Abstract
Thitarodes pui larvae have a limited distribution in the Tibetan Plateau and are the host of a parasitic fungus, Ophiocordyceps sinensis. Low temperature is a main environmental stress. However, understanding of T. pui cold adaptation mechanisms is insufficient. Delta‐9‐acyl‐CoA desaturase (D9D) is closely correlated with cold adaptation for many organisms. To further understand the cold adaptation processes in T. pui larvae, two D9Ds, TpdesatA and TpdesatB were sequenced, and expression patterns were investigated during different seasons and cold exposure (under 0°C) in the laboratory. The full lengths of two cDNAs are 1,290 bp and 1,603 bp, and the ORFs encode a polypeptide of 348 and 359 amino acids, respectively. Four transmembrane domains, three conserved histidine residues and five hydrophobic regions exist in these two sequences. The expression level of TpdesatA is up‐regulated in the long‐term cold exposure and negatively correlated with temperature in seasonal patterns. TpdesatB responds to cold temperature in short‐term cold exposure and positively corresponds temporarily in seasonal expression. Two D9Ds may have different substrate specificities, TpdesatA tends to use C16:0 and C18:0 as substrate while TpdesatB prefers C18:0. In conclusion, TpdesatA may play a very important role in T. pui cold tolerance and TpdesatB regulates function in short‐term cold exposure and content change of fatty acids in the body.
Keywords: acclimation, delta‐9‐acyl‐CoA desaturases, experimental evolution, thermal adaptation, Thitarodes pui
1. Introduction
For overwintering insects, low temperature is one of the most serious environmental stresses affecting their survival. In fact, overwintering insects survive low temperature due to a variety of physiological and biochemical adaptations (Baust & Rojas, 1985; Clark et al., 2009). Until recently, cold hardiness in insects was most often discussed in terms of cryoprotectants, membrane lipids, and heat‐shock proteins (Storey & Storey, 2012; Teets & Denlinger, 2013; Yocum, 2001). The transition of cell membrane lipids from a liquid crystalline phase to a gel phase is an important cause of cold injuries under nonfreezing conditions (Michaud & Denlinger, 2006). Further investigations revealed that accumulation of unsaturated fatty acids (UFAs) contributed to the fluidity of cellar membranes which are susceptible to cold (Khani, Moharamipour, & Barzegar, 2007; Koštá, Berkova, & Šimek, 2003; Michaud & Denlinger, 2006). In most cases, more UFAs and less saturated fatty acids (SFAs) were detected in response to cold exposure (Los & Murata, 2004; Yi, Guo, Zou, & Zhang, 2015). Studies on the composition of cell membrane lipids in many species of microorganisms, plants, and animals under different temperatures have revealed the universal occurrence of remodeling of cell membrane lipids in response to changes in ambient temperature, a phenomenon known as homeoviscous adaptation (Hazel, 1995; Teets & Denlinger, 2013). The increasing UFAs are considered to play a role in maintaining the liquid crystalline phase at low temperatures (Kayukawa, Chen, Hoshizaki, & Ishikawa, 2007).
Delta‐9‐acyl‐CoA desaturase (D9D) is an important enzyme that introduces a double bond into SFAs, and has been shown to play an essential role in cold hardiness by increasing the ratio of UFAs to SFAs in cell membranes (Hsieh & Kuo, 2005; Tiku, Gracey, Macartney, Beynon, & Cossins, 1996). Certain groups of reports demonstrated that up‐regulation of the D9D gene occurs during cold exposure. Tiku et al. (1996) indicated that transcription of the D9D gene increased tenfold in the liver of cold‐exposed carp. Similar findings were also proved in Oreochromis niloticus (Zerai, Fitzsimmons, & Collier, 2010), as well as Chanos chanos and Ctenopharyngodon idella (Hsieh & Kuo, 2005). For insects, the D9D gene was firstly proved to participate in cold adaptation mechanisms in Delia antique. In that study, twofold to tenfold up‐regulation of the D9D gene was induced in brain tissues, malpighian tubules, and the midgut when D. antique was exposed to cold (Kayukawa et al., 2007). This same result occurred in Sarcophaga crassipalpis (Rinehart, Robich, & Denlinger, 2010), Folsomia candida (Waagner, Holmstrup, Bayley, & Jesper, 2013), and Aedes albopictus (Reynolds et al. 2012). Meanwhile, another kind of D9D, which was associated with dietary alterations, was found in Cyprinus carpio and Acheta domesticus (Batcabe, Howell, Blomquist, & Borgeson, 2000; Polley et al., 2003).
Thitarodes pui (Zhang et al.) (Lepidoptera: Hepialidae) (Figure 1) was first reported as Hepialus pui (Zhang, Gu, & Liu, 2007) in China but was later moved to the genus Thitarodes (Zou, 2009; Zou, Liu, & Zhang, 2010). Larvae of Thitarodes in Southeast Asia are the host of the fungus Ophiocordyceps sinensis (Berkeley) Saccardo (Dong Chong Xia Cao in Chinese) (Winkler, 2009), which is one of the most valuable resources for traditional Chinese medicine (Buenz, Bauer, Osmundson, & Motley, 2005; Yue, Ye, Lin, & Zhou, 2013; Zhu, Halpern, & Jones, 1998). T. pui has a limited distribution of 4,100–5,000 m surrounding Mount Segrila in Tibet (Zhang et al., 2007). In this region, the average annual temperature is below 5°C and the soil is periodically frozen and thawed, and low temperature is considered a main environmental stress for T. pui (Yi, Guo, et al., 2015). Nevertheless, Thitarodes larvae can endure extreme temperatures at −12 and −20°C, but the mechanism for this cold tolerance is unclear (Yang, Li, Shu, & Yang, 1996).
Figure 1.

Adults (a) and larva (b) of Thitarodes pui
Our previous work indicated that proteins, total sugar, and total fat in the hemolymph of T. pui larvae showed negative correlation with soil temperature (Yi, Zhang, Guo, Min, & Zou, 2015). In addition, HSP90 of T. pui, rather than HSP70, responds to temperature changes and potentially plays a key role in cold tolerance (Zou, Sun, Li, & Zhang, 2011). In addition, trehalose‐6‐phosphate synthase is involved in the complicated cold adaptation process in T. pui (Min et al., 2016). To obtain a further understanding of cold adaptation, two D9D genes (TpdesatA and TpdesatB) were sequenced in T. pui larvae and their expression patterns were investigated by real‐time PCR during different seasons and cold exposure under 0°C in the laboratory. The results might serve to build a framework for comprehensively understanding the biology and molecular mechanisms of T. pui adaptation to thermal stress.
2. Materials and methods
2.1. Temperature measurement of soil with T. pui larvae
Temperature of soil at 20 cm below the surface was measured with Hobo Pro temperature and RH data logger (Model H08‐032‐08, Eco‐tech Co. LTD, USA). The data logger was set to record the temperature every 30 min, and the data were downloaded every 30 days with BoxCar Pro software (version 4.3, Onset Computer Corporation, USA) (Zou et al., 2011).
2.2. Insect collection and cold exposure regime
The investigation of seasonal expression patterns was processed from July 2008 to June 2009. In the middle of every month, six individuals of the sixth instar T. pui larvae were collected from Mt. Segrila (4,156 m, 29°37′N, 94°37′E) in the Tibetan Plateau, and these samples were used to transcription level analysis. Experiments under cold exposure were processed from July to August in 2013. More than 100 individuals of sixth instar T. pui larvae were collected in July 2013 at the same area, then fed in soil at laboratory, and the environment‐controlled at 10°C; after fifteen days, they were used to cold exposure experience. Ten individuals were used as a control group. The other were reared at 0°C (Thermo Scientific Precision, USA) and collected at different times, including short term (1, 3, 6, 12 hr), midterm (24, 48, 72 hr, 5 days), and long term (7, 10, and 15 days).
2.3. Collection of fat body
All samples were dissected to obtain fat bodies. The fat bodies isolated from two larvae were mixed and then stored in RNA protect solution (TaKaRa, Japan) at −80°C.
2.4. Cloning the full‐length cDNA of two D9D genes
Total RNA was extracted from the fat body of one individual using Trizol Reagent Kit (Invitrogen, USA) according to the manufacturer's instructions, then dissolved by 30 μl diethylpyrocarbonate (DEPC) water and stored at −80°C. The RNA was quantitated by NanoDrop 2000 (BioSpec‐mini, Shimadzu) and transferred to cDNA by using AMV reverse transcriptase (TaKaRa, Dalian, China) under the manufacturer's protocol. Degenerate primers were designed based on the conserved amino acid sequences of known D9D genes of other Lepidoptera insects (Table 1) and then used to amplify the initial segments of two D9D genes. The PCR was conducted with 30 cycles under condition of 30 s at 94°C for denaturation, 30 s at 45°C for annealing, 1 min at 72°C for extension. Specific primers for 5′‐RACE (Rapid amplification of cDNA ends) and 3′‐RACE (Table 1) were synthesized based on the initial segments of two D9D genes. The 5′ and 3′ RACE were processed using the SMART RACE cDNA Amplification Kit (Clontech, CA, USA). The PCR was placed in 50 μl volume with 30 cycles under the condition of 30 s at 94°C, 30 s at 55°C, 2 min at 72°C.
Table 1.
Primers used for cloning and expression analysis of two D9D in Thitarodes pui
| Fragment | Primer | Primer sequence (F/R) 5′→ 3′ |
|---|---|---|
| Tpdesat | desF1 | TGGGCDCACAARWSHTAYAA |
| desF2 | GAYCAYMGNATGCAYCAYAA | |
| desF3 | GAYGCBGAYCCNCAYAAYGC | |
| desR1 | TGRTAGTTGTGGAADCCYTC | |
| desR2 | TTVADRTCRTAWGCCCA | |
| TpdesatA | TdAF1 | TTTTCTCTCATATGGGCTGGC |
| TdAF2 | TCCGTCAGCCTGCTTACCCT | |
| TdAR1 | GGCTACGAAAAACGCAG | |
| TdAR2 | CTTCTGAAATGTAACGATGGGGT | |
| TdAR3 | ATAAGCCAGCCCATATGAGAGAA | |
| QdesatAF | CGTCAGCCTGCTTACCCTTG | |
| QdesatAR | GCCCGTTCGTATGATCCTCTTC | |
| TpdesatB | TdBF1 | TGATACAGACGCCGACCCG |
| TdBF2 | TGCCGCTTGTCTGCTTCATT | |
| TdBF3 | CGACCCTATCCTAGCCTTCCA | |
| TdBR1 | GGATAGGGTCGTTCTCG | |
| TdBR2 | TGTGCGGGTCGGCGTCTGTATC | |
| TdBR3 | CTATCACAGAGTCCTGGAATGC | |
| QdesatBF | TGATACAGACGCCGACCCG | |
| QdesatBR | GGCAAAATGAAGCAGACAAGC | |
| β‐Actin | QActinF | TAACCCCAAAGCGAACAGAGA |
| QActinR | GCCAAGTCCAGACGGAGAATG |
2.5. Sequence analysis
The obtained fragments of two D9D genes were assembled by DNASTAR and the ORFs were identified through ORF Finder (Thompson, Higgins, & Gibson, 1994), respectively. Amino acid sequences were deduced from the corresponding cDNA sequences by using the translation tool on the ExPASy Proteomics Web site, and analogs were searched by BLASTP at the NCBI (Zou et al., 2011). The molecular weight (MW) and isoelectric point (PI) of the deduced amino acid sequences were predicted from the ExPASy Proteomics Web site. Analysis of the transmembrane domains and hydrophobic regions were performed using Kyte–Doolittle hydropathy plots in DNASTAR. Multiple alignments were performed among TpdesatA, TpdesatB as well as the analogous amino acid sequences by DNAMAN. Finally, base on the amino acid sequences of known D9D genes in Lepidoptera and Dipteran from GenBank (Kayukawa et al., 2007), phylogenetic tree was constructed using the neighbor‐joining method in MEGA software with 1,000 bootstrap replications.
2.6. Quantitative analysis of two D9D genes
The seasonal expression and cold adaptation changes of two D9D genes were investigated through RT‐PCR in CFX96™ Real‐Time System. Two pairs of primers were designed for the quantitative analysis of two genes as well as a pair of primers for the control (β‐actin) (Table 1). The reaction was performed following the manufacturer's instructions of SYBR® Premix Ex Taq™ (TaKaRa, Dalian, China) with the conditions as followed: 3 min at 95°C followed by 40 cycles of 95°C for 15 s, 30 s at 60°C and 30 s at 72°C. The remaining curve analysis at the end of program was used to test the specificity of primers. Experimental operation was repeated three times for each group. 2−ΔΔCt method was used to determine the expression profiles of TpdesatA and TpdesatB. The relative mRNA levels of TpdesatA and TpdesatB in July and 0 hr were set as 1, respectively.
2.7. Statistical analysis
Means and variances of treatments were analyzed using SPSS program (version 19.0, IBM Inc., USA), and the relative mRNA levels of D9D in July or control group was set as 1. All data were shown as mean ± SD. The means were compared with variance (ANOVA) and Tukey's studentized range test with the level of significant difference at p < .05 and highly significant difference at p < .01.
3. Results
3.1. Sequence identification and characterization of two D9D genes
The full length of two D9D genes, TpdesatA and TpdesatB, were obtained through overlapping PCR and RACE. They are 1,290 bp and 1,603 bp, and their nucleotide sequences and deduced amino acid sequences are shown in Figure 2a,b, respectively. Their nucleotide sequences have been deposited in NCBI GenBank with accession numbers GU126468 and GU205814, respectively.
Figure 2.

Nucleotide and deduced amino acid sequences of TpdesatA (a) and TpdesatB (b) The start and stop codons were showed as bold. Four transmembrane domains and three conserved histidine residues were boxed and underlined, separately. Five hydrophobic regions were leaned and underlined
The full length of TpdesatA cDNA contains 65 bp in the 5′‐untranslated region (UTR), 1,041 bp in the open reading frame (ORF) and 184 bp in 3′‐UTR. The ORF encodes a polypeptide of 348 amino acids. The inferred molecular mass of the mature protein is 107.5 kDa with an estimated PI of 5.02 (Figure 2a). The TpdesatB includes 103 bp in 5′‐UTR, 420 bp in 3′‐UTR and 1,080 bp in an ORF encoding a polypeptide of 359 amino acids with MWs of 133.4 kDa and PI of 4.96 (Figure 2b). Otherwise, three histidine clusters can be found in two D9D (HXXXH, HXXHH, and EXXHXXHH) (Figure 2a,b). The presence of five hydrophobic regions and four transmembrane domains in TpdesatA and TpdesatB was revealed with Kyte–Doolittle hydropathy analysis. The amino acids of TpdesatA and TpdesatB were aligned to those D9D genes of other 12 species, and analysis results showed that TpdesatA is similar to Manduca sexta (65.6%), Bombyx mori (65.6%), Lampronia capitella (65.3%); TpdesatB showed a similarity with L. capitella (59.4%) and Dendrolimus punctatus (59.4%), followed by Epiphyas postvittana (59.1%). Otherwise, the similarity of TpdesatA and TpdesatB is 45.5%. Same feature sequences were also existed in others species D9D sequence (Figure 3).
Figure 3.
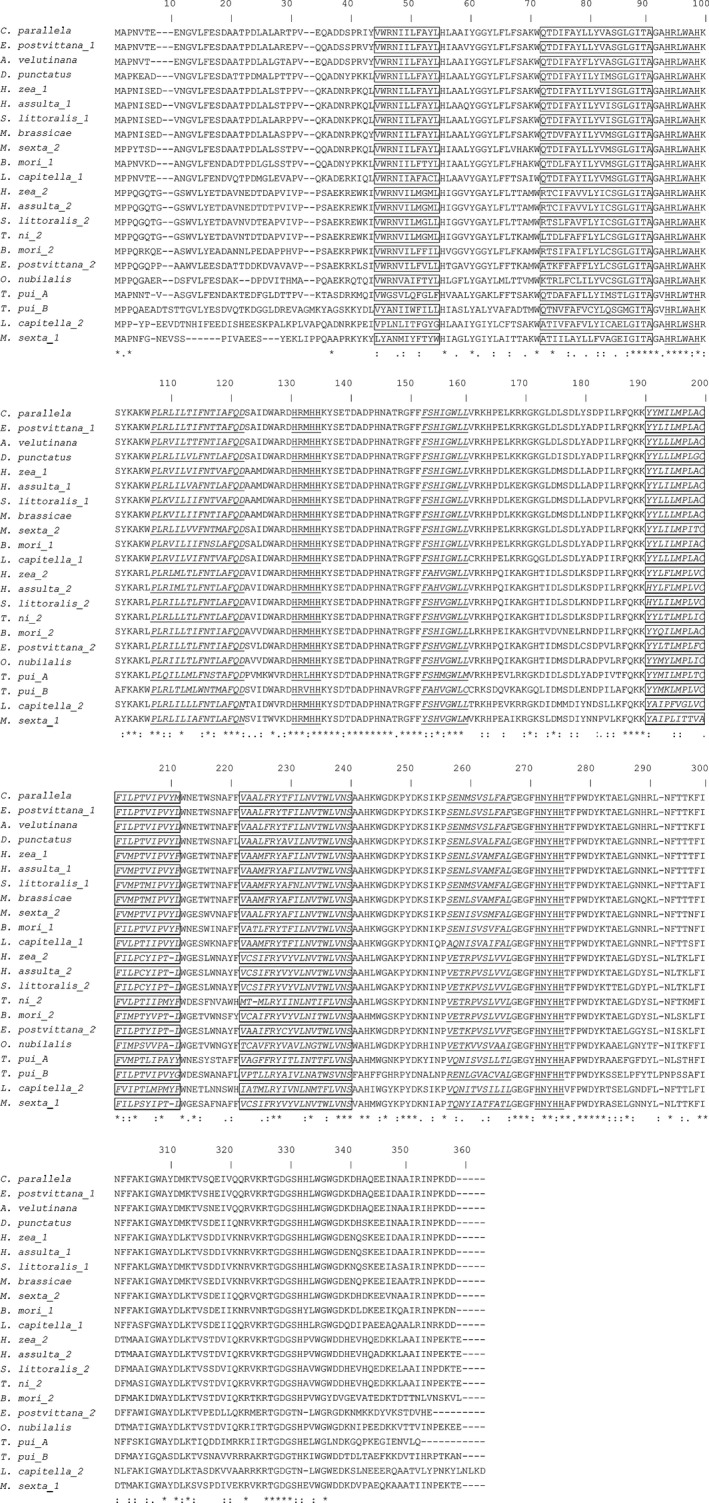
Multiple alignment of Tpdesat amino acids in insects Four transmembrane domains and three conserved histidine residues were boxed and underlined, separately. Five hydrophobic regions were leaned and underlined
A phylogenetic tree was constructed by the neighbor‐joining method, based on amino acid sequences of 20 known D9D in Lepidopteran and Dipteran. Four denatures groups with different substrate specificities were clearly identified as follows (substrate preferences are indicated in parentheses): △9 (16 > 18), △9 (16 = 18), △9 (18 > 16) and △9 (14–26), respectively. It was shown that TpdesatA gene belongs to the △9 (16 = 18) group and TpdesatB gene belongs to the △9 (18 > 16) (Figure 4).
Figure 4.

A neighbor‐joining tree of delta‐9‐acyl‐CoA desaturases in known lepidopteran and dipteran insects Bootstrap probabilities with 1,000 replicates, Genbank sequence accession numbers are given after the abbreviated species name (A. velutinana, Argyrotaenia velutinana; C. parallela, Choristoneura parallela; D. antique, Delia antique; E. postvittana, Epiphyas postvittana; H. zea, Helicoverpa zea; M. domestica, Musca domestica; O. furnacalis, O. furnacalis; O. nubilalis, Ostrinia nubilalis; P. octo, Planotortrix octo; S. littoralis, Spodoptera littoralis; T. ni, Trichoplusia ni)
3.2. Seasonal expression patterns of two D9D genes
Quantitative analysis was performed to indicate the seasonal expression patterns of two D9D genes through RT‐PCR. The soil temperature kept low level in whole year (Figure 5a). As shown in Figure 5b, the expression of TpdesatA exhibited a negative correlation with temperature (y = 4.286 − 0.227x) (r = −.388, p = .390). The transcription of TpdesatA reached the highest level in December while the temperature remained at low level. In spite of the lowest level in March and July, the expression of TpdesatA remained stable in January, May, August as well as October. Expression of TpdesatB showed a positive correlation with soil temperature (y = 0.656 + 0.035x) (r = .437, p = .326). During the investigation, the expression of TpdesatB sustained in high level in July, August, and October while it dropped and remained at low level from December to May (Figure 5c).
Figure 5.

Relative mRNA levels of TpdesatA (b) and TpdesatB (c) of Thitarodes pui in different seasons with different soil temperature (a). Data represent means ± SD from three replicate experiments (p < .05), actin gene was used as reference one
3.3. Expression patterns of two D9D genes during cold exposure under 0°C
0°C was set to explore the cold adaptation mechanism under stable cold exposure in laboratory. Significant change was detected in the expression level of TpdesatA during the cold stress (F 11, 35 = 40.777, p < .001). In the short‐term and midterm cold exposure, TpdesatA was stable, and remained at a low level from 1 hr to 5 days with no substantial change detected (Figure 6a). In the long‐term cold exposure, the expression of TpdesatA increased from 5 days to the highest level at 10 days (2.43‐fold) and slightly declined to 1.88‐fold at 15 days. Expression of TpdesatB was significantly affected by cold exposure (F 11, 35 = 109.469, p < .001), the expression of TpdesatB was up‐regulated from 6 hr (2.55‐fold) to 5 days (2.97‐fold) with a highest level appeared at 24 hr (3.59‐fold), and TpdesatB was down‐regulated before 3 hr and after 7 days.
Figure 6.

Relative mRNA levels of TpdesatA (a) and TpdesatB (b) of Thitarodes pui under different cold exposure at 0°C Data represent means ± SD from three replicate experiments. Different letters indicate significant differences (p < .05), actin gene was used as the reference one
4. Discussion
As a plateau insect with high cold tolerance, a series of physiological and biochemical mechanisms are evolved in T. pui. The proteins, total sugar, and total fat in the hemolymph as well as the fatty acid in whole body had the negative correlation with soil temperature (Yi, Guo, et al., 2015; Yi, Zhang, et al., 2015). Moreover, trehalose‐6‐phosphate synthase, HSP90 of T. pui, rather than HSP70, responds to temperature changes (Min et al., 2016; Zou et al., 2011). In this paper, TpdesatA and TpdesatB were found to have the relation with cold tolerance of T. pui.
In T. pui larvae, the lipid content changes in response to soil temperature. In phospholipids, C18:1 and C18:2, showed significant negative correlation with soil temperature. However, the fluctuation soil temperature did not cause any significant changes in any of the individual's triacylglycerols fatty acids (Yi, Guo, et al., 2015), to which the molecular mechanism remains unknown. D9D plays an essential role in cold hardiness, by increasing the ratio of unsaturated and saturated fatty acids (UFA/SFA) (Rinehart et al., 2010; Tiku et al., 1996). In this study, two D9D genes were isolated for the first time in Thitarodes insects, and their expression patterns were investigated during different seasons and cold exposure under 0°C.
The two D9D genes separately encoded 346 AA and 359 AA amino acids, which correspond to the size range in other insects from NCBI. Alignment of two D9D genes and isoforms of thirteen other insects revealed D9D genes exist in several highly conserved regions. Four transmembrane domains existed in D9D, suggesting that the sequence spans the lipid bilayer of membranes four times (Kayukawa et al., 2007; Los & Murata, 1998). Three histidine residues in these D9D genes were the highly conserved regions which are catalytically essential in desaturases (Shanklin, Whittle, & Fox, 1994). These histidine residues are suggested to combine with iron atoms at the catalytic center (Los & Murata, 1998). According to the N‐J tree that was constructed in current study, two D9D genes in T. pui occurred in two independent clades, indicating that two D9D has different substrate specificities.
D9D desaturase is a key enzyme in synthetic pathway of UFAs, contributing to the formation of C16:1, C18:1, and C18:2. And these three UFAs are crucial to sustaining the fluidity of membranes under cold conditions (Kayukawa et al., 2007; Khani et al., 2007; Miyazaki, Kayukawa, Chen, Nomura, & Ishikawa, 2006). Previous investigations had proved that D9D gene was critical for cold adaptation in fish (Tiku et al., 1996), bacteria (Sakamoto & Bryant, 1997), and plants (Vega, Del Rio, Bamberg, & Palta, 2004). In this study, the expression level of TpdesatA was up‐regulated in the long‐term cold exposure and remained at a low level at short term and midterm; this indicates that TpdesatA contributes to long‐term cold hardiness.
It was shown that seasonal expression patterns of TpdesatA exhibited a negative correlation with soil temperature (r = −.388, p = .390). Kayukawa et al. (2007) proved that the expression of D9D increased to enhance the cold hardiness in Delia antiqua through up‐regulating the abundance of C16:1 and C18:1. So far, the same results were seen in S. crassipalpis (Rinehart et al., 2010), A. albopictus (Reynolds, Poelchau, Rahman, Armbruster, & Denlinger, 2012) and F. candida (Waagner et al., 2013). At the same time, seasonal cold‐hardening is defined as cold‐hardening that requires at least days to weeks for induction (Teets & Denlinger, 2013). Therefore, we suggest that TpdesatA has contributed to seasonal cold‐hardening. In phospholipids of T. pui larvae, C18:1 was the most abundant UFAs and exhibited a weak negative correlation with soil temperature. C18:2, the second abundant UFA, was highly accumulated at early days of overwintering and fluctuated in lower levels during warmer seasons. Prewinter accumulation was also detected in C18:3 (Yi, Guo, et al., 2015). These three UFAs content change in phospholipids may associate with the regulation of TpdesatA. Moreover, C16:0 was the second abundant in triacylglycerols, with a significant negative correlation with soil temperature (Yi, Guo, et al., 2015). At the same time, TpdesatA gene was clustered in the △9 (16 = 18) group in phylogenetic tree. It indicates that TpdesatA works in seasonal cold‐hardening, and C16:0 and C18:0 served as main substrate in triacylglycerols and phospholipids, respectively.
During cold exposure at 0°C, TpdesatB up‐regulated from 6 hr to 5 days and down‐regulate after 5 days; this indicates that TpdesatB may responds to cold temperature in short‐term cold exposure. The same results were obtained in the winter diapause pupae of D. antiqua (Hao et al., 2012). In the seasonal expression pattern of TpdesatB, it remained at high levels in summer and dropped in winter. It is obvious that high temperature contributed to the transcription of TpdesatB and low temperature suppressed the process (r = .437, p = .326). Polley et al. (2003) indicated that one D9D gene (Cds 1) in carp was well expressed at 30°C and repressed at 15°C, and the regulated pattern was associated with dietary. Down‐regulation in two D9Ds was induced in Drosophila montana (desat 1) and Drosophila virilis (desat 2) by cold acclimatization (Vesala, Salminen, Laiho, Hoikkala, & Kankare, 2012), and there is also evidence that desaturases function in stress resistance (Greenberg, Moran, Coyne, & Wu, 2003). Based on the results showed above, TpdesatB seems to be not responsible for seasonal cold‐hardening. Many researchers believed that the UFAs accompanied by triacylglycerols increased and functioned as energy resources to help organism overcome cold (Joanisse & Storey, 1996; Teets & Denlinger, 2013). Therefore, TpdesatB may act as important short‐term regulate substance in cold exposure and caused the change of the proportion of fatty acids in the body. Meanwhile, TpdesatB rather than TpdesatA was clustered closer to the △9 (18 > 16) group in phylogenetic tree. It indicated that C18 served as the substrate of TpdesatB prior to C16 in the short‐term cold exposure.
Our results suggest that, during cold exposure at 0°C, TpdesatA and TpdesatB contributed to the cold tolerance in T. pui larvae. TpdesatA has contributed to cold hardiness in long term and TpdesatB in short term during cold exposure at 0°C. The seasonal expression pattern of TpdesatA exhibited a negative correlation with temperature. While TpdesatB showed another expression pattern compared to TpdesatA, it is contributed to short‐term cold hardiness and positively corresponds to temperature in seasonal expression pattern. These results indicated that TpdesatA played a very important role in seasonal cold‐hardening and TpdesatB acted as an important regulating substance in short‐term cold exposure and the proportion change of fatty acids in larvae. Different D9D have diverse substrate specificities, TpdesatA tends to use C16:0 and C18:0 as substrate, and TpdesatB prefers to C18:0.
Conflict of interest
None declared.
Acknowledgement
The research was funded by the National Natural Science Foundation of China (31160081, 31460553), Natural Science Foundation of Jiangxi Province (20151BAB204016 and 20161BBF60066), and Graduate Scientific Research Foundation of Jiangxi Province (YC2015‐S025). We would like to thank Miss Alexandra G Duffy (Purdue University, USA) for her support and comments on writing.
Min Q, Cheng S, Xi J, Xin T, Xia B, Zou Z. Differential expression patterns of two delta‐9‐acyl‐CoA desaturases in Thitarodes pui (Lepidoptera: Hepialidae) during different seasons and cold exposure. Ecol Evol. 2017;7:1909–1918. https://doi.org/10.1002/ece3.2792
References
- Batcabe, J. P. , Howell, J. D. , Blomquist, G. J. , & Borgeson, C. E. (2000). Effects of developmental age, ambient temperature, and dietary alterations on Δ12 desaturase activity in the house cricket, Acheta domesticus . Archives of Insect Biochemistry and Physiology, 44, 112–119. [DOI] [PubMed] [Google Scholar]
- Baust, J. G. , & Rojas, R. R. (1985). Review‐insect cold hardiness: Facts and fancy. Journal of Insect Physiology, 31, 755–759. [Google Scholar]
- Buenz, E. J. , Bauer, B. A. , Osmundson, T. W. , & Motley, T. J. (2005). The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis. Journal of Ethnopharmacology, 96, 19–29. [DOI] [PubMed] [Google Scholar]
- Clark, M. S. , Thorne, M. A. S. , Purać, J. , Burns, G. , Hillyard, G. , Popović, Z. D. , … Worland, M. R. (2009). Surviving the cold: Molecular analyses of insect cryoprotective dehydration in the Arctic springtail Megaphorura arctica (Tullberg). BMC Genomics, 10, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, A. J. , Moran, J. R. , Coyne, J. A. , & Wu, C. I. (2003). Ecological adaptation during incipient speciation revealed by precise gene replacement. Science, 302, 1754–1757. [DOI] [PubMed] [Google Scholar]
- Hao, Y. J. , Li, W. S. , He, Z. B. , Si, F. L. , Ishikawa, Y. K. , & Chen, B. (2012). Differential gene expression between summer and winter diapause pupae of the onion maggot Delia antiqua, detected by suppressive subtractive hybridization. Journal of Insect Physiology, 58, 1444–1449. [DOI] [PubMed] [Google Scholar]
- Hazel, J. R. (1995). Thermal adaptation in biological membranes‐is homeoviscous adaptation the explanation? Annual Review of Physiology, 57, 19–42. [DOI] [PubMed] [Google Scholar]
- Hsieh, S. L. , & Kuo, C. M. (2005). Stearoyl‐CoA desaturase expression and fatty acid composition in milkfish (Chanos chanos) and grass carp (Ctenopharyngodon idella) during cold acclimation. Comparative Biochemistry and Physiology – Part B, 141, 95–101. [DOI] [PubMed] [Google Scholar]
- Joanisse, D. R. , & Storey, K. B. (1996). Fatty acid content and enzymes of fatty acid metabolism in overwintering cold‐hardy gall insects. Physiological Zoology, 69, 1079–1095. [Google Scholar]
- Kayukawa, T. , Chen, B. , Hoshizaki, S. , & Ishikawa, Y. (2007). Upregulation of a desaturase is associated with the enhancement of cold hardiness in the onion maggot, Delia antique . Insect Biochemistry and Molecular Biology, 37, 1160–1167. [DOI] [PubMed] [Google Scholar]
- Khani, A. , Moharamipour, S. , & Barzegar, M. (2007). Cold tolerance and trehalose accumulation in overwintering larvae of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). European Journal of Entomology, 104, 385–392. [Google Scholar]
- Koštá, V. , Berkova, P. , & Šimek, P. (2003). Remodelling of membrane phospholipids during transition to diapause and cold‐acclimation in the larvae of Chymomyza costata (Drosophilidae). Comparative Biochemistry and Physiology – Part B, 135, 407–419. [DOI] [PubMed] [Google Scholar]
- Los, D. A. , & Murata, N. (1998). Structure and expression of fatty acid desaturases. Biochimica et Biophysica Acta (BBA)‐Lipids and Lipid Metabolism, 1394, 3–15. [DOI] [PubMed] [Google Scholar]
- Los, D. A. , & Murata, N. (2004). Membrane fluidity and its roles in the perception of environmental signals. Biochimica et Biophysica Acta (BBA)‐Biomembranes, 1666, 142–157. [DOI] [PubMed] [Google Scholar]
- Michaud, M. R. , & Denlinger, D. L. (2006). Oleic acid is elevated in cell membranes during rapid cold‐ hardening and pupal diapause in the flesh fly, Sarcophaga crassipalpis . Journal of Insect Physiology, 52, 1073–1082. [DOI] [PubMed] [Google Scholar]
- Min, Q. , Cheng, S. Y. , Xi, J. F. , Ma, J. , Xin, T. R. , Xia, B. , & Zou, Z. W. (2016). The expression patterns of three genes under short and long term cold exposure in Thitarodes pui (Lepidoptera: Hepialidae), a host of Ophiocordyceps sinensis . CryoLetters, 37(6), 432–439. [PubMed] [Google Scholar]
- Miyazaki, S. , Kayukawa, T. , Chen, B. , Nomura, M. , & Ishikawa, Y. (2006). Enhancement of cold hardiness by acclimation is stage‐specific in the non‐diapausing pupae of onion maggot Delia antiqua (Diptera: Anthomyiidae). European Journal of Entomology, 103, 691–694. [Google Scholar]
- Polley, S. D. , Tiku, P. E. , Trueman, R. T. , Caddick, M. X. , Morozov, I. Y. , & Cossins, A. R. (2003). Differential expression of cold‐ and diet‐specific genes encoding two carp liver 9‐acyl‐CoA desaturase isoforms. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 284, 41–50. [DOI] [PubMed] [Google Scholar]
- Reynolds, J. A. , Poelchau, M. F. , Rahman, Z. , Armbruster, P. A. , & Denlinger, D. L. (2012). Transcript profiling reveals mechanisms for lipid conservation during diapause in the mosquito, Aedes albopictus . Journal of Insect Physiology, 58, 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart, J. P. , Robich, R. M. , & Denlinger, D. L. (2010). Isolation of diapause‐regulated genes from the flesh fly, Sarcophaga crassipalpis by suppressive subtractive hybridization. Journal of Insect Physiology, 56, 603–609. [DOI] [PubMed] [Google Scholar]
- Sakamoto, T. , & Bryant, D. A. (1997). Temperature‐regulationed mRNA accumulation and stabilization for fatty acid desaturase genes in the cyanobacterium Synechococcus sp. Strain PCC 7002. Molecular Microbiology, 23, 1281–1292. [DOI] [PubMed] [Google Scholar]
- Shanklin, J. , Whittle, E. , & Fox, B. G. (1994). Eight histidine residues are catalytically essential in a membrane‐associated iron enzyme, stearoyl coa desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry, 33, 12787–12794. [DOI] [PubMed] [Google Scholar]
- Storey, K. B. , & Storey, J. M. (2012). Insect cold hardiness: Metabolic, gene, and protein adaptation. Canadian Journal of Zoology, 90, 456–475. [Google Scholar]
- Teets, N. M. , & Denlinger, D. L. (2013). Physiological mechanisms of seasonal and rapid cold‐hardening in insects. Physiological Entomology, 38, 105–116. [Google Scholar]
- Thompson, J. D. , Higgins, D. G. , & Gibson, T. J. (1994). Improved sensitivity of profile searches through the use of sequence weights and gap excision. Computer Applications in the Biosciences, 10, 19–29. [DOI] [PubMed] [Google Scholar]
- Tiku, P. E. , Gracey, A. Y. , Macartney, A. I. , Beynon, R. J. , & Cossins, A. R. (1996). Cold‐induced expression of D9‐desaturase in carp by transcriptional and posttranslational mechanisms. Science, 271, 815–818. [DOI] [PubMed] [Google Scholar]
- Vega, S. E. , Del Rio, A. H. , Bamberg, J. B. , & Palta, J. P. (2004). Evidence for the up‐ regulation of stearoyl‐ ACP (A9) desaturase gene expression during cold acclimation. American Journal of Potato Research, 81, 125–135. [Google Scholar]
- Vesala, L. , Salminen, T. S. , Laiho, A. , Hoikkala, A. , & Kankare, M. (2012). Cold tolerance and cold‐induced modulation of gene expression in two Drosophila virilis group species with different distributions. Insect Molecular Biology, 21(1), 107–118. [DOI] [PubMed] [Google Scholar]
- Waagner, D. , Holmstrup, M. , Bayley, M. , & Jesper, G. (2013). Induced cold‐tolerance mechanisms depend on duration of acclimation in the chill‐sensitive Folsomia candida (Collembola). Journal of Experimental Biology, 216, 1991–2000. [DOI] [PubMed] [Google Scholar]
- Winkler, D. (2009). Caterpillar fungus (Ophiocordyceps sinensis) production and sustainability on the Tibetan Plateau and in the Himalayas. Asian Medicine, 5, 291–316. [Google Scholar]
- Yang, D. R. , Li, C. D. , Shu, C. , & Yang, Y. X. (1996). Studies on the Chinese species of the genus Hepialus and their geographical distribution. Acta Entomologica Sinica, 39, 413–422. [Google Scholar]
- Yi, J. Q. , Guo, C. L. , Zou, Z. W. , & Zhang, G. R. (2015). Seasonal changes of fatty acid composition in Thitarodes pui larvae, a host of Ophiocordyceps sinensia . CryoLetters, 36, 205–212. [PubMed] [Google Scholar]
- Yi, J. Q. , Que, S. Q. , Xin, T. R. , Xia, B. , & Zou, Z. W. (2016). Complete mitochondrial genome of Thitarodes pui (Lepidoptera: Hepialidae). Mitochondrial DNA, 27, 109–110. [DOI] [PubMed] [Google Scholar]
- Yi, J. Q. , Zhang, G. R. , Guo, C. L. , Min, Q. , & Zou, Z. W. (2015). The study of haemolymph composition and cold tolerance in Thitarodes pui larvae. Acta Ecologica Sinica, 35, 608–615. [Google Scholar]
- Yocum, G. D. (2001). Differential expression of two HSP70 transcripts in response to cold shock, thermoperiod, and adult diapause in the Colorado potato beetle. Journal of Insect Physiology, 47, 1139–1145. [DOI] [PubMed] [Google Scholar]
- Yue, K. , Ye, M. , Lin, X. , & Zhou, Z. (2013). The genus Cordyceps: A chemical and pharmacological review. Journal of Pharmacy and Pharmacology, 15, 425–434. [DOI] [PubMed] [Google Scholar]
- Zerai, D. B. , Fitzsimmons, K. M. , & Collier, R. J. (2010). Transcriptional response of delta‐9‐desaturase gene to acute and chronic cold stress in nile tilapia, Oreochromis niloticus . Journal of the World Aquaculture Society, 41, 800–806. [Google Scholar]
- Zhang, G. R. , Gu, D. X. , & Liu, X. (2007). A new species of Hepialus (Lepidoptera, Hepialidae) from China. Acta Zootaxonomica Sinica, 32, 473–476. [Google Scholar]
- Zhu, J. S. , Halpern, G. M. , & Jones, K. (1998). The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: Part I. Journal of Alternative and Complementary Medicine, 4, 289–303. [DOI] [PubMed] [Google Scholar]
- Zou, Z. W. (2009). On the Insects of the Genus Thitarodes in Mt. Sejila of Tibet. Dissertation for DSc of Sun Yet‐sen University Guangzhou China (pp. 1–203).
- Zou, Z. W. , Liu, X. , & Zhang, G. R. (2010). Revision of taxonomic system of Hepialus (Lepidoptera, Hepialidae) currently adopted in China. Journal of Hunan University of Science & Technology (Natural Science Edition), 25, 114–120. [Google Scholar]
- Zou, Z. W. , Sun, Z. X. , Li, J. F. , & Zhang, G. R. (2011). Molecular cloning and characterization of two heat shock proteins in Thitarodes pui (Lepidoptera: Hepialidae). CryoLetters, 32(3), 225–239. [PubMed] [Google Scholar]


