Abstract
Objective: Deficits in static postural control related to chronic ankle instability (CAI) and fatigue have been investigated separately, but little evidence links these factors to performance of dynamic postural control. Our purpose was to investigate the effects of fatigue and CAI on performance measures of a dynamic postural-control task, the Star Excursion Balance Test.
Design and Setting: For each of the 3 designated reaching directions, 4 separate 5 (condition) × 2 (time) × 2 (side) analyses of variance with a between factor of group (CAI, healthy) were calculated for normalized reach distance and maximal ankle-dorsiflexion, knee-flexion, and hip-flexion angles. All data were collected in the Athletic Training Research Laboratory.
Subjects: Thirty subjects (16 healthy, 14 CAI) participated.
Measurements: All subjects completed 5 testing sessions, during which sagittal-plane kinematics and reaching distances were recorded while they performed 3 reaching directions (anterior, medial, and posterior) of the Star Excursion Balance Test, with the same stance leg before and after different fatiguing conditions. The procedure was repeated for both legs during each session.
Results: The involved side of the CAI subjects displayed significantly smaller reach distance values and knee-flexion angles for all 3 reaching directions compared with the uninjured side and the healthy group. The effects of fatigue amplified this trend.
Conclusions: Chronic ankle instability and fatigue disrupted dynamic postural control, most notably by altering control of sagittal-plane joint angles proximal to the ankle.
Keywords: Star Excursion Balance Test, lower extremity, neuromuscular control
Lateral ankle sprains are one of the most common injuries among athletes.1 After initial injury, the rate of recurrence may be as high as 80% among active individuals.2 Altered mechanical joint stability due to repeated disruptions to ankle integrity with resultant perceived and observed deficits in neuromuscular control has been described as chronic ankle instability (CAI).3
Aspects of neuromuscular control may be quantified through measures of postural control. Postural control may be classified as either static (attempting to maintain a base of support with minimal movement) or dynamic (attempting to maintain a stable base of support while completing a prescribed movement).4 Altered static postural control in the presence of CAI has been studied extensively,5,6 but investigations into the effects of CAI on dynamic postural control are limited.7–9
Fatigue may impair the proprioceptive and kinesthetic properties of joints.10 Fatigue increases the threshold of muscle spindle discharge, which disrupts afferent feedback, subsequently altering joint awareness. The detrimental effect of fatigue on static postural control has been established,11–18 but the effects on measures of dynamic postural control are unknown.
Although numerous authors have studied neuromuscular control in CAI subjects using instrumented methods,5,8,19–25 noninstrumented, clinically applicable tests to assess neuromuscular and functional deficits in patients with CAI are clearly lacking. The Star Excursion Balance Test (SEBT) is one such test that has been established as highly reliable and valid for both research and clinical purposes.26–29 It challenges the subject to maintain a stable unilateral base of support while reaching in 8 directions with the opposite leg.
Distinct muscle recruitment patterns in the stance leg (test leg) have been established for each of the 8 reaching directions, suggesting that specific neuromuscular control patterns are required to maintain the base of support during the dynamic task.30 Previous researchers have established the SEBT as valid in differentiating the dynamic postural control of those with and without CAI by demonstrating that the CAI subjects could not reach as far as the non-CAI subjects while maintaining a stable base of support.31 The neuromuscular patterns necessary to complete this dynamic postural-control task would appear to be altered in the presence of CAI, but the contributing mechanisms to this finding have not been specifically addressed.
It seems that neuromuscular control, quantified through measures of static postural control, is affected adversely by fatigue and CAI individually. One of the clinician's roles is to ensure the functional ability of an injured athlete before allowing a return to competition. This includes rehabilitation interventions to restore proper neuromuscular control to an injured joint. An athlete with CAI commonly undergoes fatiguing activities during practices and competitions.
We are not aware of any previous research into the combined effects of fatigue and CAI on dynamic postural control. The effect of the 2 conditions combined on measures of dynamic postural control is unknown. Therefore, our purpose was to examine potential deficits on reach distance and kinematic measures of the SEBT related to fatigue and CAI.
METHODS
Subjects
Thirty physically active subjects (15 males, 15 females, age = 22.3 ± 2.6 years, height = 1.74 ± 0.1 m, mass = 71.41 ± 15.67 kg) volunteered as subjects. All subjects signed an informed consent form approved by the university's institutional review board. Individuals with self-reported vestibular disorders or mild head injury in the previous 6 months were excluded from the study.
Subjects were placed into 1 of 2 groups: healthy (8 males, 8 females, age = 22.5 ± 2.4 years, height = 1.75 ± 0.1 m, mass = 71.1 ± 18.6 kg) and CAI (7 males, 7 females, age = 21.9 ± 2.9 years, height = 1.73 ± 0.1 m, mass = 71.8 ± 12.8 kg). The healthy subjects were free of any self-reported lower extremity injury in the previous 6 months. Subjects in the CAI group were free from injury to the lower extremity other than the ankle in the previous 6 months; had a history of at least 1 acute ankle sprain that resulted in swelling, pain, and temporary loss of function (but none in the previous 3 months); and a history of multiple episodes of the ankle “giving way” in the past 6 months.
Protocol
Subjects reported to the Athletic Training Research Laboratory for 5 individual sessions at least 1 week apart. We measured leg length of both legs with the subject lying supine on a plinth using a standard tape measure from the anterior superior iliac spine to the distal end of the medial malleolus at the beginning of the first session.
Subjects completed 5 testing sessions during which they performed the SEBT before and after a fatiguing condition. The 5 fatigue conditions (1 per session) were (1) isokinetic ankle fatigue, (2) isokinetic knee fatigue, (3) isokinetic hip fatigue, (4) lunging task, and (5) control (5-minute period of quiet sitting).
The SEBT consists of 8 reaching directions. Because of the time constraints related to the timing of postfatigue measures and the fact that we were concerned mainly with sagittal-plane kinematics of the stance leg, only 3 of the 8 reaching directions (anterior, medial, and posterior) of the SEBT were performed before and after each fatigue condition. The orders of the 5 testing sessions and of the reaching directions for each session were counterbalanced. Reaching distance during the SEBT was measured while sagittal-plane kinematics of the stance leg, the leg that underwent the fatiguing task, were recorded. The SEBT postfatigue testing took place immediately (approximately 15 seconds) after achieving the designated fatigue level. During each session, subjects completed the protocol using both the right and left legs; the order of stance leg was also counterbalanced. After completion of the first testing leg, subjects sat quietly for 30 minutes before beginning the identical protocol for the opposite leg.
Instrumentation
We used an isokinetic dynamometer (Biodex, Inc, Shirley, NY) to induce fatigue to the sagittal-plane movers of the hip, knee, and ankle. A metronome provided the rhythm of movement for performance of lunges. Each subject wore an adjustable weight vest (All Pro Exercise Products, Inc, Longboat, FL) that carried 10% of the subject's body weight. This weight was based on pilot work that made this task similar in length to the isokinetic fatigue tasks. Kinematic data of ankle, knee, and hip joint positions were collected using a digital video camera (Panasonic Digital Palmcorder, Panasonic Electronics, Denver, CO) sampled at 30 frames/s. Joint angles in the sagittal plane were calculated using the SMART video analysis system (ECI-Software, Inc, Boston, MA).
Fatigue
Three of the fatiguing protocols were performed on a Biodex System III isokinetic dynamometer, set for concentric-concentric function for the particular joint being tested during that session. Sagittal-plane movement patterns at the ankle (plantar flexion-dorsiflexion), knee (flexion-extension), and hip (flexion-extension) were tested. Patient positioning followed guidelines set forth by the manufacturer.
Subjects performed 5 continuous maximum trials at 60°/s for the designated movement pattern in order to determine peak torque. After a 2-minute rest, subjects repeated the movement pattern at 60°/s continuously until force production dropped below 50% of the peak torque in both directions of motion being tested. This procedure followed a protocol we used previously17 that has been established in the literature.15,18
The fourth fatiguing protocol consisted of performing a lunging task a maximum number of times. Subjects lunged forward with the leg that was to be their stance leg for the SEBT performance. The order of testing leg was counterbalanced. Subjects lunged forward the distance equal to the individual leg length that had been measured at the beginning of the first session. Pieces of tape on the floor served as the point of origin and the target reaching distance. Lunges were performed at the rate of 1 lunge per 2 seconds. A metronome established the rate of performance for the subjects. A lunge cycle was defined as having the subject reach to the target, achieve approximately 90° of hip and knee flexion in the lunging leg while maintaining an upright trunk, and return the reaching leg back to the point of origin.
Subjects were given ample practice time to ensure proper execution of the lunging task and 2 minutes to rest between practice trials and test trials. Following the protocol of Pincivero et al,32 fatigue was quantified by having subjects perform the task a maximum number of times until they could not complete the movement with proper form or were unable to meet the required rhythm for 2 repetitions in a row. Throughout the task, subjects received verbal cues to ensure proper form and verbal encouragement to continue the task until fatigued.
The fifth testing condition was for the purposes of establishing control data. No fatiguing task was implemented. Instead, the subjects sat quietly for 5 minutes between the 2 performances of the SEBT.
Star Excursion Balance Test Performance
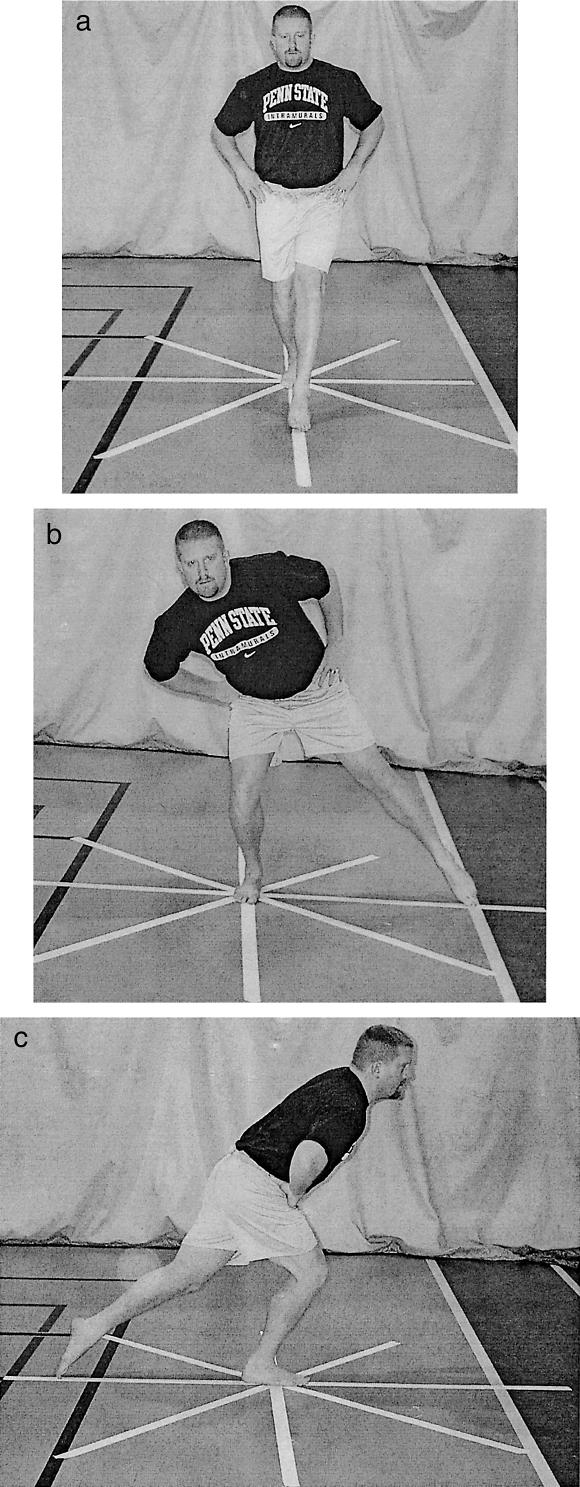
The SEBT was performed with the subjects standing in the middle of a grid formed by 8 lines made with athletic tape extending out at 45° from each other33 (Figure 1). For this project, subjects were only reaching in the anterior, medial, and posterior directions. Each subject was asked to reach as far as possible along the line, make a light touch on the line, and return the reaching leg back to the center while maintaining a single-leg stance with the other leg in the center of the grid. The subjects were instructed to make a light touch on the ground with the most distal part of the reaching foot and return to a double-leg stance without allowing the contact to affect the base of support. Subjects were instructed to keep their hands on their hips and to keep the heel of the stance leg on the ground at all times.
Figure 1. Reaching directions for the SEBT.
A, Anterior. B, Medial. C, Posterior
Reach distances were recorded by placing a mark on a length of athletic tape on the floor corresponding to the touchdown point of the subject. We recorded the reaching distance as the distance from the center of the grid to the point of maximum excursion of the reaching leg. Based on our investigation,29 reach distances were divided by leg length and multiplied by 100 to calculate a dependent variable that represents reach distance as a percentage of leg length (MAXD). A trial was discarded and repeated if the investigator felt the subject used the reaching leg for a substantial amount of support at any time, removed the foot from the center of the grid, or was unable to maintain balance on the support leg throughout the trial.
On each testing day, subjects were allowed to practice reaching in the anterior, medial, and posterior directions 6 times to minimize the learning effect.27 On the control (nonfatiguing) day, subjects were allowed 5 minutes of quiet sitting between the practice trials and test trials. During the 4 fatigue testing sessions, the designated fatigue protocol was completed between the practice trials and the test trials. Three consecutive test trials each in the anterior, medial, and posterior directions were performed. The order of reaching directions was counterbalanced.
Kinematic Analysis
The digital video camera was positioned on a tripod 8 m from the center of the SEBT grid. Subjects were positioned perpendicular to the camera lens and performed the test such that the stance leg was facing the camera. Subjects had markers placed on the base of the fifth metatarsal, calcaneus, lateral malleolus, lateral joint line of the knee, greater trochanter of the femur, and acromion of the scapula.
Joint angles at the ankle, knee, and hip were measured at the maximum reach distance indicated by touch down of the reaching leg. The SMART system calculated joint angles for hip flexion-extension, knee flexion-extension, and ankle plantar flexion-dorsiflexion. Raw data were processed with Video Wave III video processing software (MGI Software Corp, Richmond Hill, ON, Canada). Kinematic data were calculated using the SMART system.
Statistical Analysis
We calculated the means of the joint angles and normalized reach distances at maximum reach distance from the 3 trials of each performance of the SEBT for statistical analysis. The involved limb of the CAI group (ICAI) subjects was matched with the limb of the same side of the matched healthy group (IHEA) subject for the purposes of making within (ICAI versus UCAI)- and between (ICAI versus IHEA)-side comparisons. Because of the inherent differences in movement patterns among the 3 reaching directions (anterior, medial, and posterior), we did not make direct comparisons, and each direction was analyzed separately. For each reaching direction, 4 separate 5 × 2 × 2 × 2 repeated-measures analyses of variance were performed for normalized reach distance, ankle dorsiflexion, knee flexion, and hip flexion with within factors of day (control and ankle, knee, hip, and lunge fatigue), side (involved and uninvolved), and time (prefatigue and postfatigue) and a between factor of group (healthy and CAI). Scheffé post hoc testing was performed as needed. The level of significance was set a priori at 0.05 for all analyses. All statistical analyses were performed using the SPSS statistical software package (version 10.0; SPSS, Inc, Chicago, IL).
RESULTS
Anterior Reaching Direction
Reach Distance as a Percentage of Length
A significant group × side interaction (F1,28 = 5.56, P = .026) existed for MAXD in the anterior reach direction. Scheffé post hoc testing revealed that the injured side of the CAI group reached significantly less than the uninjured side (Figure 2). However, the CAI group had significantly larger normalized MAXD percentages compared with the healthy group.
Figure 2. Group × side interaction for reach distance as a percentage of leg length (MAXD) in the anterior reaching direction (*P < .05).
Knee Flexion
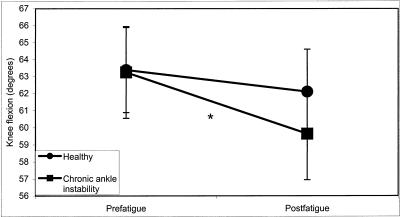
A significant group × time interaction existed for knee flexion (F1,28 = 4.68, P = .039). Post hoc testing revealed that the CAI group was more adversely affected across all fatigue conditions (Δ = −3.61°) compared with the healthy group (Δ = −1.28°) (Figure 3).
Figure 3. Group × time interaction for knee flexion in the anterior reaching direction (*P < .05).
Hip Flexion
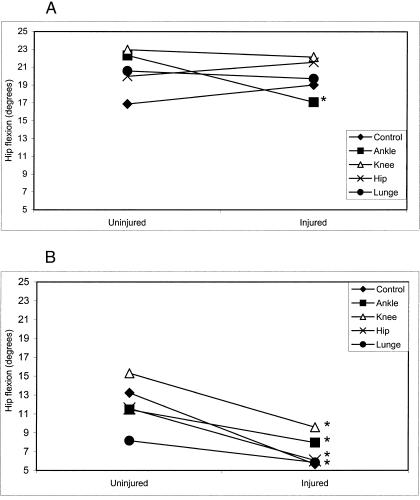
A significant group × day × side interaction (F4,112 = 2.53, P = .044) existed for hip-flexion measures. The healthy group seemed to use more hip flexion than the CAI group (Figure 4). Additionally, post hoc testing revealed that among the CAI group, significantly more hip flexion occurred on the uninjured side on all days except the lunge fatigue day.
Figure 4. Group × day × side interaction for hip flexion in the anterior reaching direction (*P < .05).
A, Healthy subjects. B, Chronic ankle instability subjects
Medial Reaching Direction
Reach Distance as a Percentage of Length
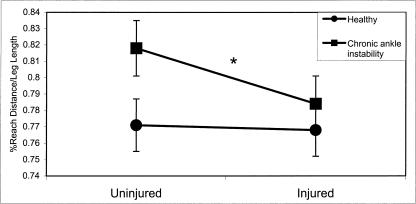
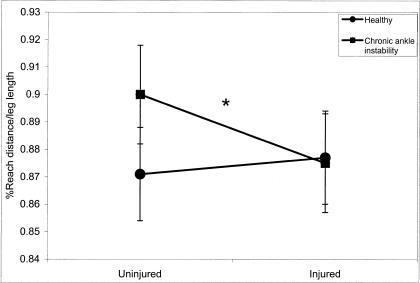
A significant group × side interaction (F1,28 = 5.88, P = .022) existed for MAXD in the medial reaching direction. Significant differences were noted between the uninjured (0.900) and injured (0.875) sides of the CAI group as well as between the uninjured sides of the CAI (0.900) and healthy (0.871) groups (Figure 5).
Figure 5. Group × side interaction for reach distance as a percentage of leg length (MAXD) in the medial reaching direction (*P < .05).
Knee Flexion
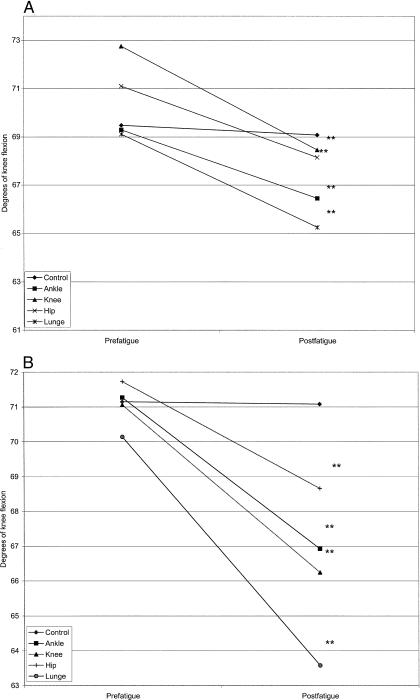
A significant 3-way interaction existed for comparisons of group, day, and time (F4,112 = 10.88, P = .045). Fatigue decreased knee flexion in both the healthy and CAI groups; however, the control day resulted in no prefatigue-postfatigue change in either group (Figure 6). On the days that a significant prefatigue-postfatigue change occurred, the changes were larger in the CAI group than the healthy group.
Figure 6. Group × day × time interaction for knee flexion in the medial direction(**P < .01).
A, Healthy subjects. B, Chronic ankle instability subjects
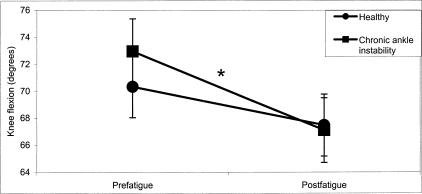
The 3-way interaction is further explained by a significant group × time interaction for knee flexion (F1,28 = 4.75, P < .001). Although a mean decrease in knee flexion was observed from prefatigue to postfatigue for both the healthy (Δ = −2.86°) and CAI (Δ = −5.86°) groups, the decrease was statistically significant only for the CAI group (Figure 7).
Figure 7. Group × time interaction for knee flexion in the medial reaching direction (*P < .05).
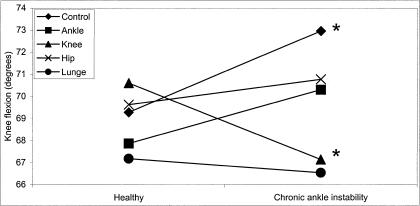
A significant group × day interaction also helps to further describe the 3-way interaction (F4,112 = 3.06, P = .020). The 2 groups experienced statistically different effects on knee flexion on the control day (CAI = 3.68° > healthy) and knee-fatigue day (healthy 3.46° > CAI) (Figure 8).
Figure 8. Group × day interaction for knee flexion in the medial direction (*P < .05).
Posterior Reaching Direction
Reach Distance as a Percentage of Length
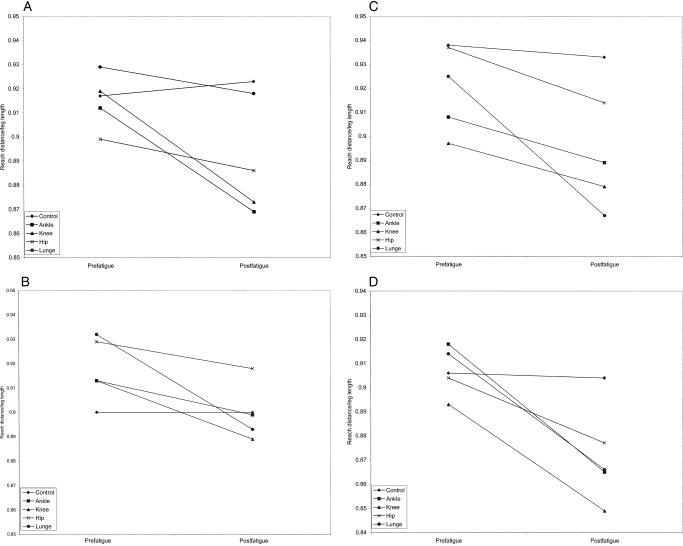
A significant group × day × side × time interaction existed for MAXD in the posterior reaching direction (F4,112 = 7.44, P < .001). It appeared that the 4 fatigue conditions had a diminishing effect on MAXD in both groups for both the uninvolved and involved sides, whereas the control day had no significant prefatigue-postfatigue effect (Figure 9). The involved side of the CAI group experienced a larger decrease in MAXD after all fatigue conditions compared with both the uninvolved side of the CAI group and the healthy group.
Figure 9. Group × day × side × time interaction for reach distance as a percentage of leg length (MAXD) in the posterior reaching direction.
A, Healthy subjects, uninvolved side. B, Healthy subjects, involved side. C, Chronic ankle instability subjects, uninvolved side. D, Chronic ankle instability subjects, involved side
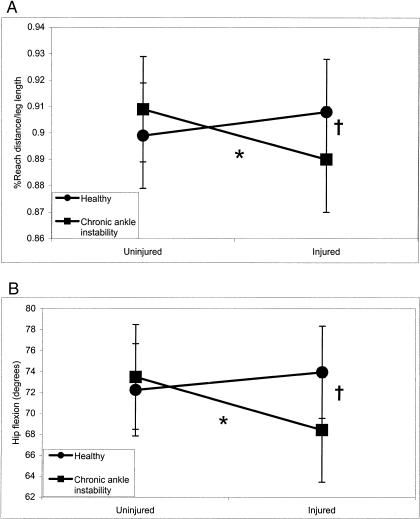
A significant group × side interaction (F1,28 = 7.01, P = .013) helps to further explain the 4-way interaction. The injured side of the CAI group had significantly less MAXD compared with the uninjured side of the CAI group as well as the matched side of the healthy group (Figure 10).
Figure 10. Group × side interaction for reach distance as a percentage of leg length (A) and hip-flexion angle (B) in the posterior reaching direction (side difference, *P < .05; group difference, †P < .05).
Hip Flexion
A significant group × side interaction for hip-flexion angle during posterior reaching was noted (F1,28 = 5.61, P = .025). Hip flexion on the injured side of the CAI group was notably less than on the uninjured side of the CAI group and the matched side of the healthy group (see Figure 10).
DISCUSSION
Chronic ankle instability and fatigue to the lower extremity adversely affected dynamic postural control as assessed by the SEBT. For all 3 reaching directions, the involved limb of the CAI group (ICAI) demonstrated less MAXD, knee flexion, and hip flexion compared with the uninjured limb (UCAI) as well as the matched limb of the healthy group (HEA).
Influence of CAI on Performance
Olmsted et al31 found similar results when comparing the performance of those with unilateral CAI with matched control subjects during completion of all 8 reaching directions of the SEBT. Normalized reaching distances were reduced on the ICAI; however, the authors did not examine potential sources of performance deficits. Our results demonstrate reduced knee and hip joint angles for ICAI occurred simultaneously with reduced MAXD, indicating a relationship between performance on the SEBT and altered neuromuscular control at the knee and hip due to ankle injury.
Documented impairments in static postural control result from musculoskeletal injury to the ankle.6,19,20,34,35 An injury that disrupts joint integrity, such as CAI, is theorized to impair afferent-efferent pathways that allow for maintenance of proprioception, kinesthesia, and ultimately neuromuscular control. Most investigators who have examined this phenomenon have focused on neuromuscular impairments only at the injured joint complex. Although this is a viable means of answering questions of impaired neuromuscular control relying primarily on single-joint neural systems, functional activities of the lower extremity do not involve single-joint movements but rather coordinated actions throughout the entire lower kinetic chain.
Proximal Joint Influences on Performance
Disruptions to one joint may create altered neural activity and compensatory muscle recruitment at other joint complexes, resulting in disrupted movement patterns. Bullock-Saxton et al24 reported alterations in electromyographic activity of hamstrings, gluteus maximus, and erector spinae as well as decreased detection to vibration in the ankle36 in subjects who had suffered a severe lateral ankle sprain. Subjects performed prone hip extensions, a non-weight-bearing, open chain task that is markedly different from the SEBT. However, the trend of alterations in neuromuscular control in the proximal joints of subjects with lateral ankle sprain is consistent across these investigations. The injured athlete may be able to complete a gross motor task, but the method of completion may be altered and less than optimal and/or efficient, creating the potential threat of reinjury.
Beckman and Buchanan7 reported an altered proximal muscle recruitment pattern in hip musculature in response to ankle inversion perturbation in the presence of pathologic ankle hypermobility. When a hypermobile ankle was forced into unexpected inversion, the contralateral gluteus medius was recruited before the ipsilateral gluteus medius, both recruitments occurring more rapidly than in the healthy subjects. The authors attributed this pattern to a hip strategy of muscle recruitment for the purpose of maintaining upright posture. Beckman and Buchanan7 hypothesized that because these observed corrective muscle recruitments are faster than allowed by peripheral neural afferent generation, the altered hip gluteus medius recruitment was due to a central nervous system adaptation. The authors believe this may have led to enhanced gamma motor neuron activity to the hip musculature, which could be categorized as a protective mechanism in the presence of pathologic ankle hypermobility.
We found reduced hip and knee flexion in the CAI group during the dynamic postural-control task, resulting in greater resistance to movement of these joints during the task. Beckman and Buchanan7 attributed the proximal musculature recruitment in their results, in part, to centrally created neural adaptations to postural control due to the rapid pace of their applied perturbation. Further supporting this theory, Konradsen21 demonstrated that the protective contraction by the peroneal muscles to resist forced inversion does not occur through peripheral feedback loops quickly enough and must, therefore, be a centrally driven response.
The CAI subjects in our study presented with reduced hip and knee flexion compared with uninjured subjects' motion during the reaching task. Whether this was due to altered muscle recruitment about these joints, a change in joint stiffness as a means of increasing stability or centrally altered neuromuscular patterns is unknown. The SEBT is performed with a self-directed pace of movement of the reaching leg, with challenges to stability of the stance leg occurring at a much slower pace than the inversion-release platforms used by Konradsen21 and Beckman and Buchanan.7 Whether the apparent lack of muscle recruitment associated with decreased proximal joint movement in our CAI group is a result of central or peripheral nervous system alteration or, more likely, a combination of both is unclear.
Central Versus Peripheral Influences on Performance
Previous researchers have demonstrated bilateral deficits in neuromuscular control after acute lateral ankle sprain.23,34 These findings suggest a centrally mediated alteration in neuromuscular control, in contrast to the early findings of Freeman,35 who showed unilateral deficits in postural control in the presence of ankle instability. Our results demonstrated a unilateral effect of CAI on performance of the SEBT, suggesting contributions from a peripheral alteration in neuromuscular control. In examining these studies, it appears that the subjects with acute lateral ankle sprains presented with bilateral deficits, whereas Freeman35 and we looked at patients with chronic instability presenting with unilateral deficits. Perhaps there is an effect of the healing process on the level of neuromuscular adaptations related to ankle instability. Future researchers should examine serial testing of postural control after acute lateral ankle instability and the development of CAI.
During static postural-control measures, input from cutaneous plantar-surface receptors is proposed to aid in afferent input in maintaining the position of the base of support.37 Ochsendorf et al15 reported improved static postural control with the use of orthotics, which they attributed partially to potential improved cutaneous input on the undersurface of the foot. Cutaneous input from the plantar surface of the foot influences electromyographic activity of the biarticulate muscles of the thigh, creating an important link to maintenance of upright posture between the foot and proximal musculature.37 This relationship to muscle activation controlling the knee and hip is significantly reduced in patients with diabetes mellitus who have suffered cutaneous deficits to the plantar surface of the foot; subsequently, they display reduced static postural control.37 It may be possible that neuromuscular deficits associated with CAI result in similar changes in proximal neuromuscular control.
Each reach direction is inherently different in its combinations of sagittal-plane movements to achieve maximum reaching distance, and direct comparisons were not made. However, it is of interest that in all directions, HEA and UCAI produced further reaching distances while simultaneously incorporating larger degrees of knee and hip flexion compared with ICAI. This relationship of knee and hip flexion follows the coupled relationship that describes normal gait patterns in healthy adults.38
Influence of Fatigue on Performance
When considering the influence of fatigue on the comparisons of CAI and performance on the SEBT, we found that the ICAI group had consistently larger prefatigue-postfatigue decreases in MAXD, knee flexion, and hip flexion compared with UCAI and HEA. Not only does CAI create a decrease in dynamic postural control compared with unaffected limbs, but the disruption of normal muscle activation seems to be amplified in the presence of fatigue.
Freeman et al6 theorized that damage to joint receptors leads to delays in afferent conduction to recruit corrective muscle contractions from efferent signals in response to perturbation, ultimately altering joint stability. Deficits in static postural control among CAI sufferers have helped to support this theory.6,19,20,34,35 Contemporary theory points to the disruption of muscle spindle activity after joint injury as possibly contributing to deficits in neuromuscular control among CAI sufferers.22 Fatigue increases the threshold of muscle spindle discharge, which disrupts the afferent feedback, subsequently altering joint awareness.10 Deficits in static postural control after induced fatigue have helped confirm this theory.11–18 Future investigators should examine the role of fatigue-altered muscle spindle activity in the maintenance of neuromuscular control in patients who have suffered joint injuries.
Few researchers have studied the relationships that CAI and fatigue have on postural control collectively, especially dynamic postural control. The performance of the SEBT, being a measure of dynamic postural control, has been assessed only in terms of MAXD,26–31,33,39 even though it requires maintenance of the center of mass through coordination, strength, and flexibility. We attempted to examine some of the mechanisms that allow maintenance of dynamic postural control by establishing that there is a reliance on optimal knee and hip flexion to achieve maximum reach, which may be construed as a measure of neuromuscular control.
In previous studies,16,17 we noted that static postural control in healthy subjects was altered by fatiguing tasks similar to what we employed in this study. Fatigue to the proximal musculature of the knee and hip created the largest increases in center-of-pressure velocity scores, leading to conclusions that maintenance of quiet upright stance in a fatigued state relies more on proximal neuromuscular control than on the previously accepted ankle strategy of distal muscle recruitment in maintaining postural control in young populations.40
In our study, subjects appeared to be relying on proximal joint control during the SEBT. When fatigue was induced, proximal joint control was disrupted, and task performance was diminished. Riemann et al41 demonstrated greater reliance on ankle activity during static postural-control measures and proximal joint motion during more challenging postural tasks. Further investigation is needed into previously described ankle and hip strategies in maintaining double-limb stance and how these theories translate to more challenging postural-control tasks and the response to fatigue.
Initially, we hypothesized that the different fatigue conditions might create altered contraction properties of the muscles at the affected joint and result in altered kinematics at that joint. We thought that, because of the altered neuromuscular control inherent to ICAI, some of the most noticeable changes would occur at the ankle. Although the relationships of fatigue and CAI to ankle dorsiflexion were statistically significant, they were not discussed because we felt the clinical significance of the problem was not worthwhile. A statistically significant change was on average less than 2° and may be easily dismissed as measurement error rather than a significant finding.
A limitation to our study is that the targeted level of fatigue achieved by our subjects was not quantified through electromyographic measures. Instead, the levels of isokinetic and functional fatigue we used were based on methods previously established in the literature. Because the posttesting measurements needed to be performed as quickly as possible, it would have taken too long to analyze electromyographic data to look for a shift in the median frequency.
CONCLUSIONS
Neuromuscular impairments that result from CAI and fatigue have been well documented, but the combined relationship of the 2 has not been investigated. Chronic ankle instability and fatigue created dynamic postural-control deficits that appear to be linked to kinematic changes at the knee and hip. Future researchers should investigate how fatigue and CAI adversely affect dynamic postural control to help enlighten us as to the best way to prevent and rehabilitate functional deficits in CAI sufferers.
Acknowledgments
Financial support for this project was received from the National Athletic Trainers' Association Doctoral Research Grant (Dallas, TX) (Phillip Gribble) and the Pennsylvania Athletic Trainers' Association Supported Research Program Award (Harrisburg, PA) (Phillip Gribble).
REFERENCES
- Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7:29–36. [PubMed] [Google Scholar]
- Yeung MS, Chan KM, So CH, Wuan WY. An epidemiological survey on ankle sprain. Br J Sports Med. 1994;28:112–116. doi: 10.1136/bjsm.28.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Athl Train. 2002;37:376–380. [PMC free article] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Frank JS. Assessment of balance control in humans. Med Prog Technol. 1990;16:31–51. [PubMed] [Google Scholar]
- Bernier JN, Perrin DH. Effect of coordination training on proprioception of the functionally unstable ankle. J Orthop Sport Phys Ther. 1998;27:264–275. doi: 10.2519/jospt.1998.27.4.264. [DOI] [PubMed] [Google Scholar]
- Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47:678–685. [PubMed] [Google Scholar]
- Beckman SM, Buchanan TS. Ankle inversion injury and hypermobility: effect on hip and ankle muscle electromyography onset latency. Arch Phys Med Rehabil. 1995;76:1138–1143. doi: 10.1016/s0003-9993(95)80123-5. [DOI] [PubMed] [Google Scholar]
- Ross SE, Guskiewicz KM, Yu B. Comparison of time to stabilization measures in functionally unstable versus stable ankles [abstract] J Athl Train. 2001;36(suppl):S–76. [Google Scholar]
- Testerman C, Vander Griend R. Evaluation of ankle instability using the Biodex Stability System. Foot Ankle Int. 1999;20:317–321. doi: 10.1177/107110079902000510. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Yuktanandana P, Pincivero D, Lephart S. Role of fatigue on proprioception and neuromuscular control. In: Lephart S, Fu F, editors. Proprioception and Neuromuscular Control in Joint Stability. Champaign, IL: Human Kinetics; 2000. pp. 375–383. [Google Scholar]
- Johnston RB, Howard ME, Cawley PW, Lossee GM. Effect of lower extremity muscular fatigue on motor control performance. Med Sci Sports Exerc. 1998;30:1703–1707. doi: 10.1097/00005768-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Lundin TM, Feuerbach JW, Grabiner MD. Effect of plantarflexor and dorsiflexor fatigue on unilateral postural control. J Appl Biomech. 1993;9:191–201. [Google Scholar]
- Miller PK, Bird AM. Localized muscle fatigue and dynamic balance. Percept Mot Skills. 1976;42:135–138. doi: 10.2466/pms.1976.42.1.135. [DOI] [PubMed] [Google Scholar]
- Nardone A, Tarantola J, Giordano A, Schieppati M. Fatigue effects on body balance. Electroencephalogr Clin Neurophysiol. 1997;105:309–320. doi: 10.1016/s0924-980x(97)00040-4. [DOI] [PubMed] [Google Scholar]
- Ochsendorf DT, Mattacola CG, Arnold BL. Effect of orthotics on postural sway after fatigue of the plantar flexors and dorsiflexors. J Athl Train. 2000;35:26–30. [PMC free article] [PubMed] [Google Scholar]
- Gribble P, Hertel J. Effect of hip and ankle muscle fatigue on unipedal postural control. J Electromyogr Kinesiol. 2004;14:641–646. doi: 10.1016/j.jelekin.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Gribble PA, Hertel J. Effect of lower-extremity muscle fatigue on postural control. Arch Phys Med Rehabil. 2004;85:589–592. doi: 10.1016/j.apmr.2003.06.031. [DOI] [PubMed] [Google Scholar]
- Ramsdell KM, Mattacola CG, Uhl TL, McCrory JL, Malone TR. Effects of two ankle fatigue models on the duration of postural stability dysfunction [abstract] J Athl Train. 2001;36(suppl):S–33. [PMC free article] [PubMed] [Google Scholar]
- Tropp H, Odenrick P, Gillquist J. Stabilometry recordings in functional and mechanical instability of the ankle joint. Int J Sports Med. 1985;6:180–182. doi: 10.1055/s-2008-1025836. [DOI] [PubMed] [Google Scholar]
- Goldie PA, Evans OM, Bach TM. Postural control following inversion injuries of the ankle. Arch Phys Med Rehabil. 1994;75:969–975. [PubMed] [Google Scholar]
- Konradsen L. Sensori-motor control of the uninjured and injured human ankle. J Electromyogr Kinesiol. 2002;12:199–203. doi: 10.1016/s1050-6411(02)00021-4. [DOI] [PubMed] [Google Scholar]
- Khin-Myo-Hla, Ishii T, Sakane M, Hayashi K. Effect of anesthesia of the sinus tarsi on peroneal reaction time in patients with functional instability of the ankle. Foot Ankle Int. 1999;20:554–559. doi: 10.1177/107110079902000903. [DOI] [PubMed] [Google Scholar]
- Isakov E, Mizrahi J. Is balance impaired by recurrent sprained ankle? Br J Sports Med. 1997;31:65–67. doi: 10.1136/bjsm.31.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock-Saxton JE, Janda V, Bullock ML. The influence of ankle sprain injury on muscle activation during hip extension. Int J Sports Med. 1994;15:330–334. doi: 10.1055/s-2007-1021069. [DOI] [PubMed] [Google Scholar]
- Caulfield BM, Garrett M. Functional instability of the ankle: differences in patterns of ankle and knee movement prior to and post landing in a single leg jump. Int J Sports Med. 2002;23:64–68. doi: 10.1055/s-2002-19272. [DOI] [PubMed] [Google Scholar]
- Kinzey SJ, Armstrong CW. The reliability of the star-excursion test in assessing dynamic balance. J Orthop Sports Phys Ther. 1998;27:356–360. doi: 10.2519/jospt.1998.27.5.356. [DOI] [PubMed] [Google Scholar]
- Hertel J, Miller S, Denegar C. Intratester and intertester reliability during the Star Excursion Balance Test. J Sport Rehabil. 2000;9:104–116. [Google Scholar]
- Earl J. University Park: Pennsylvania State University; 2002. Relationships Among Dynamic Malalignment, Neuromuscular Rehabilitation, and Patellofemoral Pain Syndrome [dissertation] [Google Scholar]
- Gribble PA, Hertel J. Predictors for performance of dynamic postural control using the Star Excursion Balance Test. Measure Phys Ed Exerc Sci. 2003;7:89–100. [Google Scholar]
- Earl J, Hertel J. Lower-extremity muscle activation during the Star Excursion Balance Tests. J Sport Rehabil. 2001;10:93–104. [Google Scholar]
- Olmsted LC, Carcia CR, Shultz SJ, Arnold BL. The effects of functional ankle instability on the performance of the Star Excursion Balance Test [abstract] J Athl Train. 2002;37(suppl):S–75. [PMC free article] [PubMed] [Google Scholar]
- Pincivero DM, Aldworth C, Dickerson T, Petry C, Shultz T. Quadriceps-hamstring EMG activity during functional, closed kinetic chain exercise to fatigue. Eur J Appl Physiol. 1999;81:504–509. doi: 10.1007/s004210050075. [DOI] [PubMed] [Google Scholar]
- Gray GW. Adrian, MI: Wynn Marketing, Inc; 1995. Lower Extremity Functional Profile. [Google Scholar]
- Tropp H, Ekstrand J, Gillquist J. Factors affecting stabilometry recordings of single limb stance. Am J Sports Med. 1984;12:185–188. doi: 10.1177/036354658401200302. [DOI] [PubMed] [Google Scholar]
- Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47:669–677. [PubMed] [Google Scholar]
- Bullock-Saxton JE. Local sensation changes and altered hip muscle function following severe ankle sprain. Phys Ther. 1994;74:17–31. doi: 10.1093/ptj/74.1.17. [DOI] [PubMed] [Google Scholar]
- van Deursen RWM, Cavanagh PR, van Ingen-Schenau G, Becker MB, Ulbrecht JS. The role of cutaneous information in a contract control task of the leg in humans. Hum Mov Sci. 1998;17:95–120. [Google Scholar]
- Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70:340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- Miller S. University Park: Pennsylvania State University; 2001. Biomechanical Analysis of the Anterior Balance Reach Test [dissertation] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Riemann BL, Myers JB, Lephart SM. Comparison of the ankle, knee, hip, and trunk corrective action shown during single-leg stance on firm, foam, and multiaxial surfaces. Arch Phys Med Rehabil. 2003;84:90–95. doi: 10.1053/apmr.2003.50004. [DOI] [PubMed] [Google Scholar]