Abstract
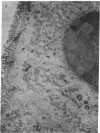
Glutathione (GSH) deficiency produced in mice by giving buthionine sulfoximine leads to severe degeneration of the epithelial cells of the jejunum and colon. This is prevented by giving GSH monoester (orally or i.p.) and also by giving GSH (orally, but not i.p.). The i.p. administration leads to high plasma levels of GSH but does not appreciably increase GSH levels in intestinal mucosa or pancreas. These and previous studies on lens, lung, lymphocytes, liver, heart, and skeletal muscle indicate that there is very little, if any, transport of intact GSH from plasma to these tissues. Cells can use extracellular GSH by a pathway involving its cleavage, uptake of products and intracellular GSH synthesis. Epithelial cells of the gastrointestinal tract may use this pathway and can also take up lumenal GSH (which arises partly from the bile) by a mechanism(s) that may involve transport of dipeptides or of GSH. It is suggested that biliary GSH normally functions in the protection of intestinal mucosa. Administration of GSH may be protective of the gastrointestinal epithelium and may also serve as a good source of cysteine moieties for intracellular GSH synthesis in the gastrointestinal tract and in other tissues. Administration of GSH delivery agents such as GSH esters is more effective than administration of GSH in increasing cellular and mitochondrial levels of GSH.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. A., Bridges R. J., Meister A. Extracellular metabolism of glutathione accounts for its disappearance from the basolateral circulation of the kidney. J Biol Chem. 1984 Dec 25;259(24):15393–15400. [PubMed] [Google Scholar]
- Abbott W. A., Griffith O. W., Meister A. Gamma-glutamyl-glutathione. Natural occurrence and enzymology. J Biol Chem. 1986 Oct 15;261(29):13657–13661. [PubMed] [Google Scholar]
- Abbott W. A., Meister A. Intrahepatic transport and utilization of biliary glutathione and its metabolites. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1246–1250. doi: 10.1073/pnas.83.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi S. A., Morse E. L., Masilamani S. S., Amin P. M. Evidence for two different modes of tripeptide disappearance in human intestine. Uptake by peptide carrier systems and hydrolysis by peptide hydrolases. J Clin Invest. 1975 Dec;56(6):1355–1363. doi: 10.1172/JCI108215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen L., Meck R., Yunis A. The inhibition of gamma-glutamyl transpeptidase from human pancreatic carcinoma cells by (alpha S,5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Res Commun Chem Pathol Pharmacol. 1980 Jan;27(1):175–182. [PubMed] [Google Scholar]
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Glutathione monoesters. Anal Biochem. 1989 Nov 15;183(1):16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Inhibition of gamma-glutamyl transpeptidase and induction of glutathionuria by gamma-glutamyl amino acids. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5029–5032. doi: 10.1073/pnas.83.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Astor M. B., Anderson M. E., Meister A. Relationship between intracellular GSH levels and hypoxic cell radiosensitivity. Pharmacol Ther. 1988;39(1-3):115–121. doi: 10.1016/0163-7258(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Berggren M., Dawson J., Moldéus P. Glutathione biosynthesis in the isolated perfused rat lung: utilization of extracellular glutathione. FEBS Lett. 1984 Oct 15;176(1):189–192. doi: 10.1016/0014-5793(84)80938-2. [DOI] [PubMed] [Google Scholar]
- Cornell J. S., Meister A. Glutathione and gamma-glutamyl cycle enzymes in crypt and villus tip cells of rat jejunal mucosa. Proc Natl Acad Sci U S A. 1976 Feb;73(2):420–422. doi: 10.1073/pnas.73.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle D., Clarke R., Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem. 1981 Mar 10;256(5):2115–2117. [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Excretion of cysteine and gamma-glutamylcysteine moieties in human and experimental animal gamma-glutamyl transpeptidase deficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3384–3387. doi: 10.1073/pnas.77.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen T. M., Brown L. A., Jones D. P. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986 Dec 15;35(24):4537–4542. doi: 10.1016/0006-2952(86)90776-8. [DOI] [PubMed] [Google Scholar]
- Hagen T. M., Jones D. P. Transepithelial transport of glutathione in vascularly perfused small intestine of rat. Am J Physiol. 1987 May;252(5 Pt 1):G607–G613. doi: 10.1152/ajpgi.1987.252.5.G607. [DOI] [PubMed] [Google Scholar]
- Hunjan M. K., Evered D. F. Absorption of glutathione from the gastro-intestinal tract. Biochim Biophys Acta. 1985 May 14;815(2):184–188. doi: 10.1016/0005-2736(85)90287-1. [DOI] [PubMed] [Google Scholar]
- Jensen G. L., Meister A. Radioprotection of human lymphoid cells by exogenously supplied glutathione is mediated by gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4714–4717. doi: 10.1073/pnas.80.15.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Hagen T. M., Jones D. P. Exogenous glutathione protects intestinal epithelial cells from oxidative injury. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4641–4645. doi: 10.1073/pnas.83.13.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Renal glutathione transport. Characteristics of the sodium-dependent system in the basal-lateral membrane. J Biol Chem. 1984 Dec 10;259(23):14508–14514. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A., Griffith O. W. Effects of methionine sulfoximine analogs on the synthesis of glutamine and glutathione: possible chemotherapeutic implications. Cancer Treat Rep. 1979 Jun;63(6):1115–1121. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. Overview--preparation and properties of mitochondria from different sources. Methods Enzymol. 1979;55:3–28. doi: 10.1016/0076-6879(79)55003-4. [DOI] [PubMed] [Google Scholar]
- Puri R. N., Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5258–5260. doi: 10.1073/pnas.80.17.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Turley R. B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986 Mar;153(2):267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Jr, Srere P. A. Organization of Krebs tricarboxylic acid cycle enzymes in mitochondria. J Biol Chem. 1985 Sep 5;260(19):10800–10805. [PubMed] [Google Scholar]
- Sies H., Koch O. R., Martino E., Boveris A. Increased biliary glutathione disulfide release in chronically ethanol-treated rats. FEBS Lett. 1979 Jul 15;103(2):287–290. doi: 10.1016/0014-5793(79)81346-0. [DOI] [PubMed] [Google Scholar]
- Singhal R. K., Anderson M. E., Meister A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987 Sep;1(3):220–223. doi: 10.1096/fasebj.1.3.2887478. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Crawford J. M., Holden S. A., Lin Y., Cathcart K. N., Luchette C. A., Flatow J. Glutathione monoethyl ester can selectively protect liver from high dose BCNU or cyclophosphamide. Cancer. 1988 Oct 1;62(7):1275–1281. doi: 10.1002/1097-0142(19881001)62:7<1275::aid-cncr2820620705>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Rosano C. L. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. J Appl Physiol (1985) 1989 Mar;66(3):1029–1034. doi: 10.1152/jappl.1989.66.3.1029. [DOI] [PubMed] [Google Scholar]
- Vistica D. T. Cellular pharmacokinetics of the phenylalanine mustards. Pharmacol Ther. 1983;22(3):379–406. doi: 10.1016/0163-7258(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Anderson M. E., Puri R. N., Jensen G. L., Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Iwauchi Y., Sugiyama S., Kuwamura T., Odaka Y., Satoh T., Kitagawa H. Transport of L-cysteine and reduced glutathione through biological membranes. Res Commun Chem Pathol Pharmacol. 1982 Aug;37(2):171–186. [PubMed] [Google Scholar]