Abstract
Purpose
Explore the potential benefits of using priming methods prior to an active hand task in the acute phase post-stroke in persons with severe upper extremity hemiparesis.
Methods
Five individuals were trained using priming techniques including virtual reality (VR) based visual mirror feedback and contralaterally controlled passive movement strategies prior to training with an active pinch force modulation task. Clinical, kinetic, and neurophysiological measurements were taken pre and post the training period. Clinical measures were taken at six months post training.
Results
The two priming simulations and active training were well tolerated early after stroke. Priming effects were suggested by increased maximal pinch force immediately after visual and movement based priming. Despite having no clinically observable movement distally, the subjects were able to volitionally coordinate isometric force and muscle activity (EMG) in a pinch tracing task. The Root Mean Square Error (RMSE) of force during the pinch trace task gradually decreased over the training period suggesting learning may have occurred. Changes in motor cortical neurophysiology were seen in the unaffected hemisphere using Transcranial Magnetic Stimulation (TMS) mapping. Significant improvements in motor recovery as measured by the Action Research Arm Test (ARAT) and the Upper Extremity Fugl Meyer Assessment (UEFMA) were demonstrated at six months post training by three of the five subjects.
Conclusion
This study suggests that an early hand-based intervention using visual and movement based priming activities and a scaled motor task allows participation by persons without the motor control required for traditionally presented rehabilitation and testing.
Keywords: Acute phase, cortical priming, virtual reality-augmented rehabilitation, cerebral vascular accident, flaccid upper extremity
Introduction
Approximately, 795,000 new or recurrent strokes occur each year in the United States.[1] Furthermore, stroke is one of the leading causes of long-term disability in the United States.[1] Proportionally more stroke survivors are left with upper extremity impairment and disability than lower extremity.[2] This may be due to greater emphasis placed on training ambulation for early mobilization, or due to more complex and multi-joint movements required by the upper extremity to interact with the environment.[3,4] In a population with mixed severity of stroke, 78% had not reached age matched upper extremity function at three months post-stroke,[5] and in severely affected individuals, only 11.6% had complete functional recovery at six months post-stroke.[6] Specifically with regards to hand function, grasp efficiency was found to be more impaired than proximal function at one year in individuals with mild to moderate stroke, with a possible explanation that alternate descending pathways such as the ipsilateral cortical and the reticulospinal tracts cannot compensate for distal fine motor control as well as they can compensate for proximal motor control.[7] Recovery of lateral corticospinal connections is needed for return of isolated distal hand function.[8]
Unfortunately, despite research in humans and animals showing that most impairment based recovery after ischemic stroke occurs in the first 1–3 months,[9] the majority of neuro-rehabilitation studies are conducted in the chronic phase.[10,11] Additionally, most of these studies enroll subjects with some active movement of the affected upper extremity.
To our knowledge, there have been only three groups that have studied virtual reality (VR) training in the acute/sub-acute phase post-stroke. Two of the three studies used dose-matched controls and noted significant improvement favoring the treatment group in upper extremity function, speed, and impairment.[12–14]
Importantly, none of these studies investigated the rehabilitation of individuals with flaccid or severely paretic upper extremities. This population is challenging because they are limited in the therapeutic tasks in which they can actively participate. Consequently, emphasis is placed on teaching compensatory movements to accomplish necessary functional tasks [10] that can lead to “learned nonuse” – a phenomenon in which the individual tapers use of the affected limb. Learned nonuse has been shown to hinder the ultimate recovery of function in the impaired limb.[15] We surmise that tasks that engage the affected extremity early after stroke, however small that engagement is, may reduce the degree of learned nonuse and potentially maximize the recovery process.
After stroke it is hypothesized that there is an imbalance in inter-hemispheric inhibitory drive and motor cortex (M1) excitability, most notably reflecting a decrease in the excitability of ipsilesional M1. These changes have been associated with reduced functional recovery.[16,17] Proof of principle studies in humans has shown that restoring this balance is associated with better outcomes.[17]
In individuals with severe paralysis in the acute phase, priming the motor system using techniques such as somatosensory input, visual feedback, repetitive TMS, or movement-based strategies might stimulate plastic reorganization (e.g., rebalancing inter-cortical asymmetries in activation and activating appropriate circuitries) and thereby promote improved motor outcomes.[16,18] One form of visual priming is VR based mirror feedback. For this, the subject watches a computer screen and moves their unaffected hand while a virtual hand representing their affected hand is actuated in real time by this movement. Beneficial effects of visual priming have been found in both healthy and stroke affected individuals. In a healthy population, ipsilateral M1 corticospinal excitability (as measured by TMS) was facilitated when unilateral hand movement was fed back visually via a regular mirror box setup.[19] Parallel results were found when visual mirror feedback was paired with repetitive motor training in a healthy group, and this facilitation was associated with improved motor performance of the untrained hand.[20,21] Similar effects have been demonstrated in persons with stroke. A study that compared VR based mirror feedback to regular mirror therapy in a sample of subjects with mild to moderate upper extremity hemiparesis found increased corticospinal excitability of the ipsilesional M1 in both treatment groups; with the VR based method showing the larger change in MEP amplitude and latency.[22] However, it is important to note that this single study does not provide definitive support that VR based visual priming is superior to traditionally presented visual priming. With regards to neural correlates, our group has demonstrated in chronic stroke that virtual mirror training activates regions in the sensory-motor cortex similar to those activated by volitional movement of the affected hand, suggesting that this type of feedback may facilitate regions that are relevant for motor control in individuals with moderate hemiparesis post-stroke.[23] Taken together, these data suggest that VR based visual mirror feedback may be a useful priming tool to increase the excitability of and sensitivity to rehabilitation activities in desired brain networks in individuals post-stroke, and perhaps aid in functional recovery of the affected arm.
Priming can also be induced using contralaterally controlled passive movement. As an example, Byblow and colleagues [24] used a method they called Active Passive Bilateral Therapy using a device that couples both hands such that active movement of one wrist produces mirror symmetric movements of the opposite wrist. A similar method was used previously by Boos et al. [25] Movement priming was found to facilitate corticomotor excitability for ≥30 min in the passive hemisphere in healthy subjects. When used with individuals post-stroke, this is hypothesized to create a period of time where plastic reorganization may be facilitated within the affected motor cortex.[24]
In another study, active passive bilateral therapy was used in individuals with chronic stroke, starting with purely passive movement of the affected hand and progressing to active movement as able. The results showed improved and sustained upper extremity function of the affected hand, increased ipsilesional M1 excitability, increased transcallosal inhibition from the ipsi- to the contralesional M1, and increased intracortical inhibition within contralesional M1.[16] More recently, the same group used this method in the sub-acute phase and noted similar neurophysiological changes along with accelerated time to functional recovery.[26]
The challenging task of rehabilitating individuals with severely affected distal upper extremities, in addition to the limited amount of prior research conducted in the acute phase using these types of techniques, makes this a ripe time to ask whether a combination of VR based visual mirror and proprioceptive/movement-based priming prior to a scaled active training task may be feasible and beneficial for those in the acute phase post-stroke and with severe paresis (Stage 1 on Hand and Arm Impairment Inventory of the Chedoke-McMaster Stroke Assessment [27]).
Specifically, this study aims to uncover the strengths and weaknesses of using visual and movement based priming techniques prior to a force modulation task in addition to usual care in persons with flaccid hemiplegia in the acute phase post-stroke. Additionally, clinical measures at six months will allow us to evaluate recovery patterns over time. This study will also elucidate the requirements for a future randomized controlled trial. We hypothesize that this unique combination of priming and training will allow for meaningful active participation in distal motor training despite severe paralysis that would render active participation with other training modalities impossible. Furthermore, we hypothesize that the hand-focused intervention will be feasible and well tolerated by individuals with severe paresis in the first month post stroke. Finally, we propose that any changes in motor recovery will be associated with changes in cortical neurophysiology as assayed via TMS mapping.
Methods
Participants and protocol
Participants
Five subjects were recruited from an acute rehabilitation department of a suburban hospital. After initial screening by the department’s physician, a physical therapist screened subjects based on the following criteria: (1) within one month post-stroke, (2) between the ages of 30 and 80, and (3) with severe upper extremity paresis (Stage 1 on Hand and Arm Impairment Inventory of the Chedoke-McMaster Stroke Assessment [27]). Exclusion criteria included: (1) severe spasticity (Modified Ashworth score of 3 or greater [28]), (2) cognitive deficits rendering them unable to follow three step commands or attend to a task for at least 10 min (based on review of the Speech Therapist’s evaluation using the Montreal Cognitive Assessment [29]), (3) hemi-spatial neglect rendering them unable to interact with an entire 24 inch computer screen (based on review of the physiatrist’s admission evaluation), (4) proprioceptive loss that renders potential subjects unable to interact with a virtual environment without looking at their hands (tested clinically by the physical therapist), and (5) unstable blood pressure and oxygen saturation responses to activity. Between January 2015 and October 2015, 80 subjects were screened by a physical therapist and five met the above criteria. Subjects ranged in age from 51 to 66 years (58.2 SD 6.91). Four of five subjects had subcortical lesions. Their initial Upper Extremity Fugl-Meyer Assessment (UEFMA) [30] scores ranged from 2 to 6 and represented reflexive and active scapular movement only (Table 1). All subjects provided written consent prior to participating in this study.
Table 1.
Demographic and baseline data.
| UEFMA Pre
|
Chedoke Pre
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age | Days Post-stroke | Lesion Site | Prox | Distal | Arm | Hand |
| S1 | Male | 55 | 9 | L Internal Capsule | 2 | 0 | 1 | 1 |
| S2 | Male | 51 | 27 | L Basal Ganglia | 6 | 0 | 1 | 1 |
| S3 | Male | 53 | 7 | R Frontal and Parietal | 4 | 0 | 1 | 1 |
| S4 | Female | 65 | 5 | L Pons | 3 | 0 | 1 | 1 |
| S5 | Male | 66 | 16 | R Pons | 4 | 0 | 1 | 1 |
Protocol
All subjects were projected to receive eight 1-h training sessions over a two-week period. All received eight sessions except S5 (received seven sessions) due to early discharge. All subjects received 15 min of each of the two priming methods (60–120 repetitions per priming protocol depending on their speed of movement) followed by 15 min of training using the force modulation pinch trace task. In addition, subjects received their standard in-patient rehabilitation program (which included positioning, range of motion, supportive devices, and functional electrical stimulation to the affected upper extremity).
Training systems and simulations
System
This VR environment was developed with Virtools 4.0 software package (Dassault Systemes, Vélizy-Villacoublay, France) and a VRPack Plug-in that communicated with an open source Virtual Reality Peripheral Network (VRPN) interface.[31]
Priming tasks
The priming involves two simulations. The first is a visual priming task consisting of a flexion task of the unaffected index finger without coupled passive movement of the affected hand. The second is a movement based priming task consisting of an extension task of the unaffected hand coupled to movement of the affected hand via an exoskeleton. The flexion task was chosen because our group has demonstrated in chronic stroke that this type of virtual mirror training activates regions in the sensory-motor cortex similar to those activated by volitional movement of the affected hand.[23] This is followed by the extension task which incorporates contralaterally controlled passive movement as a priming technique.[24] These two tasks were chosen to increase cortical excitability of the hand area of M1 immediately prior to the scaled active pinch force modulation task. This sequence was followed for all subjects.
Flexion Task Without Exoskeleton (visual based priming) [23]
The subjects wore a CyberGlove™ (Immersion, San Jose, CA) on the unaffected hand to track finger angles. They watched a computer screen that shielded their hands from view and displayed virtual feedback of the affected hand in neutral position in a first-person view. Subjects performed flexion/extension with their non-affected index finger. The goal of the movement was to flex their unaffected index finger to align the virtual finger to a visual line target. Although the subjects only moved their non-affected index finger, the visual feedback presented to them in real time appeared as if they were moving their affected finger to the goal target. The subjects spent 15 min on this priming method (60–120 repetitions depending on their speed of movement) (Figure 1).
Figure 1.
View of the computer screen from the subject’s perspective during the visual mirror flexion task.
Extension Task With Exoskeleton (movement based priming)
[25]: In addition to the above hardware, the subjects wore a CyberGrasp™ (Immersion, USA) on the affected hand that was actuated into extension by motion of the unaffected hand (e.g., the exoskeleton moved in synchrony with the unaffected hand while the subject hit a virtual ball with this hand). The exoskeleton is lightweight, fits over the hand, and assists hand extension via a system of cables affixed to the distal fingers. The amount of assistive force on the affected hand is proportional to the opening angle of the unaffected hand (Figures 2 and 3). This simulation was performed after the flexion task and all subjects spent 15 min on this task (60–120 repetitions depending on their speed of movement).
Figure 2.
View of the CyberGrasp™ (Immersion, USA) on the affected hand.
Figure 3.
View of the computer screen from the subject’s perspective during the mirror extension task.
Training intervention
Pinch Trace Task
For the training task, we have chosen an isometric pinch force task to control the vertical motion of a cursor in order to trace a sinusoidal wave on a computer screen. The advantage of this task is that the instrumentation allows for calibrating the force demands to accommodate minimal voluntary movements available to the subjects while still preserving meaningful visual feedback.[32] This incorporates principles of motor learning to enhance its efficacy as a training tool. This task was used as both a training task and an outcome measure (see below). Pinch force was measured with an ATI Nano17™force sensor (ATI Industrial Automation, Apex, NC). Subjects pinched the sensor placed between the index and thumb of the affected hand in order to control the vertical displacement of a cursor along a sinusoid that was moving along the horizontal dimension of a computer screen. Subjects’ finger position on the force sensor was inspected throughout the session to ensure that they pinched with the thumb and index finger, and did not compensate by using alternate strategies. Subjects spent 15 min on this training task.
Outcome measures
Clinical assessments
A physical therapist performed the UEFMA, the Chedoke-McMaster Stroke Impairment Inventory Stage of the arm and hand, and the Action Research Arm Test (ARAT) [33] at baseline, prior to each training session, immediately post intervention, and again six months after the intervention. The UEFMA and the Chedoke-McMaster Inventory measures were chosen as both can be used with people who have severe paresis and are recommended for use in the acute and sub-acute phase post stroke (APTA – StrokEdge).
Kinetic-pinch force and trace
Pinch force was used as an outcome measure because the sensitivity of the load sensor used is such that it can detect minute levels of force and therefore it is sensitive to small changes in motor function which could not be detected using clinical measures.
Pinch force measures the maximum voluntary force a subject can exert on a force sensor held between their paretic thumb and index finger. Larger numbers indicate stronger pinch force. Subjects were given two attempts and the largest number was used.
Pinch trace measures the ability to control pinch force between 0% and 50% of maximum pinch force in order to control the vertical motion of a cursor tracking a horizontal sine wave (duration of 1 cycle ≈ 6 s, period = 0.15 Hz) on a computer screen. Force data was collected at 100 Hz over the 4-min trace. RMSE is a measure of the difference between a sinusoid and the force values the subject actually generates. It is normalized by maximum force because the amplitude of the sinewave is determined by the maximum force generated during calibration prior to each training session. These values are very different between subjects and within each subject per training day and therefore are normalized by this maximum force calibration value in order to allow comparison between subjects and across days.
The formula is as follows:
where Force Model is the sinusoid that the subject was required to trace, Actual Force is the force generated by the subject and n is the number of samples collected over one trial. Smaller RMSE values indicate better performance.
Electromyographic (EMG) assessment
Surface EMG was recorded at 2 kHz (Delsys Trigno, Natick, MA) from the First Dorsal Interosseous (FDI) muscle, the Abductor Pollicis Brevis (APB) muscle, the Flexor Digitorum Superficialis (FDS) muscle, and the Extensor Digitorum Communis (EDC) muscle of the affected upper extremity while the subjects performed the pinch force and trace task. All data were imported into Matlab (The Mathworks, Inc., Natick, MA) for custom processing and analysis. EMG data were filtered at 20–300 Hz, full wave rectified and a Root Mean Square average was applied with a 50 ms time window.
Transcranial magnetic stimulation (TMS) mapping
To assay the neurophysiological underpinnings of performance and recovery, we measured the EMG activity of hand muscles during the pinch trace task, and mapped the motor evoked potentials (MEPs) bilaterally (using TMS) pre/post the training period.
In subjects one to four, topographic representations of finger-hand muscles were mapped bilaterally pre and post training. Motor evoked potentials were recorded from surface electrodes (Delsys Trigno, 2 kHz sampling) placed on the FDI, extensor indicis longus [EI], APB, abductor digiti minimi [ADM], flexor digitorum superficialis [FDS], and EDC muscles of the limb contralateral to the stimulated hemisphere. Subjects were seated with their arms comfortably positioned in front of them and EMG was monitored to ensure that the upper extremity was at rest. Frameless neuro-navigation (Advanced Neuro Technology) was used to monitor and record TMS coil (Magstim Rapid2, 70 mm AFC coil) position during and across sessions. All stimulation was conducted with the TMS coil held tangential to the scalp with the posterior handle 45° off the sagittal plane.[34] Mapping began by identifying the site at which the minimal stimulator output produced the strongest consistent MEPs in the contralateral FDI muscle (motor “hotspot”). Following determination of the FDI hotspot, resting motor threshold (RMT) was calculated as the minimum stimulus intensity required to elicit MEPs >50 μV in the FDI muscle for 50% of six consecutive trials.[35] A 10 × 10 cm area surrounding the motor hotspot was marked using the neuronavigation software to provide consistent map boundaries across sessions. If a reliable hotspot for the lesioned hemisphere could not be determined due to the absence of MEPs, the hotspot and map boundaries from the contralesional hemisphere were mirrored to the lesioned side and mapped at 100% of stimulator output. All mapping was performed with the subject at rest and stimulation intensity set to 110% of the determined RMT.[36] TMS pulses were delivered within the bounds with special attention paid to regions surrounding the hotspot territory.[37] For each stimulation point MEPs were calculated as the peak-to-peak amplitude of the filtered (2nd order Butterworth filter, 5–250 Hz band-pass) EMG signal 20–50 ms after the TMS pulse. MEP amplitudes were interpolated to a 10 × 10 cm mesh of 5 mm resolution centered on the M1 hotspot, using cubic surface interpolation.[38,39] Map area, the extent of the representation producing corticospinal output, was calculated as the product of the number of interpolated scalp sites eliciting MEPs >50 μV and the map resolution (0.25 cm2).[38,40–43]
Results
Five subjects were recruited for this study. They received seven or eight sessions (45 min sessions) of training. Each subject participated in the two priming tasks (visual and movement based) and one training task (pinch force modulation) at each session. All training was well tolerated without adverse side effects such as fatigue. Furthermore, the subjects were able to understand the simulations despite having no prior instructions.
Maximum pinch force pre and post priming
Maximum pinch force of the affected hand was measured pre and immediately post priming during each training session. This was used as a proxy measure of excitability changes induced from the priming. Table 2 shows the mean change (with SEM) per person. Four of the five subjects demonstrated a mean increase in change in pinch force following priming. This may reflect the hypothesized increase in excitability caused by the two priming tasks. These changes are small and would not be considered clinically significant but they may represent small positive changes in motor function elicited by a 30 min priming intervention.
Table 2.
Mean of the change in maximum force pre to post priming per subject.
| S1 | S2 | S3 | S4 | S5 | |
|---|---|---|---|---|---|
| Mean of change in maximum force | 0.042 N | −0.016 N | 0.006 N | 0.34 N | 2.9 N |
| SEM | 0.007 N | 0.17 N | 0.032 N | 0.235 N | 1.878 N |
Pinch trace task performance and EMG relationship
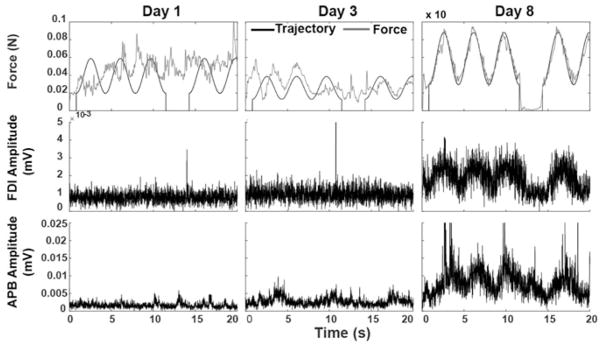
The pinch trace training task allowed active participation by these individuals despite having no observable voluntary movement of the affected hand. Figure 4 is a representative example of one subjects change in force and control over the training period while continuing to have a clinically flaccid hand. For example, initially on day 1 of training, S1 had no active hand movement and was unable to actively modulate pinch force to track a sine wave. Despite this, EMG demonstrated some minimal muscle activation of the APB muscle (Figure 4, left panel). On day 3, the same subject continued with a “flaccid” hand, however showed improved tracking ability with corresponding improvement in APB muscle EMG amplitude and pattern (Figure 4, middle panel). With continued training, this improvement increased to the last day with both APB and FDI muscles showing corresponding EMG activation (Figure 4, right panel). Subjects S2, S4, and S5 also demonstrated improved performance on this force modulation task with corresponding improvements in FDI and APB activation as measured by EMG.
Figure 4.
Force and EMG relationship for S1.
Four of the five subjects demonstrated a decrease in RMSE during the pinch trace task over time. Subject 1 started at 0.206 on day 1 and ended at 0.047 on day 8. Subject 2 started at 0.259 on day 1 and ended at 0.16 on day 8. Subject 4 started at 0.41 on day 1 and ended at 0.16 on day 8. Subject 5 started at 0.209 on day 1 and ended at 0.031 on day 7. Subject 3 could not perform the task adequately at any time point (Figure 5). The gradual decrease in RMSE over the course of the intervention demonstrated by these four subjects may reflect task learning despite their poor levels of overall motor performance.
Figure 5.
Normalized RMSE over time in days for four subjects. Subject 3 was unable to perform the task adequately therefore RMSE could not be calculated.
Maximum pinch force measured pre and post training
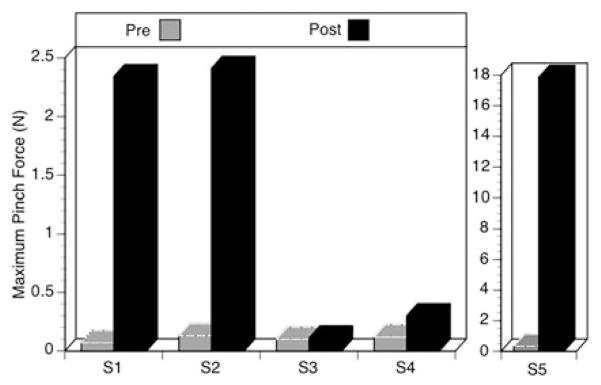
Maximum pinch force was also measured at testing sessions pre and post training. Figure 6 indicates that four of the five subjects generated an increased pinch force after the training. The maximum pinch force the first two subjects could generate increased over time from 0.07 N to 2.34 N for S1 and 0.13 N to 2.41 N for S2. S3 did not make any gains (0.10 N to 0.11 N) The last two subjects also made gains, 0.12 N to 0.30 N for S4, and 0.32 N to 17.86 N for S5 (Figure 6).
Figure 6.
Maximum pinch force pre and post training per subject. Two different graphs shown as two different scales were required.
Clinical tests measured pre/immediately post training and six months post training
Pre and post training
S1 started with a score of 2/66 at onset on the UEFMA and progressed to 29/66 by the end of training. These scores represented reflex activity initially and mostly proximal movement with some partial grasp and hand opening at the end of training. S1’s Chedoke-McMaster scores improved for both the arm and hand with more gains made proximally. Their ARAT score was a 0 at onset and improved to a 6/57 reflecting proximal movement. S2 had an initial score of 6/66 on the UEFMA reflecting reflex and proximal scapular movement. After training this improved to 13/ 66 and again the majority of gains were made proximally. S2’s Chedoke-McMaster scores improved minimally for both the arm and hand scores. Their ARAT score only improved to 3/57 after training and again this represented minimal active proximal movement. S3 scored 4/66 on the UEFMA initially (reflex and scapular movement) and made only proximal gains by the end of training. Similar gains were seen on the Chedoke-McMaster assessment. As well, their ARAT score improved minimally to a 3/57 post training. S4’s initial UEFMA score was 3/66 and improved to 9/66. This subject’s Chedoke-McMaster score changed from 1 on both the hand and arm sections initially to a 2 on the arm section post. Their ARAT score improved minimally to a 3/57 after intervention. This subject had only reflex and scapular movement at onset and made only proximal gains. Finally, S5 started with a 4/66 on the UEFMA (reflexive and scapular movement) and progressed to a 9/66. Their Chedoke-McMaster scores changed from 1 to 2 on both the arm and hand sections and their ARAT improved minimally to 3/57 (Table 3).
Table 3.
Clinical measurements.
| Chedoke arm
|
Chedoke hand
|
UEFMA proximal
|
UEFMA distal
|
ARAT
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Pre | Post | Six months | Pre | Post | Six months | Pre | Post | Six months | Pre | Post | Six months | Pre | post | Six months |
| S1 | 1 | 5 | 5 | 1 | 3 | 4 | 2 | 21 | 21 | 0 | 8 | 23 | 0 | 6 | 33 |
| S2 | 1 | 2 | 2 | 1 | 3 | 3 | 6 | 11 | 14 | 0 | 2 | 3 | 0 | 3 | 3 |
| S3 | 1 | 3 | 5 | 1 | 1 | 4 | 4 | 10 | 22 | 0 | 0 | 22 | 0 | 3 | 26 |
| S4 | 1 | 2 | 4 | 1 | 1 | 5 | 3 | 9 | 28 | 0 | 0 | 20 | 0 | 3 | 35 |
| S5 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 8 | 18 | 0 | 1 | 4 | 0 | 3 | 3 |
Six months after training
All participants made gains clinically at six months after training with subjects 1, 3, and 4 showing the greatest functional and impairment based recovery (Table 3).
TMS maps
TMS motor maps of the non-lesioned side for subjects 1–4 showed an increase in area for the FDS and EDC muscles from pre to post training (Figure 7). No MEPs were demonstrated at the lesioned hemisphere for any muscles tested at both time frames (pre and post training) in all four subjects. This increase in motor map representations in the contralesional M1 may reflect early cortical reorganization required to support the impaired hand’s function as this has been shown to correlate with improving upper extremity function in this early time post-stroke.[44]
Figure 7.
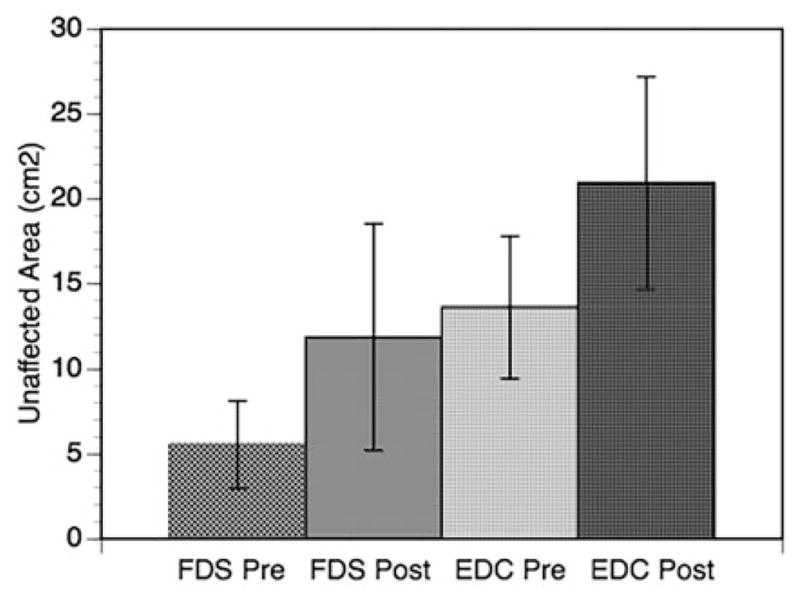
TMS motor maps pre and post training for FDS and EDC muscles for subjects 1–4 (the group average area is shown with standard error of the mean).
Discussion
There are several relevant findings from this group of subjects that provide evidence for further study. First, it may be possible to develop an intervention specifically targeted to the hand of persons with a clinically flaccid arm. Second, although this study is conducted in the very early phase post-stroke, the subjects were able to tolerate an additional 45 min of hand therapy. Third, despite having an initially flaccid upper extremity at onset, several subjects demonstrated important clinical gains at six months after training. Lastly, results from the pinch trace task demonstrated that even when subjects had no clinically measurable or observable active movement of the hand, there was some initial minimal muscle activation that could be detected by an instrumented force sensor and EMG. Notably, the force and EMG modulation improved over time (Figure 4). It may be the case that visual feedback provided by the pinch trace task increased the salience and motivation as subjects could unequivocally see that they were able to actively control the cursor despite “not being able to move their fingers”. A 2012 feasibility study using VR based gain scaling in the sub-acute phase post-stroke demonstrated that this method led to improved functional status in six subjects.[45] Furthermore it is known that increased salience of the training activity enhances neuroplasticity in both animals and humans.[46]
In the past, VR based visual mirror simulations in chronic stroke subjects have shown to activate brain areas that partially overlap those that produce active movement of the affected arm.[23] Furthermore, a study by Kang et al.[22] demonstrated greater cortical excitability of the affected hemisphere using VR based visual mirror feedback as compared to a mirror box or control. Although there is no direct evidence, it is interesting to consider that the increase in maximal pinch force in the affected hand after the priming tasks for four of the five subjects may have been related to an increase in ipsilesional cortical excitability induced by these tasks. It has been shown that priming techniques may promote plastic reorganization in response to subsequent motor training, and mirror therapies as well as the movement-based/proprioceptive task, have been suggested as priming techniques.[16,18] We hope that our priming interventions will lead to increased neural activity of the damaged cortex and promote more effective brain reorganization, which will perhaps facilitate optimal motor recovery.
Although small, the increase in maximum force noted for four of the subjects suggested an improvement in motor output in the hand over time (Figure 6). Additionally, the overall decrease in RMSE seen for the pinch trace task over the first few days with four of the subjects suggests that learning might be occurring (Figure 5). However, we acknowledge that this could be due to becoming familiar with a new task rather than true motor learning.
In this study, the pinch trace task was used both as a training method and an outcome measure. It demonstrated the benefits of objective, technologically based measurement tools. It has been noted previously that standard clinical measures used in clinical trials appear to have floor and ceiling effects and may not be sensitive to continuous change post-stroke, whereas objective measures such as kinematics and kinetics can measure small changes in motor ability without these effects in either direction.[47] The superior ability to detect small changes was demonstrated by this objective measure, as the force trace was able to detect change in motor output to a greater extent than the UEFMA. For example, S1 made distal gains in his UEFMA score however changes at the hand were only detectable with this clinical test once observable movement was present while the force sensor detected change starting on day 1.
TMS maps were obtained for four of the subjects and demonstrated an increase in map area for the FDS and EDC muscles in the unaffected hemisphere from pre to post testing. Using fMRI, Rehme et al. [44] found additional recruitment of the unaffected M1 and premotor areas over the first two weeks post acute stroke in their severely impaired subgroup that was correlated with functional recovery of the affected arm (measured with the ARAT and maximum grip force). They suggested that this reflected stroke induced early reorganization that might represent an enhanced effort by severely impaired individuals to move their affected side, and support arm function during this acute time. A similar reorganization in the unaffected hemisphere may be occurring in the subjects in this current study and merits further investigation.
Finally, establishing rehabilitation prognoses for persons with upper extremity hemiplegia has been a topic of discussion for many years. It has been suggested that persons with minimal active movement of the arm and hand be taught compensation techniques emphasizing the use of the unimpaired upper extremity.[48] More recently, Stinear et al.,[49] suggested that people post stroke without active shoulder abduction or finger extension (tested at 72 h post event) and without MEPs at two weeks post-stroke have “limited or no predicted potential for upper extremity recovery” at 12 weeks after stroke. None of the five subjects in this study demonstrated active shoulder abduction or finger extension at pretest (tested at a mean of 11.6, range 5–27 days after CVA). In addition, none demonstrated lesioned hemisphere MEPs at the extensor carpi radialis muscle during pre or post training evaluations. Contrary to their findings, three of our five subjects showed good gains in all clinical measures made at six months after training (Chedoke Inventory arm and hand progressed to 4–5, the UEFMA improved to a range of 44–48/66, and their ARAT scores increased to 26–35/57). These findings suggest that there could be potential for recovery and functional use of the upper extremity in those with severe impairment at onset that persists beyond two weeks post-stroke, and that continued attempts to develop evaluation and rehabilitation techniques to identify and maximize this potential are indicated.
In the future, we plan to conduct a larger scale randomized controlled trial to determine whether this type of early intervention changes motor and neural recovery differently as compared to usual acute rehabilitation. In monkeys, it was shown that early, skilled training of the hand post infarct led to preservation of the intact hand in the surrounding tissue and “may direct the intact tissue to take over the damaged function”.[50] It is our hope that the skilled training of the affected hand provided by the pinch force task in conjunction with the motor priming from the mirror tasks, will also elicit this type of reorganization in our subjects. The addition of TMS mapping and excitability measures for all subjects in our future work will be important to objectively characterize any changes in cortical neurophysiology that is associated with improvement in motor outcome brought upon by our interventions.
Study limitations
We were primarily interested in testing our ability to provide a scaled hand intervention in this early period post-stroke and determining if priming had any measurable effect on motor function and therefore we did not have a usual care or non-priming control. Our future study will be a randomized control trial that will address some of these limitations.
There are natural challenges to conducting such a study in the acute care setting. A major one is the confounding factor of spontaneous recovery in the early phase post-stroke. Longitudinal studies have found that there is a non-linear, predictable functional recovery that occurs post-stroke that is independent of the dosage or type of therapeutic intervention provided. This recovery is based on processes such as “restitution of the non-infarcted penumbral areas, resolution of diaschisis, and brain plasticity based on anatomical and functional reorganization of the central nervous system”.[51] However, it has also been suggested that recovery can be positively affected by the right type of training.[9] As stated previously, it is our anticipation that the task-specific training immediately after the priming techniques, introduced during this unique time period, will enhance recovery at both the impairment and functional level, as well as positively affect neuro-plasticity and motor learning.
A second challenge to the acute rehabilitation setting is the short length of stay that is seen with patients having persistent flaccidity of the affected extremities. Based on clinical experience it has been noted that due to their lack of ability to actively participate in therapeutic activities with the impaired side, patients with persistent severe hemiparesis are often discharged to a less intense rehabilitation setting faster than someone with active motor control. As we recruit more subjects in the future, the shorter length of stay for these subjects may hinder our ability to provide sufficient amounts of training.
Finally, given the multifactorial approach with this combination training protocol, one limitation of a future RCT study is that it will be difficult to assess whether any anticipated enhanced recovery is due to the combination of interventions or a single intervention. If enhanced recovery is observed with the combination training protocol, then further studies could be designed to evaluate the clinical benefits of each priming method separately in order to tease apart which method is more beneficial.
Conclusion
This initial study allowed us to demonstrate the ability to use VR based visual mirror feedback and movement based priming techniques in conjunction with a pinch trace force modulation task in the acute phase post-stroke in five people with flaccid upper extremities. Traditional rehabilitation options are limited for those with severe paresis, and may encourage compensatory movements that can potentially lead to learned nonuse. Our method allowed people without discernable motor ability distally to produce meaningful movement that could be quantified with objective measures and instrumented technology. Furthermore, three of the five subjects demonstrated important clinical gains at six months after training suggesting that developing effective rehabilitation strategies for this group is warranted. We suggest that this combination training protocol based on principles of neuroplasticity and motor learning will affect neural recovery in a positive manner and help individuals with poorer prognosis. We will evaluate this with future research involving a larger scaled prospective randomized control trial.
IMPLICATIONS FOR REHABILITATION.
Rehabilitation of individuals with severely paretic upper extremities after stroke is challenging due to limited movement capacity and few options for therapeutic training.
Long-term functional recovery of the arm after stroke depends on early return of active hand control, establishing a need for acute training methods focused distally.
This study demonstrates the feasibility of an early hand-based intervention using virtual reality based priming and scaled motor activities which can allow for participation by persons without the motor control required for traditionally presented rehabilitation and testing.
Acknowledgments
The authors thank the clerical, nursing, and rehabilitation staff of the Acute Rehabilitation Department at St. Joseph’s Hospital, Wayne, NJ for their assistance with subject recruitment, scheduling, and ongoing support.
Funding
NIH, 10.13039/100000002 [HD 58301] NIH, 10.13039/100000002 [K01-HD059983, R01-NS085122-01].
Footnotes
Disclosure statement
There are no declarations of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aprile I, Rabuffetti M, Padua L, et al. Kinematic analysis of the upper limb motor strategies in stroke patients as a tool towards advanced neurorehabilitation strategies: a preliminary study. Biomed Res Int. 2014;2014:636123, 8. doi: 10.1155/2014/636123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan PW, Goldstein LB, Horner RD, et al. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25:1181–1188. doi: 10.1161/01.str.25.6.1181. [DOI] [PubMed] [Google Scholar]
- 5.Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil. 1999;21:258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- 6.Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 7.Lang CE, Wagner JM, Edwards DF, et al. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair. 2006;20:444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- 8.Krakauer JW. Arm function after stroke: from physiology to recovery. Semin Neurol. 2005;25:384–395. doi: 10.1055/s-2005-923533. [DOI] [PubMed] [Google Scholar]
- 9.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol. 2013;26:609–616. doi: 10.1097/WCO.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krakauer JW, Carmichael ST, Corbett D, et al. Getting neuro-rehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinear C, Ackerley S, Byblow W. Rehabilitation is initiated early after stroke, but most motor rehabilitation trials are not: a systematic review. Stroke. 2013;44:2039–2045. doi: 10.1161/STROKEAHA.113.000968. [DOI] [PubMed] [Google Scholar]
- 12.Yin CW, Sien NY, Ying LA, et al. Virtual reality for upper extremity rehabilitation in early stroke: a pilot randomized controlled trial. Clin Rehabil. 2014;28:1107–1114. doi: 10.1177/0269215514532851. [DOI] [PubMed] [Google Scholar]
- 13.Saposnik G, Teasell R, Mamdani M, et al. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke. 2010;41:1477–1484. doi: 10.1161/STROKEAHA.110.584979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva Cameirao M, Bermudez IBS, Duarte E, et al. Virtual reality based rehabilitation speeds up functional recovery of the upper extremities after stroke: a randomized controlled pilot study in the acute phase of stroke using the rehabilitation gaming system. Restor Neurol Neurosci. 2011;29:287–298. doi: 10.3233/RNN-2011-0599. [DOI] [PubMed] [Google Scholar]
- 15.Kitago T, Krakauer JW. Motor learning principles for neuro-rehabilitation. In: Barnes MP, Good DC, editors. Handbook of clinical neurology, neurological rehabilitation. Vol. 110. New York (NY): Elsevier B.V; 2013. pp. 93–104. [DOI] [PubMed] [Google Scholar]
- 16.Stinear CM, Barber PA, Coxon JP, et al. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131:1381–1390. doi: 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- 17.Hummel FC, Cohen LG. Noninvasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 18.Pomeroy V, Aglioti SM, Mark VW, et al. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabil Neural Repair. 2011;25:33S–43S. doi: 10.1177/1545968311410942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res. 2005;163:118–122. doi: 10.1007/s00221-005-2226-9. [DOI] [PubMed] [Google Scholar]
- 20.Nojima I, Mima T, Koganemaru S, et al. Human motor plasticity induced by mirror visual feedback. J Neurosci. 2012;32:1293–1300. doi: 10.1523/JNEUROSCI.5364-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lappchen CH, Ringer T, Blessin J, et al. Optical illusion alters M1 excitability after mirror therapy: a TMS study. J Neurophysiol. 2012;108:2857–2861. doi: 10.1152/jn.00321.2012. [DOI] [PubMed] [Google Scholar]
- 22.Kang YJ, Park HK, Kim HJ, et al. Upper extremity rehabilitation of stroke: facilitation of corticospinal excitability using virtual mirror paradigm. J Neuroeng Rehabil. 2012;9:71. doi: 10.1186/1743-0003-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleh S, Adamovich SV, Tunik E. Mirrored feedback in chronic stroke: recruitment and effective connectivity of ipsilesional sensorimotor networks. Neurorehabil Neural Repair. 2014;28:344–354. doi: 10.1177/1545968313513074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byblow WD, Stinear CM, Smith MC, et al. Mirror symmetric bimanual movement priming can increase corticomotor excitability and enhance motor learning. PLoS One. 2012;7:33882. doi: 10.1371/journal.pone.0033882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boos A, Qiu Q, Fluet GG, et al. Haptically facilitated bimanual training combined with augmented visual feedback in moderate to severe hemiplegia. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:3111–3114. doi: 10.1109/IEMBS.2011.6090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinear CM, Petoe MA, Anwar S, et al. Bilateral priming accelerates recovery of upper limb function after stroke: a randomized controlled trial. Stroke. 2014;45:205–210. doi: 10.1161/STROKEAHA.113.003537. [DOI] [PubMed] [Google Scholar]
- 27.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 28.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 30.Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 31.Adamovich SV, Fluet GG, Mathai A, et al. Design of a complex virtual reality simulation to train finger motion for persons with hemiparesis: a proof of concept study. J Neuroeng Rehabil. 2009;6:28. doi: 10.1186/1743-0003-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fluet GG, Merians AS, Qiu Q, et al. Robots integrated with virtual reality simulations for customized motor training in a person with upper extremity hemiparesis. A case Study. J Neurol Phys Ther. 2012;36:79–86. doi: 10.1097/NPT.0b013e3182566f3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 34.Littmann AE, McHenry CL, Shields RK. Variability of motor cortical excitability using a novel mapping procedure. J Neurosci Methods. 2013;214:137–143. doi: 10.1016/j.jneumeth.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler AJ, Kahn S, Wolf SL, et al. Finger extensor variability in TMS parameters among chronic stroke patients. J Neuroeng Rehabil. 2005;2:10. doi: 10.1186/1743-0003-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngomo S, Leonard G, Moffet H, et al. Comparison of trans-cranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. J Neurosci Methods. 2012;205:65–71. doi: 10.1016/j.jneumeth.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Niskanen E, Julkunen P, Saisanen L, et al. Group-level variations in motor representation areas of thenar and anterior tibial muscles: Navigated Transcranial Magnetic Stimulation Study. Hum Brain Mapp. 2010;31:1272–1280. doi: 10.1002/hbm.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borghetti D, Sartucci F, Petacchi E, et al. Transcranial magnetic stimulation mapping: a model based on spline interpolation. Brain Res Bull. 2008;77:143–148. doi: 10.1016/j.brainresbull.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Weiss C, Nettekoven C, Rehme AK, et al. Mapping the hand, foot and face representations in the primary motor cortex – retest reliability of neuronavigated TMS versus functional MRI. Neuroimage. 2013;66:531–542. doi: 10.1016/j.neuroimage.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 40.Bastings EP, Greenberg JP, Good DC. Hand motor recovery after stroke: a transcranial magnetic stimulation mapping study of motor output areas and their relation to functional status. Neurorehabil Neural Repair. 2002;16:275–282. doi: 10.1177/154596802401105207. [DOI] [PubMed] [Google Scholar]
- 41.Wassermann EM, McShane LM, Hallett M, et al. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- 42.Sparing R, Buelte D, Meister IG, et al. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008;29:82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flament D, Goldsmith P, Buckley CJ, et al. Task dependence of responses in first dorsal interosseous muscle to magnetic brain stimulation in man. J Physiol. 1993;464:361–378. doi: 10.1113/jphysiol.1993.sp019639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehme AK, Fink GR, von Cramon DY, et al. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011;21:756–768. doi: 10.1093/cercor/bhq140. [DOI] [PubMed] [Google Scholar]
- 45.Shiri S, Feintuch U, Lorber-Haddad A, et al. Novel virtual reality system integrating online self-face viewing and mirror visual feedback for stroke rehabilitation: rationale and feasibility. Top Stroke Rehabil. 2012;19:277–286. doi: 10.1310/tsr1904-277. [DOI] [PubMed] [Google Scholar]
- 46.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 47.van Kordelaar J, van Wegen EE, Nijland RH, et al. Assessing longitudinal change in coordination of the paretic upper limb using on-site 3-dimensional kinematic measurements. Phys Ther. 2012;92:142–151. doi: 10.2522/ptj.20100341. [DOI] [PubMed] [Google Scholar]
- 48.Barreca SR, Bohannon RW, Charness AL, et al. Management of the post stroke hemiplegic arm and hand: treatment recommendations of the 2001 Consensus Panel. Heart and Stroke Foundation of Ontario. 2002 [Google Scholar]
- 49.Stinear CM, Barber PA, Petoe M, et al. The PREP algorithm predicts potential for upper limb recovery post stroke. Brain. 2012;135:2527–2535. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- 50.Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 51.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]