Abstract
The prognosis of metastatic breast cancer is always very poor. Thus, it is urgent to develop novel drugs with less toxicity against metastatic breast cancer. A new drug (XC-591) derived from benzothiazole-2-thiol was designed and synthesized in our lab. In this study, we tried to assess effects of XC-591 treatment on primary breast cancer and pulmonary metastasis in 4T1 mice model. Furthermore, we tried to discover its possible molecular mechanism of action. MTT experiment showed XC-591 had significant anti-cancer activity on diverse cancer cells. Furthermore, XC-591 significantly suppressed the proliferation of 4T1 cells by colony formation assay. The in vivo results displayed that XC-591 could inhibit the growth and metastasis in 4T1 model. Moreover, histological analysis revealed that XC-591 treatment increased apoptosis, inhibited proliferation and angiogenesis in vivo. In addition, XC-591 did not contribute to obvious drug associated toxicity during the whole study. Molecular mechanism showed XC-591 could inhibit RhoGDI, activate caspase-3 and decrease phosphorylated Akt. The present data may be important to further explore this kind of new small-molecule inhibitor.
Keywords: benzothiazole-2-thiol derivative, breast cancer, proliferation, metastasis, apoptosis
INTRODUCTION
Breast cancer incidence and mortality have been increasing in China. It has been estimated that 268,600 Chinese women developed breast cancer and 69,500 died of breast cancer in the year 2015 [1]. Therapeutic options such as chemotherapy, radiotherapy, hormonal therapy, and targeted therapies are often used to treat patients with breast cancer [2]. In the last decades, early detection and proper treatment have helped reduce breast cancer mortality. However, distant metastases are still the main cause of breast cancer associated deaths [3]. Thus, it is urgent to develop novel drugs with less toxicity against metastatic breast cancer. Considering safety and increasing anti-cancer efficacy, small-molecule inhibitors are being deeply investigated as a new treatment method.
Benzothiazole derivatives have diverse biological functions, including anti-inflammatory, anticonvulsant, antimicrobial and antitumor activities [4, 5]. But previous studies used 2-aminobenzothiazoles or 2-arylbenzothiazoles to design benzothiazole derivatives. In our lab, we used the benzothiazole-2-thiol as a functional group to develop a new series of compounds. Among these small-molecule compounds, XC-591 had obvious anti-tumor activity on diverse cancer cells in vitro, which was more effective than cisplatin (a platinum based chemotherapeutic drug) [6]. However, the exact anti-cancer effects of XC-591 in vivo have not been reported. In this study, we attempted to investigate the effect of XC-591 on suppressing both murine breast cancer and pulmonary metastases. In addition, possible molecular mechanism of action was also studied.
RESULTS
RhoGDI was increased in cancer cells
Western blot was conducted to check RhoGDI expression in cancer cell lines (including 4T1 and A549) and normal cell lines (including MM3MG and HK2). RhoGDI was obviously increased in two cancer cell lines, when compared with two normal cells (Figure 1A).
Figure 1.
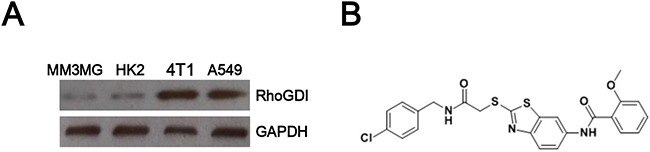
A. RhoGDI expression was increased in cancer cells. B. The chemical structure of XC-591.
The cytotoxicity effect of XC-591 on tumor cells
RhoGDI specific inhibitor XC-591 was synthesized in our lab and the chemical structure was shown in Figure 1B. As shown in Table 1, XC-591 inhibited proliferation of many cancer cells (including CT26, 4T1, LLC, B16, MethA, A549, HeLa and DU145). Howerver, no apparently toxicity on normal cells (including MM3MG, HK2 and LO2) was observed (Table 1). Compared with other tumor cells, 4T1 was more sensitive to XC-591 than others and thus was selected for further study.
Table 1. The cytotoxicity effect of XC-591 on tumor cells and normal cells.
| Cell line | Cell type | IC50 (μM)(mean±S.D.) |
|---|---|---|
| CT26 | Mouse colon carcinoma cell line | 4.3±0.8 |
| 4T1 | Mouse mammary tumor cell line | 1.2±0.3 |
| LLC | Mouse Lewis lung carcinoma cell line | 6.5±0.4 |
| B16 | Mouse melanoma cell line | 5.6±0.9 |
| MethA | Mouse fibrosarcoma cell line | 12.3±2.8 |
| A549 | Human lung carcinoma cell line | 2.4±0.7 |
| HeLa | Human cervical cancer cell line | 4.3±1.6 |
| DU145 | Human prostate cancer cell line | 1.9±1.1 |
| MM3MG | Mouse mammary normal epithelial cell line | >40 |
| HK2 | Human normal kidney cell line | >40 |
| LO2 | Human normal liver cell line | >40 |
Each cell line was treated with XC-591 for 48 h. Cell viability was detected by MTT assay. Data are expressed as the mean±S.D. from three independent experiments.
XC-591 suppressed proliferation of 4T1
Colony formation assay demonstrated that there was a marked decrease in the colony number from 364±46.4 in the untreated 4T1 cells to 189±35.2 and 56±12, respectively, in 1.25 and 2.5 μM XC-591 treated tumor cells (P<0.05) (Figure 2). These results showed that the proliferative activity of 4T1 cells was inhibited by XC-591 in a dose-dependent way.
Figure 2. XC-591 could inhibit proliferation of 4T1 in vitro.
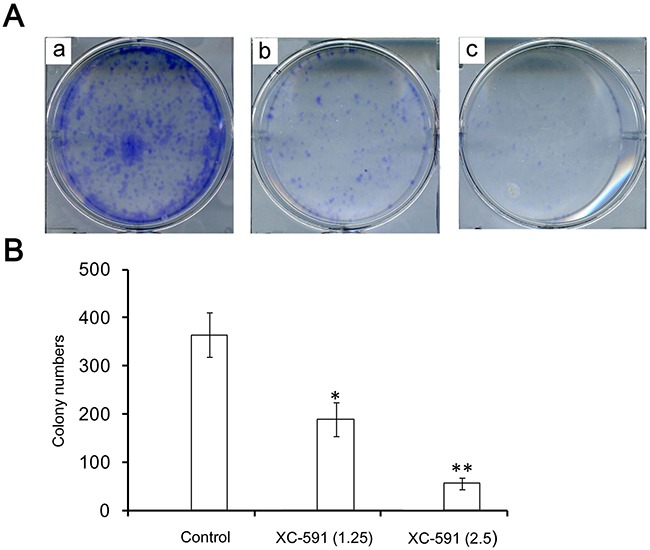
A. Representative pictures from colony formation assays. a: untreated group; b: 1.25 μM XC-591 treated group; c: 2.5 μM XC-591 treated group. B. Colony quantification of 4T1 cells treated with 0, 1.25 and 2.5μM of XC-591, respectively. * indicates P < 0.05, ** indicates P < 0.01.
Anti-tumor effect of XC-591 in vivo
As shown in Figure 3A, it is obvious that the treatment with XC-591 resulted in primary tumor growth regression of 48.5% and 79%, in 50 mg/kg XC-591 treated group and 100 mg/kg XC-591 treated group respectively, when compared with the untreated group. The similar results about tumor weight were also observed. We found the average weight of the tumors was decreased by 46.9% and 71.8% in 50 mg/kg XC-591 treated group and 100 mg/kg XC-591 treated group, respectively, when compared with the untreated control (Figure 3B).
Figure 3. Anti-tumor effect of XC-591 in vivo.
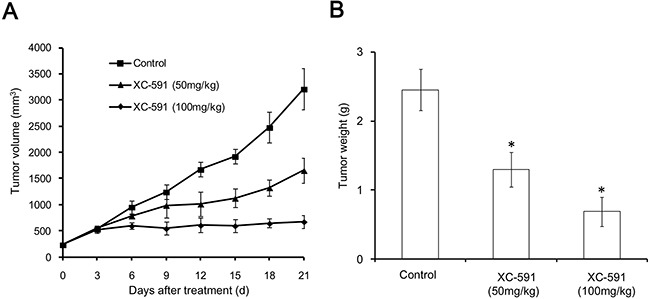
A. Tumor growth curves. B. Tumor weight. * indicates P < 0.05.
XC-591 inhibited the metastasis of 4T1 from the primary site to lungs in vivo
As shown in Figure 4 and Table 2, our data showed that the untreated group had a median of 40.5 metastases/lungs (range, 28-56 metastases). However, the median of 50 mg/kg XC-591 treated group was only 19.5 metastases/lungs (range, 13–27 metastases). Furthermore, mice treated with 100 mg/kg XC-591 had a median of 6 metastases/lungs (range, 0–8 metastases). More importantly, 3 mice with no visible lung metastases were observed in the group treated with 100 mg/kg XC-591 (Table 2). Thus, XC-591 could inhibit primary tumor growth and distant metastases in 4T1 model.
Figure 4. Representative pictures from metastatic lungs in mouse 4T1 model.
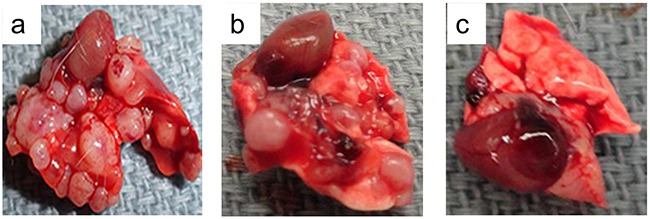
a: untreated group; b: 50 mg/kg XC-591 treated group; c: 100 mg/kg XC-591 treated group.
Table 2. Lung metastatic nodules of each group.
| Groups | Median no. of metastases (range)/lung | % metastasis-free mice |
|---|---|---|
| Control | 40.5 (28-56) | 0 |
| XC-591(50mg/kg) | 19.5 (13-27) * | 0* |
| XC-591(100mg/kg) | 6 (0-8)* | 30* |
*significantly (P < 0.05) differs from the control groups according to Mann-Whitney test.
Inhibition of proliferation (PCNA) and increase of apoptosis (TUNEL)
Tumor sections of each group were stained with anti-PCNA antibody and TUNEL reagent in order to evaluate proliferation and apoptosis rate. Compared with the control group, XC-591 significantly decreased percentages of PCNA-positive nuclei (Figure 5A&5B). Meanwhile we could find a large area of necrosis in the sections of both XC-591 treated groups (Figure 5A). Moreover, in a concentration-dependent way, XC-591 increased percentage of TUNEL-positive nuclei, when compared with the untreated group (Figure 5C&5D). Thus, XC-591 could directly inhibit proliferation and induce apoptosis in vivo.
Figure 5. PCNA immunohistochemistry, TUNEL analysis and CD31 staining.
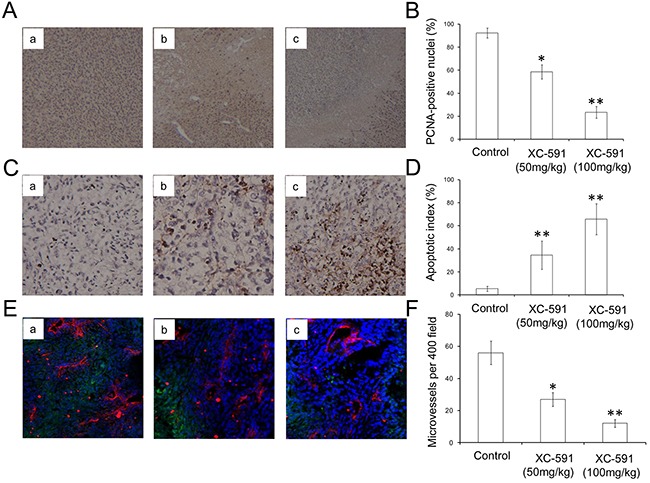
A. Representative pictures from PCNA immunohistochemistry. a: untreated group; b: 50 mg/kg XC-591 treated group; c: 100 mg/kg XC-591 treated group. B. Quantified values shown were the average percentage of PCNA-positive nuclei. C. Representative pictures from TUNEL. a: untreated group; b: 50 mg/kg XC-591 treated group; c: 100 mg/kg XC-591 treated group. D. Percent apoptosis in each group. E. Shown are representative sections from CD31 staining. a: untreated group; b: 50 mg/kg XC-591 treated group; c: 100 mg/kg XC-591 treated group. F. Quantified values shown were MVD in each group. * indicates P < 0.05, ** indicates P < 0.01.
XC-591 inhibited angiogenesis in vivo
As shown in Figure 5E&5F, compared with the untreated group with high MVD, lower MVD could be observed in XC-591 treated groups. Thus, these data showed that XC-591 could inhibit angiogenesis.
Toxicity assessment
To assess the possible adverse effects of XC-591, weight of mice was monitored every 3 days during the experiment. No weight changes were found after XC-591 treatment (Figure 6). In addition, no ruffled fur or toxic death was observed in the XC-591 treated groups. Furthermore, as shown in Table 3, XC-591 treatment did not contribute to blood toxicity and liver toxicity (P>0.05, respectively).
Figure 6. Body weight curves of each group.
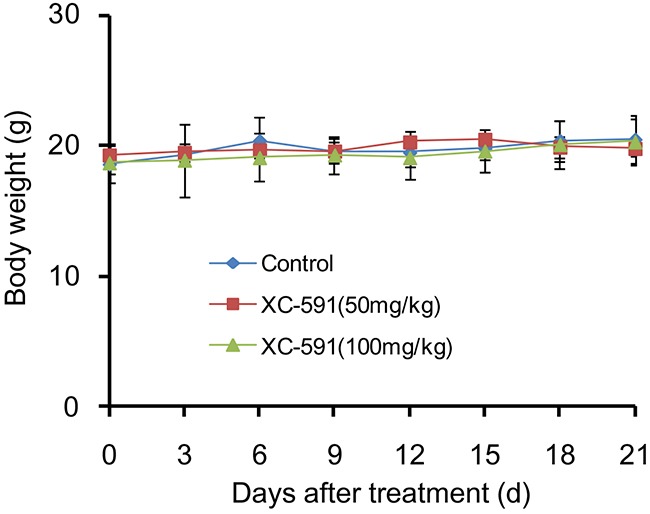
There were no significant differences in body weight among the three groups (P>0.05).
Table 3. The blood toxicity of XC-591 treatment.
| Treatment | RBC (×1012/L) | HGB (g/L) | WBC (×109/L) | PLT (×109/L) | ALT (U/L) | AST(U/L) |
|---|---|---|---|---|---|---|
| Control | 7.6±0.3 | 138.2±13.6 | 7.3±0.5 | 543.7±18.9 | 32.6±2.3 | 98.6±6.8 |
| XC-591(50mg/kg) | 7.7±0.7 | 136.1±17.3 | 7.6±0.8 | 523.7±16.7 | 36.4±4.5 | 96.3±12.3 |
| XC-591(100mg/kg) | 7.5±0.5 | 141.2±15.4 | 7.5±0.9 | 537.9±21.5 | 35.3±3.5 | 96.4±6.4 |
Data are expressed as the mean±S.D.
XC-591 inhibited RhoGDI, activated caspase-3 and reduced phosphorylated Akt
The predicted drug target protein of XC-591 is Rho GDP-dissociation inhibitor 1 (RhoGDI). The protein expression level of RhoGDI was examined by western blot after treatment with XC-591. As shown in Figure 7, RhoGDI was inhibited obviously in the XC-591 treated 4T1 cells.
Figure 7. XC-591 inhibited RhoGDI, activated caspase-3 and reduced phosphorylated Akt.
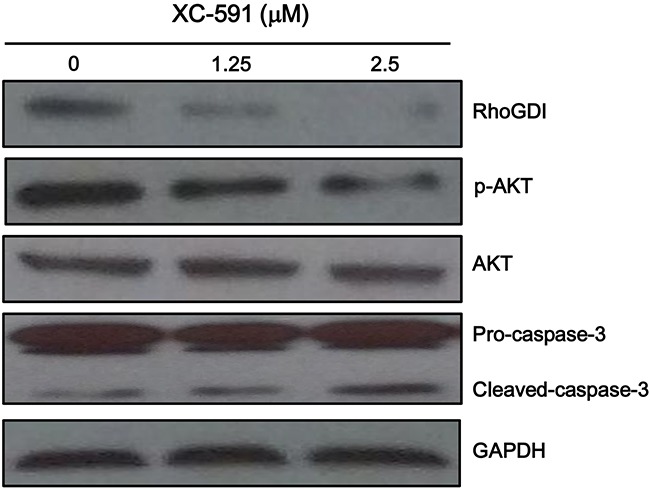
4T1 cells were treated with XC-591 at various concentrations for 48 h and then were analyzed the expression levels of RhoGDI, p-AKT, AKT, pro-caspase-3 and cleaved caspase-3 by western blot.
We investigated whether Akt was involved in XC-591-mediated proliferation inhibitory effect. Our result showed XC-591 clearly decreased phosphorylated Akt (Figure 7). Furthermore, we attempted to study the impact of XC-591 on the activation of caspase-3. XC-591 treatment contributed to an obvious increase of cleaved caspase-3 (Figure 7).
DISCUSSION
Because an obvious improvement of cancer molecular biology has been achieved recently, novel small molecule targeted drugs, which target key proteins of oncogenic pathways, have shown good effects in the management of many cancers [7–9].
In this study, we attempted to study the biological activities of XC-591, a new benzothiazole-2-thiol derivative, in detail. XC-591 showed obvious anti-proliferative activity on many cancer cells by MTT assay. Compared with other tumor cells, murine mammary tumor cell line 4T1 was more sensitive to XC-591 than others and thus was chosen for further study. XC-591 administered p.o. displayed a marked antitumor activity in 4T1 tumor models. The histological analysis showed XC-591 significantly reduced percentages of PCNA-positive nuclei, increased percentages of TUNEL positive nuclei and inhibited angiogenesis in a concentration-dependent way. During the whole experiment, no potential toxicity induced by XC-591 treatment was observed. In addition, in order to further study the molecular mechanism, we used western blot to examine the expression level of RhoGDI (predicted drug target protein), cleaved caspase-3 and phosphorylated Akt. The data showed that XC-591 could inhibit RhoGDI, activate caspase-3 and reduce phosphorylated Akt.
Rho family GTPases work in diverse cellular functions, including morphology, migration, gene transcription and cell cycle [10]. RhoGDI can inhibit nucleotide exchange and membrane association to down-regulate activities of Rho family GTPases [11]. RhoGDI is overexpressed in diverse human cancers, including lung cancer, melanoma, ovarian cancer and breast cancer [12–14]. The increased RhoGDI is associated with radiochemotherapy resistance and poor progonosis [15]. For example, a comparative proteomic study showed that RhoGDI was increased in oral squamous cell carcinoma and validated as an independent prognostic factor [16]. Furthermore, RhoGDI was identified as a metastasis-associated protein in colon and prostate cancer [17]. In addition, it has been reported that RhoGDI could stimulate the transcriptional activity of estrogen receptor α(ERα) via a RhoGTPase-dependent pathway that acts on estrogen receptor co-activators GRIP1 and CBP/p300 in breast cancer [18]. RhoGDI1 could increase both ligand-dependent and -independent ERα activity in breast cancer [19]. Thus, RhoGDI is a very promising anti-cancer target protein in breast cancer.
The important role of RhoGDI in tumor growth is supported by a number of important experimental observations. The suppression of RhoGDI alone by siRNA has a profound inhibitory effect on the growth of hepatocellular carcinoma [20]. Overexpressed RhoGDI can inhibit the induction of apoptosis by cytotoxic drugs in breast cancer cells. Silencing of RhoGDI by siRNA can increase the sensitivity of chemotherapy drugs, such as etoposide, doxorubicin and so on [21, 22]. These findings mentioned above showed that it is a good method to treat cancer by down-regulating RhoGDI. However, until now, no specific RhoGDI small molecule inhibitors have been designed and studied.
In conclusion, we developed a new benzothiazole-2-thiol derivative called XC-591. It showed good anti-cancer activity without obvious toxicity in vitro and in vivo. The molecular mechanism study showed that XC-591 could inhibit its target protein RhoGDI, and thus activated caspase-3 and decreased phosphorylated Akt. The present data may be useful for further exploration of this new small-molecule inhibitor in the treatment of breast cancer.
MATERIALS AND METHODS
Cell culture
Mouse colon carcinoma cell line CT26, mouse mammary tumor cell line 4T1, mouse Lewis lung carcinoma cell line LLC, mouse melanoma cell line B16, mouse fibrosarcoma cell line MethA, human lung carcinoma cell line A549, human cervical cancer cell line HeLa, human prostate cancer cell line DU145, mouse mammary normal epithelial cell line MM3MG and human normal kidney cell line HK2 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Human normal liver cell line LO2 was purchased from Cell biology of shanghai institute, shanghai, china. They were cultured in DMEM (Life Technologies, Bedford, MA, USA) or RPMI 1640 (Life Technologies).
Synthesis of XC-591
The route adapted for the synthesis of compound XC-591 was reported before [6].
MTT assay
Cells were seeded in 96-well plates and cultured for 24 h, followed by XC-591 treatment for 48 h. According to the instructions, MTT was done as reported before [6].
Colony formation assay
4T1 cells were plated and then treated with 0, 1.25 and 2.5μM of XC-591, respectively. After 7-10 days of incubation, the cells were stained with 0.5% crystal violet in absolute ethanol and colonies were counted under dissection microscope.
In vivo tumor experiment
To study the antitumor activities of XC-591 in vivo, 4T1 mice mammary tumor metastatic models were established. In brief, 1 × 105 4T1 cells were subcutaneously injected into the right dorsal flank of 6 to 8 weeks old female BALB/c mice. The tumor-bearing mice were randomly put into the following three groups (ten mice per treatment group) and each mouse received the corresponding treatment by intragastric administration once daily: (a) control group; (b) XC-591, 50 mg/kg; (c) XC-591, 100 mg/kg. Tumor volumes were evaluated according to the following formula: tumor volume (mm3) = 0.52 × length × width2. After sacrificed, tumor net weight was weighed. Autopsy was performed to assess the number and diameter of the metastatic nodules of lung.
Immunohistochemistry
The primary antibody for PCNA was purchased from Santa Cruz Biotechnology (sc-7907, Santa Cruz, CA, USA). The primary antibody for CD31 was purchased from Abcam (ab28364, Abcam, Cambridge, United Kingdom). According to instructions of the Envision System-HRP method (DakoCytomation Inc, Carpinteria, CA, USA), tumor sections were stained.
TUNEL assay
According to the instructions, TUNEL (terminal deoxynucleotidyl transferase mediated dUTP nick-end labeling) was completed to assess the percentage of apoptotic cells within tumors. Percent apoptosis was determined by counting the number of apoptotic cells and dividing by the total number of cells in the field (5 high power fields/slide).
Toxicity assessment
To study the potential side effects in the XC-591 treated mice, they were continuously observed for relevant indexes such as weight loss, ruffled fur, diarrhea, anorexia, skin ulcer or toxic deaths. After sacrificed, various organs (lung, liver, kidney, heart, and spleen) were stained with H&E, and observed by two pathologists in a blinded manner. The levels of serum ALT and AST were determined with an automatic multifunction-biochemical analyzer. Complete blood counts and differentials were measured within each sample using an Abbott CELL-DYN 3700 hematology analyzer (Abbott Laboratories, Abbott Park, IL, USA).
Western blot
The western blot was done as previously described [7]. The primary antibodies for RhoGDI and Akt/p-Akt were purchased from Cell Signaling Technology (Beverly, MA, USA). The primary antibodies for caspase-3 and GAPDH were acquired from Santa Cruz Biotechnology.
Statistical analysis
Statistical analysis of the differences in tumor volume, tumor net weight, animal weight, percentages of apoptosis, percentages of PCNA-positive nuclei and microvessel density were done using one-way analysis of variance (ANOVA). P< 0.05 was considered statistically significant. Statistical analysis of the differences in pulmonary metastasis was analyzed using the Mann-Whitney test. P < 0.05 was considered statistically significant.
Acknowledgments
The work was supported by National Natural Sciences Foundation of China (81402494 and 81672386) and Science and technology support program of sichuan province (2015SZ0076 and 2014HH0063) and supported by international visiting program for excellent young scholars of SCU.
Footnotes
CONFLICTS OF INTEREST
None.
REFERENCES
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical Diagnosis and Management of Breast Cancer. J Nucl Med. 2016;57:9S–16S. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- 3.Fouad TM, Kogawa T, Liu DD, Shen Y, Masuda H, El-Zein R, Woodward WA, Chavez-MacGregor M, Alvarez RH, Arun B, Lucci A, Krishnamurthy S, Babiera G, Buchholz TA, Valero V, Ueno NT. Overall survival differences between patients with inflammatory and noninflammatory breast cancer presenting with distant metastasis at diagnosis. Breast Cancer Res Treat. 2015;152:407–416. doi: 10.1007/s10549-015-3436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchinson I, Chua MS, Browne HL, Trapani V, Bradshaw TD, Westwell AD, Stevens MF. Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl)benzothiazoles. J Med Chem. 2001;44:1446–1455. doi: 10.1021/jm001104n. [DOI] [PubMed] [Google Scholar]
- 5.Kok SH, Gambari R, Chui CH, Yuen MC, Lin E, Wong RS, Lau FY, Cheng GY, Lam WS, Chan SH, Lam KH, Cheng CH, Lai PB, Yu MW, Cheung F, Tang JC, et al. Synthesis and anti-cancer activity of benzothiazole containing phthalimide on human carcinoma cell lines. Bioorg Med Chem. 2008;16:3626–3631. doi: 10.1016/j.bmc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Shi XH, Wang J, Zhou T, Xu YZ, Huang TT, Li YF, Zhao YL, Yang L, Yang SY, Yu LT, Wei YQ. Synthesis, structure-activity relationships and preliminary antitumor evaluation of benzothiazole-2-thiol derivatives as novel apoptosis inducers. Bioorg Med Chem Lett. 2011;21:1097–1101. doi: 10.1016/j.bmcl.2010.12.124. [DOI] [PubMed] [Google Scholar]
- 7.Peng X, Xie G, Wang Z, Lin H, Zhou T, Xiang P, Jiang Y, Yang S, Wei Y, Yu L, Zhao Y. SKLB-163, a new benzothiazole-2-thiol derivative, exhibits potent anticancer activity by affecting RhoGDI/JNK-1 signaling pathway. Cell Death Dis. 2014;5:e1143. doi: 10.1038/cddis.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Li Z, Yang J, Jiang Y, Chen Z, Ali Z, He N, Wang Z. Cell-specific biomarkers and targeted biopharmaceuticals for breast cancer treatment. Cell Prolif. 2016;49:409–420. doi: 10.1111/cpr.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MX Jiahao Huanga, Gaoc Piyao, Yed Yu, Liue Yun, Zhaof Yang, Wenjing Luog, Zhian Lingd, Yunfei Caoa, Sen Zhanga, Feng Gaoa, Weizhong Tang. Antiproliferative effects of formononetin on human colorectal cancer via suppressing cell growth in vitro and in vivo. PROCESS BIOCHEMISTRY. 2015;50:912–917. [Google Scholar]
- 10.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 11.Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poland J, Schadendorf D, Lage H, Schnolzer M, Celis JE, Sinha P. Study of therapy resistance in cancer cells with functional proteome analysis. Clin Chem Lab Med. 2002;40:221–234. doi: 10.1515/CCLM.2002.037. [DOI] [PubMed] [Google Scholar]
- 13.Sinha P, Kohl S, Fischer J, Hutter G, Kern M, Kottgen E, Dietel M, Lage H, Schnolzer M, Schadendorf D. Identification of novel proteins associated with the development of chemoresistance in malignant melanoma using two-dimensional electrophoresis. Electrophoresis. 2000;21:3048–3057. doi: 10.1002/1522-2683(20000801)21:14<3048::AID-ELPS3048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Wang H, Li J, Liu Y, Ding Y. Overexpression of Rho GDP-dissociation inhibitor alpha is associated with tumor progression and poor prognosis of colorectal cancer. J Proteome Res. 2008;7:3994–4003. doi: 10.1021/pr800271b. [DOI] [PubMed] [Google Scholar]
- 16.Chiang WF, Ho HC, Chang HY, Chiu CC, Chen YL, Hour TC, Chuang SJ, Wu YJ, Chen HR, Chen JH, Liu SY, Lu CL, Chen JY. Overexpression of Rho GDP-dissociation inhibitor alpha predicts poor survival in oral squamous cell carcinoma. Oral Oncol. 2011;47:452–458. doi: 10.1016/j.oraloncology.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T, Okamura T, Nagano K, Imai S, Abe Y, Nabeshi H, Yoshikawa T, Yoshioka Y, Kamada H, Tsutsumi Y, Tsunoda S. Rho GDP-dissociation inhibitor alpha is associated with cancer metastasis in colon and prostate cancer. Pharmazie. 2012;67:253–255. [PubMed] [Google Scholar]
- 18.Su LF, Knoblauch R, Garabedian MJ. Rho GTPases as modulators of the estrogen receptor transcriptional response. J Biol Chem. 2001;276:3231–3237. doi: 10.1074/jbc.M005547200. [DOI] [PubMed] [Google Scholar]
- 19.Su LF, Wang Z, Garabedian MJ. Regulation of GRIP1 and CBP Coactivator activity by Rho GDI modulates estrogen receptor transcriptional enhancement. J Biol Chem. 2002;277:37037–37044. doi: 10.1074/jbc.M111607200. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Wang B, Liao Q, An H, Li W, Jin X, Cui S, Zhao L. Overexpression of RhoGDI, a novel predictor of distant metastasis, promotes cell proliferation and migration in hepatocellular carcinoma. FEBS Lett. 2014;588:503–508. doi: 10.1016/j.febslet.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Zhang Y, Dagher MC, Rho Shacter E. GDP dissociation inhibitor protects cancer cells against drug-induced apoptosis. Cancer Res. 2005;65:6054–6062. doi: 10.1158/0008-5472.CAN-05-0175. [DOI] [PubMed] [Google Scholar]
- 22.Storvold GL, Andersen TI, Perou CM, Frengen E. siRNA: a potential tool for future breast cancer therapy? Crit Rev Oncog. 2006;12:127–150. doi: 10.1615/critrevoncog.v12.i1-2.70. [DOI] [PubMed] [Google Scholar]


