28 (47) | 0.04 |
| Race/ethnicity | 0.01 | ||
| Non-Hispanic white | 20 (44) | 35 (58) | |
| Non-Hispanic black | 7 (16) | 3 (5) | |
| Hispanic | 4 (9) | 14 (23) | |
| Other | 14 (31) | 8 (13) | |
| Parental history of asthma | 9 (20) | 11 (18) | 0.99 |
| Prenatal history | |||
| Maternal antibiotic use during pregnancy | 7 (16) | 6 (10) | 0.58 |
| Maternal antibiotic use during labor | 14 (31) | 17 (28) | 0.93 |
| Maternal smoking during pregnancy | 3 (7) | 0 (0) | 0.15 |
| Past medical history | |||
| Mode of birth | 0.47 | ||
| Vaginal birth | 26 (58) | 40 (67) | |
| C-section | 19 (42) | 20 (33) | |
| Prematurity (32–37 weeks) | 5 (11) | 5 (8) | 0.89 |
| History of eczema | 7 (16) | 9 (15) | 0.99 |
| Previous breathing problems before enrollment | 0 (0) | 0 (0) | -* |
| Systemic antibiotic use before enrollment | 6 (13) | 6 (10) | 0.83 |
| Systemic corticosteroid use before enrollment | 0 (0) | 0 (0) | -* |
| Home environmental characteristics | |||
| Smoking exposure at home | 3 (7) | 0 (0) | 0.15 |
| Mostly breastfed for the first 3 months of age | 32 (71) | 49 (82) | 0.30 |
| Ever attended daycare | 10 (22) | 4 (7) | 0.04 |
| Number of siblings in the household | -* | ||
| 0 | 0 (0) | 60 (100) | |
| 1 | 36 (80) | 0 (0) | |
| 2 | 5 (11) | 0 (0) | |
| 3 | 3 (7) | 0 (0) | |
| 4 | 1 (2) | 0 (0) | |
| Microbiota characteristics | |||
| Nasal microbiota | |||
| Number of genera, median (IQR) | 11 (8–22) | 23 (16–30) | <0.001 |
| Shannon index, median (IQR) | 0.63 (0.22–1.03) | 1.30 (0.92–1.76) | <0.001 |
| Microbiota profiles | |||
| Moraxella-dominant profile | 34 (76) | 11 (18) | <0.001† |
| Corynebacterium/Dolosigranulum-dominant profile | 8 (18) | 30 (50) | - |
| Mixed profile | 3 (7) | 19 (32) | 0.48† |
| Fecal microbiota | |||
| Number of genera, median (IQR) | 16 (11–21) | 14 (10–18) | 0.051 |
| Shannon index, median (IQR) | 1.40 (1.08–1.87) | 1.30 (0.98–1.70) | 0.35 |
| Microbiota profiles | |||
| Escherichia-dominant profile | 11 (24) | 22 (37) | - |
| Bifidobacterium-dominant profile | 22 (49) | 19 (32) | 0.08‡ |
| Enterobacter-dominant profile | 12 (27) | 19 (32) | 0.65‡ |
Data are no. (%) of infants unless otherwise indicated. Percentages may not equal 100 because of missingness or rounding IQR, interquartile range
Not computed
Computed by unadjusted multinomial regression model with Corynebacterium/Dolosigranulum-dominant profile as reference group
Computed by unadjusted multinomial regression model with Escherichia-dominant profile as reference group
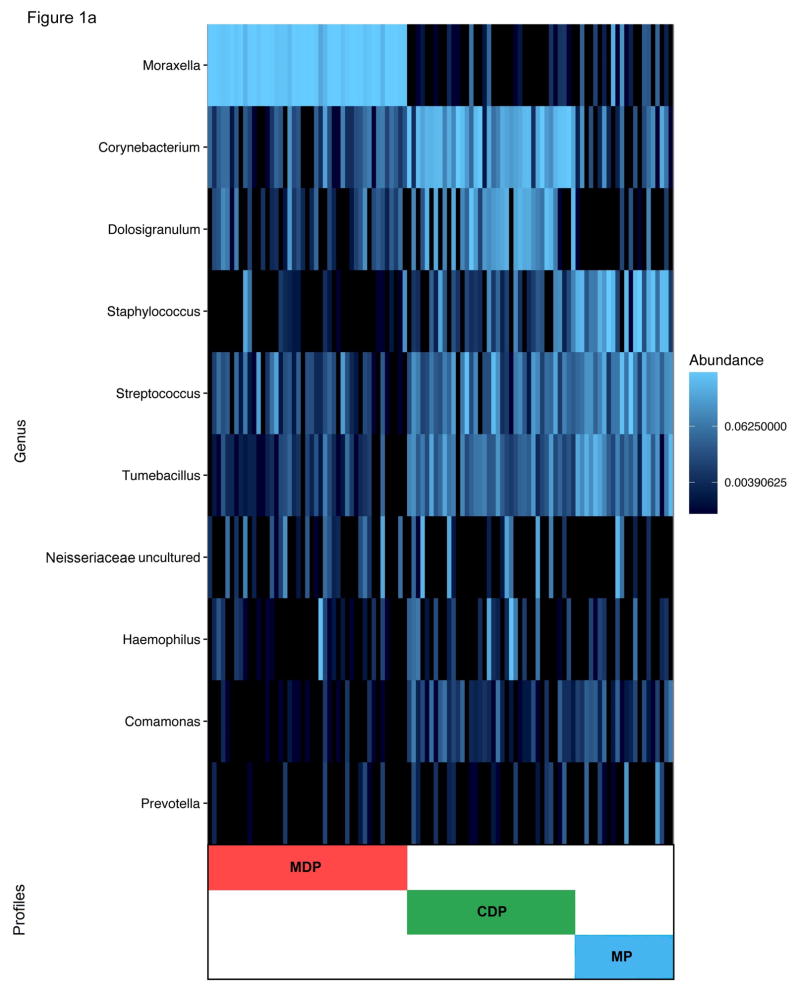
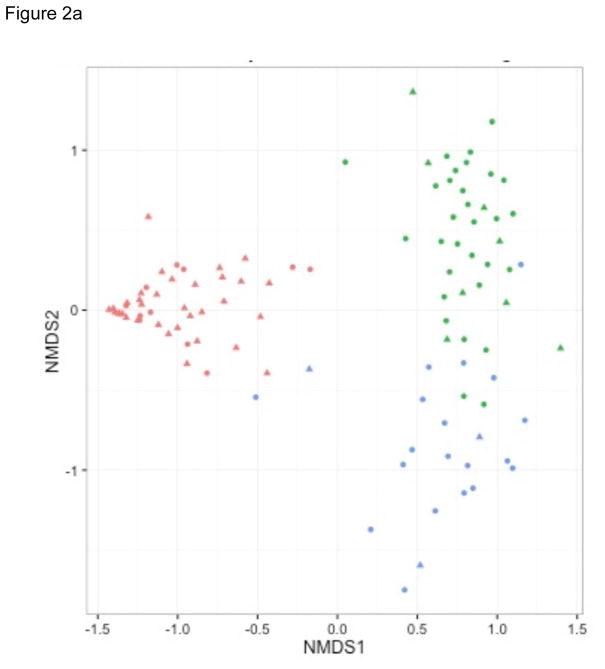
We analyzed nasal airway samples from 105 infants by 16S rRNA gene sequencing. All 105 yielded high quality sequence data (rarefaction cutoff, 1,066 reads per sample). The sequencing identified 21 phyla and 306 genera. The nasal airway microbiota was dominated by three genera – Moraxella (37%), Corynebacterium (17%), and Staphylococcus (9%) – followed by Dolosigranulum (8%) and Streptococcus (8%). Partitioning around medoids clustering of nasal airway microbiota identified three distinct microbiota profiles: 1) Moraxella-dominant (43%), 2) Corynebacterium/Dolosigranulum-dominant (36%), and 3) mixed (21%) (Figure 1a). The first two profiles were dominated either by Moraxella or Corynebacterium/Dolosigranulum (co-dominant) genus. By contrast, the mixed profile had the highest relative abundance of Staphylococcus, Streptococcus, and Tumebacillus (Benjamini-Hochberg adjusted P<0.05; Table 2). The nonmetric multidimensional scaling (NMDS) plots also demonstrated that the infants cluster together according to their nasal airway microbiota profile (Figure 2a).
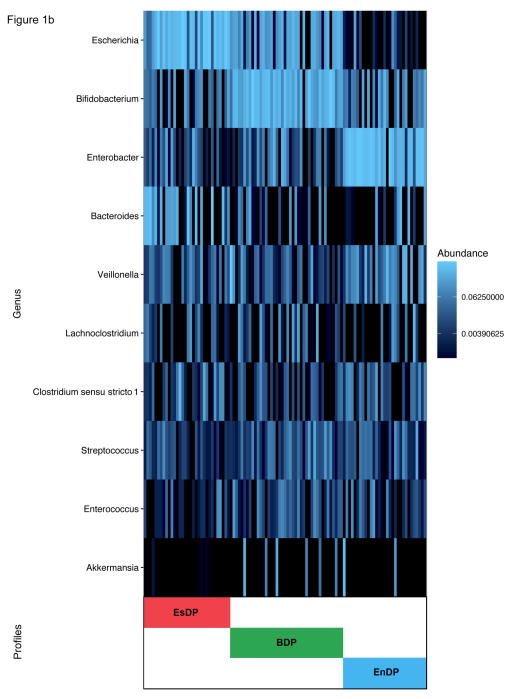
Figure 1. Clustering and Composition in Nasal Airway and Fecal Microbiota in 105 Infants.
All nasal airway and fecal microbiota profiles of 105 infants were clustered using the partitioning around medoids clustering method with the use of Bray-Curtis distance. The optimal number of clusters was identified by using the gap statistic. To obtain further information about the bacterial composition of samples within microbiota profiles, the ten most abundant genera present in an adjacent heatmap were displayed. A) Nasal airway microbiota. Colored bars indicate three microbiota profiles: Moraxella-dominant (red), Corynebacterium/Dolosigranulum-dominant (green), and mixed (blue). MDP = Moraxella-dominant profile; CDP= Corynebacterium/Dolosigranulum-dominant profile; MP = mixed profile. B) Fecal microbiota. Colored bars indicate three microbiota profiles: Escherichia-dominant (red), Bifidobacterium -dominant (green), and Enterobacter-dominant (blue). EsDP = Escherichia-dominant profile; BDP = Bifidobacterium-dominant profile; EnDP = Enterobacter-dominant profile.
Table 2.
Richness, Alpha-diversity and Relative Abundance by Nasal Airway Microbiota Profile
| Moraxella-dominant profile n=45 (43%) | Corynebacterium/Dolosigranulum-dominant profile n=38 (36%) | Mixed profile n=22 (21%) | P-value | |
|---|---|---|---|---|
| Richness, median (IQR) | ||||
| Number of genera | 11 (7–17) | 23 (17–33) | 27 (15–29) | <0.001 |
| Alpha-diversity, median (IQR) | ||||
| Shannon index | 0.51 (0.18–0.84) | 1.32 (1.04–1.70) | 1.70 (1.01–2.01) | <0.001 |
| Relative abundance of 10 most common genera, mean (SD) | ||||
| Moraxella | 0.85 (0.17) | 0.00 (0.01) | 0.03 (0.09) | 0.003* |
| Corynebacterium | 0.03 (0.05) | 0.41 (0.28) | 0.05 (0.08) | 0.003* |
| Dolosigranulum | 0.02 (0.04) | 0.20 (0.21) | 0.01 (0.02) | 0.003* |
| Staphylococcus | 0.01 (0.06) | 0.03 (0.06) | 0.36 (0.32) | 0.003* |
| Streptococcus | 0.03 (0.06) | 0.09 (0.15) | 0.17 (0.23) | 0.02* |
| Tumebacillus | 0.01 (0.02) | 0.06 (0.08) | 0.17 (0.17) | 0.003* |
| Neisseriaceae uncultured | 0.02 (0.06) | 0.04 (0.11) | 0.03 (0.12) | 0.98* |
| Haemophilus | 0.02 (0.10) | 0.04 (0.13) | 0.00 (0.01) | 0.98* |
| Comamonas | 0.00 (0.00) | 0.01 (0.02) | 0.01 (0.02) | 0.01* |
| Prevotella | 0.00 (0.00) | 0.00 (0.01) | 0.02 (0.06) | 0.15* |
Abbreviations: IQR, interquartile range; SD, standard deviation
Benjamini-Hochberg adjusted P-value accounting for multiple comparisons
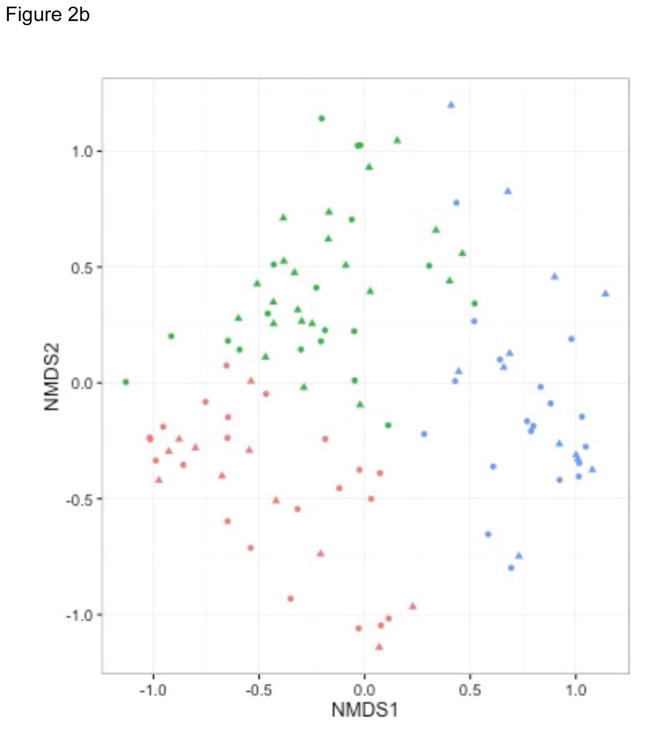
Figure 2. Nonmetric Multidimensional Scaling Plots on Nasal Airway and Fecal Microbiota.
To show the differences in nasal airway and fecal microbiota among 105 infants, nonmetric multidimensional scaling (NMDS) plots based on the Bray-Curtis distance between all infants were generated. Each dot represents the overall bacterial community in each subject. Shapes indicate the status of siblings at household: infants with sibling (triangle) and those without (circle). A) Nasal airway microbiota. Colors indicate three microbiota profiles: Moraxella-dominant (red), Corynebacterium/Dolosigranulum-dominant (green), and mixed (blue). B) Fecal microbiota. Colors indicate three microbiota profiles: Escherichia-dominant (red), Bifidobacterium-dominant (green), and Enterobacter-dominant (blue).
Nasal airway microbiota profiles differed between infants with siblings and those without. For instance, infants with siblings were more likely to have a Moraxella-dominant profile than a Corynebacterium/Dolosigranulum-dominant profile (76% vs. 18%; Table 1) while those without siblings had the opposite pattern (18% vs. 50%), corresponding to a relative risk ratio (RRR) of 11.6 (95%CI, 4.12–32.6; P<0.001; Table 3). This significant finding persisted in the multivariable model, (adjusted RRR, 18.9; 95%CI, 5.30–67.3; P<0.001) and in the sensitivity analysis modeling the number of siblings as ordinal variable (adjusted RRR per each incremental sibling, 6.05; 95%CI, 2.26–16.2; P<0.001). Furthermore, in the stratified analyses by mode of delivery (Table 4) and by feeding status (Table 5), the results did not change materially across the strata. In addition to the differences in nasal microbiota profiles, infants with siblings had a significantly lower bacterial richness (P<0.001) and alpha-diversity index (Shannon index, P<0.001) compared to those without (Table 1).
Table 3.
Associations between Having Siblings in Household and Microbiota Profiles
| Microbiota and models | RRR (95% CI) | P-value |
|---|---|---|
| Nasal microbiota | ||
| Unadjusted model | ||
| Moraxella-dominant profile | 11.6 (4.12–32.6) | <0.001 |
| Corynebacterium/Dolosigranulum-dominant profile | Reference | - |
| Mixed profile | 0.59 (0.14–2.51) | 0.48 |
| Adjusted model* | ||
| Moraxella-dominant profile | 18.9 (5.30–67.3) | <0.001 |
| Corynebacterium/Dolosigranulum-dominant profile | Reference | - |
| Mixed profile | 0.62 (0.14–2.85) | 0.54 |
| Sensitivity analysis*† | ||
| Moraxella-dominant profile | 6.05 (2.26–16.2) | <0.001 |
| Corynebacterium/Dolosigranulum-dominant profile | Reference | - |
| Mixed profile | 0.44 (0.11–1.75) | 0.24 |
| Fecal microbiota | ||
| Unadjusted model | ||
| Escherichia-dominant profile | Reference | - |
| Bifidobacterium-dominant profile | 2.32 (0.90–5.98) | 0.08 |
| Enterobacter-dominant profile | 1.26 (0.45–3.51) | 0.65 |
| Adjusted model‡ | ||
| Escherichia-dominant profile | Reference | - |
| Bifidobacterium-dominant profile | 2.90 (1.03–8.20) | 0.04 |
| Enterobacter-dominant profile | 1.50 (0.44–5.12) | 0.51 |
| Sensitivity analysis†‡ | ||
| Escherichia-dominant profile | Reference | - |
| Bifidobacterium-dominant profile | 1.98 (0.94–4.15) | 0.07 |
| Enterobacter-dominant profile | 1.24 (0.51–2.99) | 0.64 |
RRR, relative risk ratio; CI, confidence interval
Adjusted for age, sex, maternal antibiotic use during pregnancy, maternal smoking, and history of systemic antibiotic use in infancy
Modeling the number of siblings as ordinal variable
Adjusted for age, sex, maternal antibiotic use during pregnancy, maternal smoking, breast-feeding status, and history of systemic antibiotic use in infancy
Table 4.
Unadjusted Associations between Having Siblings in Household and Microbiota Profiles by Mode of Delivery*
| Groups and microbiota profiles | RRR (95% CI) | P-value |
|---|---|---|
| Nasal microbiota | ||
| Vaginal delivery (n=66) | ||
| Moraxella-dominant profile | 12.7 (3.31–48.5) | <0.001 |
| Corynebacterium/Dolosigranulum-dominant profile | Reference | - |
| Mixed profile | 0.25 (0.03–2.41) | 0.23 |
| C-section (n=39) | ||
| Moraxella-dominant profile | 10.3 (2.00–52.7) | 0.005 |
| Corynebacterium/Dolosigranulum-dominant profile | Reference | - |
| Mixed profile | 1.83 (0.22–15.3) | 0.58 |
| Fecal microbiota | ||
| Vaginal delivery (n=66) | ||
| Escherichia-dominant profile | Reference | - |
| Bifidobacterium-dominant profile | 1.59 (0.49–5.14) | 0.44 |
| Enterobacter-dominant profile | 1.09 (0.31–3.88) | 0.89 |
| C-section (n=39) | ||
| Escherichia-dominant profile | Reference | - |
| Bifidobacterium-dominant profile | 4.28 (0.80–22.9) | 0.09 |
| Enterobacter-dominant profile | 1.67 (0.28–9.82) | 0.57 |
RRR, relative risk ratio; CI, confidence interval
Multivariable-adjustment was not performed given the decreased sample sizes
Table 5.
Unadjusted Associations between Having Siblings in Household and Microbiota Profiles in 83 Infants Aged <6 Months by Feeding Status
| Groups and microbiota profiles | RRR (95% CI) | P-value |
|---|---|---|
| Nasal microbiota | ||
| Breast-feeding (n=61) | ||
| Moraxella-dominant profile | 21.0 (4.58–96.3) | <0.001 |
| Corynebacterium/Dolosigranulum-dominant profile | Reference | - |
| Mixed profile | 1.27 (0.18–8.79) | 0.81 |
| Formula-feeding (n=22) | ||
| Moraxella-dominant profile | 5.79 (4.06–8.24) | <0.001 |
| Corynebacterium/Dolosigranulum-dominant profile | Reference | - |
| Mixed profile | 7.39 (0.61–89.5) | 0.20 |
| Fecal microbiota | ||
| Breast-feeding (n=61) | ||
| Escherichia-dominant profile | Reference | - |
| Bifidobacterium-dominant profile | 2.17 (0.57–8.19) | 0.25 |
| Enterobacter-dominant profile | 1.60 (0.41–6.21) | 0.50 |
| Formula-feeding (n=22) | ||
| Escherichia-dominant profile | Reference | - |
| Bifidobacterium-dominant profile | 2.00 (0.18–22.01) | 0.57 |
| Enterobacter-dominant profile | 1.25 (0.12–13.2) | 0.85 |
RRR, relative risk ratio; CI, confidence interval
Multivariable-adjustment was not performed given the decreased sample sizes
All 105 fecal samples yielded high quality reads (rarefaction cutoff, 1,470 reads per sample). The sequencing identified 7 phyla and 108 genera. The fecal microbiota was composed primarily of three genera – Escherichia (23%), Bifidobacterium (20%), Enterobacter (17%) – followed by Bacteroides (9%) and Veillonella (6%). Partitioning around medoids clustering of fecal microbiota identified three distinct microbiota profiles: 1) Escherichia-dominant (31%), 2) Bifidobacterium-dominant (39%), and 3) Enterobacter-dominant (30%) (Figure 1b). The Bifidobacterium-dominant profile had the highest bacterial richness (P=0.005) and Shannon index (P=0.02; Table 6). The NMDS plots also demonstrated that the infants cluster together according to their fecal microbiota profile (Figure 2b). Additionally, there was a borderline significant association between nasal airway microbiota profiles and fecal microbiota profiles within subjects (P=0.07 by marginal homogeneity test; Table 7). Fecal microbiota profiles differed between infants with siblings and those without. For example, infants with siblings were more likely to have a Bifidobacterium-dominant profile than an Escherichia-dominant profile (49% vs. 24%; Table 1) while those without siblings had the opposite pattern (32% vs. 37%), corresponding to an adjusted RRR of 2.90 (95%CI, 1.03–8.20; P=0.04; Table 3). In the sensitivity analysis, infants with a higher number of siblings had a non-significantly higher RRR to have a Bifidobacterium-dominant profile (adjusted RRR per each incremental sibling, 1.98; 95%CI, 0.94–4.15; P=0.07). In the stratified analyses by mode of delivery (Table 4) and by feeding status (Table 5), while the statistical power is limited owing to the small sample sizes, the direction of association (i.e., the point estimates of RRR) did not change across the strata.
Table 6.
Richness, Alpha-diversity, and Relative Abundance by Fecal Microbiota Profile
| Escherichia-dominant profile n=33 (31%) | Bifidobacterium-dominant profile n=41 (39%) | Enterobacter-dominant profile n=31 (30%) | P-value | |
|---|---|---|---|---|
| Richness, median (IQR) | ||||
| Number of genera | 13 (10–20) | 17 (12–21) | 11 (9–15) | 0.005 |
| Alpha-diversity, median (IQR) | ||||
| Shannon index | 1.16 (0.82–1.72) | 1.62 (1.18–1.90) | 1.26 (0.97–1.61) | 0.02 |
| Relative abundance of 10 most common genera, mean (SD) | ||||
| Escherichia | 0.46 (0.29) | 0.20 (0.15) | 0.01 (0.05) | 0.003* |
| Bifidobacterium | 0.06 (0.08) | 0.38 (0.21) | 0.10 (0.12) | 0.003* |
| Enterobacter | 0.04 (0.09) | 0.06 (0.10) | 0.47 (0.24) | 0.003* |
| Bacteroides | 0.21 (0.25) | 0.03 (0.07) | 0.05 (0.13) | 0.003* |
| Veillonella | 0.06 (0.14) | 0.03 (0.05) | 0.12 (0.14) | 0.11* |
| Lachnoclostridium | 0.02 (0.04) | 0.04 (0.08) | 0.03 (0.09) | 0.99* |
| Clostridium sensu stricto 1 | 0.04 (0.10) | 0.01 (0.02) | 0.04 (0.05) | 0.89* |
| Streptococcus | 0.01 (0.02) | 0.04 (0.06) | 0.03 (0.07) | 0.89* |
| Enterococcus | 0.01 (0.03) | 0.03 (0.04) | 0.02 (0.04) | 0.99* |
| Akkermansia | 0.00 (0.00) | 0.02 (0.08) | 0.03 (0.11) | 0.99* |
Abbreviations: IQR, interquartile range; SD, standard deviation
Benjamini-Hochberg adjusted P-value accounting for multiple comparisons
Table 7.
Within-subject Association Between Nasal Airway Microbiota Profiles and Fecal Microbiota Profiles*
| Nasal airway microbiota profile (n) | Sum | ||||
|---|---|---|---|---|---|
| Moraxella-dominant profile | Corynebacterium/Dolosigranulum-dominant profile | Mixed profile | |||
| Fecal microbiota profile (n) | Escherichia-dominant profile | 10 | 13 | 10 | 33 |
| Bifidobacterium-dominant profile | 24 | 11 | 6 | 41 | |
| Enterobacter-dominant profile | 11 | 14 | 6 | 31 | |
|
|
|||||
| Sum | 45 | 38 | 22 | 105 | |
Marginal homogeneity test, P=0.07
DISCUSSION
In this study of 105 community-based healthy infants, we identified three nasal airway microbiota profiles and three fecal microbiota profiles. We also observed, both before and after adjusting for potential confounders, associations between having siblings in the household and two microbiota profiles: Moraxella-dominant nasal microbiota profile and Bifidobacterium-dominant fecal microbiota profile. Additionally, there was a non-significant association between nasal airway microbiota profiles and fecal microbiota profiles within the subjects.
Within the sparse literature on airway microbiota in infants, our findings are in agreement with those in the Childhood Asthma Study.26 By applying 16S rRNA gene sequencing on the nasopharyngeal samples from 234 Australian infants at high-risk of atopy, the authors reported that co-habiting with siblings was associated with higher abundances of Moraxella genus in the nasopharynx. This finding is of particular interest because a recent cohort study of 60 Dutch infants found that early (1.5 to 6 months) colonization by Moraxella in the upper airway is associated with a lower frequency of respiratory infection in early childhood,27 which is a major risk factor of incident asthma.28 Although the underlying mechanism remains to be elucidated, these findings collectively suggest that cohabiting with siblings and Moraxella-dominant airway microbiota profile in early childhood, via fewer lower respiratory infections thereby protecting against airway damage and remodeling during the critical window of lung development, lead to a lower risk of incident asthma. However, Bisgaard et al., by applying a culture-dependent approach on the hypopharyngeal aspirate from 312 healthy neonates in Denmark, found that colonization of the airways with M. catarrhalis (as well as S. pneumoniae or H. influenzae) was associated with a higher risk of incident asthma by age 5 years.29 These data, including the apparent discrepancies, underscore the importance of high-quality longitudinal studies examining the complex interplay between early-life environmental exposures, the airway microbiome, and asthma pathogenesis.
Our data also demonstrated that infants with siblings had a significantly lower bacterial richness and alpha-diversity index in the nasal airway. Although there has been only one study using a culture-independent approach that have examined the relationship between cohabiting siblings in the household and upper airway microbiota (the Childhood Asthma Study26), this previous study did not examine bacterial richness or alpha-diversity. Our novel finding is intriguing because it is generally considered that a higher bacterial richness and diversity is favorable. Yet, recent studies have demonstrated that a higher bacterial diversity may be associated with diseases.16,30,31 For instance, Huang et al. reported that patients with asthma had a higher bacterial richness and diversity in their airway when compared to healthy controls.30 These data collectively suggest that the structure of microbiota (and likely the function of microbiota), rather than bacterial richness or diversity in the airway niche, plays an important role in the development of respiratory diseases.
Previous studies on fecal microbiota in infants have demonstrated inconsistent associations between the presence of siblings and microbial composition – e.g., having siblings is associated with increased abundances of Bifidobacterium (similar to our finding),11,32 Faecalibacterium,9 and Bacteroides,33 and decreased abundances of Peptostreptococcaceae.10 Potential explanations for this inconsistency include differences in study population, setting, and laboratory techniques for microbial identification (e.g., culture,34 bacterial PCR32,33), or any combinations of these factors. Nevertheless, our data build on prior research linking the presence of siblings to the human microbiota, and extend them by examining both airway and fecal microbiota using the 16S rRNA gene sequencing approach in U.S. infants.
The mechanisms linking the presence of siblings in the household to unique microbiota profiles in infants is likely multifactorial. The original explanation was the exposure to microbes from older siblings through direct contact or intermediate reservoirs in the home environment (e.g., house dust and maternal microbiota).6 Alternatively, the presence of siblings may contribute, via in-utero immunological programming (“birth order effect”33), to perturbation of microbiota in infants. These two possibilities are not mutually exclusive.
Our study has potential limitations. First, the cross-sectional analysis was unable to examine the association between the microbiota and the development of childhood asthma. To address this important question, the children are being followed longitudinally, with airway and fecal sampling at multiple time-points. Second, airway microbiota was examined in nasal airway samples since lower airway sampling in asymptomatic infants is both technically and ethically challenging. Nevertheless, prior research has reported a strong correlation between upper and lower airway microbiology in children.35 In addition, one may surmise that the anterior nasal swab sampling is susceptible to contamination with skin microbiota compared to “deeper” sampling methods (e.g., nasopharyngeal aspirates), our samples demonstrated that Moraxella genus was the most abundant genera and that Staphylococcus genus accounted for only 9% of microbiota. The observed relative abundance of Staphylococcus was also consistent with the recent study of Australian infants – Staphylococcus accounted for 10% of their nasopharyngeal microbiota.26 Therefore, it is unlikely that potential contamination with skin microbiota would have substantially affected our results. Third, 16S rRNA gene sequencing precluded us from assessing the functional capacity of microbiota. We hope to address this important issue in future work using metagenomic and metatranscriptomic approaches. Fourth, our inferences might be biased by unmeasured confounders (e.g., use of complementary food in older infants). In addition, because of the relatively small number of subjects within microbiota profiles, we were unable to adjust for all sets of potential confounders that were measured in the study. However, the significant associations persisted after controlling for clinically and biologically important covariates. Finally, this single-year study recruited infants from one hospital, which may limit our generalizability to other settings.
Conclusions
In summary, in this cross-sectional study of community-based healthy infants, we identified three nasal airway microbiota profiles and three fecal microbiota profiles. We also found that infants living with siblings are more likely to have a Moraxella-dominant nasal microbiota profile and a Bifidobacterium-dominant fecal microbiota profile. Our findings should facilitate further epidemiologic and mechanistic investigation of the interplay between early-life environmental exposures, the microbiome, and asthma pathogenesis, which, in turn, could offer a new avenue (e.g., microbiome modification) for the primary prevention of childhood asthma.
Acknowledgments
This study was supported by the grants U01 AI-087881, R01 AI-114552, R01 AI-108588, and R21 HL-129909 from the National Institutes of Health (Bethesda, MD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Pedro A. Piedra, MD (Baylor College of Medicine), Ashley F. Sullivan, MS, MPH (Massachusetts General Hospital), the staff at the Massachusetts General Hospital Pediatric Group Practice, and all of the study families for their contributions to the study.
Footnotes
Competing Interests: Drs. Ajami and Petrosino own shares of Diversigen Inc., a microbiome research company. The other authors have no financial relationships relevant to this article to disclose.
Authors’ Contributions: KH carried out the statistical analysis, drafted the initial manuscript, and approved the final manuscript as submitted. RWL conceptualized and designed the study, enrolled the subjects, drafted the initial manuscript, and approved the final manuscript as submitted. JMM and CAC conceptualized and designed the study, obtained the funding, reviewed and revised the manuscript, and approved the final manuscript as submitted. NJA and JFP generated the microbiome data, carried out the initial statistical analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. RGF was involved in the patient enrollment, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. JAE carried out the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
References
- 1.Strachan DP, Ait-Khaled N, Foliaki S, et al. Siblings, asthma, rhinoconjunctivitis and eczema: a worldwide perspective from the International Study of Asthma and Allergies in Childhood. Clin Exp Allergy. 2015;45(1):126–136. doi: 10.1111/cea.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu P, Feldman AS, Rosas-Salazar C, et al. Relative Importance and Additive Effects of Maternal and Infant Risk Factors on Childhood Asthma. PLoS One. 2016;11(3):e0151705. doi: 10.1371/journal.pone.0151705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma? A review. J Epidemiol Community Health. 2002;56(3):209–217. doi: 10.1136/jech.56.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343(8):538–543. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 6.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nature Rev Immunol. 2010;10(12):861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 7.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135(1):25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laursen MF, Zachariassen G, Bahl MI, et al. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol. 2015;15:154. doi: 10.1186/s12866-015-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad MB, Konya T, Maughan H, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Imuunol. 2013;9(1):15. doi: 10.1186/1710-1492-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap GC, Chee KK, Hong PY, et al. Evaluation of stool microbiota signatures in two cohorts of Asian (Singapore and Indonesia) newborns at risk of atopy. BMC Microbiol. 2011;11:193. doi: 10.1186/1471-2180-11-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsland BJ, Trompette A, Gollwitzer ES. The Gut-Lung Axis in Respiratory Disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150–156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K, Linnemann RW, Avadhanula V, et al. Detection of respiratory syncytial virus and rhinovirus in healthy infants. BMC Res Note. 2015;8(1):718. doi: 10.1186/s13104-015-1695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GM, Salomon JA, Friedman JF, et al. Illness transmission in the home: a possible role for alcohol-based hand gels. Pediatrics. 2005;115(4):852–860. doi: 10.1542/peds.2004-0856. [DOI] [PubMed] [Google Scholar]
- 15.Lambert SB, Ware RS, Cook AL, et al. Observational Research in Childhood Infectious Diseases (ORChID): a dynamic birth cohort study. BMJ Open. 2012;2(6) doi: 10.1136/bmjopen-2012-002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa K, Linnemann RW, Mansbach JM, et al. The fecal microbiota profile and bronchiolitis in infants. Pediatrics. 2016 doi: 10.1542/peds.2016-0218. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Human Microbiome Project C. A framework for human microbiome research. Nature. 2012;486(7402):215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 21.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Method. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 22.Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic acids research. 2013;41(Database issue):D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J Royal Stat Soc. 2001;63(2):411–423. [Google Scholar]
- 25.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teo Shu M, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;13(17):704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biesbroek G, Tsivtsivadze E, Sanders EA, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190(11):1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa K, Mansbach JM, Camargo CA., Jr Infectious pathogens and bronchiolitis outcomes. Exp Rev Anti Infect Ther 4. 2014;12(7):817–828. doi: 10.1586/14787210.2014.906901. [DOI] [PubMed] [Google Scholar]
- 29.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 30.Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–381. e371–373. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 33.Penders J, Gerhold K, Stobberingh EE, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132(3):601–607. e608. doi: 10.1016/j.jaci.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 34.Adlerberth I, Strachan DP, Matricardi PM, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120(2):343–350. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184(8):957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]