Figure 6. Intense heat stress induces Bcl-2 ubiquitination via superoxide activity in HUVEC cells.
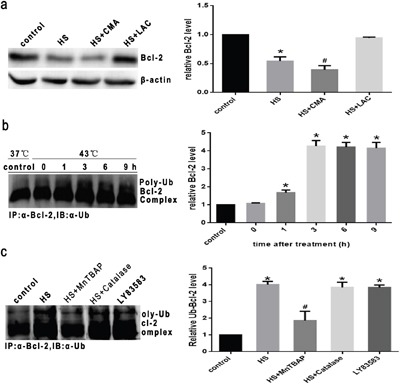
a. Cells were pretreated with the proteasome inhibitor lactacystin (LAC, 10μM) or lysosome inhibitor concanamycin A (CMA, 1μM) for 1h prior to heat stress at 43°C for 2h, and further incubated at 37°C for 6h. Western blot analysis of Bcl-2 protein expression. b. Cells were pretreated with LAC (10μM) for 1 h to prevent proteasomal degradation of Bcl-2, then cultured at 43°C for 2h, and incubated at 37°C for different lengths of times as indicated (0h, 1h, 3h, 6h or 9h). c. Cells were pretreated with LAC (10μM) for 1h, and in the presence or absence of MnTBAP (100μM) or Catalase (1000 U/μl) for 0.5h prior to heat stress (43°C) for 2h, and further incubated at 37°C for 6h. LY83583 (10μM) was used as positive control. Cell lysates were immunoprecipitated with anti-Bcl-2 antibody and the immune complexes were analyzed for ubiquitin by Western blotting. Each value represents the mean ± SD of three separate experiments, *P < 0.05, compared to control group (37°C), #P < 0.05, compared to heat stress group (43°C).
