Highlights
-
•
The first field use of MenAfriVac's new label allowed the vaccine to be kept at up to 40 °C for up to 4 days.
-
•
155,000 people were vaccinated using the CTC approach in the Meningitis A campaign in northern Benin in 2012.
-
•
98.7% of supervisors and 100% of vaccinators would prefer to conduct their next campaign using CTC.
-
•
They saw CTC benefits as: more people vaccinated, no need to return to health centre every night, reduced logistic burden.
-
•
Taking advantage of the flexibility offered by CTC opens the door for the implementation of new immunization strategies.
Keywords: Immunization, Vaccine, Campaign, Cold chain, Controlled temperature chain, Thermostability, Africa, Outreach, Out of the cold chain, Logistics
Abstract
Background
In October 2012, the Meningococcal A conjugate vaccine MenAfriVac was granted a label variation to allow for its use in a controlled temperature chain (CTC), at temperatures of up to 40 °C for not more than four days. This paper describes the first field use of MenAfriVac in a CTC during a campaign in Benin, December 2012, and assesses the feasibility and acceptability of the practice.
Methods
We implemented CTC in one selected district, Banikoara (target population of 147,207; 1–29 years of age), across 14 health facilities and 150 villages. We monitored the CTC practice using temperature indicators and daily monitoring sheets. At the end of the campaign we conducted a face-to-face survey to assess vaccinators’ and supervisors’ experience with CTC.
Findings
A mix of strategies were implemented in the field to maximize the benefits from CTC practice, depending on the distance from health centre to populations and the availability of a functioning refrigerator in the health centre. Coverage across Banikoara was 105.7%. Over the course of the campaign only nine out of approx. 15,000 vials were discarded due to surpassing the 4 day CTC limit and no vial was discarded because of exposure to a temperature higher than 40 °C or due to the Vaccine Vial Monitor (VVM) reaching its endpoint. Overall confidence and perceived usefulness of the CTC approach were very high among vaccinators and supervisors.
Interpretation
Vaccinators and supervisors see clear benefits from the CTC approach in low income settings, especially in hard-to-reach areas or where cold chain is weak. Taking advantage of the flexibility offered by CTC opens the door for the implementation of new immunization strategies to ensure all those at risk are protected.
1. Introduction
Despite immunization being one of public health's most effective and cost-friendly interventions, over 20 million children worldwide are under vaccinated, and remain at risk of vaccine preventable diseases each year [1]. The need to continually keep vaccines in a 2–8 °C cold chain is a major constraining factor for achieving universal immunization coverage and impacts the choice of vaccination strategies and activities, especially in the ‘last mile’, from health centre to vaccinee.
Many of the vaccines used today have some inherent stability in addition to what is currently reflected on their label [2], [3], [4]. Reflecting that stability on the product label would allow for limited use of the vaccine outside of the cold chain, without the constraints of needing to maintain 2–8 °C at all times.
The cold chain in the last mile is particularly labour intensive during immunization campaigns, such as those conducted across sub-saharan Africa against Meningitis A. Given the size of the target populations for MenAfriVac – up to 70% of the population, all those aged 29 years and under [5], [6] – the logistical challenges in maintaining the cold chain, from faltering electricity, poorly functioning or absent equipment, to ice pack production capacity, are significant.
In October 2012, the Meningococcal A conjugate vaccine MenAfriVac was granted a label variation by the national regulatory authority in its country of manufacture and pre-qualified by WHO to allow for its use in a controlled temperature chain (CTC), at temperatures of up to 40 °C for not more than four days. This marks the first time a vaccine used in developing countries has been granted authorization to be used at ambient temperature. This paper evaluates the first use of the flexibility offered by MenAfriVac's new label during a mass vaccination campaign in Benin.
2. Objectives
The study aimed to capture the first field experience using MenAfriVac in a CTC, to evaluate whether the implementation of CTC – rather than a traditional 2–8 °C cold chain – during a mass campaign is feasible, acceptable to health care workers, and to identify the benefits and challenges of the approach.
3. Methods
3.1. Study site
The study took place in the district of Banikoara in Northern Benin as part of the sub-National Meningitis A vaccination campaign held from November 15–25, 2012. Banikoara is a rural area, made up of 150 villages and hamlets, divided into nine administrative zones. There is one rural hospital, one district health centre, nine smaller health centres and three dispensaries. The population is 210,296 (as of 2012), 70% of which are estimated to be 29 years of age or younger (target population = 147,207). Banikoara was selected as the site for this pilot study by the Ministry of Health in Benin, using criteria developed by WHO's Immunization Practices Advisory Committee as part of their guidance on the implementation of CTC campaigns for MenAfriVac [7]. During this campaign, Banikoara used a mixture of fixed site and mobile/outreach teams to vaccinate the population; all vaccination activities conducted in Banikoara were conducted using the CTC approach.
3.2. The vaccine
MenAfriVac is a Meningitis A polysaccharide conjugate vaccine designed for use across the sub-Saharan African meningitis belt. It comes in a 10-dose vial, with a separate diluent which contains an aluminium adjuvant, which is sensitive to freezing. As is standard for vaccines procured through UN agencies, the vaccine comes with a Vaccine Vial Monitor (VVM) on its label [8].
The original label for MenAfriVac stated that the vaccine should be kept between 2 and 8 °C at all times. As with all vaccines, these storage and use conditions on the vaccine's label were approved as part of the vaccine's licensure by the national regulatory authority in the country where the vaccine is manufactured, in this case India. In October 2012, based on scientific laboratory studies and analyses submitted by the vaccine manufacturer (Serum Institute of India), MenAfriVac's regulatory agency of record (India) and WHO both approved a revision to the label which states that MenAfriVac and its diluent can “be stored at up to 40 °C for not more than four days immediately prior to administration, provided the vaccine has not reached its expiry date and the vaccine vial monitor is still valid, Unopened vials should be discarded at the end of the four days at up to 40 °C. Reconstituted vaccine should be used within six hours after reconstitution, otherwise discarded.”
3.3. Implementation of CTC
In order to ensure the vaccine is safe and effective at all times when used in a CTC, vaccination teams, comprised of one nurse and two volunteers relied on two indicators: the VVM, affixed to the label of the vaccine, and a peak temperature threshold indicator – a small laminated card with a heat sensitive sticker that changed colour immediately upon being exposed to 40 °C, placed inside each vaccine carrier. Unlike the VVM, which gradually changes colour over time to reflect cumulative exposure to heat, the peak temperature threshold indicator is binary, and changes colour instantly if exposed to temperatures of 40 °C, without a gradual change. Teams were instructed to check this card at the start of their day, upon arrival at their vaccination site, and prior to opening each new vial throughout the day. If they found that either the VVM or the peak threshold indicator had changed colour, they were advised to stop using the vaccines and contact their supervisor immediately.
In addition to the standard pre-campaign training conducted in all campaign areas in Benin, training was provided in Banikoara on CTC prior to the campaign. This included explanations of what CTC is, how to use the threshold indicator, a review of all forms to complete and how to read the VVM, training on adverse events following immunization as well as ‘scenario planning’, on how to take advantage of the flexibility provided by CTC.
Teams were asked to complete a CTC monitoring form daily as follows: before departing the health centre, on arrival at the vaccination site, on administration of the last dose of vaccine and on return to the health centre. Teams recorded the time each of these activities took place, the number of vials they had with them at that point, and the status of the peak threshold indicator.
At the end of each day, when teams returned to the health centre, any vials that they had taken with them for the day but not used were marked with a line on the label, indicating one day of CTC exposure. All the marked vials from all teams were consolidated and stored at ambient temperature overnight, and were the first vials distributed to the teams for use the next day. Teams were instructed to use the marked vials first. From the second day of the campaign, teams indicated the number of marked and unmarked vials they took with them at the start of each day on their CTC monitoring form.
As this was the first use of CTC in a mass campaign, and in order to ensure the tools were being properly used, six additional supervisors were recruited to oversee campaign activities and provide support to vaccinators.
3.4. Data collection
The data on coverage, vaccine wastage and adverse events following immunization were collected using standard Ministry of Health issued forms.
Data on CTC specific vaccine wastage was collected through the specially designed CTC monitoring form, described above. At the end of the campaign a survey was conducted to evaluate the CTC practice among the vaccinators and supervisors in Banikoara. The survey was pre-tested with vaccinators prior to being administered. The survey included 20 multiple choice and short answer questions.
4. Results
Three different CTC scenarios were implemented in the campaign, based on the situation found in Banikoara.
The first scenario was the most standard option, used by all three dispensaries and seven of the health centres. It involved keeping the vaccines in the standard cold chain at the health centre. This meant the vaccine was transported from the district level to the health centre using the cold chain and placed into the fridge at district level. On the first morning of the campaign, vaccination teams arrived at the health centre and retrieved their vaccines. The vaccines were placed into a standard vaccine carrier, without icepacks, marking the beginning of the CTC practice.
The second scenario was used in two health centres to enable access to remote communities with no reliable electricity or power source, accessible only by difficult to navigate roads. In other non-CTC campaigns, teams had to return each night to the health centre to maintain the cold chain, limiting their ability to reach the most remote areas. With the CTC practice, the teams collected their vaccines from the health centre, as described above, and set out for the remote villages. However rather than coming back each night, they stayed in the villages for three days, enabling them to ensure better vaccination coverage of the population.
The third scenario involved starting CTC at the point when the vaccines were transported from the district to the health centre level. This was used in the one health centre that did not have any functional cold chain equipment. While in previous campaigns they had to make a daily trek to the district capital to collect their vaccine, during this campaign vaccines were transported from district to the health centre in a CTC, and then stored in a CTC for four days, at which point a new drop off of vaccines was needed.
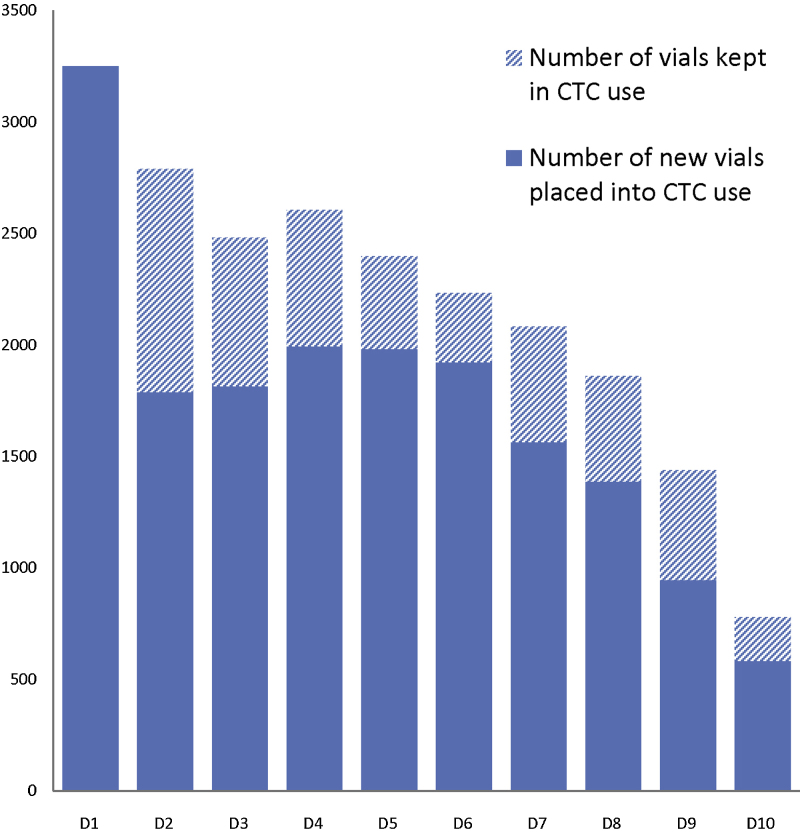
In the scenario one, the vaccinators prioritized the use of vials of vaccine which had been taken out of the cold chain and placed in a CTC on previous days but which had not been used; on average, one third (33.5%) of the daily vial quantity taken out for the day came from unused vials from the day(s) before. A summary of the number of vials kept for multiple days across all scenarios can be found in Fig. 1. We observed that on the first day of the campaign, after the CTC training, health centre staff took out more vials than were necessary to reach the days’ target population in an attempt to prevent vaccination teams running out of vaccines, which had occurred in previous campaigns. Following supervisory visits by district staff, the health centre staff removed only the estimated quantity of vaccine needed, plus a small buffer.
Fig. 1.
Number of vials taken out for vaccination activities per day by cold chain storage status, across all scenarios of CTC use, Banikoara district.
Vaccination coverage in the district was high, with 155,596 people vaccinated at the end of the campaign, equivalent to a coverage rate of 105.7%. This proportion is comparable to the results seen in the other zones of Benin: the overall coverage in the country was 104.7%. In Banikoara, the average time for a health care worker to reach their vaccination site was 36 min and 85% of the teams used motorbikes for transport. Each team vaccinated on average 318 persons a day (range 249–433).
Over the course of the campaign, 15,570 vials of MenAfriVac were used. Nine vials were discarded due to surpassing the 4 day CTC limit, five vials at day 4 and four vials on the last day. No VVMs reached their endpoint. One vial was reported as broken. No indicators reached 40 °C and no vial was discarded because of exposure to a temperature higher than 40 °C.
4.1. Survey
A total of 21 supervisors and 77 vaccinators were surveyed, 92.2% of which had conducted outreach vaccination activities as part of the campaign. Overall confidence and perceived usefulness of the CTC approach were very high among both groups (Table 1).
Table 1.
Health worker confidence in and perception of CTC.
| % Vaccinators (n = 77) | % Supervisors (n = 21) | |
|---|---|---|
| Confidence in CTC | ||
| Entirely confident | 69.3 | 52.4 |
| Confident but need to respect the limits | 29.3 | 47.6 |
| Limited confidence | 1.3 | 1.0 |
| Usefulness of CTC | ||
| Very useful | 74.0 | 81.0 |
| Relatively useful | 26.0 | 19.0 |
| Not useful | 0 | 0 |
Most of the participants felt that the CTC practice was more useful for outreach sessions (Table 2). Health staff identified the top benefits as allowing them to vaccinate more people per day, reduced weight of the vaccine carrier, not needing to return to the health centre every night and not needing to freeze ice packs.
Table 2.
Health worker perception of the usefulness of the CTC approach.
| Vaccinators | Supervisors | ||
|---|---|---|---|
| CTC is the most useful in which strategy? | Fixed vaccination site | 1.3% | 0 |
| Outreach vaccination site | 39.0% | 66.7% | |
| Both outreach and fixed sites | 59.7% | 33.3% |
| % Finding the criteria useful or very useful | Ranking for ‘most useful’ | % Finding the criteria useful or very useful | Ranking for ‘most useful’ | |
|---|---|---|---|---|
| Allows to vaccinate more persons | 98.7 | 1 | 100 | 1 |
| Vaccine carrier is lighter | 97.4 | 2 | 100 | 2 |
| No need to freeze the ice pack | 96.1 | 3 | 100 | 4 |
| Less wastage | 96.1 | 4 | 85.7 | 5 |
| No need to come back every night | 97.4 | 5 | 100 | 3 |
More than half of the interviewees (52.4% of supervisors and 54.1% of vaccinators) felt that there was no risk associated with CTC. Those that spoke of risks often raised what can more accurately be termed as concerns, usually about the ability to respect the CTC limits; very few were about efficacy, adverse events or wastage (Table 3).
Table 3.
Health worker perception of the risks associated with CTC.
| % Staff indicating a concern |
||
|---|---|---|
| Vaccinators (n = 77) | Supervisors (n = 21) | |
| Surpassing 40 °C | 31.2 | 47.6 |
| Surpassing 4 days | 27.3 | 19.0 |
| Higher rate of AEFI | 5.2 | 0 |
| Vaccine less effective | 1.3 | 4.8 |
| Higher wastage | 0 | 0 |
The main difficulties in implementing CTC were identified as reading the indicator and managing the quantity of vaccine that should be taken out of the fridge. A small proportion of staff indicated that avoiding exposing the vaccine to the sun was a challenge (Table 4).
Table 4.
Health worker perception on the level of difficulty associated with CTC practice.
| % of vaccinators (n = 77) | % of supervisors (n = 21) | ||
|---|---|---|---|
| Reading the peak temperature threshold indicator | Easy | 81.8 | 90.0 |
| Midlevel difficulty | 16.9 | 10.0 | |
| Difficult | 1.3 | 0 | |
| Vaccine management | Easy | 80.5 | 75.0 |
| Midlevel difficulty | 19.5 | 20.0 | |
| Difficult | 0 | 5.0 | |
| Avoid the sun | Easy | 94.8 | 81.0 |
| Midlevel difficulty | 5.2 | 19.0 | |
| Difficult | 0 | 0 | |
98.7% of supervisors and 100% of vaccinators indicated that, if given a choice, they would prefer to conduct their next campaign in a CTC rather than using the traditional cold chain. Asked which vaccines they would most like to see licensed for CTC use, most vaccinators and supervisors cited other vaccines used in campaigns, with polio (44%), measles (40% and yellow fever (29%) the most commonly cited.
5. Discussion
Over the course of the campaign, 155,000 people were vaccinated with MenAfriVac in a CTC. This marks the first time since the establishment of EPI that a campaign was conducted using a vaccine with on-label guidance for use beyond the 2–8 °C standard cold chain range. As per the coverage rates attained, the campaign was successful in reaching the target population. The 2013 disease surveillance across Benin—including in the CTC area—supports this, with no cases of Meningitis A reported in the vaccinated population [9].
Cold chain has been a limiting factor since the inception of the EPI. The need to keep vaccines between 2 and 8 °C at all times currently drives the way immunization strategies are developed and implemented. This study provides a first example of the types of benefits that could be seen from removing that constraint, especially for immunization campaigns and other outreach based strategies.
While the rigorous regulatory reviews provided assurance as to the efficacy of the vaccine, the pilot provides critical validation of the acceptability of the practice by health care workers. In addition to the survey results which indicated a strong preference for CTC when feasible, the CTC approach also has the potential to have a positive impact on the provision of other primary health care initiatives, freeing up health care worker time and resources to keep other regular primary care services operational (often cancelled during campaigns) [10], rather than managing cold chain and ice pack production logistics [11].
In addition, while the original six EPI vaccines were very sensitive to heat, many new vaccines—including the MenAfriVac diluent—are damaged by exposures to freezing temperatures while remaining stable at higher temperatures for longer periods of time. Studies have shown that freezing is a particular risk during transport and outreach [12]. The CTC practice removes the risk of freezing during these activities at the ‘last mile’.
As with any new practice, there were several challenges noted with the CTC implementation. The biggest of these was the need to discard unused vials after four days in a CTC, rather than having the ability to return them to the fridge. This required close supervision by health care workers and district health staff, and if staff are not well trained, could lead to increases in vaccine wastage.
Once trained, vaccinators found the peak threshold temperature cards easy to use. However the need to ensure the vaccines are always kept with an indicator provides an additional difficulty, and vial level peak threshold indicators should be considered. Caution must be exercised around storage of the indicator cards prior to use. During the study two indicator cards changed colour when kept in pockets of supervisors where temperatures surpassed 40 °C.
Finally, while CTC implementation does not require the use of cold boxes, their use during this study allowed us to protect vaccines from high temperatures (reported ambient temperatures reached 39 °C) and direct sunlight, and they remain a known ‘signal’ of vaccination activities within the community.
Although MenAfriVac is not the only vaccine to be kept outside the 2–8 °C range, it is the first vaccine approved with this type of variation by WHO, and this study marks the first demonstration of potential benefits from this type of use in low income setting. This landmark decision opens the door for the development of new immunization strategies and approaches to ensure the vaccine reaches all those who are at risk, not just those reached by a cold chain. However in order to achieve CTC vaccine labels, close collaboration with manufacturers, regulatory experts and WHO technical staff is essential. The data that is necessary for these types of variations is not yet systematically generated, and collaboration to define the parameters for which additional testing should follow in order to apply for a variation is essential [13].
As the current CTC work aims to take advantage of existing stability without requiring reformulation, the length of time available in a CTC is likely to be constrained by the limited stability of today's vaccines. This means CTC will likely provide benefits in the very last mile, rather than alleviate cold chain capacity issues higher up in the supply chain. However, further work to assess full impact on health care workers, coverage and potential cost savings from the approach is needed.
In the longer term, combining the CTC workstream with other more upstream efforts on vaccine development and thermostability, and generating the data necessary to achieve a CTC license systematically, have the potential to enable routine EPI services without cold chain for longer periods of time and should be explored.
Funding
The operational costs of the campaign were covered within the standard new vaccine introduction support window to the Government of Benin by the Global Alliance for Vaccine and Immunization; project Optimize, a WHO/PATH collaboration funded by the Bill & Melinda Gates Foundation, provided additional specific funding for training, supervision and the evaluation.
Acknowledgements
The authors wish to extend their sincere thanks to the following: For operational and planning support, the Ministry of Health in Benin, the WHO country office in Benin, especially Dr. Aristide Sousou and Dr. Jose Biey; AMP Benin, in particular Philippe Jaillard. Regulatory support and expertise from Maria Baca-Estrada, Tong Wu, Dean Smith and their colleagues at Health Canada; and from Carmen Rodriguez and Nora Dellepiane at WHO, Quality Safety and Standards team. Programmatic input from WHO's Immunization Practices Advisory Committee, and in particular the working group on CTC. For their guidance and support, the authors extend their thanks to Monique Berlier and Jean-Marie Preaud at PATH, France and to Marie-Pierre Preziosi and Michel Zaffran at WHO, Geneva.
References
- 1.vol. 87, 44. 2012. pp. 421–436. (Weekly Epidemiological Record). http://www.who.int/wer/2012/wer8744.pdf [accessed 20.10.13] [Google Scholar]
- 2.Zipursky S., Boualam L., Cheikh D.O., Fournier-Caruana J., Hamid D., Janssen M. Assessing the potency of oral polio vaccine kept outside of the cold chain during a national immunization campaign in Chad. Vaccine. 2011;29:5652–5656. doi: 10.1016/j.vaccine.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Milstien J., Galazka A.M., Kartoglu U., Zaffran M. 2014. Temperature sensitivity of vaccines. World Health Organization. Dept. of Immunization, Vaccines and Biologicals. http://www.who.int/iris/handle/10665/69387#sthash.PkPIJOEY.dpuf [accessed 4.01.14] [Google Scholar]
- 4.Sutanto A., Suarnawa I.M., Nelson C.M., Stewart T., Soewarso T.I. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77(2.) [PMC free article] [PubMed] [Google Scholar]
- 5.LaForce F.M., Konde K., Viviani S., Preziosi M.P. The Meningitis Vaccine Project. Vaccine. 2007;25S:A97–A100. doi: 10.1016/j.vaccine.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 6.Djingarey M.H., Barry R., Bonkoungou M., Tiendrebeogo S., Sebgo R., Kandolo D. Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine. 2012;30S:B40–B45. doi: 10.1016/j.vaccine.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 7.2013. Use of MenAfriVac™ (meningitis A vaccine) in a controlled temperature chain (CTC) during campaigns. http://www.who.int/immunization/documents/WHO_IVB_13.04_5_6/en/index.html [access 31.10.13] [Google Scholar]
- 8.World Health Organization-UNICEF . WHO; Geneva: 2007. WHO-UNICEF Policy Statement on the Implementation of Vaccine Vial Monitors: The Role of Vaccine Vial Monitors in Improving Access to Immunization. [WHO/IVB/070.04] [Google Scholar]
- 9.2013. Meningitis Weekly Bulletin, September 2–9. http://www.meningvax.org/epidemic-updates.php [accessed 23.10.13] [Google Scholar]
- 10.Verguet S., Jassat W., Bertram M.Y., Tollman S.M., Murray C.J., Jamison D.T., Hofman K.J. Impact of supplemental immunisation activity (SIA) campaigns on health systems: findings from South Africa. J Epidemiol Community Health. 2013;67:947–952. doi: 10.1136/jech-2012-202216. [DOI] [PubMed] [Google Scholar]
- 11.Lydon P., Zipursky S., Tevi-Benissan C., Djingarey M.H., Gbedonou P., Youssouf B. 2014. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad Publication: Bulletin of the World Health Organization. Article ID: BLT.13.123471 http://www.who.int/bulletin/online_first/13-123471.pdf [accessed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dipika M., Robertson J., Garrison M., Newland S., Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine. 2007;25:3980–3986. doi: 10.1016/j.vaccine.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Smith D., Ferguson M., Krause P., Wu T., Baca-Estrada M., Conrad C. 2012. WHO/Health Canada Drafting Group Meeting on Scientific and Regulatory Considerations on the Stability Evaluation of Vaccines under Controlled Temperature Chain. Final Report, December 6. http://who.int/biologicals/areas/vaccines/controlledtemperaturechain/en/index.html [accessed 5.12.13] [Google Scholar]