Abstract
Purpose
The aim of this study was to explore the choice of modality for diagnosis, treatments, and consequences of anastomotic leakage.
Methods
This is a retrospective study of consecutive patients who underwent surgery that included a colorectal anastomosis due to colorectal cancer, diverticulitis, inflammatory bowel disease (IBD), or benign polyps.
Results
A total of 600 patients were included during 2010–2012, and 60 (10%) had an anastomotic leakage. It took in mean 8.8 days (range 2–42) until the anastomotic leakage was diagnosed. A total of 44/60 of the patients with a leakage had a CT scan of the abdomen; 11 (25%) were initially negative for anastomotic leakage. Among all leakages, the anastomosis was taken down in 45 patients (76.3%). All patients with a grade B leakage (n = 6) were treated with antibiotics, and two also received transanal drainage. The overall complication rate was also significantly higher in those with leakage (93.3 vs. 28.5%, p < 0.001), and it was more common with more than three complications (70 vs. 1.5%, p < 0.001). There was a higher mortality in the leakage group.
Conclusion
This study demonstrated that one fourth of the CT scans that were executed were initially negative for leakage. Most patients with a grade C leakage will not have an intact anastomosis. An anastomotic leakage leads to significantly more severe postoperative complications, higher rate of reoperations, and higher mortality. An earlier relaparotomy instead of a CT scan and improved postoperative surveillance could possibly reduce the consequences of the anastomotic leakage.
Keywords: Colrectal surgery, Anastomotic leakage, Postoperative complications
Introduction
Anastomotic leakage remains a severe complication after abdominal surgery with considerable morbidity and mortality [1–11]. The frequency ranges from 1.8 to 19.2% and depends partly on different risk factors [4, 12–20]. Risk factors for leakage have been extensively studied, and the most frequent factors mentioned are male sex, high age, a low anastomosis, malignant disease, high American Society of Anesthesiologists (ASA) score, long operation time, emergency operation, preoperative radiotherapy, and perioperative blood loss or transfusion [4, 13, 18, 21–26]. There is no universal grading of the leakages, but the definition proposed by Rahbari et al. is often used for rectal cancer and consists of a three-grade scale. Grade A requires no therapeutic intervention; grade B includes active intervention without laparotomy, and if laparotomy is required, the leakage is classified as grade C [27]. The diagnostic methods commonly used when a leakage is suspected are CT scan, contrast enema, endoscopic examination, and reoperation [28]. The leakage may be diagnosed at different time points postoperatively, and there are theories that early and late leakages are different entities. One suggestion is that a later diagnosed leakage only has more subtle symptoms, and thus, more is accurately described as discrete than late [29–33]. Treatment of an anastomotic leakage differs with the severity and the location of the anastomosis. Often, there is a high frequency of permanent stoma after a reoperation and anastomotic take down. Salvage of the anastomosis is more common in grade A and B leakages with the treatment consisting of drainage and/or antibiotics [3, 34–36]. Despite the increased knowledge of an anastomotic leakage, there is still a need for studies in an unselected cohort of patients receiving surgery for both benign and malignant diseases, to try to improve results after the anastomotic leakage has occurred.
The aim of this study was to explore the choice of modality for diagnosis, treatments, and consequences of anastomotic leakage in colorectal surgery in an unselected population.
Methods
Study design
This is a retrospective study of a consecutive series of patients, over 16 years old, between the first of January 2010 to the 30 June 2012, who underwent colorectal surgery that included an anastomosis due to colorectal cancer, diverticulitis, inflammatory bowel disease (IBD), or benign polyps. All patients were treated at the Sahlgrenska University Hospital/Östra in Sweden, that serves approximately 700,000 inhabitants. The Nordic Medico-Statistical Committee (NOMESCO) Classification of Surgical Procedures version 1.9 was used to identify all patients. End follow-up was set to 6 May 2014 or the date of death. The median time of follow-up was 32 months (interquartile range (IQR) = 16).
Included variables
The medical records were studied, and data was collected including patient-related information such as demography (date of birth, sex, weight, height) and ASA classification. The following comorbidity was registered: diabetes mellitus, hypertension, other cardiovascular disease (heart failure, heart attack, angina pectoris, or heart valve diseases), neurologic disease (stroke, epilepsy), and chronic obstructive pulmonary disease (COPD)/asthma. The diagnoses were identified using the International Statistical Classifications of Diseases and Related Health Problems 10 (ICD-10 codes). In addition to medical records, information was extracted from a health declaration that the patients filled in prior to surgery. Perioperative and postoperative variables including timing of surgery, type of operation, blood loss, hospital stay, complications (using the Clavien-Dindo classification system [37]), reoperations, and mortality were included in the database.
Exclusion criteria
Exclusion criteria were resection without an anastomosis to the colon or rectum, when the surgery was considered a reoperation, reversal of a stoma, ileo pouch-anal anastomosis, and patients who moved shortly after the procedure (thus, no follow-up was possible) and when records had missing data from the surgery.
Definitions
Anastomotic leakage was defined as any clinical signs of leakage, confirmed by radiological examination, endoscopy, clinical examination of the anastomosis (i.e., palpation of the anastomosis), or reoperation. The leakages were graded retrospectively according to the system proposed by Rahbari et al. [27]. Anastomosis takedown was defined as an interruption of the continuity of the bowel and the formation of a stoma. The blood loss was the volume noted by the anesthetic nurse during surgery. The surgical approach was divided into three groups: laparoscopy, laparotomy, and conversions from laparoscopy to open surgery, but in statistical calculations, the converted group is in the laparoscopic group as intention to treat. Anastomosis not taken down, salvage was defined as preservation of the bowel continuity with repair of the anastomosis or conservative treatment with or without drainage or antibiotics. Death was recorded within 30 and 90 days from index surgery. Time to diagnosis of a leakage was calculated as days between the index operation and diagnosis of the leakage with reoperation or CT abdomen or CT rectal contrast or with endoscopy or when fecal containing fluid was seen in the drainages. Total hospital stay included a second admission to hospital if the cause was anastomotic leakage or complications thereof. A stoma was counted as permanent if it was present at the end of follow-up time.
Statistical analysis
The statistical calculations were performed using the IBM SPSS Statistics version 22.0. The study is mainly descriptive, and therefore, univariate statistical calculations were used. Chi-squared tests (categorical variables) or Mann-Whitney tests (continuous variables such as BMI or blood loss) were applied in comparison of groups. Fishers exact test was used if the number of categorical observations were fewer than five. Mean with range or median with interquartile range was used as descriptive statistics. Significance was defined as p value <0.05.
Results
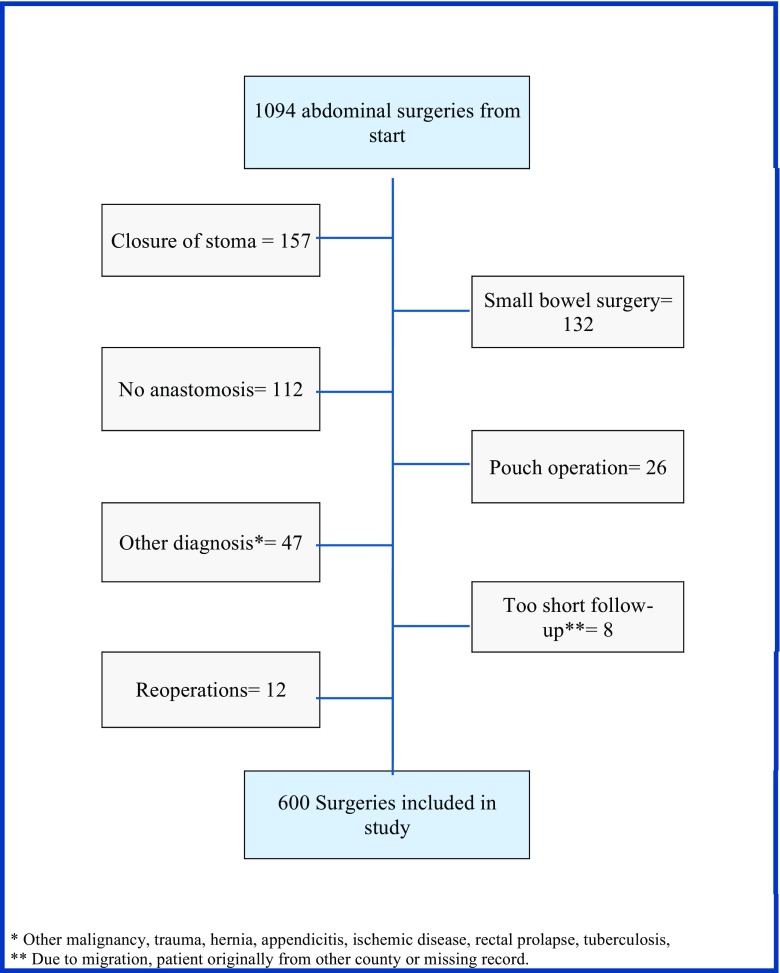
A total of 1094 patients were identified; after exclusion, 600 consecutive patients who underwent a colorectal surgical procedure that included a primary anastomosis due to colorectal cancer, diverticulitis, IBD, or benign polyps were included (Fig. 1). Median age was 68.4 years (IQR 18), and there were slightly more women (50.8%) than men. Malignant disease was the reason for surgery in 487 (81.2%), and among these, 396 were colon cancer and 91 were rectal cancer. Sixty patients were found to have an anastomotic leakage resulting in an overall incidence of 10%. Anastomotic leakages were more common in rectal resections with a stapled anastomosis and when a defunctioning stoma was used, see Table 1 for details.
Fig. 1.
Flowchart of exclusion
Table 1.
Patient characteristics and anastomotic leakage
| Anastomotic leakage n = 60 | No anastomotic leakage n = 540 | Rate of anastomotic leakage | Comparison regarding anastomotic leakage | OR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Age median (interquartile range) | 67.3 (16) | 68.6 (18) | −5.0, 2.4 | 0.485 | ||
| Gender | Male/female | 1.5 (0.9, 2.6) | 0.134 | |||
| Male | 35 | 260 | 11.9% | |||
| Female | 25 | 280 | 8.2% | |||
| ASA score (missing = 6) | ASA I–II/ASA III–IV | 1.1 (0.6, 2.0) | 0.747 | |||
| ASA I–II | 44 | 381 | 10.4% | |||
| ASA III–IV | 16 | 153 | 9.5% | |||
| BMI (missing = 6) | BMI ≤25/>25 | 1.2 (0.7, 2.1) | 0.495 | |||
| ≤25 | 32 | 260 | 11.0% | |||
| >25 | 28 | 274 | 9.3% | |||
| Diagnosis | Malignant/benign | 1.4 (0.6, 2.8) | 0.423 | |||
| Malignant disease | 51 | 436 | 10.5% | |||
| Benign disease | 9 | 104 | 8.0% | |||
| Comorbiditya | Comorbidity yes/no | 1.3 (0.7, 2.3) | 0.349 | |||
| 0 | 22 | 232 | 8.7% | |||
| 1 | 31 | 250 | 11.0% | |||
| ≥2 | 7 | 58 | 10.8% | |||
| Smoking (missing = 3) | Smoking yes/no | 1.9 (0.9, 3.9) | 0.066 | |||
| Yes | 11 | 56 | 16.4% | |||
| No | 49 | 481 | 9.2% | |||
| Timing | Elective/emergency | 1.4 (0.6, 3.4) | 0.423 | |||
| Elective | 54 | 466 | 10.4% | |||
| Emergency | 6 | 74 | 7.5% | |||
| Surgical approach | Laparoscopy and converted/openb | 0.9 (0.5, 1.9) | 0.836 | |||
| Open | 49 | 435 | 10.1% | |||
| Laparoscopy | 9 | 71 | 11.3% | |||
| Conversion to Laparotomy | 2 | 34 | 5.6% | |||
| Surgical procedures | 0.02 | |||||
| Right hemicolectomy | 17 | 225 | 7.0% | |||
| Left hemicolectomy | 5 | 63 | 7.4% | |||
| Sigmoid colectomy | 11 | 117 | 8.6% | |||
| Rectal resection | 22 | 95 | 18.8% | |||
| Total colectomy | 2 | 14 | 12.5% | |||
| Other colonic anastomosisc | 3 | 26 | 10.3% | |||
| Anastomosis technique (missing 7) | Stapled/hand-sewn | 2.8 (1.5, 5.1) | 0.001 | |||
| Stapled | 42 | 259 | 14.0% | |||
| Hand-sewn | 15 | 276 | 5.5% | |||
| Stoma | Stoma/no stoma | 2.8 (1.5, 5.2) | <0.001 | |||
| Defunctioning stoma | 18 | 71 | 20.2% | |||
| No defunctioning stoma | 42 | 469 | 8.2% | |||
| Perioperative blood loss (missing 28) | >300/<300 ml | 1.6 (0.9, 2.9) | 0.097 | |||
| >300 ml | 37 | 274 | 11.9% | |||
| <300 ml | 21 | 254 | 7.6% | |||
| Blood loss (ml) mean (range) (missing 14) | 516 (0–2200) | 426 (0–4000) | 0.534 | |||
aComorbidity: diabetes mellitus, hypertension, cardiovascular disease, cerebral disease, kidney disease, asthma/chronic obstructive pulmonary disease
b“Conversion to laparotomy” is counted in the laparoscopy group when comparison is made
cOther colonic anastomosis: transverse colectomy and other non-standard colectomies
Diagnosis of anastomotic leakage
The time until diagnosis was in mean 8.8 days (range 2–42). CT scan was the most common diagnostic method with a total of 44/60. Of these, 11 (25%) were negative and 33 (75%) positive for anastomotic leakage. Although numerical differences indicating both shorter time to diagnosis (4.3 vs. 9.3 days) and shorter hospital stay (22 vs. 29.9 days) for patients diagnosed at surgery compared to all other diagnostic methods, this was not statistically significant (Table 2). A total of 12/60 (20%) patients were diagnosed with leakage after readmission.
Table 2.
Diagnostic method of the anastomotic leakage
| Diagnosed at reoperation n = 7 | Other diagnostic methodsa n = 53 | OR (95% Cl) | p value | |
|---|---|---|---|---|
| Days to leakage diagnosis, mean (range) | 4.3 (2–9) | 9.3 (2–42) | 0.075 | |
| Days in hospital, mean (range) | 22 (5–46) | 29.9 (4–101) | 0.321 | |
| 30-day mortality n (%) | 0 | 3 (6.4%) | 1.3 (1.1, 1.5) | 1.000 |
| 90-day mortality n (%) | 0 | 5 (10.6%) | 1.3 (1.1, 1.5) | 0.575 |
aCT abdomen, CT with rectal contrast, endoscopic, or combination of more than one method
Treatment of anastomotic leakage
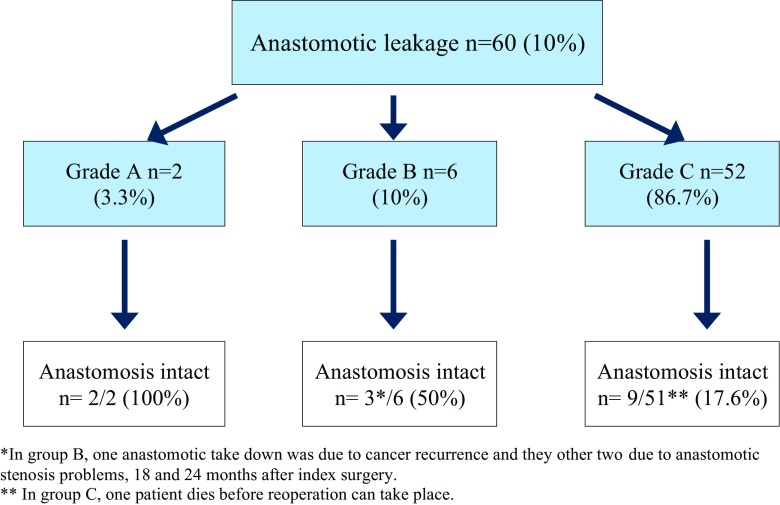
One patient, with a grade C anastomotic leakage, died before surgery could take place. Among all leakages, grades A–C, the anastomosis was taken down in 45 patients (76.3%) and bowel continuity was intact in 14 patients (23.7%). Two patients in the grade B group later had their anastomosis taken down due to anastomotic stenosis and one due to local recurrence of cancer (Fig. 2). All patients in grade B group (n = 6) were treated with antibiotics; two also received transanal drainage.
Fig. 2.
Flowchart over anastomotic leakages
Postoperative complications and reoperations
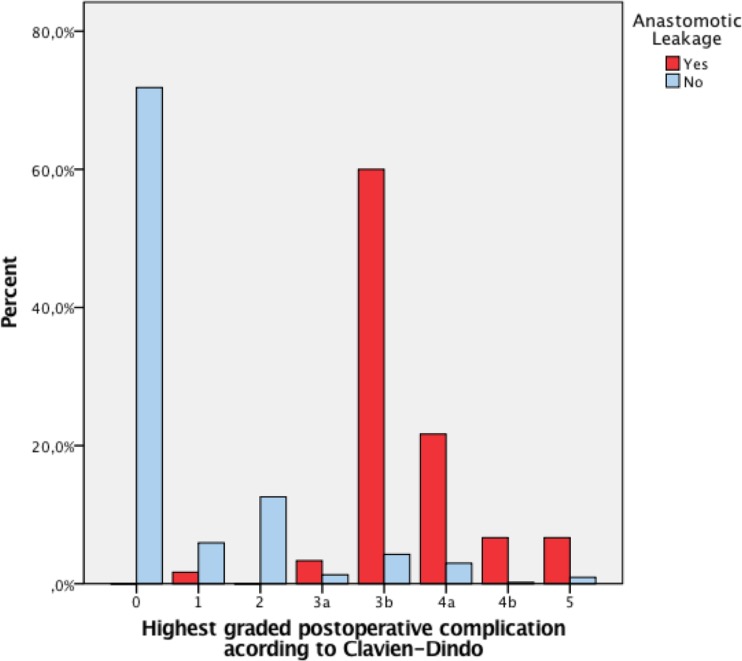
It was more common with a reoperation in patients with an anastomotic leakage compared to patients without leakage: 91.7% (n = 55) vs. 5.4% (n = 29) (p < 0.001). The overall complication rate was also significantly higher in those with leakage (93.3 vs. 28.5%, p < 0.001), and it was more common with more than three complications (70 vs. 1.5%, p < 0.001). However, the rate of wound infection and pneumonia did not differ between the groups; for details, see Table 3. More severe complications according to Clavien-Dindo were seen in patients with anastomotic leakage compared to patients without leakage (Fig. 3).
Table 3.
Postoperative complications and morbidity
| Anastomotic leakage (n = 60) | No anastomotic leakage (n = 540) | OR (CI 95%) | p value | |
|---|---|---|---|---|
| Days in hospital, mean (range) | 29.0 (4–101) | 9.4 (2–54) | 14.5, 24.7 | <0.001 |
| 30-day mortality n (%) | 3 (5.0%) | 3 (0.6%) | 9.4 (1.9, 47.8) | 0.015 |
| 90-day mortality n (%) | 5 (8.3%) | 11 (2.0%) | 4.4 (1.5, 13.0) | 0.004 |
| Reoperation within 12 months n (%) | 55 (91.7%) | 29 (5.4%) | 193.9 (72.1, 521.1) | <0.001 |
| ≥3 postoperative complications n (%) | 42 (70.0%) | 8 (1.5%) | 155.2 (63.7, 377.9) | <0.001 |
| Number of patients with one or more complications other than anastomotic leakage n (%) | 56 (93.3%) | 154 (28.5%) | 35.1 (12.5, 98.4) | <0.001 |
| Wound infection n (%) | 5 (8.3%) | 35 (6.5%) | 1.3 (0.5, 3.5) | 0.585 |
| Wound dehiscence n (%) | 5 (8.3%) | 14 (2.6%) | 3.4 (1.2, 9.8) | 0.016 |
| Abscess n (%) | 27 (45%) | 13 (2.4%) | 33.2 (15.7, 70.2) | <0.001 |
| Fistula n (%) | 6 (10%) | 4 (0.7%) | 14.9 (4.1, 54.4) | <0.001 |
| Bleeding n (%) | 6 (10%) | 18 (3.3%) | 3.2 (1.2, 8.5) | 0.012 |
| Pneumonia n (%) | 4 (6.7%) | 12 (2.2%) | 3.1 (1.0, 10.1) | 0.066 |
| Sepsis n (%) | 5 (8.3%) | 6 (1.1%) | 8.1 (2.4, 27.4) | <0.001 |
| Other infectiona n (%) | 12 (20%) | 34 (6.3%) | 3.7 (1.8, 7.7) | <0.001 |
| Cardiovascular complicationsb n (%) | 9 (15%) | 18 (3.3%) | 5.1 (2.2, 12.0) | <0.001 |
| Respiratory complications n (%) | 13 (21.7%) | 4 (0.7%) | 37.1 (11.6, 118.2) | <0.001 |
| Renal failure n (%) | 4 (6.7%) | 2 (0.4%) | 19.2 (3.4, 107.3) | 0.001 |
| Other complicationsc n (%) | 15 (25%) | 29 (5.4%) | 5.9 (3.0, 11.8) | <0.001 |
aUrinary infection, candida, or unknown source to infection
bMyocardial infarction, hypotension, heart failure, atrial fibrillation
cSevere pain, stoma-related complications, pancreatitis, peripheral neural damage, embolism, and intestinal obstruction
Fig. 3.
Postoperative complications—Clavien-Dindo
Mortality
There was a significant increase in mortality among patients with anastomotic leakage. Thirty day mortality was 5% in the leakage group compared to 0.6% in the none leakage group (p 0.015). Similarly, the 90-day mortality was higher, 8.3 vs. 2% (p 0.004). All five patients who died within 90 days in the leakage group had a grade C leakage and required surgery, two men and three women, all with colorectal cancer and severe comorbidity. See Table 2 for mortality in relationship to diagnostic method.
Discussion
The rate of anastomotic leakage varies in studies, but almost always, the incidence is higher in the rectal resections, and our study confirms that. Overall, the leakage rate was 10% but in rectal resections 18.8%. In procedures with stapled anastomosis and defunctioning stomas, the leakage rates were high, but both were strongly correlated to rectal resections; thus, no conclusions regarding stapled anastomoses can be drawn from this study.
We found that almost one fourth of all CT scans were negative in patients who later were diagnosed with anastomotic leakage. The low sensitivity of these often-used diagnostic methods has been confirmed in other studies [28, 38, 39]. When the CT scan was positive for leakage, it took 8.5 days in mean before leakage was confirmed, compared to 4.3 days in patients who were diagnosed during a reoperation. This may be due to a more ill patient in the surgical group, but it may also illustrate that a negative CT scan seems to mislead the treating physician. This raises the question if we use CT scan too often in early leakages, maybe a second look in the operating theater would be preferable. Maybe postoperative surveillance scores and protocol for leakage diagnosis should be used more often such as routine measurement of C-reactive protein or procalcitonin [40–42]. In previous studies, a question has been raised if there are two different kinds of leakages, one early and another type that present itself later. This is somewhat confirmed in our cohort as 20% (12/60) of our patients had their leakage diagnosed after discharge and at a readmission (1 leakage in grade A, 2 in grade B, and 9 in grade C). This can of course also be influenced by the increased use of enhanced recovery programs discharging patients early [43].
Most patients with a leakage had many other postoperative complications and underwent surgery. More than three fourths (76.3%) of the patients had underwent surgery with the anastomosis taken down, is it possible to reduce this number? Sirois-Giguère et al. describe in an observational study on anastomotic leakage in rectal cancer surgery that 16 out of 37 patients (43%) were treated with transanal drainage with comparable results as the abdominal reintervention group [34]. In our leakage group, we had only 2/22 patients with rectal resections treated with transanal drainage, and perhaps it is possible to retain the anastomosis this way; however, the functional results must be evaluated [1]. In a nationwide study on colon cancer surgery, Krarup et al. describes that in grade C leakages, salvage of the anastomosis was possible in 14.6% (n = 74) with small defects or intraoperative findings similar to Hinchey I–II [36]. In our cohort, 9/51 (17.6%) of the patient with grade C leakages had salvage of the anastomosis, and this is somewhat higher than in the Krarup study. The fact that mortality was higher in patients with an anastomotic leakage is not new [44]. However, most patients that died due to anastomotic leakage had severe comorbidity and a malignant disease. All these confirm that anastomotic leakage has major effect on the patients’ life, morbidity, and mortality.
The unselected population with both malign and benign surgery is the strength of our study. We studied the charts in detail, and that is an advantage compared to a registry-based study where details to this extent are difficult to retrieve. However, a retrospective study has limitations in that the data is already existing; the patients’ medical records cannot be redesigned nor can missed information be recreated. However, one of the strengths with a retrospective study is that neither surgeons nor patients know that they are subjects of research. This study includes consecutive patients and is limited to one hospital, and the results are therefore representative for this specific geographic region.
Conclusion
This study demonstrated that one fourth of all the CT scans that were executed were initially negative for leakage, possibly delaying the diagnosis. Most patients with a grade C leakage will not have an intact anastomosis. An anastomotic leakage leads to significantly more severe postoperative complications, higher rate of reoperations, and higher mortality. An earlier relaparotomy instead of a CT scan and improved postoperative surveillance could possibly reduce the consequences of the anastomotic leakage.
Acknowledgements
The authors would like to express their gratitude to the staff at Scandinavian Surgical Outcomes Research Group (SSORG) in Gothenburg, Sweden. The study is grateful for the following financial support: The Swedish Cancer Society CAN 2013/500, Sahlgrenska University Hospital, agreement concerning research and education of doctors, ALFGBG-366481, ALFGBG-526501, ALFGBG-493341, the Magnus Bergvall’s Foundation, the Swedish Society of Medicine, and the Assar Gabrielsson Foundation.
Compliance with ethical standards
The Ethical Review Board in Gothenburg, Sweden, approved the research project EPN 647-14. This paper has not been submitted for publication elsewhere.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ashburn JH, Stocchi L, Kiran RP, et al. Consequences of anastomotic leak after restorative proctectomy for cancer: effect on long-term function and quality of life. Dis Colon rectum. 2013;56(3):275–280. doi: 10.1097/DCR.0b013e318277e8a5. [DOI] [PubMed] [Google Scholar]
- 2.Di Cristofaro L, Ruffolo C, Pinto E, et al. Complications after surgery for colorectal cancer affect quality of life and surgeon-patient relationship. Color Dis. 2014;16(12):O407–O419. doi: 10.1111/codi.12752. [DOI] [PubMed] [Google Scholar]
- 3.Thornton M, Joshi H, Vimalachandran C, et al. Management and outcome of colorectal anastomotic leaks. Int J Color Dis. 2011;26(3):313–320. doi: 10.1007/s00384-010-1094-3. [DOI] [PubMed] [Google Scholar]
- 4.Buchs NC, Gervaz P, Secic M, et al. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Color Dis. 2008;23(3):265–270. doi: 10.1007/s00384-007-0399-3. [DOI] [PubMed] [Google Scholar]
- 5.Mirnezami A, Mirnezami R, Chandrakumaran K, et al. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(5):890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 6.Law WL, Choi HK, Lee YM, et al. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11(1):8–15. doi: 10.1007/s11605-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 7.McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92(9):1150–1154. doi: 10.1002/bjs.5054. [DOI] [PubMed] [Google Scholar]
- 8.Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon rectum. 2005;48(5):1021–1026. doi: 10.1007/s10350-004-0869-4. [DOI] [PubMed] [Google Scholar]
- 9.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, et al. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259(5):930–938. doi: 10.1097/SLA.0b013e3182a6f2fc. [DOI] [PubMed] [Google Scholar]
- 10.Kube R, Mroczkowski P, Granowski D, et al. Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur J Surg Oncol. 2010;36(2):120–124. doi: 10.1016/j.ejso.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Nachiappan S, Askari A, Malietzis G, et al. The impact of anastomotic leak and its treatment on cancer recurrence and survival following elective colorectal cancer resection. World J Surg. 2015;39(4):1052–1058. doi: 10.1007/s00268-014-2887-2. [DOI] [PubMed] [Google Scholar]
- 12.Bakker IS, Grossmann I, Henneman D, et al. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg. 2014;101(4):424–432. doi: 10.1002/bjs.9395. [DOI] [PubMed] [Google Scholar]
- 13.Krarup PM, Jorgensen LN, Andreasen AH, et al. A nationwide study on anastomotic leakage after colonic cancer surgery. Color Dis. 2012;14(10):e661–e667. doi: 10.1111/j.1463-1318.2012.03079.x. [DOI] [PubMed] [Google Scholar]
- 14.Matthiessen P, Hallbook O, Rutegard J, et al. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246(2):207–214. doi: 10.1097/SLA.0b013e3180603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertelsen CA, Andreasen AH, Jorgensen T, et al. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Color Dis. 2010;12(1):37–43. doi: 10.1111/j.1463-1318.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen MT, Wibe A, Norstein J, et al. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Color Dis. 2005;7(1):51–57. doi: 10.1111/j.1463-1318.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 17.Alves A, Panis Y, Bouhnik Y, et al. Risk factors for intra-abdominal septic complications after a first ileocecal resection for Crohn’s disease: a multivariate analysis in 161 consecutive patients. Dis Colon rectum. 2007;50(3):331–336. doi: 10.1007/s10350-006-0782-0. [DOI] [PubMed] [Google Scholar]
- 18.Boccola MA, Buettner PG, Rozen WM, et al. Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single-institution analysis of 1576 patients. World J Surg. 2011;35(1):186–195. doi: 10.1007/s00268-010-0831-7. [DOI] [PubMed] [Google Scholar]
- 19.Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon rectum. 2006;49(11):1719–1725. doi: 10.1007/s10350-006-0703-2. [DOI] [PubMed] [Google Scholar]
- 20.Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208(2):269–278. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Pommergaard HC, Gessler B, Burcharth J, et al. (2014) Preoperative risk factors for anastomotic leakage after resection for colorectal cancer: a systematic review and meta-analysis. Colorectal Dis [DOI] [PubMed]
- 22.Matthiessen P, Hallbook O, Andersson M, et al. Risk factors for anastomotic leakage after anterior resection of the rectum. Color Dis. 2004;6(6):462–469. doi: 10.1111/j.1463-1318.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 23.Kruschewski M, Rieger H, Pohlen U, et al. Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer. Int J Color Dis. 2007;22(8):919–927. doi: 10.1007/s00384-006-0260-0. [DOI] [PubMed] [Google Scholar]
- 24.Konishi T, Watanabe T, Kishimoto J, et al. Risk factors for anastomotic leakage after surgery for colorectal cancer: results of prospective surveillance. J Am Coll Surg. 2006;202(3):439–444. doi: 10.1016/j.jamcollsurg.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Komen N, Dijk JW, Lalmahomed Z, et al. After-hours colorectal surgery: a risk factor for anastomotic leakage. Int J Color Dis. 2009;24(7):789–795. doi: 10.1007/s00384-009-0692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jestin P, Pahlman L, Gunnarsson U. Risk factors for anastomotic leakage after rectal cancer surgery: a case-control study. Color Dis. 2008;10(7):715–721. doi: 10.1111/j.1463-1318.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 27.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147(3):339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Hirst NA, Tiernan JP, Millner PA, et al. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Color Dis. 2014;16(2):95–109. doi: 10.1111/codi.12411. [DOI] [PubMed] [Google Scholar]
- 29.Floodeen H, Hallbook O, Rutegard J, et al. Early and late symptomatic anastomotic leakage following low anterior resection of the rectum for cancer: are they different entities? Color Dis. 2013;15(3):334–340. doi: 10.1111/j.1463-1318.2012.03195.x. [DOI] [PubMed] [Google Scholar]
- 30.Maeda H, Okamoto K, Namikawa T, et al. Rarity of late anastomotic leakage after low anterior resection of the rectum. Int J Color Dis. 2015;30(6):831–834. doi: 10.1007/s00384-015-2207-9. [DOI] [PubMed] [Google Scholar]
- 31.Hyman N, Manchester TL, Osler T, et al. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245(2):254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morks AN, Ploeg RJ, Sijbrand Hofker H, et al. Late anastomotic leakage in colorectal surgery: a significant problem. Color Dis. 2013;15(5):e271–e275. doi: 10.1111/codi.12167. [DOI] [PubMed] [Google Scholar]
- 33.Tan WP, Hong EY, Phillips B, et al. Anastomotic leaks after colorectal anastomosis occurring more than 30 days postoperatively: a single-institution evaluation. Am Surg. 2014;80(9):868–872. [PubMed] [Google Scholar]
- 34.Sirois-Giguere E, Boulanger-Gobeil C, Bouchard A, et al. Transanal drainage to treat anastomotic leaks after low anterior resection for rectal cancer: a valuable option. Dis Colon Rectum. 2013;56(5):586–592. doi: 10.1097/DCR.0b013e31827687a4. [DOI] [PubMed] [Google Scholar]
- 35.Blumetti J, Chaudhry V, Cintron JR, et al. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg. 2014;38(4):985–991. doi: 10.1007/s00268-013-2340-y. [DOI] [PubMed] [Google Scholar]
- 36.Krarup PM, Jorgensen LN, Harling H. Management of anastomotic leakage in a nationwide cohort of colonic cancer patients. J Am Coll Surg. 2014;218(5):940–949. doi: 10.1016/j.jamcollsurg.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 37.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kauv P, Benadjaoud S, Curis E, et al. (2015) Anastomotic leakage after colorectal surgery: diagnostic accuracy of CT. Eur Radiol [DOI] [PubMed]
- 39.Kornmann VN, van Ramshorst B, Smits AB, et al. Beware of false-negative CT scan for anastomotic leakage after colonic surgery. Int J Color Dis. 2014;29(4):445–451. doi: 10.1007/s00384-013-1815-5. [DOI] [PubMed] [Google Scholar]
- 40.den Dulk M, Witvliet MJ, Kortram K, et al. The DULK (Dutch leakage) and modified DULK score compared: actively seek the leak. Color Dis. 2013;15(9):e528–e533. doi: 10.1111/codi.12379. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Granero A, Frasson M, Flor-Lorente B, et al. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon rectum. 2013;56(4):475–483. doi: 10.1097/DCR.0b013e31826ce825. [DOI] [PubMed] [Google Scholar]
- 42.Singh PP, Zeng IS, Srinivasa S, et al. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101(4):339–346. doi: 10.1002/bjs.9354. [DOI] [PubMed] [Google Scholar]
- 43.Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg. 2013;37(2):285–305. doi: 10.1007/s00268-012-1787-6. [DOI] [PubMed] [Google Scholar]
- 44.Gessler B, Bock D, Pommergaard HC, et al. Risk factors for anastomotic dehiscence in colon cancer surgery-a population-based registry study. Int J Color Dis. 2016;31(4):895–902. doi: 10.1007/s00384-016-2532-7. [DOI] [PubMed] [Google Scholar]