Abstract
The cell surface membrane-bound mucin protein MUC4 promotes tumorigenicity, aggressive behavior, and poor outcomes in various types of epithelial carcinomas, including pancreatic, breast, colon, ovarian, and prostate cancer. This review summarizes the theories and findings regarding MUC4 function, and its role in epithelial carcinogenesis. Based on these insights, we developed an outline of the processes and mechanisms by which MUC4 critically supports the propagation and survival of cancer cells in various epithelial organs. MUC4 may therefore be a useful prognostic and diagnostic tool that improves our ability to eradicate various forms of cancer.
Keywords: MUC4, carcinoma, epithelial, tumorigenicity
INTRODUCTION
Mucins are high-molecular-weight epithelial glycoproteins that can be subcategorized into secretory, gel-forming, or membrane-anchored [1]. Mucins serve as the primary line of defense against extracellular disruptions, including pathogen infection, pH changes, and ion influx [1–5].
MUC4 is a transmembrane mucin that belongs to the membrane-anchored class of mucins, and its expression is tissue-dependent [6]. MUC4 is also a tumor-associated antigen, whose overexpression is observed in various epithelial malignancies, such as pancreatic adenocarcinoma and breast, lung, ovarian, and prostate cancer. The methylation and histone deacetylation profiles of the MUC4 5′-untranslated region (UTR) suppress MUC4 expression [7, 8]. The presence of MUC4 in absorptive, ciliated, and goblet cells suggests it can be both membrane-anchored and secreted [6]. When mucous layer reinforcement is needed, MUC4 undergoes proteolytic cleavage at a conserved putative GDPH proteolytic cleavage site to become a secreted, non-gel-forming mucin [9].
The multiple cellular functions directed by the sequence, structure, and glycosylation of MUC4 suggest it promotes the progression of various forms of epithelial carcinoma [3, 10]. MUC4 expression has an inverse relationship with the survival rates of epithelial carcinoma patients [11, 12]. Thus, MUC4 represents a potential diagnostic and prognostic target.
MUC4 STRUCTURE AND FUNCTIONS
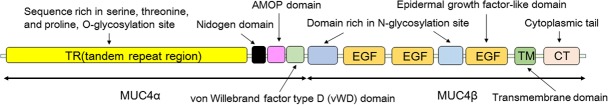
MUC4, which is homologous to rat sialomucin complex (SMC)/Muc4, is abundantly in carbohydrates [13]. The hundreds of carbohydrates bound to MUC4 are O-glycosidically linked to the protein backbone, which possesses tandem repeat peptides primarily composed of serine, threonine, and proline [6]. Carbohydrate moieties, primarily N-acetylglucosamine, galactose, fucose, N-acetylgalactosamine, and sialic acid, are the most prominent branches of MUC4. The MUC4 gene is located on chromosomal locus 3q29 [14], and encodes the large 930 kDa polypeptide precursor apomucin, which is composed of subunits MUC4α and MUC4β [15]. MUC4α is the 500-850 kDa, extracellular, hyper-glycosylated subunit; MUC4β is the 80-90 kDa, membrane-bound subunit that contains EGF-like domains and many N-glycosylation sites (Figure 1). The MUC4 precursor is enzymatically separated to form the final heterodimeric, bi-functional, cell surface glycoprotein, which varies in size from 4,468 to 8,468 amino acid residues [15].
Figure 1. A schematic view of the MUC4 protein architecture.
MUC4 protein is composed of two subunits, MUC4α and MUC4β. MUC4α is the extracellular and hyper-glycosylated subunit (500-850 kDa), and MUC4β is the membrane-bound subunit that contains EGF-like domains and many N-glycosylation sites (80-90 kDa).
The transmembrane domain of MUC4 allows the glycoprotein to participate in intracellular processes and interact with extracellular molecules and receptors via its large, mucin-like N-terminus protruding from the cell surface [16, 17]. In contrast to other membrane-anchored mucins, MUC4 contains a von Willebrand factor type D domain, a nidogen domain, and an adhesion-associated domain (Figure 1) [18, 19]. MUC4 has twenty-four variant forms, resulting from alternative splicing events that lead to differences in glycosylation and the variable number of tandem repeats (VNTR) domain [6, 20–23]. O-glycosidically linked oligosaccharides are distinguished by their core type, backbone type, and peripheral structures, all of which contribute to the filamentous conformation of the MUC4 extracellular domain [6]. In addition, O-linked carbohydrates contain sialic acid and sulfate residues that add a negative charge to the N-terminus of the glycoprotein. In the filamentous conformation, the extracellular domain of MUC4 extends 1.12-2.12 μm above the cell surface and interacts with extracellular molecules that bypass the initial mucosal barrier covering the epithelium [24].
The MUC4 ectodomain, which possesses the filamentous structure of O-glycosylated tandem repeats, contributes to the protective mucous layer by forming a physical barrier involved in growth and survival signaling. These MUC4 functions are exploited by various carcinomas to promote the propagation and survival of tumor cells [25]. The high concentration of carbohydrates in MUC4, whose characteristic filamentous structure extends well above the glycocalyx, is crucial for glycoprotein survival. MUC4-bound oligosaccharides facilitate resistance to degradation in the gastrointestinal tract, in addition to their role in mediating the recognition of extracellular lectins and antibodies [6]. The MUC4 extracellular domain, which is represented by the heavily O-glycosylated and filamentous apomucin, is responsible for navigating the extracellular environment via ligand binding [26, 27].
ErbB2, a cognate receptor tyrosine kinase also referred to as HER2 in humans and neu in rodents, binds to the EGF-like motifs of MUC4 to facilitate signal transduction, cell proliferation, and cell survival [4, 9, 24, 26, 28]. This cis interaction is restricted to cellular surfaces on which signal transduction occurs via the formation of an ErbB2-MUC4 complex [24, 27, 29]. MUC4 binding to ErbB2 starts with ErbB3 binding to neuregulin (NRG), followed by the formation of a heteromeric complex between NRG and ErbB2. The formation of this complex leads to the phosphorylation-mediated activation of ErbB2, which then binds to MUC4, forming a tetrameric MUC4-ErbB2-ErbB3-NRG complex. MUC4 stabilizes this complex by preventing its internalization [16, 24, 30, 31]. MUC4 can initiate and/or potentiate downstream MAPK signaling associated with differentiation and proliferation by imposing a protection mechanism for cell polarization [32].
Along with cell protection, MUC4 is also involved in extracellular factor-cell communication, cell proliferation, and adhesion. Its roles are most notably intertwined with the molecular mechanisms underlying the neoplastic progression and metastasis of various forms of epithelial carcinoma (Figure 2). MUC4 functions are initiated upon the activation of p27(kip), a cell cycle inhibitor [26, 33]. The MUC4-ErbB2-ErbB3-NRG complex activates the protein kinase B (PKB)/Akt and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways to induce cell proliferation and inhibit apoptosis (Figure 2) [33]. The formation of the tetrameric MUC4-ErbB2-ErbB3-NRG complex leads to the hyper-phosphorylation of ErbB2. This phosphorylation enables the downstream activation of the phosphoinositide-3 kinase (PI3K)-Akt and Ras-ERK pathways, which induce a loss of cell polarity in tumor cells. In addition, the increased activation of these pathways results in the transcriptional activation of cyclin D1, leading to increased cell proliferation [24, 26]. MUC4 also facilitates cellular adhesion and subsequent binding to the endothelium and activates immunosuppressive responses to tumor cells. Decreased MUC4 expression is associated with reduced cell proliferation and motility and increased cellular aggregation [34].
Figure 2. The functions and roles of MUC4 in various signaling transduction pathways.
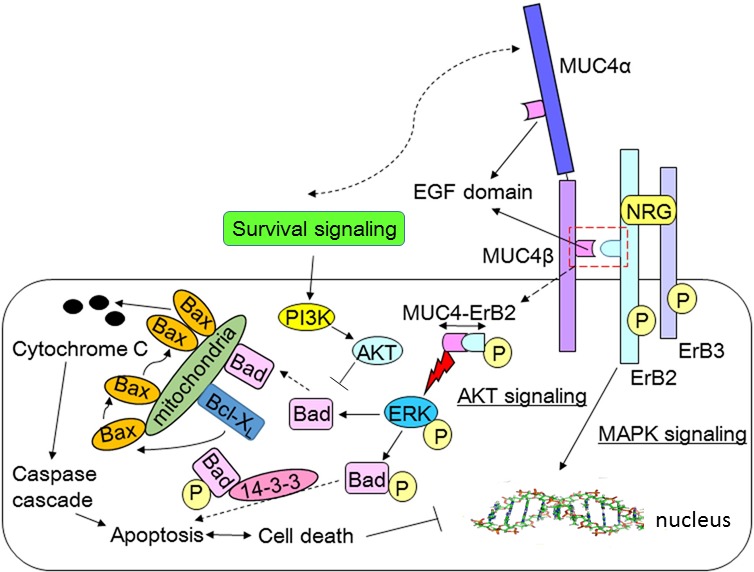
ErbB3 binds with neuregulin (NRG), followed by the formation of a heteromeric complex between NRG and ErbB2. The formation of this complex leads to the phosphorylation-mediated activation of ErbB2. The activated ErbB2 binds to the EGF-like motifs of MUC4, forming a tetrameric MUC4-ErbB2-ErbB3-NRG complex. The MUC4-ErbB2-ErbB3-NRG complex activates the protein kinase B (PKB)/Akt and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways to induce cell proliferation and inhibit apoptosis. The formation of the tetrameric MUC4-ErbB2-ErbB3-NRG complex leads to the hyper-phosphorylation of ErbB2. That phosphorylation enables the downstream activation of the phosphoinositide-3 kinase (PI3K)-Akt and Ras-ERK pathways, which induce a loss of cell polarity in tumor cells. In addition to activating growth and survival signals in pancreatic cancer cells, MUC4 deactivates pro-apoptotic proteins. In prostate cancer cells, ErbB2/HER2 activates ERK to phosphorylate and deactivate the protein Bad, which is then unable to deactivate the anti-apoptotic proteins Bcl-2 and Bcl-XL. In response to gemcitabine, MUC4 promotes the phosphorylation of Bad via its stimulation of HER2 and activation of ERK. These effects ultimately lead to the suppression of both cytochrome C release and cancer cell apoptosis
MUC4 levels inversely correlate with the 5′-UTR DNA methylation level in various cancer cell lines. MUC4 gene expression depends on the methylation status of CpG motifs near the transcriptional start site of MUC4 [7, 8]. In addition to DNA methylation, histone modification suppresses MUC4 expression. Histones correlated with MUC4 expression are commonly deacetylated in pancreatic and gastric epithelial cancer cell lines [7, 8, 31, 35].
FACTORS REGULATING MUC4 EXPRESSION
Overexpression of MUC4 in human tumor cells promotes anti-adhesive functions and represses the anti-tumor functions of the immune system [36]. MUC4 expression is up-regulated by various factors. This includes PEA3, an Ets family member (E26 transcription factor) that is involved in proliferation, differentiation, and transformation. The IFN-γ inflammatory pathway increases MUC4 expression via STAT-1 up-regulation [37, 38]. Transforming growth factor (TGF)-β participates in pancreatic carcinogenesis by activating MUC4 expression via the MAPK, PI3K, and protein kinase A (PKA) signaling pathways. All-trans-retinoic acid (RA) treatment increases MUC4 expression via RA receptor-α and TGF-β2 [26, 27, 32, 38–42].
Additional potent promoters of MUC4 include the transcription factors hepatocyte nuclear factor (HNF)4α11, forkhead box A (FOXA)1/FOXA2, GATA-4/-5/-6, and caudal-related homeobox (CDX)-1/-2 [43]. These factors stimulate cell differentiation in gut endoderm- and pancreas-derived tissues during embryonic development [15]. This differentiation network is involved in pancreatic development and is thought to increase mucin expression during carcinogenesis in a similar manner to its activities during embryonic growth.
MUC4 levels follow the cyclic expression patterns of hormones associated with the menstrual cycle [34]. MUC4 is overexpressed in cervical cancer tissues, with the highest level of MUC4 expression in high-grade dysplasia. The differences in MUC4 expression patterns between dysplastic and normal endocervical epithelia may be useful as a diagnostic marker for predicting cervical cancer [2, 44–46]. MUC4 expression is a marker of good prognosis in upper aerodigestive tract carcinomas, but a marker of poor prognosis in ovarian carcinomas [47, 48].
Metastasis is induced by MUC4, which triggers the dissociation of tumor cells from the primary tumor site by blocking surface adhesion molecule binding, integrin-mediated cell adhesion, and homotypic cell-cell interactions [49]. Elevated MUC4 expression increases aggression of breast cancer, including decreased binding of breast cancer tissues to extracellular structures such as laminin, fibronectin, and collagen and reduced cell-cell interactions among these structures [50, 51]. Furthermore, the expression of MUC4 in breast cancer cells decreases apoptosis 5-10-fold relative to nonmalignant breast cells. This demonstrates that the primary function of MUC4 is to promote tumorigenesis by suppressing apoptosis via alterations of signal transduction processes ranging from modification of cell surface interaction sites to regulation of protein synthesis [25, 47]. In breast cancer, the post-transcriptional regulation of MUC4 is lost when cancer cells become unresponsive to TGF signals, most likely as a result of ErbB2 overexpression [27, 31, 47, 51].
CHANGES IN MUC4 EXPRESSION IN CANCER CELLS
Epithelial invasion is triggered by the anti-adhesive activities of MUC4, which leads to the disassociation of tumor cells from the primary tumor site [49]. The anti-recognition activity of MUC4 promotes tumor growth by enabling the evasion of both immune surveillance and apoptosis suppression. As a result, tumor propagation is accelerated, and tumor cells migrate to the bloodstream. These events are facilitated by the sialyl-oligosaccharides on MUC4 and ultimately result in tumor cell invasion and endothelial transmigration. Cancer stem cells and MUC4 contribute to cancer relapse and progression by assisting in the initiation, growth, and recurrence of various carcinomas [52].
As a consequence of the stress induced by carcinogenesis and malignant cellular transformation, a loss of cell polarity occurs in association with activation of cellular proliferation and survival systems [2]. The process underlying the onset and maintenance of cell polarity loss is termed the epithelial-mesenchymal transition (EMT) [53, 54]. MUC4 stimulates the EMT via its effect on β-catenin, which is known to promote cell-cell adhesions through its association with cadherin complexes in the cell membrane. β-catenin also acts together with TCF as a transcriptional co-activator to induce the EMT in tumor cells upon its translocation to the nucleus. The EMT is initiated by a decrease in E-cadherin expression and an increase in vimentin filaments. These changes are followed by cellular transformation from an immotile, polarized state to a motile, mesenchymal state. Cells also adopt a spindle-like conformation rather than their original cobblestone-like morphology, and lose the expression of various differentiation markers and cell-cell junction proteins. All of these factors contribute to an overall increase in migratory capacity, a decrease in the expression of epithelial markers such as E-cadherin, and an enhancement of local invasiveness [55].
In tumor cells accustomed to long-term EMT, MUC4 freely binds with molecules that normally do not appear on the non-apical surface, which contributes to a sustained state of reduced cell polarity [56]. For example, ErbB2 is normally sequestered in epithelial cells by the MUC4-ErbB2 complex, but upon the loss of cell polarity and tight junctions, ErbB3 becomes accessible for binding to ErbB2. This leads to the activation of the ErbB2 signaling pathway and to the reduced binding of ErbB2-specific antibodies [57]. The EMT is promoted and maintained via the ErbB2-mediated disruption of the Par complex. This leads to the loss of function of cellular tight junctions, thus inducing unresponsiveness to cell-cell/cell-extracellular factor communication [2].
MUC4 expression in normal pancreatic tissue is typically undetectable, but as healthy cells transform into carcinogenic cells, MUC4 expression increases (Table 1) [58]. In the case of ovarian cancer, the level of MUC4 is actually not predictive of the disease [59]. MUC4 expression is associated with the malignant transformation of ovarian epithelial cells into cancer cells. Therefore, a reduction in MUC4 expression during the late stage of ovarian cancer indicates a greater likelihood of survival [52, 59]. This is in contrast to the majority of forms of epithelial carcinoma, which show abnormally high rates of MUC4 expression that increase with disease progression. The above trends suggest a possible relationship between MUC4 expression and the development of metastasis in ovarian epithelial cells. The specific and aberrant patterns of MUC4 expression during the progressive stages of various forms of epithelial carcinoma implicate MUC4 as a novel potential biomarker and therapeutic target [15].
Table 1. MUC4 expression in different cancers.
| Cancer tissue | Normal tissue | Detection method | |
|---|---|---|---|
| Pancreatic cancer | High***/High* | Under detectable level | Q, R, I, W, C etc [37, 89, 90] |
| Ovarian cancer | High**/High* | - | R, I, W etc [49, 52, 60] |
| Breast cancer | High** | Under detectable level | Q, R, I, W etc [51, 91, 92] |
| Lung cancer | High***/High* | Detectable/High* | R, I, W etc [24, 56, 93] |
| Colon cancer | High*** | High** | Q, R, I, W etc [71, 94] |
| Prostate cancer | High** | High* | Q, R, I etc [95–97] |
| Head-neck cancer | High* | Detectable | I, W etc [77, 98–100] |
| Cervical cancer | High*** | Detectable/High* | I, S etc [44–46] |
*** The expression of MUC4 can be used as a prognostic marker; ** the expression of MUC4 is very high; * the expression of MUC4 is higher than the threshold for detection; / MUC4 expression changes at different stages of cancer progression. Q-quantitative real-time PCR; R-real time PCR; W-western blot; I-immunohistochemistry; S-Semi-quantitative RT-PCR; C-Chromatin immune-precipitation assays (ChIP) etc.
Tumor cells overexpress aberrantly glycosylated mucins that are secreted into the bloodstream and can serve as tumor markers [30]. Alterations in mucin glycosylation during cancer most likely results from glycosyltransferase activity or post-translational modifications in the specific apomucin residues that can be glycosylated. As a result of these alterations, mucins become more easily detectable in ovarian and breast cancer patients. Whereas ovarian cancer cells express several different mucin glycoproteins during metastasis (MUC2, MUC3 and MUC5B), non-malignant ovarian cells do not express these mucins. In completely metastatic ovarian cancer cells, aberrant expression of MUC4 implies mucins are correlated with the malignant transformation of cells during specific stages of metastatic progression [52, 60].
In pancreatic cancer, MUC4 expression is increased, especially throughout the stages of tumor development leading to carcinoma [58]. MUC4 or apomucin transcription is induced in pancreatic intraepithelial neoplasia lesions and intraductal papillary mucinous neoplasms. Increased expression of MUC4 induced by mutant K-ras (a small GTPase of the Ras superfamily) correlates with the activation of the ERK, JNK, and NF-κB signaling pathways. This transcriptional up-regulation leads to increased expression of MUC4 in pancreatic adenocarcinoma [38]. MUC4 is the most differentially expressed gene in pancreatic ductal adenocarcinoma (PDC), and its expression level differed between each stage of cancer progression [58, 61]. In addition, the MUC4-ErbB2-ERK pathway has been shown to contribute to the characteristic resistance of PDC to gemcitabine by inhibiting the activation of intrinsic apoptosis in cancer cells [62, 63].
MUC4 associates with the host receptor of enterotoxigenic Escherichia coli [64–68]. Moreover, E. coli strains from phylogroup B2 harboring a pks+ island can produce a peptide-polyketide hybrid compound termed colibactin, which can trigger premature and transmissible senescence in mammalian cells, resulting in colon cancer [69]. MUC4 promotes intestinal cell proliferation during tumorigenesis, and mice deficient in MUC4 exhibited reduced tumor burden compared with WT mice [70]. Therefore, it is necessary to investigate the relationship between MUC4 and colibactin, and focusing on MUC4 might reveal a new strategy to prevent colon cancer [60, 71].
MUC4 AND CANCER TREATMENT
Thymoquinone (TQ) down-regulates MUC4 expression via the proteasomal pathway, and induces apoptosis in pancreatic cancer cells by activating the c-Jun N-terminal kinase (JNK) and p38 MAPK pathways [72]. Both of these pathways are stimulated by MUC4, and are used by cancer cells to proliferate, to defend against the immune response, and to promote the invasion of distal epithelial tissue [72]. A decrease in the MUC4 level increased the rate of apoptosis in cancer cells, decreased their motility, and reduced overall epithelial cell migration [72–76]. MUC4 overexpression is correlated with the proliferation and cellular senescence of head and neck squamous cell carcinoma (HNSCC) cells, and down-regulating MUC4 expression may serve as a promising therapeutic approach for treating HNSCC patients [77]. MUC4 down-regulation improves the efficacy of gemcitabine to eradicate pancreatic carcinoma cell masses [78].
In addition to decreasing cell migration and motility, down-regulation of MUC4 expression hampers the evasion of cancer cells from apoptosis and immune surveillance [72]. The MUC4 promoter contains many Smad-binding sites. TGF-β is a pleiotropic cytokine that is involved in gene expression through its activation of Smad proteins [27, 79]. In the early phases of carcinogenesis, TGF-β inhibits cellular growth, but in later phases, it contributes to the progression of tumor metastasis. TGF-β also suppresses MUC4 expression post-transcriptionally through the proteasomal pathway, and activates the JNK and p38 MAPK pathways[79].
These findings regarding MUC4 suppression/repression suggest the potential of this glycoprotein as a key target of successful cancer therapy. MUC4-based vaccines induced strong antigen-specific immune responses in mice [80]. A dendritic cell (DC)-based vaccine, using cells transduced with an adenovirus encoding the universal DR-restricted Th helper epitope (PADRE) combined with HLA-A1(/A2) restricted epitopes, may be a potential strategy for the immunotherapy of MUC4-associated tumors [81].
As mitochondrial number increases, apoptosis rates normally increase [10]. This is because mitochondria release factors that induce apoptosis, primarily cytochrome c. However, mini-MUC4-transfected pancreatic cancer cells showed increased mitochondrial mass without the expected enhancement of apoptosis. In poorly differentiated pancreatic cell lines, MUC4 overexpression likely leads to the sequestration of apoptogenic factors in the mitochondrial inter-membrane space due to changes in the polarization state of the mitochondrial membrane. This inhibits apoptosis without preventing the increase in the number of mitochondria [10, 31, 63, 82].
In addition to activating growth and survival signals in pancreatic cancer cells, MUC4 deactivates pro-apoptotic proteins by inducing the unresponsiveness of cancer cells to apoptotic signals [49, 83]. In prostate cancer cells, ErbB2/HER2 activates ERK to phosphorylate and deactivate Bad, which is then in turn unable to deactivate the anti-apoptotic proteins Bcl-2 and Bcl-XL. These events suppress the responses to intrinsic mitochondrial apoptotic pathway activity (Figure 2) [83]. In response to gemcitabine, MUC4 promotes the phosphorylation of Bad via its stimulation of HER2 and its activation of ERK. These effects ultimately lead to the suppression of both cytochrome C release and cancer cell apoptosis [63, 82–84].
MUC4 overexpression increases the phosphorylation and activation of a non-receptor tyrosine kinase termed focal adhesion kinase (FAK), as well as the Akt and ERK pathways. These events promote downstream HER2 signaling and alter the actin network to increase the motility of ovarian cancer cells. Other factors contributing to the motility of MUC4-transfected cells are filopodia, lamellipodia, and microspikes, which assist in propelling cell movement [74]. In pancreatic cancer cells, MUC4 up-regulation causes ultra-structural changes that promote tumorigenicity via proliferation and modified interactions with the extracellular matrix [30].
In ovarian cancer development, the loss of cell polarity enables atypical protein-protein interactions, allowing MUC4 to activate ErbB2/HER2 expression. This was determined by silencing MUC4 using antisense RNA, which results in a decrease in ErbB2/HER2 expression, which leads to decreases in the ovarian cancer cell motility rate and overall invasiveness [26, 31, 52, 74, 85, 86].
CONCLUSIONS
The multiple functions of MUC4 originating from its abundant polymorphic capabilities, its glycosylation, the flexibility of its protein backbone structure, and the repeats in its VNTR region are among the primary influencers of cancer cell growth [4, 31]. As components of cellular signal transduction mechanisms, cell propagation, apoptosis pathways, and anti-adhesive and EMT regulation systems, the functions of MUC4 are understandably highly controlled during tumorigenesis and are hijacked by tumor cells to promote proliferation, dissociation, and metastasis to distal regions [1, 17, 25, 34, 48].
The expression of MUC4 is tissue and organ specific, and alterations to its mRNA expression, protein backbone structure, or glycosylation profile lead to the malignant transformation of organ tissue cells. The distribution of MUC4 changes in the stratified epithelium, and its expression is aberrant in cancers of the upper aerodigestive tract. However, in such forms of cancer, the presence of MUC4 indicates a more favorable prognosis. Additionally, the distribution of overexpressed MUC4 is altered in muco-epidermoid cancers of the salivary gland. In both low- and high-grade forms of these cancers, MUC4 expression is correlated with an increased survival rate and a reduced recurrence rate. However, in lung adenocarcinoma, particularly in stage 1A cases, high expression of MUC4 indicates a reduced survival rate compared to similar cases with low MUC4 expression [25].
In biliary tract cancer, there is a roughly 1.9-fold increase in MUC4 mRNA levels, and increased MUC4 expression is associated with a decreased survival rate among these patients [2, 87]. Although MUC4 is undetectable in normal bile duct cells, its expression is detectable in the larger bile ducts of intra- and extrahepatic cholangiocarcinomas, as well as ductal adenocarcinomas of the pancreas [88].
Understanding the MUC4 interactome may improve the evaluation of specific predictive markers and the selection of the MUC4 pathway inhibitors in controlling cancer progression.
Acknowledgments
This study was supported by grants from the Chinese National Science Foundation Grant (No. 30571374, 30771603, 31072136, 31270171, 31672579), and State Key Laboratory of Veterinary Biotechnology Foundation (SKLVBF2017), the Genetically Modified Organisms Technology Major Project of China (2014ZX08006-001B), the 948 program grant No. 2011-G24 from the Ministry of Agriculture of the People's Republic of China, a project founded by the Priority Academic Program of Development Jiangsu High Education Institution, and the Innovative Research Team In University “PCSIRT” (IRT0978), a fund of excellent doctoral dissertations from Yangzhou University.
Footnotes
CONFLICTS OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
- 1.Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35:236–45. doi: 10.1016/j.tibs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nature reviews Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Seuningen I, Vincent A. Mucins: a new family of epigenetic biomarkers in epithelial cancers. Expert Opin Med Diagn. 2009;3:411–27. doi: 10.1517/17530050902852697. [DOI] [PubMed] [Google Scholar]
- 4.Jonckheere N, Skrypek N, Frenois F, Van Seuningen I. Membrane-bound mucin modular domains: from structure to function. Biochimie. 2013;95:1077–86. doi: 10.1016/j.biochi.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Belley A, Keller K, Gottke M, Chadee K. Intestinal mucins in colonization and host defense against pathogens. Am J Trop Med Hyg. 1999;60:10–5. doi: 10.4269/ajtmh.1999.60.10. [DOI] [PubMed] [Google Scholar]
- 6.Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert JP. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338(Pt 2):325–33. doi: 10.1042/bj3380325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada N, Nishida Y, Tsutsumida H, Goto M, Higashi M, Nomoto M, Yonezawa S. Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. Br J Cancer. 2009;100:344–51. doi: 10.1038/sj.bjc.6604845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent A, Ducourouble MP, Van Seuningen I. Epigenetic regulation of the human mucin gene MUC4 in epithelial cancer cell lines involves both DNA methylation and histone modifications mediated by DNA methyltransferases and histone deacetylases. Faseb j. 2008;22:3035–45. doi: 10.1096/fj.07-103390. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–81. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moniaux N, Chaturvedi P, Varshney GC, Meza JL, Rodriguez-Sierra JF, Aubert JP, Batra SK. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97:345–57. doi: 10.1038/sj.bjc.6603868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HK, Cho MS, Kim TH. Prognostic significance of muc4 expression in gallbladder carcinoma. World J Surg Oncol. 2012;10:224. doi: 10.1186/1477-7819-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn's disease. Gastroenterol Res Pract. 2013;2013:431231. doi: 10.1155/2013/431231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porchet N, Pigny P, Buisine MP, Debailleul V, Degand P, Laine A, Aubert JP. Human mucin genes: genomic organization and expression of MUC4, MUC5AC and MUC5B. Biochem Soc Trans. 1995;23:800–5. doi: 10.1042/bst0230800. [DOI] [PubMed] [Google Scholar]
- 14.Gross MS, Guyonnet-Duperat V, Porchet N, Bernheim A, Aubert JP, Nguyen VC. Mucin 4 (MUC4) gene: regional assignment (3q29) and RFLP analysis. Ann Genet. 1992;35:21–6. [PubMed] [Google Scholar]
- 15.Jonckheere N SN, Van Seuningen I. Mucins and pancreatic cancer. Cancers. 2010:1794–812. doi: 10.3390/cancers2041794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funes M, Miller JK, Lai C, Carraway KL, 3rd, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281:19310–9. doi: 10.1074/jbc.M603225200. [DOI] [PubMed] [Google Scholar]
- 17.Carraway K, C L Carraway. A Carothers MUC4 and membrane receptors in cancer. Mucins and Cancer. 2013:68–81. doi: 10.2217/fmeb2013.13.69. [DOI] [Google Scholar]
- 18.Duraisamy S, Ramasamy S, Kharbanda S, Kufe D. Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 AND MUC16. Gene. 2006;373:28–34. doi: 10.1016/j.gene.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Constantinou PE, Danysh BP, Dharmaraj N, Carson DD. Transmembrane mucins as novel therapeutic targets. Expert review of endocrinology & metabolism. 2011;6:835–48. doi: 10.1586/eem.11.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escande F, Lemaitre L, Moniaux N, Batra SK, Aubert JP, Buisine MP. Genomic organization of MUC4 mucin gene. Towards the characterization of splice variants. Eur J Biochem. 2002;269:3637–44. doi: 10.1046/j.1432-1033.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- 21.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–206. doi: 10.2741/a579. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury A, Moniaux N, Ringel J, King J, Moore E, Aubert JP, Batra SK. Alternate splicing at the 3′-end of the human pancreatic tumor-associated mucin MUC4 cDNA. Teratog Carcinog Mutagen. 2001;21:83–96. doi: 10.1002/1520-6866(2001)21:1<83::aid-tcm8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Moniaux N, Escande F, Batra SK, Porchet N, Laine A, Aubert JP. Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur J Biochem. 2000;267:4536–44. doi: 10.1046/j.1432-1327.2000.01504.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–76. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Carraway KL, Theodoropoulos G, Kozloski GA, Carothers Carraway CA. Muc4/MUC4 functions and regulation in cancer. Future Oncol. 2009;5:1631–40. doi: 10.2217/fon.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonckheere N, Skrypek N, Merlin J, Dessein AF, Dumont P, Leteurtre E, Harris A, Desseyn JL, Susini C, Frenois F, Van Seuningen I. The mucin MUC4 and its membrane partner ErbB2 regulate biological properties of human CAPAN-2 pancreatic cancer cells via different signalling pathways. PLoS One. 2012;7:e32232. doi: 10.1371/journal.pone.0032232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonckheere N, Perrais M, Mariette C, Batra SK, Aubert JP, Pigny P, Van Seuningen I. A role for human MUC4 mucin gene, the ErbB2 ligand, as a target of TGF-beta in pancreatic carcinogenesis. Oncogene. 2004;23:5729–38. doi: 10.1038/sj.onc.1207769. [DOI] [PubMed] [Google Scholar]
- 28.Karg A, Dinc ZA, Basok O, Ucvet A. MUC4 expression and its relation to ErbB2 expression, apoptosis, proliferation, differentiation, and tumor stage in non-small cell lung cancer (NSCLC) Pathol Res Pract. 2006;202:577–83. doi: 10.1016/j.prp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Kaur S, Sharma N, Krishn SR, Lakshmanan I, Rachagani S, Baine MJ, Smith LM, Lele SM, Sasson AR, Guha S, Mallya K, Anderson JM, Hollingsworth MA, et al. MUC4-mediated regulation of acute phase protein lipocalin 2 through HER2/AKT/NF-kappaB signaling in pancreatic cancer. Clin Cancer Res. 2014;20:688–700. doi: 10.1158/1078-0432.ccr-13-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–20. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yonezawa S, Higashi M, Yamada N, Yokoyama S, Kitamoto S, Kitajima S, Goto M. Mucins in human neoplasms: clinical pathology, gene expression and diagnostic application. Pathol Int. 2011;61:697–716. doi: 10.1111/j.1440-1827.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 32.Ramsauer VP, Pino V, Farooq A, Carothers Carraway CA, Salas PJ, Carraway KL. Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell. 2006;17:2931–41. doi: 10.1091/mbc.E05-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CA, Carraway KL. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene. 2002;21:7524–32. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- 34.Giuntoli RL, 2nd, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546–50. [PubMed] [Google Scholar]
- 35.Zhu Y, Zhang JJ, Zhu R, Zhu Y, Liang WB, Gao WT, Yu JB, Xu ZK, Miao Y. The increase in the expression and hypomethylation of MUC4 gene with the progression of pancreatic ductal adenocarcinoma. Med Oncol. 2011;28(Suppl 1):S175–84. doi: 10.1007/s12032-010-9683-0. [DOI] [PubMed] [Google Scholar]
- 36.Price-Schiavi SA, Andrechek E, Idris N, Li P, Rong M, Zhang J, Carothers Carraway CA, Muller WJ, Carraway KL. Expression, location, and interactions of ErbB2 and its intramembrane ligand Muc4 (sialomucin complex) in rat mammary gland during pregnancy. J Cell Physiol. 2005;203:44–53. doi: 10.1002/jcp.20200. [DOI] [PubMed] [Google Scholar]
- 37.Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y, Ganti AK, Batra SK. Targeting EGF-receptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6:5164–81. doi: 10.18632/oncotarget.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasseur R, Skrypek N, Duchene B, Renaud F, Martinez-Maqueda D, Vincent A, Porchet N, Van Seuningen I, Jonckheere N. The mucin MUC4 is a transcriptional and post-transcriptional target of K-ras oncogene in pancreatic cancer. Implication of MAPK/AP-1, NF-kappaB and RalB signaling pathways. Biochim Biophys Acta. 2015;1849:1375–84. doi: 10.1016/j.bbagrm.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Damera G, Xia B, Sachdev GP. IL-4 induced MUC4 enhancement in respiratory epithelial cells in vitro is mediated through JAK-3 selective signaling. Respir Res. 2006;7:39. doi: 10.1186/1465-9921-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamy V, Bousserouel S, Gosse F, Minker C, Lobstein A, Raul F. Lupulone triggers p38 MAPK-controlled activation of p53 and of the TRAIL receptor apoptotic pathway in human colon cancer-derived metastatic cells. Oncol Rep. 2011;26:109–14. doi: 10.3892/or.2011.1273. [DOI] [PubMed] [Google Scholar]
- 41.Miyahara N, Shoda J, Ishige K, Kawamoto T, Ueda T, Taki R, Ohkohchi N, Hyodo I, Thomas MB, Krishnamurthy S, Carraway KL, Irimura T. MUC4 interacts with ErbB2 in human gallbladder carcinoma: potential pathobiological implications. Eur J Cancer. 2008;44:1048–56. doi: 10.1016/j.ejca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Chapela PJ, Broaddus R, Hawkins SM, Lessey BA, Carson DD. Cytokine Stimulation of MUC4 Expression in Human Female Reproductive Tissue Carcinoma Cell Lines and Endometrial Cancer. J Cell Biochem. 2015;116:2649–57. doi: 10.1002/jcb.25213. [DOI] [PubMed] [Google Scholar]
- 43.Jonckheere N, Vincent A, Perrais M, Ducourouble MP, Male AK, Aubert JP, Pigny P, Carraway KL, Freund JN, Renes IB, Van Seuningen I. The human mucin MUC4 is transcriptionally regulated by caudal-related homeobox, hepatocyte nuclear factors, forkhead box A, and GATA endodermal transcription factors in epithelial cancer cells. J Biol Chem. 2007;282:22638–50. doi: 10.1074/jbc.M700905200. [DOI] [PubMed] [Google Scholar]
- 44.Baker AC, Eltoum I, Curry RO, Stockard CR, Manne U, Grizzle WE, Chhieng D. Mucinous expression in benign and neoplastic glandular lesions of the uterine cervix. Arch Pathol Lab Med. 2006;130:1510–1515. doi: 10.1043/1543-2165(2006)130[1510:meiban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 45.Narayan G, Bourdon V, Chaganti S, Arias-Pulido H, Nandula SV, Rao PH, Gissmann L, Durst M, Schneider A, Pothuri B, Mansukhani M, Basso K, Chaganti RS, et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer. 2007;46:373–84. doi: 10.1002/gcc.20418. [DOI] [PubMed] [Google Scholar]
- 46.Munro EG, Jain M, Oliva E, Kamal N, Lele SM, Lynch MP, Guo L, Fu K, Sharma P, Remmenga S, Growdon WB, Davis JS, Rueda BR, et al. Upregulation of MUC4 in cervical squamous cell carcinoma: pathologic significance. Int J Gynecol Pathol. 2009;28:127–33. doi: 10.1097/PGP.0b013e318184f3e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carraway KL, Idris N. Regulation of sialomucin complex/Muc4 in the female rat reproductive tract. Biochem Soc Trans. 2001;29:162–6. doi: 10.1042/0300-5127:0290162. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty S, Jain M, Sasson AR, Batra SK. MUC4 as a diagnostic marker in cancer. Expert Opin Med Diagn. 2008;2:891–910. doi: 10.1517/17530059.2.8.891. [DOI] [PubMed] [Google Scholar]
- 49.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–6. doi: 10.1158/0008-5472.can-06-3114. [DOI] [PubMed] [Google Scholar]
- 50.Workman HC, Miller JK, Ingalla EQ, Kaur RP, Yamamoto DI, Beckett LA, Young LJ, Cardiff RD, Borowsky AD, Carraway KL, Sweeney C, Carraway KL., 3rd The membrane mucin MUC4 is elevated in breast tumor lymph node metastases relative to matched primary tumors and confers aggressive properties to breast cancer cells. Breast Cancer Res. 2009;11:R70. doi: 10.1186/bcr2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukhopadhyay P, Lakshmanan I, Ponnusamy MP, Chakraborty S, Jain M, Pai P, Smith LM, Lele SM, Batra SK. MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS One. 2013;8:e54455. doi: 10.1371/journal.pone.0054455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ponnusamy MP, Seshacharyulu P, Vaz A, Dey P, Batra SK. MUC4 stabilizes HER2 expression and maintains the cancer stem cell population in ovarian cancer cells. J Ovarian Res. 2011;4:7. doi: 10.1186/1757-2215-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 55.Zhi X, Tao J, Xie K, Zhu Y, Li Z, Tang J, Wang W, Xu H, Zhang J, Xu Z. MUC4-induced nuclear translocation of beta-catenin: a novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014;346:104–13. doi: 10.1016/j.canlet.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 56.Gao L, Liu J, Zhang B, Zhang H, Wang D, Zhang T, Liu Y, Wang C. Functional MUC4 suppress epithelial-mesenchymal transition in lung adenocarcinoma metastasis. Tumour Biol. 2014;35:1335–41. doi: 10.1007/s13277-013-1178-0. [DOI] [PubMed] [Google Scholar]
- 57.Lakshmanan I, Seshacharyulu P, Haridas D, Rachagani S, Gupta S, Joshi S, Guda C, Yan Y, Jain M, Ganti AK, Ponnusamy MP, Batra SK. Novel HER3/MUC4 oncogenic signaling aggravates the tumorigenic phenotypes of pancreatic cancer cells. Oncotarget. 2015;6:21085–99. doi: 10.18632/oncotarget.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–6. doi: 10.1309/7y7n-m1wm-r0yk-m2va. [DOI] [PubMed] [Google Scholar]
- 59.Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, Moniaux N, Batra SK. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19:1386–94. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 60.Ponnusamy MP, Lakshmanan I, Jain M, Das S, Chakraborty S, Dey P, Batra SK. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene. 2010;29:5741–54. doi: 10.1038/onc.2010.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radhakrishnan P, Mohr AM, Grandgenett PM, Steele MM, Batra SK, Hollingsworth MA. MicroRNA-200c modulates the expression of MUC4 and MUC16 by directly targeting their coding sequences in human pancreatic cancer. PLoS One. 2013;8:e73356. doi: 10.1371/journal.pone.0073356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skrypek N, Duchene B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2012;28(32):1714–23. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101:1155–61. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sargeant HR, Miller HM, Shaw M-A. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Molecular Immunology. 2011;48:2113–21. doi: 10.1016/j.molimm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Fontanesi L, Bertolini F, Dall'olio S, Buttazzoni L, Gallo M, Russo V. Analysis of Association Between the MUC4 g.8227C>G Polymorphism and Production Traits in Italian Heavy Pigs Using a Selective Genotyping Approach. Anim Biotechnol. 2012;23:147–55. doi: 10.1080/10495398.2011.653462. [DOI] [PubMed] [Google Scholar]
- 66.Joller D, Jorgensen CB, Bertschinger HU, Python P, Edfors I, Cirera S, Archibald AL, Burgi E, Karlskov-Mortensen P, Andersson L, Fredholm M, Vogeli P. Refined localization of the Escherichia coli F4ab/F4ac receptor locus on pig chromosome 13. Anim Genet. 2009;40:749–52. doi: 10.1111/j.1365-2052.2009.01881.x. [DOI] [PubMed] [Google Scholar]
- 67.Peng QL, Ren J, Yan XM, Huang X, Tang H, Wang YZ, Zhang B, Huang LS. The g.243A>G mutation in intron 17 of MUC4 is significantly associated with susceptibility/resistance to ETEC F4ab/ac infection in pigs. Anim Genet. 2007;38:397–400. doi: 10.1111/j.1365-2052.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 68.Rampoldi A, Jacobsen MJ, Bertschinger HU, Joller D, Burgi E, Vogeli P, Andersson L, Archibald AL, Fredholm M, Jorgensen CB, Neuenschwander S. The receptor locus for Escherichia coli F4ab/F4ac in the pig maps distal to the MUC4-LMLN region. Mamm Genome. 2011;22:122–9. doi: 10.1007/s00335-010-9305-3. [DOI] [PubMed] [Google Scholar]
- 69.Secher T, Samba-Louaka A, Oswald E, Nougayrede JP. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS One. 2013;8:e77157. doi: 10.1371/journal.pone.0077157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das S, Rachagani S, Sheinin Y, Smith LM, Gurumurthy CB, Roy HK, Batra SK. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene. 2016;35:2645–54. doi: 10.1038/onc.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krishn SR, Kaur S, Smith LM, Johansson SL, Jain M, Patel A, Gautam SK, Hollingsworth MA, Mandel U, Clausen H, Lo WC, Fan WT, Manne U, et al. Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: Prospective pathological implication(s) for early diagnosis of colon cancer. Cancer Lett. 2016;374:304–14. doi: 10.1016/j.canlet.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, Batra SK. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9:1419–31. doi: 10.1158/1535-7163.mct-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–20. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 74.Ponnusamy MP, Singh AP, Jain M, Chakraborty S, Moniaux N, Batra SK. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br J Cancer. 2008;99:520–6. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–8. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–30. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 77.Macha MA, Rachagani S, Pai P, Gupta S, Lydiatt WM, Smith RB, Johansson SL, Lele SM, Kakar SS, Farghaly H, Lee JH, Meza J, Ganti AK, et al. MUC4 regulates cellular senescence in head and neck squamous cell carcinoma through p16/Rb pathway. Oncogene. 2015;34:1698–708. doi: 10.1038/onc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soto P, Price-Schiavi SA, Carraway KL. SMAD2 and SMAD7 involvement in the post-translational regulation of Muc4 via the transforming growth factor-beta and interferon-gamma pathways in rat mammary epithelial cells. J Biol Chem. 2003;278:20338–44. doi: 10.1074/jbc.M301886200. [DOI] [PubMed] [Google Scholar]
- 80.Cai H, Palitzsch B, Hartmann S, Stergiou N, Kunz H, Schmitt E, Westerlind U. Antibody induction directed against the tumor-associated MUC4 glycoprotein. Chembiochem. 2015;16:959–67. doi: 10.1002/cbic.201402689. [DOI] [PubMed] [Google Scholar]
- 81.Wei J, Gao W, Wu J, Meng K, Zhang J, Chen J, Miao Y. Dendritic cells expressing a combined PADRE/MUC4-derived polyepitope DNA vaccine induce multiple cytotoxic T-cell responses. Cancer Biother Radiopharm. 2008;23:121–8. doi: 10.1089/cbr.2007.0427. [DOI] [PubMed] [Google Scholar]
- 82.Bafna S, Singh AP, Moniaux N, Eudy JD, Meza JL, Batra SK. MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 2008;68:9231–8. doi: 10.1158/0008-5472.CAN-08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pino V, Ramsauer VP, Salas P, Carothers Carraway CA, Carraway KL. Membrane mucin Muc4 induces density-dependent changes in ERK activation in mammary epithelial and tumor cells: role in reversal of contact inhibition. J Biol Chem. 2006;281:29411–20. doi: 10.1074/jbc.M604858200. [DOI] [PubMed] [Google Scholar]
- 84.Bax DA, Haringsma J, Einerhand AW, van Dekken H, Blok P, Siersema PD, Kuipers EJ, Kusters JG. MUC4 is increased in high grade intraepithelial neoplasia in Barrett's oesophagus and is associated with a proapoptotic Bax to Bcl-2 ratio. J Clin Pathol. 2004;57:1267–72. doi: 10.1136/jcp.2004.017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kozloski GA, Carraway CA, Carraway KL. Mechanistic and signaling analysis of Muc4-ErbB2 signaling module: new insights into the mechanism of ligand-independent ErbB2 activity. J Cell Physiol. 2010;224:649–57. doi: 10.1002/jcp.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Theodoropoulos G, Carraway CA, Carraway KL. MUC4 involvement in ErbB2/ErbB3 phosphorylation and signaling in response to airway cell mechanical injury. J Cell Biochem. 2009;107:112–22. doi: 10.1002/jcb.22106. [DOI] [PubMed] [Google Scholar]
- 87.Matull WR, Andreola F, Loh A, Adiguzel Z, Deheragoda M, Qureshi U, Batra SK, Swallow DM, Pereira SP. MUC4 and MUC5AC are highly specific tumour-associated mucins in biliary tract cancer. Br J Cancer. 2008;98:1675–81. doi: 10.1038/sj.bjc.6604364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmuck RB, de Carvalho-Fischer CV, Neumann C, Pratschke J, Bahra M. Distal bile duct carcinomas and pancreatic ductal adenocarcinomas: postulating a common tumor entity. Cancer Med. 2016;5:88–99. doi: 10.1002/cam4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang X, Wang X, Lu SM, Chen C, Wang J, Zheng YY, Ren BH, Xu L. Clinicopathological and prognostic significance of MUC4 expression in cancers: evidence from meta-analysis. Int J Clin Exp Med. 2015;8:10274–83. [PMC free article] [PubMed] [Google Scholar]
- 90.Jonckheere N, Lahdaoui F, Van Seuningen I. Targeting MUC4 in pancreatic cancer: miRNAs. Oncoscience. 2015;2:799–800. doi: 10.18632/oncoscience.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koo DH, Lee HJ, Ahn JH, Yoon DH, Kim SB, Gong G, Son BH, Ahn SH, Jung KH. Tau and PTEN status as predictive markers for response to trastuzumab and paclitaxel in patients with HER2-positive breast cancer. Tumour Biol. 2015;36:5865–71. doi: 10.1007/s13277-015-3258-9. [DOI] [PubMed] [Google Scholar]
- 92.Li G, Zhao L, Li W, Fan K, Qian W, Hou S, Wang H, Dai J, Wei H, Guo Y. Feedback activation of STAT3 mediates trastuzumab resistance via upregulation of MUC1 and MUC4 expression. Oncotarget. 2014;5:8317–29. doi: 10.18632/oncotarget.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon KY, Ro JY, Singhal N, Killen DE, Sienko A, Allen TC, Zander DS, Barrios R, Haque A, Cagle PT. MUC4 expression in non-small cell lung carcinomas: relationship to tumor histology and patient survival. Arch Pathol Lab Med. 2007;131:593–8. doi: 10.1043/1543-2165(2007)131[593:MEINCL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 94.Algamas-Dimantov A, Yehuda-Shnaidman E, Peri I, Schwartz B. Epigenetic control of HNF-4alpha in colon carcinoma cells affects MUC4 expression and malignancy. Cell Oncol (Dordr) 2013;36:155–67. doi: 10.1007/s13402-012-0123-3. [DOI] [PubMed] [Google Scholar]
- 95.Singh AP, Chauhan SC, Bafna S, Johansson SL, Smith LM, Moniaux N, Lin MF, Batra SK. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66:421–9. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 96.Legrier ME, de Pinieux G, Boye K, Arvelo F, Judde JG, Fontaine JJ, Bara J, Poupon MF. Mucinous differentiation features associated with hormonal escape in a human prostate cancer xenograft. Br J Cancer. 2004;90:720–7. doi: 10.1038/sj.bjc.6601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cozzi PJ, Wang J, Delprado W, Perkins AC, Allen BJ, Russell PJ, Li Y. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis. 2005;22:565–73. doi: 10.1007/s10585-005-5376-z. [DOI] [PubMed] [Google Scholar]
- 98.Mahomed F. Recent advances in mucin immunohistochemistry in salivary gland tumors and head and neck squamous cell carcinoma. Oral Oncol. 2011;47:797–803. doi: 10.1016/j.oraloncology.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 99.Kusafuka K, Muramatsu K, Iida Y, Mori K, Miki T, Suda T, Fuke T, Kamijo T, Onitsuka T, Nakajima T. MUC expression in adenosquamous carcinoma of the head and neck regions of Japanese patients: immunohistochemical analysis. Pathol Int. 2014;64:104–14. doi: 10.1111/pin.12144. [DOI] [PubMed] [Google Scholar]
- 100.Hamada T, Wakamatsu T, Miyahara M, Nagata S, Nomura M, Kamikawa Y, Yamada N, Batra SK, Yonezawa S, Sugihara K. MUC4: a novel prognostic factor of oral squamous cell carcinoma. Int J Cancer. 2012;130:1768–76. doi: 10.1002/ijc.26187. [DOI] [PubMed] [Google Scholar]