Abstract
The efficient recruitment of immune cells is a vital cornerstone of our defense against infections and a key challenge of immunotherapeutic applications. It relies on the ability of chemotaxing cells to prioritize their responses to different stimuli. For example, immune cells are known to abandon gradients of host-cell-produced cytokines in favor of complement-derived anaphylatoxins, which then guide the cells toward nearby pathogen surfaces. The aptitude to triage stimuli depends on the cells’ specific sensitivities to different chemoattractants. We here use human neutrophils as uniquely capable biodetectors to map out the anaphylatoxic cloud that surrounds microbes in the presence of host serum. We quantify the neutrophil sensitivity in terms of the ratio between the chemoattractant concentration c and the production rate j0 of the chemoattractant at the source surface. An integrative experimental/theoretical approach allows us to estimate the c/j0-threshold at which human neutrophils first detect nearby β-glucan surfaces as c/j0 ≈ 0.0044 s/μm.
Main Text
How does an immune cell cope with situations in which it faces multiple chemotactic stimuli? How does the cell decide on a particular response? Such questions touch on the core of our mechanistic understanding of immune-cell behavior, and have inspired the paradigm that immunotaxis comprises an intricate spatiotemporal hierarchy of distinct chemotactic processes (1, 2, 3, 4, 5, 6). The systematic dissection of this hierarchy is an enormous interdisciplinary challenge that requires, among others, quantitative analyses of the stimulus-specific sensitivity of the responding cells.
Complement-mediated chemotaxis has emerged as a universal, short-range homing mechanism by which chemotaxing immune cells can implement a last-minute course correction toward pathogenic bacteria and fungi. Recent single-cell experiments have validated human neutrophils as uniquely capable biodetectors of minuscule amounts of complement-derived anaphylatoxins in the proximity of microbial and model pathogens (Fig. 1) (6, 7, 8). But the question just how sensitive these immune cells are was not addressed by earlier studies.
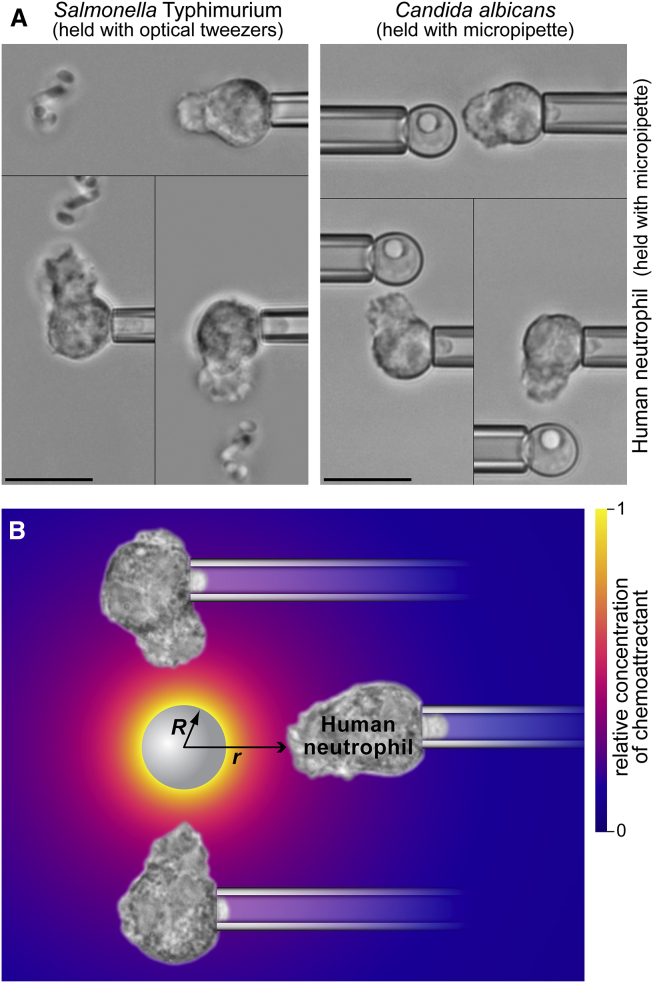
Figure 1.
Human neutrophils as biodetectors. (A) Neutrophils report the presence of nearby bacterial and fungal pathogens by extending directed pseudopods. Repositioning the target allows us to triple-check the specificity of the response. Scale bars denote 10 μm. (B) Chemoattractant anaphylatoxins like C5a are produced by the host’s complement system on the surface of foreign particles and released. Neutrophils detect these anaphylatoxins through G-protein-coupled receptors (e.g., the C5a receptor CD88).
We here use an integrative theoretical/experimental strategy to tackle this difficult question. A recently found closed-form solution of the appropriate reaction-diffusion problem (V.H., E.A.F., and W.D. Simpson, unpublished data) predicts the spatiotemporal distribution of anaphylatoxins as a function of the time t and the radial distance from the source, Δr = r − R (Fig. 1 B; Supporting Material). In the considered scenario, the source of chemoattractant is a sphere of radius R that, at time t = 0, starts releasing anaphylatoxins at a constant rate given by the boundary flux j0. The chemoattractant is redistributed in the surrounding infinite space by diffusion. A realistic estimate gives a diffusion coefficient of D ≈ 130 μm2/s for the dominant anaphylatoxin C5a (V.H., E.A.F., and W.D. Simpson, unpublished data). We further model the deactivation of chemoattractant by carboxypeptidases as an irreversible removal reaction with a typical kinetic off-rate constant of k ≈ 0.011 s−1 (V.H., E.A.F., and W.D. Simpson, unpublished data). This removal process is vital because it prevents potentially dangerous overstimulation of the host organism by an unchecked buildup of chemoattractant.
We apply this model to single-cell/single-target experiments in which individual human neutrophils are exposed to anaphylatoxins produced at the surface of fungal model particles in the presence of autologous serum (added to the experiment buffer at 20%; Fig. 1 B). The most suitable targets for our purpose turned out to be β-glucan particles. These particles tended to cluster, which allowed us to use a variety of targets with different sizes but the same chemical surface composition (Fig. 2). The ability to vary R, a key control parameter in our analysis, considerably increased the robustness of our results.
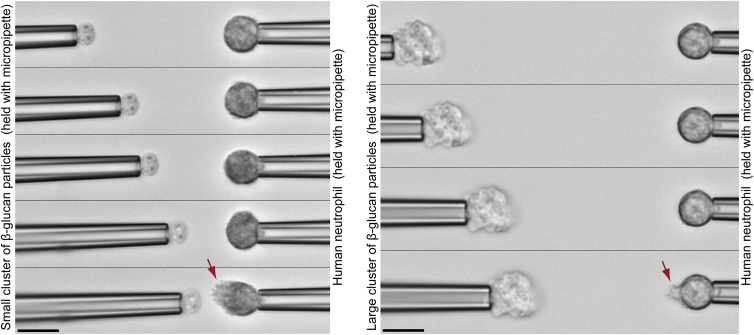
Figure 2.
Measurement of the response distance of human neutrophils to clusters of β-glucan particles. Two sequences of video images demonstrate how the cell-target distance is stepwise reduced every 2 min (see also Movie S1). The distance at which the cell first forms a pseudopod directed toward the cluster of β-glucan particles (video snapshot at the bottom) is the sought response distance. The two panels confirm that the response distance depends strongly on the target size. Scale bars denote 10 μm. To see this figure in color, go online.
This integrative strategy suggests two possible approaches to characterize the sensitivity of human neutrophils in their role as biosensors. First, one could measure the time lag from placing a target particle in the cell’s proximity to the onset of the formation of a pseudopod directed toward the target. However, this lag includes not only the sought time required to reach the concentration threshold triggering the cell response, but also the time it takes a quiescent neutrophil to subsequently start up its internal actin-remodeling machinery. The latter time is not accurately known, nor is it negligibly small. For example, the typical time lag from placing a zymosan particle near a neutrophil to the first sign of a newly forming chemotactic pseudopod was found to be on the order of ∼60 s (9). Similar results were obtained for bacterial and fungal pathogens (6, 8). On the other hand, for the typical cell-target distances (Δr ≈ 5 μm) and target sizes (R ≈ 2.5 μm) used in those experiments, Eq. S4 in the Supporting Material predicts that the concentration of chemoattractant at the front of the cell rises to near-steady-state values in <5 s. Thus, it is the time required for cell activation rather than for the formation of the anaphylatoxic cloud that dominates the measured time lag in this case. Consequently, this type of analysis is not suited to characterize the cells’ sensitivity.
The second, more promising type of analysis is based on the determination of the maximum cell-target distance that triggers a cell response. In experiments specifically designed for this purpose, we stepwise reduced the cell-target distance, giving the cell enough time (∼2 min) at each distance to sample the local near-steady-state concentration of chemoattractant. We performed >50 recognition experiments with β-glucan particles of different sizes (Fig. 2; Movie S1) and recorded the yes/no answers to the question whether or not a response was triggered at a particular distance. We then superimposed these data onto a concentration map that predicted the profile of the anaphylatoxic cloud as a function of Δr and R at the 2-min time point (Fig. 3 A).
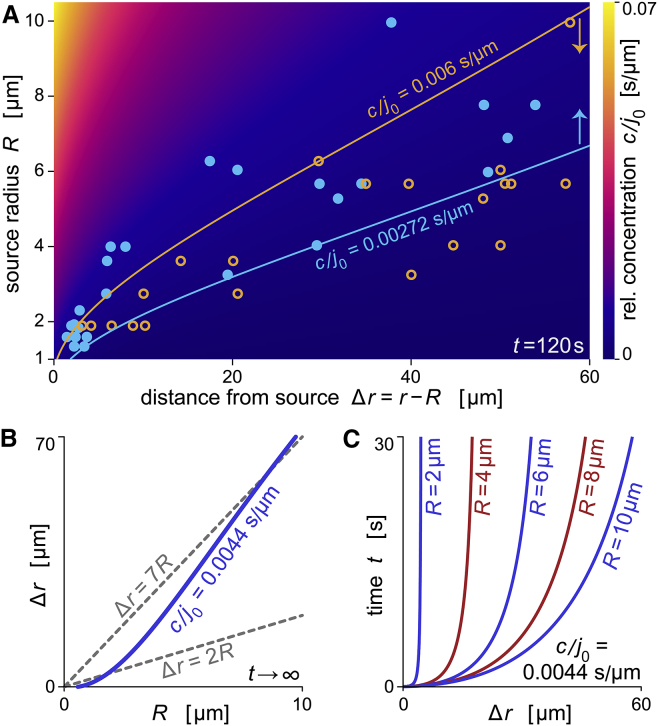
Figure 3.
Calibration of the sensitivity of human neutrophils to C5a. (A) Density map of the relative concentration c/j0 as a function of Δr and R calculated at t = 120 s. Overlaid open circles (orange) show the locations of experiments where no neutrophil response was observed. The contour line of constant concentration at c/j0 = 0.006 s/μm (orange line) marks the upper concentration limit of the no-response data. Solid circles (light blue) show the locations of experiments where neutrophils did detect anaphylatoxins. The contour line at c/j0 = 0.00272 s/μm (light-blue line) marks the lower concentration limit of the data corresponding to positive responses. (B) Response distance Δr as a function of the source size R for the concentration threshold c/j0 = 0.0044 s/μm. Here, this relationship is shown for the steady state. Dashed lines with slopes of 2 and 7 are included for comparison. (C) Time required to reach the critical concentration c/j0 = 0.0044 s/μm as a function of Δr for five different source sizes.
Two lines of constant concentration included in Fig. 3 A subdivide this map into three regions: one where recognition always occurred; one where recognition never occurred; and an intermediate region where some, but not all, neutrophils detected the chemoattractant. For each cell-target pair, the source-size-dependent recognition distance was smaller than the respective value of the right boundary of the intermediate region (blue line), and in almost all cases it was larger than the value of the left boundary (orange line). Accordingly, the concentration threshold that triggered the formation of a chemotactic pseudopod can be assumed to lie within the range of concentrations bounded by the two lines. We choose the average (c/j0 ≈ 0.0044 s/μm) of the relative concentration values of these contour lines as a representative estimate of this threshold. This estimate is specific to β-glucan surfaces exposed to 20% serum, because j0, and hence c/j0, depends on these features. Yet as long as the targets had similar sizes, we did not observe noticeable differences between the chemotactic responses of neutrophils to β-glucan particles, Candida albicans cells, clusters of Salmonella Typhimurium, and other targets. Therefore, we believe that the critical concentration of c/j0 ≈ 0.0044 s/μm is a representative estimate for the surfaces of all of these types of target.
Based on this estimate, we can characterize the effective spatial extent and dynamics of the anaphylatoxic cloud surrounding these target surfaces under near-physiological conditions. Fig. 3 B shows that for c/j0 ≈ 0.0044 s/μm, the effective spatial reach of the anaphylatoxic cloud spans ∼1–3.5 source diameters for the depicted target-size range. The smaller spatial reach of ∼1 source diameter is typical for target sizes up to R ≈ 2.5 μm, whereas a reach of ∼3.5 source diameters is characteristic for target sizes in the R-range from ∼7.5 to 10 μm. Finally, Fig. 3 C confirms that the anaphylatoxic cloud forms rapidly compared to typically observed cell-response times. For example, at a cell-target distance of one source diameter, the critical concentration of chemoattractant is reached in <∼3 s for all considered source sizes.
We caution that, due to natural cell-to-cell variability of live human neutrophils, the measured c/j0-threshold should be viewed as a very rough estimate. The c/j0-values of the two lines in Fig. 3 A could be taken as best-case error margins of this estimate. But because of other uncertainties (Supporting Material; V.H., E.A.F., and W.D. Simpson, unpublished data), the error margins could be larger. We conservatively view the value of c/j0 ≈ 0.0044 s/μm as an order-of-magnitude estimate.
The ultimate completion of the calibration of immune cells as detectors of chemoattractant requires knowledge of the value of the source flux j0. We hope to be able to estimate this value for β-glucan surfaces in future work, which will then allow us to pinpoint the absolute concentration threshold of anaphylatoxins required to trigger a chemotactic response by human neutrophils. In conclusion, this study demonstrates how superb experimental control over one-on-one encounters between immune cells and pathogenic targets (10), in conjunction with realistic mathematical modeling, provides key insights into mechanisms of vital cellular behavior that are inaccessible to traditional biological methods.
Author Contributions
E.A.F. and V.H. designed the experiments and analyzed the data; E.A.F. performed the experiments; and V.H. developed the theory, prepared the figures, the movie, and the Letter.
Acknowledgments
This work was supported by National Institutes of Health, grant No. R01 GM098060.
Editor: Jennifer Curtis.
Footnotes
Supporting Materials and Methods and one movie are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30104-2.
Supporting Material
The video illustrates how a human neutrophil and a cluster of β-glucan particles (both held at the tip of micropipettes) are initially placed at a large distance from each other. The cell-target distance is then stepwise reduced approximately every 2 min. The distance at which the neutrophil first forms a pseudopod directed toward the cluster of β-glucan particles marks the threshold at which the cell detects the presence of anaphylatoxins produced at the target surface. The real-time duration of this experiment was approximately 14 min.
References
- 1.Zhelev D.V., Alteraifi A.M., Chodniewicz D. Controlled pseudopod extension of human neutrophils stimulated with different chemoattractants. Biophys. J. 2004;87:688–695. doi: 10.1529/biophysj.103.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heit B., Robbins S.M., Kubes P. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat. Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 3.Chou R.C., Kim N.D., Luster A.D. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 5.Zabel B.A., Rott A., Butcher E.C. Leukocyte chemoattractant receptors in human disease pathogenesis. Annu. Rev. Pathol. 2015;10:51–81. doi: 10.1146/annurev-pathol-012513-104640. [DOI] [PubMed] [Google Scholar]
- 6.Lee C.-Y., Thompson G.R., 3rd, Heinrich V. Coccidioides endospores and spherules draw strong chemotactic, adhesive, and phagocytic responses by individual human neutrophils. PLoS One. 2015;10:e0129522. doi: 10.1371/journal.pone.0129522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinrich V., Lee C.-Y. Blurred line between chemotactic chase and phagocytic consumption: an immunophysical single-cell perspective. J. Cell Sci. 2011;124:3041–3051. doi: 10.1242/jcs.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wangdi T., Lee C.-Y., Bäumler A.J. The Vi capsular polysaccharide enables Salmonella enterica serovar typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 2014;10:e1004306. doi: 10.1371/journal.ppat.1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mankovich A.R., Lee C.-Y., Heinrich V. Differential effects of serum heat treatment on chemotaxis and phagocytosis by human neutrophils. PLoS One. 2013;8:e54735. doi: 10.1371/journal.pone.0054735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinrich V. Controlled one-on-one encounters between immune cells and microbes reveal mechanisms of phagocytosis. Biophys. J. 2015;109:469–476. doi: 10.1016/j.bpj.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video illustrates how a human neutrophil and a cluster of β-glucan particles (both held at the tip of micropipettes) are initially placed at a large distance from each other. The cell-target distance is then stepwise reduced approximately every 2 min. The distance at which the neutrophil first forms a pseudopod directed toward the cluster of β-glucan particles marks the threshold at which the cell detects the presence of anaphylatoxins produced at the target surface. The real-time duration of this experiment was approximately 14 min.