Abstract
Some studies found that there was a significant association between asthma and the risk of lung cancer. However, the results are inconclusive. Therefore, we performed a meta-analysis. We searched the electronic databases for all relevant articles. Odds ratio (OR) with 95% confidence interval (CI) were used to calculate the strength of the association between asthma and lung cancer risk. Asthma was significantly associated with the increased risk of lung cancer (OR = 1.44; 95% CI 1.31–1.59; P < 0.00001; I2 = 83%). Additionally, asthma patients without smoking also had the increased lung cancer risk. In the subgroup analysis of race and gender, Caucasians, Asians, male, and female patients with asthma showed the increased risk of lung cancer. However, asthma was not significantly associated with lung adenocarcinoma risk. In the stratified analysis by asthma definition, significant associations were found between asthma and lung cancer in self-reported subgroup, questionnaire subgroup, and register databases subgroup. However, no significant association was observed in physician-diagnosed asthma subgroup. In conclusion, this meta-analysis suggested that asthma might be significantly associated with lung cancer risk.
Keywords: asthma, lung cancer, association, meta-analysis
INTRODUCTION
Lung cancer is one of the most common malignant tumors affecting millions of people around the world. It is acknowledged that smoking is the most important risk factor of lung cancer [1]. Sun et al. suggested that approximately 25% of lung cancer cases worldwide are not attributable to tobacco use [2]. This fact indicated that other factors might contribute to susceptibility to lung cancer.
Asthma is one of the most common diseases of childhood, with an estimated global prevalence of 10% among children aged 6–7 [3]. It is a condition characterized by chronic inflammation of the lungs, presenting with airway hyper-reactivity, excessive mucous formation, and respiratory obstruction. The chronic inflammation had an important role in the cancer development [4]. Thus, the chronic inflammation in conditions such as asthma might lead to lung cancer development. Some studies found that there was a significant association between asthma and the risk of lung cancer. However, the results are inconclusive [5–22]. Therefore, we performed a meta-analysis to determine the association between asthma and lung cancer risk.
RESULTS
Characteristics of eligible studies
The detailed literature search strategy was showed in Figure 1. A total of 884 potential studies were identified by preliminary searching. After carefully review, 18 studies were included in this meta-analysis [5–22]. Four studies reported two independent studies. Thus, 22 studies with 16375202 subjects were included. Table 1 presents the main characteristics of these studies, such as first author's name, year of publication, ethnicity, gender, age, asthma definition, lung cancer definition, duration of follow-up, sample size, and adjustment. The quality of the studies was high.
Figure 1. The selection of included studies.
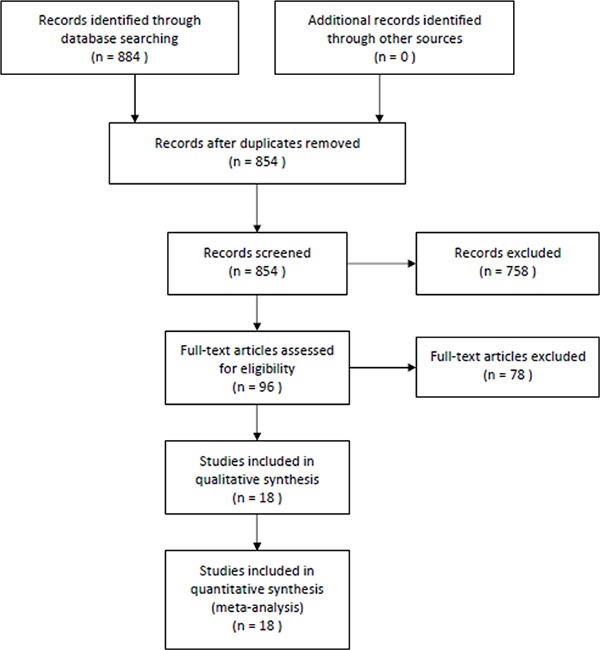
Table 1. Characteristics of the included studies.
| First author | Year | Race | Gender | Age (y) | Definition of asthma | Definition of lung cancer | Follow-up years | Samle size | Covariants | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Alderson | 1974 | Mixed | Mixed | 25–59 | Self-reported | Obtain from register databases | 21 | 1892 | No | 7 |
| Reynolds 1 | 1987 | Mixed | Male | NA | Self-reported | Obtain from register databases | 18 | 3117 | Age, smoking | 9 |
| Reynolds 2 | 1987 | Mixed | Female | NA | Self-reported | Obtain from register databases | 18 | 3708 | Age, smoking | 9 |
| Mills | 1992 | Caucasian | Mixed | NA | Questionnaire | Mailed questionnaires and tumor registries | 6 | 34198 | Age, sex, smoking history, and time since last physician contact | 9 |
| Vesterinen | 1993 | Caucasian | Mixed | NA | Obtain from register databases | Obtain from register databases | 7 | 35126 | No | 7 |
| Eriksson | 1995 | Caucasian | Mixed | 16–79 | Physician-diagnosed | Obtain from register databases | 14 | 6593 | No | 7 |
| Huovinen | 1997 | Caucasian | Male | > 18 | Self-reported | Obtain from register databases | 16 | 14654 | Age, smoking, social class, pets, dogs, chronic bronchitis, dyspnoea, hay fever | 9 |
| Boffetta 1 | 2002 | Caucasian | Male | > 20 | Obtain from register databases | Obtain from register databases | 8.5 | 42663 | Duration of follow-up, calendar year at entry, age, other diagnoses, emphysema, chronic bronchitis | 9 |
| Boffetta 2 | 2002 | Caucasian | Female | > 20 | Obtain from register databases | Obtain from register databases | 8.5 | 50323 | Duration of follow-up, calendar year at entry, age, other diagnoses, emphysema, chronic bronchitis | 9 |
| Talbot-Smith 1 | 2003 | Caucasian | Male | NA | Physician-diagnosed | Obtain from register databases | 19 | 124 | Age, smoking | 8 |
| Talbot-Smith 2 | 2003 | Caucasian | Female | NA | Physician-diagnosed | Obtain from register databases | 19 | 155 | Age, smoking | 8 |
| Vandentorren | 2003 | Mixed | Mixed | 25–59 | Questionnaire | Obtain from register databases | 25 | 14286 | Age, sex, educational level, smoking habit, occupational exposure and forced expiratory volume in one second | 9 |
| Littman | 2004 | Mixed | Mixed | 44–74 | Self-reported | Obtain from register databases | 9.1 | 17698 | Sex, exposure cohort, study arm, education, body mass index, years smoked and years smoked squared, average number of cigarettes smoked per day and average number of cigarettes smoked per day squared, and all other lung diseases, and stratified by smoking status | 9 |
| Turner | 2005 | Mixed | Mixed | > 30 | Self-reported | Obtain from register databases | 19 | 26097 | Age, gender, race, smoking, education, marital status, body mass index, occupational exposures, beer, wine, and liquor consumption, chronic bronchitis, emphysema, tuberculosis, intakes of vegetables, fruit, fiber, and fat, and passive smoking | 9 |
| Brown | 2006 | Mixed | Mixed | 50–89 | Self-reported | Self-reported | 9 | 8896 | Age, smoking, sociodemographics | 8 |
| Gonzalez-Perez | 2006 | Mixed | Mixed | 20–79 | Self-reported | Mailed questionnaires | 7 | 18792 | Age, sex, calendar year, body mass index, alcohol intake, smoking status, prior comorbidities, health services utilization, use of aspirin, and paracetamol | 8 |
| Ji | 2009 | Mixed | Mixed | NA | Obtain from register databases | Obtain from register databases | 40 | 140425 | No | 7 |
| Colak | 2015 | Mixed | Mixed | 56 | Self-reported | Obtain from register databases | 9.4 | 94097 | Age, sex, body mass index, familial pre-disposition for asthma, allergy, childhood asthma, hay fever, or eczema, use of asthma medication, occupational exposure to dust and/or fumes, daily exposure to passive smoking, physical activity in leisure-time, education, annual household income, and cumulative tobacco consumption | 8 |
| Huang 1 | 2015 | Asian | Male | > 20 | Obtain from register databases | Obtain from register databases | 4 | 8002536 | Lung diseases, low income, age, comorbidities, urbanization and geographic area | 9 |
| Huang 2 | 2015 | Asian | Female | > 20 | Obtain from register databases | Obtain from register databases | 4 | 7216488 | Lung diseases, low income, age, comorbidities, urbanization and geographic area | 9 |
| Fan | 2016 | Asian | Mixed | 40–9 | Physician-diagnosed | Chest radiography, physician-diagnosed, obtain from register databases | 9 | 9295 | Age, sex, education, smoking, arsenic level, radon level, prior comorbidities | 8 |
| Pirie | 2016 | Mixed | Female | 56 | Questionnaire | Obtain from register databases | 14 | 634039 | Age, region, deprivation quintile, height | 7 |
NA, not available.
Association of asthma and risk of lung cancer
As shown in Figure 2, asthma was significantly associated with the increased risk of lung cancer (odds ratio (OR) = 1.44; 95% confidence interval (CI) 1.31–1.59; P < 0.00001; I2 = 83%). Additionally, asthma patients without smoking also had the increased lung cancer risk (OR = 1.28; 95% CI 1.10–1.50; P = 0.002; I2 = 0%). In the subgroup analysis of race, both Caucasians and Asians with asthma showed the same results (OR = 1.53; 95% CI 1.37–1.72; P < 0.00001; I2 = 56%; OR = 1.52; 95% CI 1.15–2.01; P < 0.00001; I2 = 93%). In the stratified analysis by gender, both male and female patients with asthma showed the increased risk of lung cancer (OR = 1.38; 95% CI 1.31–1.46; P < 0.00001; I2 = 24%; OR = 1.68; 95% CI 1.45–1.95; P < 0.00001; I2 = 63%). However, asthma was not significantly associated with lung adenocarcinoma risk (OR = 1.01; 95% CI 0.69–1.50; P = 0.95; I2 = 45%). In the stratified analysis by asthma definition, significant associations were found between asthma and lung cancer in self-reported subgroup (OR = 1.23; 95% CI 1.03–1.48; P = 0.02; I2 = 53%), questionnaire subgroup (OR = 1.32; 95% CI 1.12–1.57; P = 0.001; I2 = 0%), and register databases subgroup (OR = 1.60; 95% CI 1.42–1.79; P < 0.00001; I2 = 91%). However, no significant association was observed in physician-diagnosed asthma subgroup (OR = 1.26; 95% CI 0.96–1.65; P = 0.10; I2 = 0%). The results were showed in Table 2.
Figure 2. Meta-analysis of the association between asthma and lung cancer.
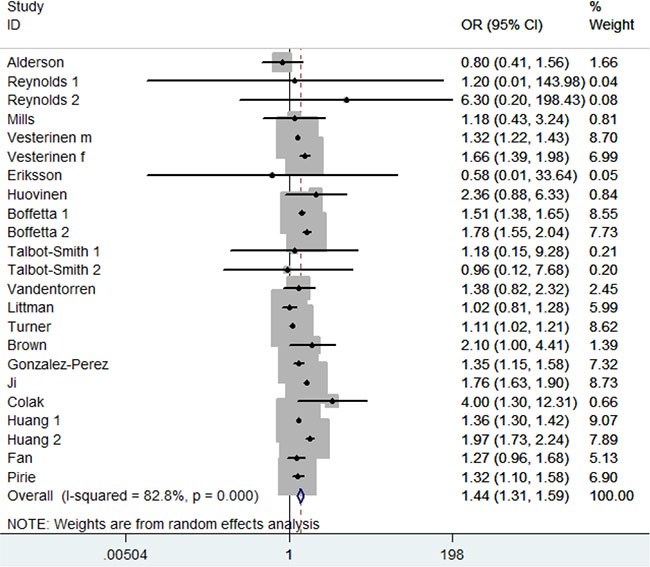
Table 2. Results of this meta-analysis.
| No. of study | OR (95% CI) | P Value for meta-analysis | I2 (%) | P Value for subgroup analysis | |
|---|---|---|---|---|---|
| Overall lung cancer risk | 23 | 1.44 (1.31–1.59) | < 0.00001 | 83 | |
| Non-smoker | 4 | 1.28 (1.10–.50) | 0.002 | 0 | |
| Subgroup analysis | |||||
| Race | 0.95 | ||||
| Caucasian | 9 | 1.53 (1.37–1.72) | < 0.00001 | 56 | |
| Asian | 3 | 1.52 (1.15–2.01) | < 0.00001 | 93 | |
| Gender | 0.02 | ||||
| Male | 6 | 1.38 (1.31–1.46) | < 0.00001 | 24 | |
| Female | 6 | 1.68 (1.45–1.95) | < 0.00001 | 63 | |
| Lung adenocarcinoma | 2 | 1.01 (0.69–1.50) | 0.95 | 45 | |
| Definition of asthma | 0.05 | ||||
| Self-reported | 9 | 1.23 (1.03–1.48) | 0.02 | 53 | |
| Questionnaire | 3 | 1.32 (1.12–1.57) | 0.001 | 0 | |
| Physician-diagnosed | 4 | 1.26 (0.96–1.65) | 0.10 | 0 | |
| Register databases | 7 | 1.60 (1.42–1.79) | < 0.00001 | 91 |
In the sensitive analysis, similar data were observed after sequentially excluding each study (Figure 3). Furthermore, when the studies without adjustment were excluded, the result was still statistically significant (OR = 1.43, 95% CI 1.28–1.60, P < 0.00001; I2 = 80%). In addition, after excluding the studies without adjusting smoking and age, the result was not changed (OR = 1.29, 95% CI 1.12–1.48, P = 0.0004; I2 = 27%). The results were showed in Table 3.
Figure 3. Sensitivity analysis of the association between asthma and lung cancer.
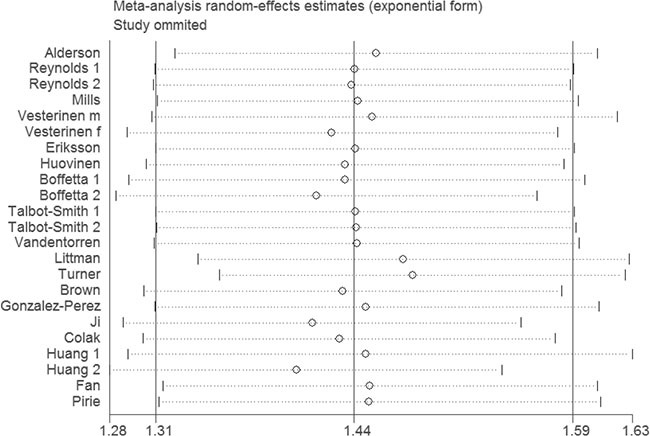
Table 3. Results of sensitivity analysis.
| No. of study | OR (95% CI) | P Value | I2 (%) | |
|---|---|---|---|---|
| Studies with adjustment | 18 | 1.43 (1.28–1.60) | < 0.00001 | 80 |
| Adjustment with smoking and age | 12 | 1.29 (1.12–1.48) | 0.0004 | 27 |
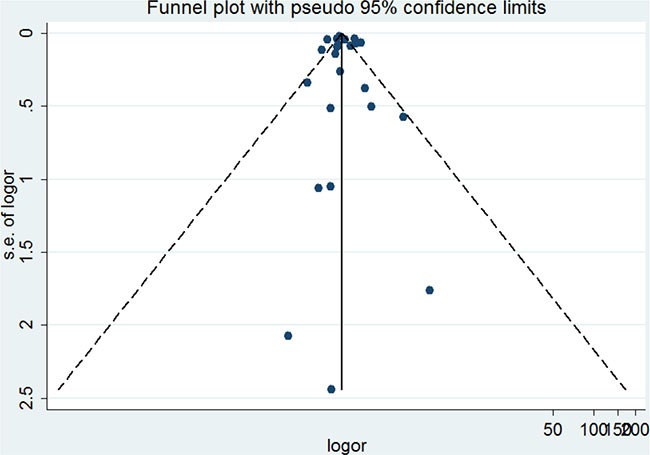
The publication bias of the included studies was assessed by the funnel plot and Egger's test. The funnel plot was symmetric (Figure 4). Egger's test showed no significant publication bias (P = 0.683).
Figure 4. Funnel plot of the association between asthma and lung cancer.
DISCUSSION
This present meta-analysis investigating the relationship between asthma and lung cancer risk. Eighteen studies with a total of 16375202 individuals were included in this meta-analysis. Prior asthma was significantly associated with lung cancer risk. In the subgroup analysis of race, both Caucasians and Asians with asthma showed the same results. In the stratified analysis by gender, both male and female patients with asthma showed the increased risk of lung cancer. Smoke habit was a recognized risk factor for lung cancer. However, asthma patients without smoking also had the increased lung cancer risk. These results suggested that asthma might be an independent risk factor for lung cancer. Lung cancer is classified small cell lung cancer and adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [23]. Asthma was not significantly associated with lung adenocarcinoma risk in this meta-analysis. Future studies should be performed to assess the association between asthma and other pathological types of lung cancer. In the stratified analysis by asthma definition, significant associations were found in self-reported subgroup, questionnaire subgroup, and register databases subgroup. However, no significant association was observed in physician-diagnosed asthma subgroup. Only four studies were included in this subgroup. A positive association could therefore not be ruled out, because studies with small sample sizes may have had insufficient statistical power to detect any slight effect. To determine the stability of the result, we did sensitivity analysis. Removal of each study did not change the result, suggesting the reliability of our result. The adjusted ORs could be used to overcome some of the confounding within the observational studies. Thus, we did sensitivity analysis by excluding the studies without adjustment. The result was still statistically significant.
Asthma is a disease of chronic airway inflammation characterized by recurrent episodes of wheezing, dyspnea, chest tightness, and cough. Inflammatory cell types, such as T and B lymphocytes, mast cells, eosinophils, basophils, neutrophils and dendritic cells, as well as structural cell types including epithelial and mesenchymal cells involved in airway inflammation [24]. Inflammation also plays a pivotal role in the pathogenesis of lung cancer. Ballaz and Mulshine indicated that chronic inflammation contributes to the process of lung carcinogenesis [25]. Azad et al. suggested that chronic inflammation-induced production of reactive oxygen/nitrogen species in the lung may predispose individuals to lung cancer [26].
This meta-analysis was stable and reliable. First, sensitivity analyses revealed that the results were robust. Second, no significant publication bias was found in this meta-analysis. Third, the sample size of this meta-analysis was large enough. Several limitations should be noted. First, other races, such as African, were not included. Second, heterogeneity was high in this meta-analysis. Third, subgroup analysis based on study level variables could be due to the potential for ecological fallacy. Therefore, more studies are required to confirm the results of this meta-analysis.
In conclusion, this meta-analysis suggested that asthma might be significantly associated with lung cancer risk.
MATERIALS AND METHODS
Publication search
Two authors (YLQ and JL) searched Pubmed, Embase, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases (www.wanfangdata.com.cn). We also checked American Society of Clinical Oncology (ASCO) meeting abstracts to find grey literature (http://meetinglibrary.asco.org/abstracts). Last search was updated in 10 Nov, 2016. We searched the bibliographies of identified studies and narrative reviews for additional citations. No language and time restriction were imposed in this meta-analysis. The detailed search strategy is showed in the Supplementary Material.
Study selection
Two authors (YLQ and JL) searched the titles and abstracts obtained from the initial electronic search for potentially relevant studies for full review. Two authors (YLQ and LXZ) then assessed the full text of the retrieved studies to determine whether the study met the inclusion criteria. Studies included in this meta-analysis should meet the following criteria: (1) study design: prospective cohort study, cross-sectional study, and longitudinal study; (2) population: individuals without lung cancer; (3) exposure: asthma or wheeze; (4) comparison: individuals without asthma or wheeze; (5) outcome: relative risk (RR), hazard ratio (HR), or OR with corresponding 95% CI of lung cancer risk in overall population and in non-smokers. If serial studies of the same population from the same group were reported, the largest study was included. Reviews, meta-analyses, letters, and editorial articles were all excluded. We resolved disagreement by consensus.
Data extraction and qualitative assessment
Two investigators (YLQ and JL) extracted the data independently. The following data were collected from each study: first author's name, year of publication, ethnicity, gender, age, asthma definition, lung cancer definition, duration of follow-up, sample size, and adjustment. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of included studies.
Statistical analysis
ORs with 95% CIs were used to calculate the strength of the association between asthma and lung cancer risk. Random effects model was used in this meta-analysis. Heterogeneity among studies was examined with I2 statistic and Q statistic. Subgroup analysis was carried out by smoke status, ethnicity, gender, subtype of lung cancer, and definition of asthma. Relative influence of each study on the pooled estimate was assessed by omitting one study at a time for sensitivity analysis. Sensitivity analysis was also performed by excluding studies without adjustment, studies without adjustment of age and smoking. Funnel plot and Begg's test were employed to evaluate publication bias. All statistical tests were performed using the STATA 11.0 software (Stata Corporation, College Station, TX). A P value < 0.05 was considered statistically significant, except for tests of heterogeneity where a level of 0.10 was used. All tests were two-sided.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
ACKNOWLEDGMENTS AND FUNDING
This work is supported by The army youth development program in medical technology (16QNP014).
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 2.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 3.Koppelman GH, Stine OC, Xu J, Howard TD, Zheng SL, Kauffman HF, Bleecker ER, Meyers DA, Postma DS. Genome-wide search for atopy susceptibility genes in Dutch families with asthma. J Allergy Clin Immunol. 2002;109:498–506. doi: 10.1067/mai.2002.122235. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderson M. Mortality from malignant disease in patients with asthma. Lancet. 1974;2:1475–7. doi: 10.1016/s0140-6736(74)90217-7. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds P, Kaplan GA. Asthma and cancer. Am J Epidemiol. 1987;125:539–40. doi: 10.1093/oxfordjournals.aje.a114561. [DOI] [PubMed] [Google Scholar]
- 7.Mills PK, Beeson WL, Fraser GE, Phillips RL. Allergy and cancer: organ site-specific results from the Adventist Health Study. Am J Epidemiol. 1992;136:287–95. doi: 10.1093/oxfordjournals.aje.a116494. [DOI] [PubMed] [Google Scholar]
- 8.Vesterinen E, Pukkala E, Timonen T, Aromaa A. Cancer incidence among 78,000 asthmatic patients. Int J Epidemiol. 1993;22:976–82. doi: 10.1093/ije/22.6.976. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson NE, Holmén A, Högstedt B, Mikoczy Z, Hagmar L. A prospective study of cancer incidence in a cohort examined for allergy. Allergy. 1995;50:718–22. doi: 10.1111/j.1398-9995.1995.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 10.Huovinen E, Kaprio J, Vesterinen E, Koskenvuo M. Mortality of adults with asthma: a prospective cohort study. Thorax. 1997;52:49–54. doi: 10.1136/thx.52.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boffetta P, Ye W, Boman G, Nyré n. Lung cancer risk in a population-based cohort of patients hospitalized for asthma in Sweden. Eur Respir J. 2002;19:127–33. doi: 10.1183/09031936.02.00245802. [DOI] [PubMed] [Google Scholar]
- 12.Talbot-Smith A, Fritschi L, Divitini ML, Mallon DF, Knuiman MW. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am J Epidemiol. 2003;157:606–12. doi: 10.1093/aje/kwg020. [DOI] [PubMed] [Google Scholar]
- 13.Vandentorren S, Baldi I, I Annesi Maesano, Charpin D, Neukirch F, Filleul L, Cantagrel A, Tessier JF. Long-term mortality among adults with or without asthma in the PAARC study. Eur Respir J. 2003;21:462–7. doi: 10.1183/09031936.03.00030303. [DOI] [PubMed] [Google Scholar]
- 14.Littman AJ, Thornquist MD, White E, Jackson LA, Goodman GE, Vaughan TL. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control. 2004;15:819–27. doi: 10.1023/B:CACO.0000043432.71626.45. [DOI] [PubMed] [Google Scholar]
- 15.Turner MC, Chen Y, Krewski D, Ghadirian P, Thun MJ, Calle EE. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005;162:212–21. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 16.Brown DW, Young KE, Anda RF, Felitti VJ, Giles WH. Re: asthma and the risk of lung cancer. findings from the Adverse Childhood Experiences (ACE) Cancer Causes Control. 2006;17:349–50. doi: 10.1007/s10552-005-0420-5. [DOI] [PubMed] [Google Scholar]
- 17.González-Pérez A, Fernández-Vidaurre C, Rueda A, Rivero E, García Rodríguez LA. Cancer incidence in a general population of asthma patients. Pharmacoepidemiol Drug Saf. 2006;15:131–8. doi: 10.1002/pds.1163. [DOI] [PubMed] [Google Scholar]
- 18.Ji J, Shu X, Li X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in hospitalised asthma patients. Br J Cancer. 2009;100:829–33. doi: 10.1038/sj.bjc.6604890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Çolak Y, Afzal S, Nordestgaard BG, Lange P. Characteristics and Prognosis of Never-Smokers and Smokers with Asthma in the Copenhagen General Population Study. A Prospective Cohort Study. Am J Respir Crit Care Med. 2015;192:172–81. doi: 10.1164/rccm.201502-0302OC. [DOI] [PubMed] [Google Scholar]
- 20.Huang JY, Jian ZH, Nfor ON, Ku WY, Ko PC, Lung CC, Ho CC, Pan HH, Huang CY, Liang YC, Liaw YP. The effects of pulmonary diseases on histologic types of lung cancer in both sexes: a population-based study in Taiwan. BMC Cancer. 2015;15:834. doi: 10.1186/s12885-015-1847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan Y, Jiang Y, Hu P, Chang R, Yao S, Wang B, Li X, Zhou Q, Qiao Y. Modification of association between prior lung disease and lung cancer by inhaled arsenic: A prospective occupational-based cohort study in Yunnan, China. J Expo Sci Environ Epidemiol. 2016;26:464–70. doi: 10.1038/jes.2016.22. [DOI] [PubMed] [Google Scholar]
- 22.Pirie K, Peto R, Green J, Reeves GK, V; Beral, Million Women Study Collaborators Lung cancer in never smokers in the UK Million Women Study. Int J Cancer. 2016;139:347–54. doi: 10.1002/ijc.30084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–92. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012;11:958–72. doi: 10.1038/nrd3792. [DOI] [PubMed] [Google Scholar]
- 25.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5:46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 26.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


