Abstract
Channelrhodopsins (ChR1 and ChR2) are light-activated ion channels that enable photomobility of microalgae from the genus Chlamydomonas. Despite common use of ChR2 in optogenetics for selective control and monitoring of individual neurons in living tissue, the protein structures remain unresolved. Instead, a crystal structure of the ChR chimera (C1C2), an engineered combination of helices I–V from ChR1, without its C-terminus, and helices VI–VII from ChR2, is used as a template for ChR2 structure prediction. Surprisingly few studies have focused in detail on the chimera. Here, we present atomistic molecular dynamics studies of the closed-state, non-conducting C1C2 structure and protonation states. A new and comprehensive characterization of interactions in the vicinity of the gating region of the pore, namely between residues E90, E123, D253, N258, and the protonated Schiff base (SBH), as well as nearby residues K93, T127, and C128, indicates that the equilibrated C1C2 structure with both E123 and D253 deprotonated closely resembles the available crystal structure. In agreement with experimental studies on C1C2, no direct or water-mediated hydrogen bonding between an aspartate and a cysteine (D156-O…S-C128) that would define a direct-current gate in C1C2 was observed in our simulations. Finally, we show that a single hydrogen bond between a glutamic acid (E90) and an asparagine (N258) residue suffices to keep the gate of C1C2 closed and to disable free water and ion passage through the putative pore, in contrast to the double bond proposed earlier for ChR2. We anticipate that this work will provide context for studies of both the gating process and water and ion transport in C1C2, and will spark interest in further experimental studies on the chimera.
Introduction
Channelrhodopsin-1 (ChR1) and channelrhodopsin-2 (ChR2) are light-activated ion channels that enable photomobility of microalgae from the genus Chlamydomonas toward or away from the conditions optimal for photosynthetic growth (1). Both ChRs accommodate retinal, a polyene chromophore that links covalently to a lysine residue (K257 (ChR2 numbering)) on helix VII via a protonated Schiff base (SBH) (Fig. 1 a) (2). Photo-activation of ChRs (λmax (ChR1) = 500 nm and λmax (ChR2) = 470 nm) causes a cascade of events that lead to opening of the channel and non-selective cation permeation (H+, Na+, K+, and Ca2+). Channelrhodopsins have become a common tool used in optogenetics, a biological method in which membranes of excitable cells (e.g., neurons, muscle cells) are enhanced with light-sensitive ion channels and membrane depolarization is selectively controlled using light of specific wavelengths. Despite the diverse applications of ChR1 (3) and ChR2 (4, 5, 6) in optogenetics and some information about intermediates in the ChR2 photocycle (described briefly below), little is known about the molecular mechanisms of action of ChRs. This problem is partly attributable to challenges in determining the atomistic structures of ChR1 and ChR2.
Figure 1.
(a) Schematic representation of the seven-helix transmembrane regions of ChR with all-trans retinal bound to the seventh helix (HVII) via a SBH. In the C1C2 chimera, helices I–V (gray) derive from ChR1, and helices VI–VII (blue) from ChR2. (b) Photocycle of ChR2. To see this figure in color, go online.
Conductance, point-mutation, and spectroscopy studies (1, 7, 8, 9, 10) predict the presence of four photointermediates, P1–P4, in the photocycle of ChR2 (Fig. 1 b). The sequence of conformational changes within ChR2 is initiated by light-induced all-trans to 13-cis retinal isomerization. The resulting changes to the hydrogen-bond network cause conformational changes in the transmembrane region of the channel and yield formation of a non-conducting photointermediate, P1. Deprotonation of the Schiff base (SB) and protonation of aspartic acid D253 initializes H+ transfer and release to the extracellular side of the membrane, assisted by formation of the P2 pre-open intermediate. It is speculated that the protein at this point is still in a non-conducting state, but there is little structural information about the two initial intermediates.
Formation of the open-state ChR2, P3, is associated with re-protonation of the SB from the cytoplasmic side of the protein, minor changes within the side chains of a few amino acids in the vicinity of the glutamic acid residue E90, and entry of bulk water into the previously dry transmembrane region. From the open, conducting P3 intermediate, there are two possible routes to pore closing (Fig. 1 b): 1) the fast route (10 ms), which leads directly to the ground/closed state; or 2) desensitization, a long-lasting (20–25 s) inactivation process that is observed under continuous illumination conditions. The latter path originates from ChR2 temporarily occupying a late low-conducting state, P4, or a mixture of P4 and conducting states. In either case, the return to the ChR2 ground state is a consequence of the 13-cis to all-trans retinal isomerization and retrieval of the non-conducting properties.
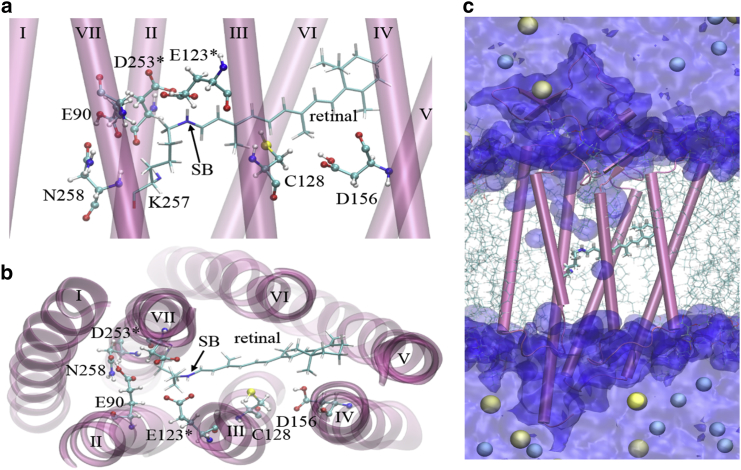
Recently, Kato et al. (11) reported an x-ray structure resolved to 2.3 Å of an engineered combination of ChR1 and ChR2, called C1C2 chimera. The protein was crystallized as a fully dark-adapted dimer, each subunit being in a non-conductive state. The 342-residue region of monomeric C1C2 consists of an engineered combination of helices I–V from ChR1, without its C-terminus, and helices VI–VII from ChR2 (Fig. 1 a). The putative pore is localized among helices I–III and VII. Helix II is lined with acidic glutamic acid residues E90, E97, E101, and E123, thus suggesting that ion selectivity and conductance depend on this helix. Despite the structural similarities between the well-studied proton-pumping bacteriorhodopsin (1) and ChR, little is known about the underlying molecular mechanism of light-induced channel gating that regulates non-selective cation permeation (H+, Na+, K+, and Ca2+) via the putative ion channel of C1C2 ChR.
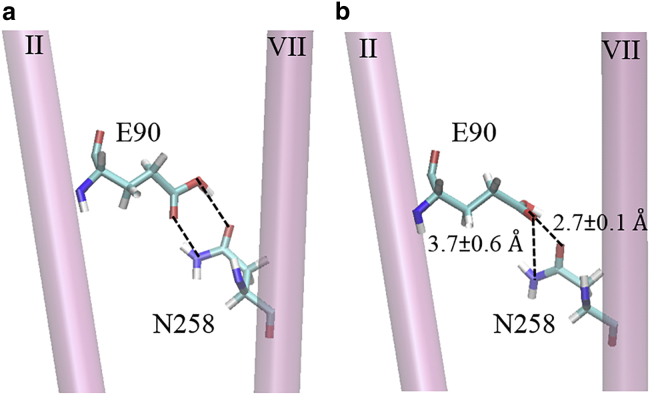
Recent time-resolved Fourier transform infrared spectroscopic and molecular dynamics (MD) studies (12, 13) of the wild-type ChR2 in both dark-adapted (closed) and pre-open (non-conducting) states suggest an E90-helix-II-tilt (EHT) model to explain channel gating (Fig. 2, a and b). According to the former study, the protonated glutamic acid E90 on helix II (HII) and asparagine N258 on helix VII (HVII) form a double hydrogen bond, an inner gate. After the all-trans to 13-cis isomerization of retinal, the E90-N258 interaction supposedly weakens. Helices II and VII disconnect and hydrophilicity increases in the central section of the pore due to deprotonation and rearrangement of E90 toward E83.
Figure 2.
(a) Side and (b) top views of functionally important residues of C1C2 (sticks and spheres), including E90 and N258 (EHT gating model), C128 and D156 (the DC gate), D253 (the deprotonated acceptor of the SB proton, SBH), and E123 (close neighbor of D253 and the SBH). Water, lipids, and ions are not shown. Asterisks (∗) denote deprotonated residues. (c) Simulation snapshot of the cross section of the equilibrated tertiary structure of the wild-type ChR chimera C1C2 (tubes represent α-helices), with all-trans retinal bound to K257 (sticks) surrounded by lipids (transparent sticks), water (blue surface), Na+ (yellow spheres), and Cl− (cyan spheres). Water spontaneously enters from the extracellular side, but does not permeate past the narrowest region of the pore, which is found in the vicinity of E90 and N258. To see this figure in color, go online.
Experimental studies of the dark-state, wild-type ChR2 suggest that a hydrogen bond between the side chains of aspartic acid D156 and cysteine C128 is crucial for on/off kinetics of the conducting state (14, 15). This so-called aspartic acid - cysteine (DC) gate is located in the vicinity of the retinal chromophore (Fig. 2, a and b). A single point mutation, D156C, results in a ChR2 variant that exhibits an extended lifetime of the conducting state, high expression in host cells, improved subcellular localization, elevated retinal affinity, and superior photocurrent amplitudes when compared with all other published variants (16). In the C1C2 chimera, however, the direct D156-OH…HS-C128 interaction was not observed either in crystal (11) or previously simulated (17) structures. Conversely, a bridging water, or formation of an indirect DC gate, has been suggested for ChR2 (11, 18).
Computer simulations can provide detailed information about networks of inter- and intramolecular interactions crucial for molecular function. Here, we present an extensive MD study of closed-state C1C2 with all-trans retinal in a physiologically relevant environment (lipid bilayer, water, and ions). We focus on analysis of the functionally important residues in the non-conducting, closed-state C1C2 that affect pore opening and water access to the transmembrane region, and initiate proton transport in ChRs. Those residues include the pairs C128 and D156, E90 and N258, and the SB (retinal and K257 bond) and its proposed proton acceptor, D253. Throughout this article, all residues follow the ChR2 numbering scheme unless otherwise stated. Our analysis is based on a total of 12 equilibrated systems containing closed C1C2, lipid bilayer, water, and ions (Na+ and Cl−). First, we varied the components of the lipid bilayer to determine which lipid composition affects the C1C2 protein’s secondary structure the least. Next, we performed eight separate simulations of protein with altered protonation states among these functionally important residues: E123, D156, D253 (Fig. 2). The glutamate E123 and aspartate D253 are proximal to the protonated SB, SBH, and both have been proposed to be the SBH proton acceptor in ChR2. We found that the protein with deprotonated E123 and D253 closely matches the crystal structure reported by Kato et al. (11). To help decipher the network of interactions among functionally important residues in the non-conducting C1C2 chimera, we analyzed the atomistic, time-dependent properties of the closed-state protein equilibrated in the representative environment.
Materials and Methods
We based our simulations on a recently resolved crystal structure of the non-conducting ChR chimera in the electronic ground state (C1C2 with all-trans retinal (Protein Data Bank (PDB): 3UG9)) (11). With the aid of the I-TASSER web server (19, 20, 21), we generated the missing secondary structure of C1C2 (see the Supporting Material for details). We chose to work with a seven-transmembrane-monomer fold (∼300 residues), which has been shown experimentally to retain photocurrents unaffected by the truncation (22). We initially assigned C1C2 protonation based on Kato et al.’s (11) crystallographic study of C1C2 and predicted for the closed-state ChR2 (18): SBH, protonated D253, and all glutamates except E90 on helix II deprotonated. In this protonation state, we noticed that the side chains of the protein equilibrated in the representative environment consisting of lipids, water, and ions did not align well with the crystal structure (Fig. 3). This motivated us to vary protonation states among key residues and study the differences among intramolecular interactions in systems I–VIII (Table S1).
Figure 3.
(a) Backbone overlay of the crystal structure of truncated C1C2 (PDB: 3UG9 (blue)) and C1C2 predicted by I-TASSER (red). (b and c) Steric overlay of the functionally important residues, including the SB (SBH) and all-trans retinal, in PDB: 3UG9 (sticks) and the modeled C1C2 after equilibration for 120 ns (bonds and spheres), where (b) D253 and E123 were deprotonated (system VII) and (c) D253 was deprotonated and E123 was neutral (system VI). The analysis of steric overlay of side chains and median pKa values showed higher agreement between equilibrated system VII (b) and the C1C2 crystal structure. Thus, we chose to investigate equilibrated system VII (b) further. To see this figure in color, go online.
We used the CHARMM-GUI (23) software to create a simulation box containing a reconstructed C1C2 immersed in lipid bilayers with ∼200 (∼100 per layer) molecules of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphatidylcholine (DSPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC), or 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), TIP3P water, and ions (Na+ and Cl−) to neutralize the protein net charge and provide 0.15 M salt concentration. The total number of atoms in each studied system was ∼96,000. Periodic boundary conditions were used in all directions to mimic bulk conditions. Electrostatic interactions were calculated using particle mesh Ewald with a real-space cutoff distance of 15 Å and grid width of 1 Å. The switching distance for non-bonded electrostatics and van der Waals interactions was 13 Å. The size of the simulation box (95 × 95 × 125 Å) was large enough to prevent interactions between the protein and its periodic images (see Fig. S3).
MD simulations of the ChR chimera in lipid environments were performed in the constant-particle-number and isothermal-isobaric ensemble (NpT) using the NAMD code (24). The time step was set to 1 fs. The Langevin piston method (25) was used to maintain a constant pressure of 1 atm. The temperature, set to 300 K (C1C2 in DPPC, DSPD, and POPC) as well as 324 K (C1C2 in DPPC) and 368 K (C1C2 in DSPC), was controlled using Langevin dynamics with a coupling coefficient of 1 ps−1. Our choice of environmental conditions derives from general interest in the protein’s behavior at diverse temperatures. Structural studies have been carried out in a range of temperatures from cryogenic (26, 27) to physiological (e.g., neuron excitability studies (5, 6)). Also, progression among the intermediates of the ChR photocycle is thermally driven. System equilibration was divided into steps I–IV, each followed by 1000 energy-minimization steps. Step I involves relaxation of the lipid tails with protein, lipid headgroups, and ions fixed (0.5 ns). Step II consists of relaxation of the system with protein heavy atoms harmonically restrained (1 kcal/mol/Å, duration 1 ns). Step III involves equilibration with protein constraints released (100 ns). Step IV comprises the production runs (additional 20 ns). System equilibration was evaluated by monitoring the root mean-square deviation (RMSD) of transmembrane sections of the protein with respect to the initial structure. In equilibrated systems, the RMSD values plateau to a constant value (Fig. S1). All systems simulated at temperatures above the lipid melting point reached equilibrium energy values at ∼5 ns. Equilibration of the systems with DPPC and DSPC lipids simulated at 300 K was not observed until 20 and 120 ns, respectively. Nevertheless, each system was simulated for a total of 120 ns.
The CHARMM36 force field (28) was chosen as it reliably reproduces protein NMR properties when calculated from MD simulations (29), and it allows for the use of tensionless ensembles in MD simulations of lipid bilayers. Force fields used previously, CHARMM 27 and 27r, overestimated the surface tension (30–40 dyn/cm) in fluid-phase bilayers at the experimentally determined surface area per lipid, causing shrinking of lipid bilayers. CHARMM36 allows for simulations of stable bilayers with or without proteins (30). The all-trans retinal parameters were taken from prior works (31, 32, 33, 34, 35).
Protein structures and properties were calculated from statistical averages of molecular coordinates obtained from the last 20 ns of the MD production runs. The results were analyzed using visual MD (VMD), home-built Tcl/Tk, and Python-based programs that use the MDAnalysis toolkit (36). Representative frames were chosen as the simulation frames with the lowest RMSD value (protein backbone) with respect to the equilibrated protein structure averaged over the last 20 ns of 120-ns-long simulations. The pKa values of ionizable C1C2 chimera residues were calculated for each frame of the production run with PROPKA software (37, 38). PROPKA is a fast, empirical method for calculating pKa values of ionizable groups as a function of perturbations arising from desolvation effects and intra-protein interactions such as hydrogen bonds and Coulombic interactions. The latter is a long-distance (up to 10 Å), pairwise interaction between ionizable residues dependent on the chemical nature of the residues (acidic or basic), their distance from each other, and their distance from the surface of the protein (buried ratios). Unless stated otherwise, we report the values of pKa, number of water molecules in the protein’s pore, and distances and angles averaged over the last 20 ns of MD simulations. See the Supporting Material for the time evolution of the properties listed above.
Results
Validation of the predicted C1C2 fold
The truncated C1C2 chimera structure deposited in the PDB: 3UG9 consists of residues 1–342. Due to the high disorder in the loop region, residues 24–48, 110–117, and 343–356 are invisible in the electron density map. To predict the positions of the missing residues, we submitted the sequence of 350 N-terminal amino acids of C1C2 to I-TASSER (19, 20, 21), a protein structure prediction server. The resulting overlay is shown in Fig. 3.
The confidence score (Cscore) for estimating the quality of the C1C2 model predicted by I-TASSER was −0.49. The Cscore value is in the higher range [−5, 2], thus indicating a higher confidence in the structural prediction. As suggested by Roy et al. (20), most protein models with Cscore values >−1.5 have a correct fold. The template modeling score (TMscore) is a measure of the similarity between two tertiary protein structures. The value of the TMscore is independent of the protein’s local errors (e.g., misalignment of highly flexible tails, and loops) and varies between 0 and 1, where 1 indicates a perfect match between the two investigated structures. The TMscore of C1C2 rebuilt via I-TASSER was 0.834, indicating a model of highly homologous topology with the truncated C1C2 crystal structure.
Upon visual inspection, a nearly perfect alignment of the transmembrane region of C1C2 with the crystal structure was observed. The most misaligned regions are the protein’s termini (Fig. 3 a, red helix and coil regions extending beyond the blue structure of the crystal). Those regions were discarded before immersing the protein in a lipid bilayer and aqueous solvent and initiating the MD simulations. We also tested the reproducibility of the I-TASSER structure predictions. For details, see Fig. S2.
Choice of the lipid bilayer
We investigated the influence of the lipid tail length (16–18 carbon atoms (Table 1)) and number of unsaturated carbon-carbon bonds (0–2) on the stability of the transmembrane region of the closed ChR chimera. The C1C2 chimera was immersed in homogeneous bilayers consisting of various lipids with phosphatidylcholine headgroups: DPPC, DSPC, POPC, or DOPC, 0.15 M sodium chloride (NaCl), and water. DPPC and DSPC were chosen to verify the correctness of the CHARMM36 force field and the behavior of the systems at temperatures below and above the lipid melting temperatures. We chose to test POPC because it is one of the most common lipids used in MD simulations. DOPC was selected because its hydrophobic tail, which consists of 18 carbon atoms and one unsaturated carbon-carbon bond per tail, is similar to monoolein, the compound used to grow C1C2 crystals (11). Each protein was equilibrated for 120 ns in a homogeneous lipid bilayer and solvent using the NAMD code.
Table 1.
Properties of C1C2 in Water, Ions, and Homogeneous Lipid Bilayers of Varying Melting Temperatures
| Number of C Atoms in Tail | Number of C=C Bonds | TMELT (K) | TSIMULATION (K) | RMSD (Å) | RGYR (Å) | |
|---|---|---|---|---|---|---|
| Initial structure | 18.3 | |||||
| DPPC | 16 | 0 | 314 | 300 | 1.9 | 19.1 |
| 324 | 2.2 | 19.3 | ||||
| DSPC | 18 | 0 | 328 | 300 | 1.5 | 18.8 |
| 368 | 2.4 | 19.1 | ||||
| POPC | 16 and 18 | 1 | 271 | 300 | 1.7 | 18.9 |
| DOPC | 18 | 2 | 290 | 300 | 1.4 | 18.9 |
Bilayers were equilibrated at temperatures, TSIMULATION, ranging from 300 to 368 K. A standard deviation of 1 K in temperature was noted for all systems. RMSD and radius of gyration, RGYR, were calculated for helical portions of C1C2 from representative frames with respect to the initial (predicted) structure of the protein. Representative frames were chosen as the simulation frames with the lowest RMSD value (protein backbone) with respect to the equilibrated protein structure averaged over the production run (the last 20 ns of 120-ns-long simulations). Timelines of the protein’s RMSD values with respect to the corresponding pre-equilibrated structures are shown in Fig. S1. TMELT, melting temperature.
Simulations of the systems at 300 K, that is, below the melting points of DPPC (TMELT = 314 K) and DSPC (TMELT = 328 K), yielded formation of gel-like lipid patches (Fig. S4). Such local ordering in DPPC and DSPC lipid bilayers at 300 K confirmed the suitability of the CHARMM36 force field and the correctness of the simulation parameter choice. Local ordering of DPPC around C1C2 was accompanied by changes in the equilibrium conformation, measured as an increase in the protein’s radius of gyration, RGYR (calculated for the helical transmembrane region of C1C2 only), to 19.1 Å compared with 18.3 Å for the initial structure (Table 1). Those changes correspond to shifts in the positions of helices and crowding in the transmembrane region. C1C2 simulated in DSPC at 300 K reveals the smallest helix dislocation (RGYR = 18.8 Å) among all analyzed systems when compared with the initial structure (RGYR = 18.3 Å). However, the protein’s geometry may be constricted by the local organization of the nearby lipids simulated below TMELT(DSPC). The largest protein distortions were observed in the systems simulated at temperatures >300 K; backbone RMSD values of C1C2 in DPPC (TSIMULATION = 324 K) and DSPC (TSIMULATION = 368 K) were 2.25 and 2.44, respectively. C1C2 in POPC and DOPC simulated above the lipid melting points were among the least affected by the lipid type and temperature choice. Hence, we chose DOPC for the simulation studies of closed-state C1C2, pursued next.
Characterization of the closed-state C1C2
The protonation states of key amino acid residues can affect protein structure and function (39). In classical MD simulations, the protonation states of residues do not change spontaneously. Nevertheless, protonation states of specific residues can be modified manually and the consequent adjustment of the local environment tracked. Our goal here is to protonate or deprotonate residues of interest and determine which of the studied systems (I–VIII) most closely resembles the crystal structure reported by Kato et al. (11). Therefore, our study of C1C2 included equilibration of eight structures (I–VIII) in representative environments, each differing in the protonation of the functionally important residues E123, D156, and D253.
To assess the results from varied protonation states, RMSD values most often are calculated for backbone atoms and indicate global dissimilarities among aligned protein structures. In the case of fine structural differences introduced by varying the positions of hydrogen atoms, however, RMSDs between the simulated protein and the crystal structure may not be the most effective measure of protein alignment. Therefore, we decided first to compare pKa values among the key residues of the equilibrated systems with the corresponding ones from the crystal structure reported by Kato et al. (11). The results, statistical averages, and variations over the last 20 ns of MD simulations for systems I–VIII are shown in Fig. S7, Table S1, and Table 2.
Table 2.
Protonation States and pKa Values of Functionally Important Residues of C1C2
| E90 | K257 | K93 | E123 | D156 | C128 | D253 | |
|---|---|---|---|---|---|---|---|
| A | 4.50 | 10.50 | 4.50 | 3.80 | 9.00 | 3.80 | |
| B | 9.31 | 5.83 | 3.21 | ||||
| C | 9.25 | 14.39 | 12.97 | 5.62 | 7.75 | 13.53 | 2.96 |
| VI | 11.21 | 15.37 | 13.89 | 10.69 | 7.71 | 14.85 | 7.57 |
| 8.62∗∗ | 14.12∗∗ | 12.21∗∗ | 8.92∗∗ | 6.79∗∗ | 13.11∗∗ | 2.06∗∗ | |
| 8.51∗∗ | 14.13∗∗ | 12.24∗∗ | 9.08∗∗ | 6.83∗∗ | 13.06∗∗ | 2.02∗∗ | |
| 6.26 | 12.66 | 10.22 | 1.40 | 5.55 | 11.47 | 0.91 | |
| 0.77 | 0.43 | 0.47 | 0.98 | 0.34 | 0.62 | 0.50 | |
| ∗ | |||||||
| VII | 10.03 | 15.04 | 13.69 | 5.98 | 8.12 | 14.83 | 5.84 |
| 8.79∗∗ | 14.03∗∗ | 11.59∗∗ | 3.94∗∗ | 7.28∗∗ | 13.24∗∗ | 2.78∗∗ | |
| 8.83∗∗ | 14.08∗∗ | 11.59∗∗ | 4.46∗∗ | 7.29∗∗ | 13.26∗∗ | 2.18∗∗ | |
| 7.13 | 11.61 | 10.79 | 1.33 | 6.12 | 11.90 | 0.75 | |
| 0.43 | 0.38 | 0.30 | 1.11 | 0.26 | 0.38 | 1.24 | |
| ∗ | ∗ |
The asterisk (∗) denotes deprotonated residues. For example, in system VI, D253 is deprotonated; in system VII, both E123 and D253 are deprotonated. Row A: pKa values of the isolated residues. Row B shows pKa values reported by Kato et al. (11), calculated using PROPKA2.0. Row C shows pKa values calculated here for C1C2 in PDB: 3UG9 using PROPKA3.1. The same software version (3.1) was used for the pKa calculations for systems I–VIII (see Table S1). Here, we report the maximum, mean and median (indicated by double asterisks), minimum, and standard deviation of pKa values calculated for each frame of the 20 ns of equilibrated production run (the last 20 ns of 120-ns-long simulations) for systems VI and VII.
In assessing the pKa results, it should be noted that the values reported by Kato et al. (11) for a single structure (PDB: 3UG9) used PROPKA software version 2.0. For comparison, we used PROPKA3.1 to calculate pKa values of the same crystal structure. The differences between the values obtained using PROPKA2.0 and those obtained using PROPKA3.1 ranged between 0.06 and 0.25 pKa units (Table 2), well below the typical standard deviation of pKa values calculated from our MD production runs (the last 20 ns of 120-ns-long simulations). Due to the empirical nature of the PROPKA code, the absolute pKa values should not be compared, but rather the trends should be analyzed.
The variations in protonation of residues E123, D156, and D253 had little effect on the pKa values of many residues. We focus attention first on the putative ion channel of ChRs, located along helix II. This helix contains negatively charged residues E82, E83, E97, and E101, which point toward the interior of the protein, allowing water to enter the pore. The discontinuity of water in the protein’s closed state is due to the presence of protonated E90 on helix II, which forms a gate disabling unrestricted water and ion permeation through the protein (Fig. 2 c). In our study, E90 was kept protonated in all eight simulated systems to model the closed channel. With the exception of system IV (described in Table S2), the observed median pKa (E90) values ranged between 8.03 and 9.62 and appeared not to vary remarkably from 9.31, the pKa value reported by Kato et al. (11), or 9.25, the value calculated using PROPKA3.1, for the crystal structure. The pKa values of 12.97 and 14.39 for the neighboring residues, lysines K93 and K257, respectively, resembled values calculated for the unequilibrated crystal structure of C1C2. A similar trend appeared away from helix II, where the median pKa values of the DC-gate residues (D156 and C128) remained close to 7.75 and 13.53 in each of the systems studied. These observations suggest that the networks of interactions among residues E90, K93, K257, D156, and C128 were not strongly affected by changes in the E123, D156, and D253 protonation states.
In contrast to the residues relevant to the putative ion channel and DC gate, the pKa values of residues E123 and D253 varied strongly (by up to ∼7 pKa units) depending on the equilibrated system under study. The steric superimposition of the functionally important residues of the closed-state C1C2 crystal (PDB: 3UG9) and the C1C2 structure predicted by I-TASSER is shown in Fig. 3 a. C1C2 with both E123 and D253 deprotonated (system VII) and C1C2 with E123 protonated and D253 deprotonated (system VI), both equilibrated in DOPC, water, and ions at 300 K for 120 ns, are shown in Fig. 3, b and c, respectively. All-trans retinal and the side chains of functionally important residues E90 and N258 of the EHT model, C128 and D156 of the DC model, and E123 and D253 (both deprotonated) were found to overlay accurately (Fig. 3 b). The backbone average RMSD of the protein structure (without inclusion of the flexible N-terminal tail) throughout the last 20 ns of the 120-ns-long simulations was 0.5 ± 0.1 Å. For comparison, we found an RMSD value of 1.7 Å between the protein extracted from a representative simulation frame and the pre-equilibrated structure of system VII (Fig. 3 b). The RMSD value of 1.7 Å accounts for the contribution from flexible loops that link transmembrane helices and extend into the solvent, but it also indicates that the equilibrated and crystal structures are structurally similar. We chose to investigate equilibrated system VII (with both E123 and D253 deprotonated) further because the pKa values for functionally important residues and the side-chain positions in this system aligned the best with the corresponding properties in the crystal structure.
The extended open-state lifetime of a D156C ChR2 mutant (16) suggests that the D156-C128 hydrogen bond (DC gate) plays a key role in ChR kinetics. The predicted C1C2 chimera structure presented here was based on the crystal structure of wild-type C1C2 (11), where the distances between the sulfur atom (S) of the C128 thiol group (SH) and carboxyl oxygen atoms (O) of protonated D156 are 4.4 Å and 4.6 Å, respectively, and the SH group of C128 was associated with the π-electron system of the all-trans retinal chromophore (Fig. 3 b). After equilibration, the mean separations between the sulfur of C128 and the oxygen of D156 as a proton acceptor (C128-SH…O-D156) or donor (C128-S…HO-D156) remain large at 4.6 ± 0.3 Å or 4.8 ± 0.4 Å. Both distances extended beyond the maximum length of a conventional hydrogen bond (40). The mean values of the D156-O-D156-H-C128-S and D156-O-C128-H-C128-S angles were 157.4 ± 11.6 and 61.7 ± 14.0°, respectively. Moreover, no water molecules were found to bridge C128 and D156. Those results exclude the existence of the direct and indirect DC gate in our model. The high value of the C128 median ionization constant, pKa (C128) = 13.26 (Table 2), predicted by the PROPKA software (41), originates from hydrogen bonding between side chains of C128 with D156 and T127, as well as Coulombic interactions with D156, K273, K93, E123, E253, and E90, and weak backbone hydrogen bonding of C128 with itself.
The EHT gating model (Fig. 4) suggests the presence of a double hydrogen bond between E90 and N258 in the closed ChR2 protein. Throughout the simulations of the dark-adapted C1C2, the mean distances between the side chains of protonated E90 and N258 were r(O…O) = 2.7 ± 0.1 Å and r(O…N) = 3.7 ± 0.6 Å, respectively. The mean values of the E90-O-E90-H…N258-O and E90-O…N258-H-N258-N angles were 160.2 ± 10.4 and 100.8 ± 14.9°, respectively. The weak O…N hydrogen bond, indicated by the large deviation from a linear donor-proton-acceptor angle and typical hydrogen bond (donor-acceptor) distance cutoff of 3.0 Å (40), dissociated frequently. Serine S63 competed for the hydrogen bonding with E90. Additionally, significant electrostatic influences of D253, K93, E123, and K257 on E90 were observed. Those interactions contributed to a significant increase of the pKa (E90) value from 4.5 in an isolated amino acid (Table 2) to the median value of 8.83 in equilibrated C1C2 (Fig. S7; Table 2). A similarly high value, pKa (E90) = 9.31, was reported for the crystal structure of C1C2 described by Kato et al. (11). That behavior suggests that the O…O interaction between E90 and N258 alone can hold helices II and VII together, thus preventing pore opening.
Figure 4.
(a) The EHT gating model in ChR2 predicts a double E90-O…N258-O and E90-O…N258-N bond. In the crystal structure of C1C2, the corresponding distances are 2.86 Å and 3.01 Å. (b) In the equilibrated C1C2, system VII, the mean distances between the side chains of protonated E90 and N258 were r(O…O) = 2.7 ± 0.1 Å and r(O…N) = 3.7 ± 0.6 Å, respectively. This suggests that the O…O interaction between E90 and N258 alone can hold helices II and VII of C1C2 together, thus preventing pore opening. To see this figure in color, go online.
During equilibration of the closed C1C2 protein in the lipid bilayer and electrolyte solution, we first allowed the water to relax and enter the constrained protein (see Materials and Methods for details of the simulation setup). An average of 32 water molecules (Fig. S5) entered the putative transmembrane channel region from the extracellular side of the protein, but they did not form a continuous pathway (Fig. 2 c). The discontinuity of water in the vicinity of the protonated E90 is consistent with previous modeling studies (12, 13). The average separation between the centers of mass of helices HII and HVII was measured in three locations: the vicinity of the extracellular, central, and intracellular parts of the protein’s pore, yielding 14.7 ± 0.3 Å, 9.9 ± 0.2 Å, and 9.4 ± 0.2 Å, respectively. The separation of the helices was commensurate with a wide, water-filled opening of the pore toward the extracellular side of the protein and a narrow, dry region in the intracellular region of the pore (Fig. 2 c). Narrowing of the pore in the vicinity of the gating region (E90-N258), the close proximity of helices, and the presence of hydrophobic residues such as bulky Y70 at the intracellular side of the pore prevented water molecules from continuously filling the pore of closed C1C2.
In the ChR2 photocycle (Fig. 1 b), deprotonated aspartic acid D253 is believed to accept the SB proton. In our simulations of C1C2 (Fig. 3 b), the mean separation between the nitrogen atom of the all-trans retinal SB, SBH, and the deprotonated D253 centered around 3.3 ± 0.3 Å and the SB-NH….O-D253 angle took on a mean value of 92.5 ± 16.5° in the equilibrated closed C1C2 protein. Similarly, the mean distance between the SBH nitrogen atom and the oxygen of deprotonated E123 was 2.8 ± 0.2 Å and the SB-NH….O-E123 angle was 158.9 ± 15.9°. The median pKa values for deprotonated D253 and E123 were 2.18 and 4.46 (system VII in Table 2), ∼1 pKa unit lower compared with the values calculated for the resolved C1C2 crystal structure. It should be noted, however, that during the course of the production run, the pKa values of the two residues fluctuated between 1.1 and 5.9 pKa units (Fig. 5), suggesting highly mobile side chains for these residues. The median separation between carbon atoms of carboxylate ions of E123 and D253 during the 20 ns production run was 5.1 Å, and both side chains experienced strong, mutual Coulombic interactions. Moreover, E123 and D253 were affected by interactions with neighboring K253, E97, K273, and R120. Additionally, E123 showed a propensity for forming hydrogen bonds with T127 and W124, which affected the E123-O…HN-SB interaction by significantly decreasing the strength of the side-chain hydrogen bond. Despite the previous suggestion that D253 is the SB proton acceptor during the ChR2 photocycle (7), our study suggests that in C1C2, the side chains of deprotonated D253 and E123 may compete for the SB proton.
Figure 5.
pKa values of functionally important residues of C1C2, (a) E123 and (b) D253 (both deprotonated). Individual pKa values (black line) and the running average of pKa values (red line) were calculated for each frame of the 20 ns equilibrated production run (the last 20 ns of 120-ns-long simulations) using PROPKA3.1. pKa standard deviations of 1.11 and 1.24 pKa units were found for deprotonated E123 and D253, respectively. To see this figure in color, go online.
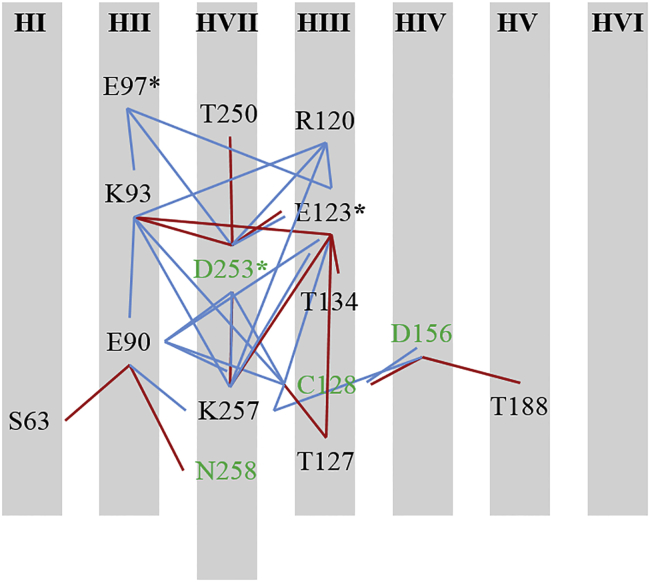
Based on results from the PROPKA calculations and geometric considerations (distances and angles among residues), we mapped selected key interactions branching out from the SB (denoted as K257) and its proton acceptor (E123 or D253), the EHT gate (E90-N258), and the DC gate (D156-C128). All those residues are found in the vicinity of the C1C2 ion channel and retinal molecule (Fig. 6); thus, retinal isomerization can be expected to influence the network of interactions leading to pore opening. The least involved in the network of interactions were helices HI, HV (originally from ChR1), and HVI (ChR2). In addition to the interactions listed above between E90-N258, D156-C128, and the SB and its proton acceptors, two more residue pairs link HII, HIII, and HVII, the three helices shown experimentally to form the key network among functionally important residues. Those residues are D253-O…N-K93 and E123-O…HN-K93, which form hydrogen bonds at 2.7 ± 0.1 Å, 112.4 ± 13.8°, and 2.6 ± 0.1 Å, 160.1 ± 8.8°, respectively.
Figure 6.
Schematic diagram of the side-chain hydrogen bonds (red lines), Coulombic interactions (blue lines), and backbone interaction with itself (green) most commonly observed among functionally important residues of C1C2 equilibrated in a lipid bilayer and electrolyte solution. The putative ion channel in ChRs has been located along HII. In C1C2, the majority of interactions spanned helices II and III (originally from ChR1), as well as helix VII (from ChR2). HVII is depicted as the longest of the helices, in agreement with the crystal structure of C1C2, where HVII extends toward the intracellular side of the protein. The asterisks (∗) denote deprotonated residues. To see this figure in color, go online.
Discussion
We equilibrated multiple wild-type, closed-state C1C2 chimera structures in a physiologically representative environment of lipid bilayer (DOPC) and electrolyte solution. All proteins consisted of deprotonated residues E82, E83, E97, and E101 along helix II and protonated E90—a component of the EHT gate in the central region of the pore. The structures differed in protonation of the functionally important residues E123, D156, and D253. We compared the mean separations among side-chain atoms, median pKa values of functionally important residues, and alignment of the representative structures extracted from each of the simulations. We determined that the equilibrated C1C2 with deprotonated E123 and D253 (system VII in Table 2) resembles the crystal structure of C1C2 reported by Kato et al. (11) most closely. That motivated further study of the atomistic details of system VII, as discussed below.
The double hydrogen bond between protonated E90 on HII and N258 on HVII in the dark-adapted ChR2 was recently indicated to be an inner gate (12, 13). Upon all-trans to 13-cis isomerization of retinal, the E90 deprotonates and the E90-N258 interaction supposedly weakens, causing disconnection of helices II and VII as well as an increase in hydrophilicity in the central section of the pore. This EHT model (13) predicts a reaction path for formation of the continuous water pore through the transmembrane section of the protein; a prerequisite for ion transport. However, the EHT model is not yet well understood. We tested the EHT mechanistic model in wild-type C1C2 via all-atom MD studies. Our simulations showed that the average distance between oxygen and nitrogen atoms of side chains of E90 and N258 fluctuated around r(O…O) = 2.7 ± 0.1 Å and r(O…N) = 3.7 ± 0.6 Å. This difference suggests that the presence of a single OH…O hydrogen bond between E90 and N258 sufficed to keep the gate in C1C2 closed.
The presence of the DC gate has been demonstrated in Fourier transform infrared spectroscopy studies by Nack et al. (14). In the dark state of ChR2, the side chains of D156 and C128 form a hydrogen bond that links helices IV and III, respectively. A single point mutation, D156C, results in a ChR2 variant of high expression and long open state due to the slow channel closure (16). Although the existence and functional relevance of the DC gate in ChR2 is well supported, the nature of the hydrogen bond between C128 and D156 is still an open issue. Nevertheless, the presence of a direct or water-mediated C128-S…O-D156 interaction has not been observed in the crystal structure of C1C2. In C1C2 analyzed here, a bridging water between residues 128 and 156 was not found. The thiol group of C128 most commonly formed a hydrogen bond with the side chain of the neighboring T127, rarely with D156. According to PROPKA simulations, C128 was also affected by the electrostatic potential originating from residues found in the vicinity of the gating region of the pore, namely E90, K93, E123, D156, and D253. Both C128 and T127 reside in the immediate vicinity of the all-trans retinal. Interestingly, the backbone oxygen of the deprotonated E123 was found forming an intra-helical hydrogen bond with T127 at 2.8 ± 0.2 Å and 158.2 ± 18.8°. It can be expected that upon the all-trans to 13-cis isomerization of the chromophore, the polyene chain displacement could affect the C128-T127 hydrogen bonding and, consequently, influence the network of interactions among the SB proton acceptor (E123 or D253) and other residues involved in the channel gating.
In this study, both deprotonated residues E123 and D253 were found to form hydrogen bonds with the SB nitrogen. Moreover, the two residues were incorporated into the largest number of contacts, both Coulombic and hydrogen bonds, within the network of functionally important interactions (Fig. 6). Those contacts also translated into large fluctuations in pKa values for both residues (Fig. 5), which, as stated in Materials and Methods, were calculated as neighbor-induced perturbations. E123 of ChRs corresponds to D85, a SB proton acceptor in H. salinarum bacteriorhodopsin (22, 42). However, point-mutation studies on E123 (22, 43) in ChR2 show that the residue is not compulsory for the protein’s function, suggesting that D253 acts as the proton acceptor from the SB also in C1C2. Although in bacteriorhodopsin D96 was suggested as a SB proton donor (44), the corresponding residue in C1C2 is still unknown. To better understand the most probable proton transfer pathway and to gain additional insight into the treatment of the C1C2 protein’s protonation and its effect on intramolecular interactions, future studies will include constant pH simulations (45, 46).
Based on the existing knowledge of the ChR2 photocycle and our study of pairwise interactions in the closed-state non-conducting ChR chimera, C1C2, we have deciphered an extended network of potentially correlated interactions among key residues in the latter (Fig. 6). We expect the network of hydrogen bonds and Coulombic interactions among E90, E123, D253, N258, and the SBH, as well as contributions from K93, C128, and T127, to play a key role in the C1C2 photocycle. In the early stage of C1C2 photoactivation, the C128…T127 pair, which resides near the polyene retinal moiety, would be affected by the isomerization of retinal from all-trans to 13-cis. Changes in the vicinity of T127, which forms a hydrogen bond with E123, could affect Coluombic interaction between E123 and D253. Those two residues reside in the vicinity of the SBH, and both have been suggested as SBH proton acceptors (7). Re-protonation of the SB in the pre-gating stage of the ChR2 photocycle is expected via residues not yet specified. Finally, we found that K93 interacts with E123 and D253. Studies on the possible involvement of K93 in the proton transfer pathway are underway.
Conclusions
With steadily growing interest in the application of ChRs in optogenetics, there have been a surprising number of unanswered questions regarding the atomistic details of the action of these proteins. Although most research on the medical applications of ChRs has focused on ChR2, the structure of the protein has not been resolved. Most of the current theories regarding ChR2 function have been derived from ChR2 homology models built based on the crystal structure of C1C2 chimera (PDB: 3UG9). Surprisingly few detailed studies address C1C2 itself. To gain a better understanding of the C1C2 structure-versus-function relationships, we conducted atomistic MD simulations of the closed state, wild-type C1C2 in a physiologically representative environment of lipid bilayer and electrolyte solution.
Out of the eight equilibrated proteins with varied protonation states of key residues (E123, D156, and D253), we found that the structure with E123 and D253 deprotonated closely matches the crystal structure of C1C2 chimera reported by Kato et al. (11). We discovered that the gating in C1C2 could be achieved by a single E90-OH…N258-O hydrogen bond, not a double E90-OH…N258-O and E90-O…N258-NH bond, as previously suggested for ChR2. We suspect that D253 is the probable SB proton acceptor, but E123 should not be excluded from consideration. In agreement with previous studies on C1C2, our simulations show no proof of the existence of a direct or water-bridged interaction between the all-trans retinal-neighboring DC-gate components, D156 and C128. Instead, the side chain of C128 formed hydrogen bonds with T127, a residue in close vicinity to E123.
We have deciphered key interactions among the functionally important residues in the gating region and in the vicinity of the all-trans retinal chromophore. We anticipate that this work will provide context for studies of the gating process as well as water and ion transport in C1C2, and spark interest in further experimental studies on the chimera. In future work, we will study pore opening and further investigate the contributions of the key residues to structural rearrangements leading to solvent transport through the C1C2 pore.
Author Contributions
M.R.V. and S.B.R. conceived the study. S.B.R and S.W.R. supervised the project. M.R.V. performed research and, together with G.G., analyzed data. G.G. contributed analytic tools. M.R.V. drafted the manuscript. S.B.R. and S.W.R. assisted in interpretation of data and writing the manuscript.
Acknowledgments
We gratefully acknowledge support by the Louisiana Optical Network Institute (LONI), the National Science Foundation award no. CHE-1301072, the Defense Threat Reduction Agency (DTRA), and the Sandia National Laboratories’ LDRD program. We also thank Andriy Anishkin for fruitful discussions and for providing the scripts to calculate pKa values. This work was performed, in part, at the Center for Integrated Nanotechnologies, a U.S. Department of Energy, Office of Basic Energy Sciences user facility at Los Alamos National Laboratory (contract DE-AC52-06NA25396) and Sandia National Laboratories (Contract DE-Ac04-94AL85000). Sandia National Laboratories is a multi-mission laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation, for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000.
Editor: Carmen Domene.
Footnotes
Seven figures and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30144-3.
Supporting Material
References
- 1.Lórenz-Fonfría V.A., Heberle J. Channelrhodopsin unchained: structure and mechanism of a light-gated cation channel. Biochim. Biophys. Acta. 2014;1837:626–642. doi: 10.1016/j.bbabio.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Spudich J.L., Yang C.S., Spudich E.N. Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 2000;16:365–392. doi: 10.1146/annurev.cellbio.16.1.365. [DOI] [PubMed] [Google Scholar]
- 3.Berthold P., Tsunoda S.P., Hegemann P. Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell. 2008;20:1665–1677. doi: 10.1105/tpc.108.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yizhar O., Fenno L.E., Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Macé E., Caplette R., Dalkara D. Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV Restores ON and OFF visual responses in blind mice. Mol. Ther. 2015;23:7–16. doi: 10.1038/mt.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruegmann T., van Bremen T., Sasse P. Optogenetic control of contractile function in skeletal muscle. Nat. Commun. 2015;6:7153. doi: 10.1038/ncomms8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lórenz-Fonfría V.A., Resler T., Heberle J. Transient protonation changes in channelrhodopsin-2 and their relevance to channel gating. Proc. Natl. Acad. Sci. USA. 2013;110:E1273–E1281. doi: 10.1073/pnas.1219502110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radu I., Bamann C., Heberle J. Conformational changes of channelrhodopsin-2. J. Am. Chem. Soc. 2009;131:7313–7319. doi: 10.1021/ja8084274. [DOI] [PubMed] [Google Scholar]
- 9.Ritter E., Stehfest K., Bartl F.J. Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy. J. Biol. Chem. 2008;283:35033–35041. doi: 10.1074/jbc.M806353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamann C., Kirsch T., Bamberg E. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J. Mol. Biol. 2008;375:686–694. doi: 10.1016/j.jmb.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 11.Kato H.E., Zhang F., Nureki O. Crystal structure of the channelrhodopsin light-gated cation channel. Nature. 2012;482:369–374. doi: 10.1038/nature10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer K., Kuhne J., Gerwert K. In channelrhodopsin-2 Glu-90 is crucial for ion selectivity and is deprotonated during the photocycle. J. Biol. Chem. 2012;287:6904–6911. doi: 10.1074/jbc.M111.327700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhne J., Eisenhauer K., Bartl F. Early formation of the ion-conducting pore in channelrhodopsin-2. Angew. Chem. Int. Ed. Engl. 2015;54:4953–4957. doi: 10.1002/anie.201410180. [DOI] [PubMed] [Google Scholar]
- 14.Nack M., Radu I., Heberle J. The DC gate in Channelrhodopsin-2: crucial hydrogen bonding interaction between C128 and D156. Photochem. Photobiol. Sci. 2010;9:194–198. doi: 10.1039/b9pp00157c. [DOI] [PubMed] [Google Scholar]
- 15.Bamann C., Gueta R., Bamberg E. Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry. 2010;49:267–278. doi: 10.1021/bi901634p. [DOI] [PubMed] [Google Scholar]
- 16.Dawydow A., Gueta R., Kittel R.J. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA. 2014;111:13972–13977. doi: 10.1073/pnas.1408269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamiya M., Kato H.E., Hayashi S. Structural and spectral characterizations of C1C2 channelrhodopsin and its mutants by molecular simulations. Chem. Phys. Lett. 2013;556:266–271. [Google Scholar]
- 18.Watanabe H.C., Welke K., Elstner M. Towards an understanding of channelrhodopsin function: simulations lead to novel insights of the channel mechanism. J. Mol. Biol. 2013;425:1795–1814. doi: 10.1016/j.jmb.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Yan R., Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat. Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy A., Kucukural A., Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel G., Szellas T., Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jo S., Kim T., Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 24.Phillips J.C., Braun R., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feller S.E., Zhang Y., Brooks B.R. Constant pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 1995;103:4613–4621. [Google Scholar]
- 26.Müller M., Bamann C., Kühlbrandt W. Light-induced helix movements in channelrhodopsin-2. J. Mol. Biol. 2015;427:341–349. doi: 10.1016/j.jmb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Ito S., Kato H.E., Kandori H. Water-containing hydrogen-bonding network in the active center of channelrhodopsin. J. Am. Chem. Soc. 2014;136:3475–3482. doi: 10.1021/ja410836g. [DOI] [PubMed] [Google Scholar]
- 28.Best R.B., Zhu X., Mackerell A.D., Jr. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J., MacKerell A.D., Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 2013;34:2135–2145. doi: 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klauda J.B., Venable R.M., Pastor R.W. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tajkhorshid E., Paizs B., Suhai S. Conformational effects on the proton affinity of the Schiff base in bacteriorhodopsin: a density functional study. J. Phys. Chem. B. 1997;101:8021–8028. [Google Scholar]
- 32.Tajkhorshid E., Suhai S. Influence of the methyl groups on the structure, charge distribution, and proton affinity of the retinal Schiff base. J. Phys. Chem. B. 1999;103:5581–5590. [Google Scholar]
- 33.Tajkhorshid E., Baudry J., Suhai S. Molecular dynamics study of the nature and origin of retinal’s twisted structure in bacteriorhodopsin. Biophys. J. 2000;78:683–693. doi: 10.1016/S0006-3495(00)76626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nina M., Roux B., Smith J.C. Functional interactions in bacteriorhodopsin: a theoretical analysis of retinal hydrogen bonding with water. Biophys. J. 1995;68:25–39. doi: 10.1016/S0006-3495(95)80184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baudry J., Crouzy S., Smith J.C. Quantum chemical and free energy simulation analysis of retinal conformational energetics. J. Chem. Inf. Comput. Sci. 1997;37:1018–1024. [Google Scholar]
- 36.Michaud-Agrawal N., Denning E.J., Beckstein O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011;32:2319–2327. doi: 10.1002/jcc.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Søndergaard C.R., Olsson M.H.M., Jensen J.H. Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J. Chem. Theory Comput. 2011;7:2284–2295. doi: 10.1021/ct200133y. [DOI] [PubMed] [Google Scholar]
- 38.Olsson M.H.M., Søndergaard C.R., Jensen J.H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011;7:525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- 39.Jiao D., Rempe S.B. Combined density functional theory (DFT) and continuum calculations of pKa in carbonic anhydrase. Biochemistry. 2012;51:5979–5989. doi: 10.1021/bi201771q. [DOI] [PubMed] [Google Scholar]
- 40.Arunan E., Desiraju G.R., Nesbitt D.J. Defining the hydrogen bond: an acount (IUPAC technical report) Pure Appl. Chem. 2011;83:1619–1636. [Google Scholar]
- 41.Rostkowski M., Olsson M.H., Jensen J.H. Graphical analysis of pH-dependent properties of proteins predicted using PROPKA. BMC Struct. Biol. 2011;11:6. doi: 10.1186/1472-6807-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braiman M.S., Mogi T., Rothschild K.J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988;27:8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- 43.Gunaydin L.A., Yizhar O., Hegemann P. Ultrafast optogenetic control. Nat. Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 44.Wolf S., Freier E., Gerwert K. Infrared spectral marker bands characterizing a transient water wire inside a hydrophobic membrane protein. J. Chem. Phys. 2014;141:22D524. doi: 10.1063/1.4902237. [DOI] [PubMed] [Google Scholar]
- 45.Chen W., Shen J.K. Effects of system net charge and electrostatic truncation on all-atom constant pH molecular dynamics. J. Comput. Chem. 2014;35:1986–1996. doi: 10.1002/jcc.23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y., Chen W., Shen J. Mechanism of pH-dependent activation of the sodiumproton antiporter NhaA. Nat. Commun. 2016;7:12940. doi: 10.1038/ncomms12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.