Abstract
Introduction: Epoetin alfa (Eprex®) is a subcutaneous, injectable formulation of short half-life recombinant human erythropoietin (rHuEPO). To current knowledge there are no published studies regarding the stability of rHuEPO once repackaging occurs (r-EPO) for clinical trial purposes. Materials and methods: We assessed EPO concentration in Eprex® and r-EPO syringes at 0, 60, 90, and 120 days after repackaging in polypropylene syringes. R-EPO was administered to 56 patients taking part in a clinical trial in Friedreich Ataxia. Serum EPO levels were measured at baseline and 48 h after r-EPO administration. Results: No differences were found between r-EPO and Eprex® syringes, but both globally decreased in total EPO content during storage at 4 °C. Patients receiving r-EPO had similar levels in EPO content as expected from previous trials in Friedreich Ataxia and from pharmacokinetics studies in healthy volunteers. Discussion: We demonstrate that repackaging of EPO does not alter its concentration if compared to the original product (Eprex®). This is true both for repackaging procedures and for the stability in polypropylene tubes. The expiration date of r-EPO can be extended from 1 to 4 months after repackaging, in accordance with pharmacopeia rules.
Keywords: Erythropoietin, Repackaging, Polypropylene, EPO, Pharmacopeia
1. Introduction
Erythropoietin (EPO) is a 34 KDa glycoprotein, synthesized by the kidney that stimulates erythropoiesis acting on erythrocyte precursors in the bone marrow (Jelkmann, 1992). Recombinant Human Erythropoietin (rHuEPO) is used for treatment in anemic patients with chronic renal failure in whom the endogenous production of EPO is impaired. It is used in patients with chemotherapy-induced anemia (Galli et al., 2015), reduces the need for blood transfusions in patients that undergo surgery, and can also be used for patients at risk for perioperative transfusions with anticipated significant blood loss (McGirr et al., 2014).
Epoetin alfa (Eprex®) is a subcutaneous, injectable formulation and short half-life recombinant human erythropoietin. It is produced by recombinant DNA technology in Chinese Hamster Ovarian cells.
There are no published studies regarding the stability of EPO, once repackaging occurs. Even the stability of the protein in polypropylene tubes has never been tested.
In our study, Eprex® was aseptically repackaged in 1 mL polypropylene syringes and stored at 4 °C for up to 120 days, and quantitative tests were performed. Repackaged EPO (r-EPO) was administered to patients taking part to a clinical trial, and serum levels were measured in order to confirm the clinical utility of repackaging procedures.
2. Materials and methods
2.1. Preparation of syringes
Commercially available Eprex® 40,000 IU/1 mL was purchased from Janssen-Cilag (Milano, Italy), a subsidiary of Johnson & Johnson (New Brunswick, NJ, USA). Each Eprex® batch used for tests had at least 18 months of shelf life before expiration date. Eprex® was delivered to “Farmacia Ettore Florio snc”, an ISO 9001:2008 pharmacy, at controlled temperature.
We performed drug-repackaging procedures following the Italian pharmacopeia rules. For each preparation, Eprex® syringes were manually emptied in a sterile glass container under a laminar flow hood. Eprex® was aspirated in 1 mL syringes (BD Micro-fine, 29G × 12.7 mm, Becton Dickinson) for a total volume of 1 mL (40,000 IU of EPO) or 0.75 mL (30,000 IU of EPO), based on clinician’s request. Environment air was checked during repackaging procedure using a portable particle counter (Solair 3100, Lighthouse, Fremont, CA, USA).
The whole process was performed slowly in order to avoid the introduction of air bubbles during the transfer phase. The syringes were then sealed in vacuum sterile bags in order to maintain sterility. Ten percent of each production, with a minimum of four syringes, was kept aside for microbiological sterility testing, as prescribed by the pharmacopeia sterility assay 2.6.1. This was performed at the Experimental Medicine Department of the Second University of Naples, Italy.
2.2. Stability assay
For stability evaluation, eight 1 mL r-EPO syringes, from the same batch preparation, were stored at 4 °C for future assays for each time-point. In parallel, the same amount of syringes of Eprex® was retained at the moment of repackaging for comparison. Both Eprex® and r-EPO syringes were stored at 4 °C up to 120 days.
The stability of r-EPO and EPREX® was assessed by enzyme-linked immunosorbent assay (ELISA) to detect human EPO (Quantikine IVD Erythropoietin ELISA, DEP00; R&D systems, Minneapolis USA) was used to assess the EPO content, following manufacturer’s instructions.
The content of the syringes was expelled into 2-mL Eppendorf tubes. Two serial dilutions of 1:100 and a final dilution of 1:40 were performed in order to obtain a final dilution of 1:400000. This matched the assay range of 2, 5–200 mIU/mL, with an expected final concentration of 100 mIU/mL. Results from ELISA measurements were multiplied by the dilution factor for final results analysis.
2.3. Clinical trial design
The trial was approved form the local Ethics Committee (256/11), registered at www.clinicaltrials.gov (NCT01493973), and EUDRACT (2011-006156-37). The trial was performed in accordance with the Declaration of Helsinki, European guidelines CPMP/ICH/135/95, and Italian law D.M.15/07/1997. All patients gave written informed consent before any activity was linked to the clinical trial.
r-EPO was administered to patients taking part in a randomized, placebo-controlled trial, to assess the effect of EPO on exercise capacity in Friedreich Ataxia (FRDA) patients (Saccà et al., 2016). The trial is part of an extensive effort in identifying drugs able to ameliorate the biochemical deficits of FRDA (Boesch et al., 2007, Boesch et al., 2008, Boesch et al., 2014, Saccà et al., 2011). Briefly, 56 patients were randomized to receive r-EPO (1200 IU/kg body weight subcutaneously) or equivalent dose of placebo in a 1:1 ratio. Serum samples were obtained immediately before the administration of r-EPO and 48 h later. Samples were frozen at −80 °C until analysis. Serum concentrations of EPO were measured using the same ELISA kit as specified. Further details on the trial can be obtained at www.clinicaltrials.gov (NCT01493973).
2.4. Statistical analysis
For stability assays, data were analyzed using a General Linear Model for repeated measures (GLM-RM). Within-subjects factor was time, and four time-points were set. These included baseline, 60, 90, and 120 days. Production method (Eprex® or r-EPO) was set as the between-subjects factor. Simple repeated measure contrast was used with baseline as the reference category. We calculated estimated marginal means for the production and time interaction. Bonferroni correction was applied for all repeated analysis. P values less than 0.05 were considered significant. For serum EPO concentration, we used a GLM-RM to analyze the effect of time and r-EPO administration in the same way as the stability assay. In addition, the effect of repackaging-to-administration time was set as a covariate in order to test its effect on serum EPO levels. Statistical analysis was performed using SPSS version 22.0.0.1 running on MAC OSX 10.10.4.
3. Results
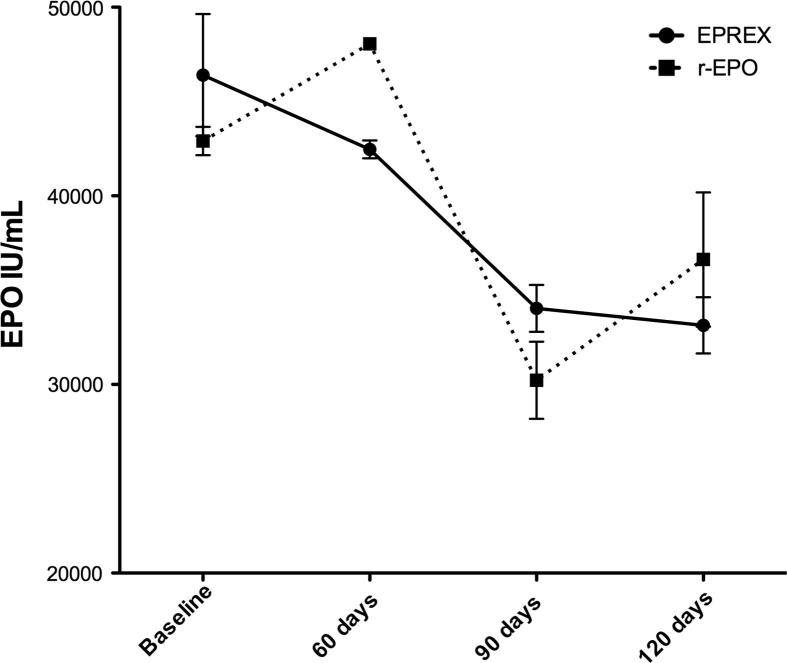
During storage at 4 °C both Eprex® and r-EPO globally decreased in total EPO content. Global mean ± SD for both Eprex® and r-EPO was 44355 ± 4734 IU/mL at baseline, 45262 ± 3121 at 60 days, 32129 ± 3737 at 90 days, and 34296 ± 3696 at 120 days (overall significance p < 0.01). The significance was driven by the contrast between baseline and the 90 days time-point (Table 1 and Fig. 1; p = 0.007).
Table 1.
Eprex® and r-EPO values are indicated as global mean ± SD; time and time ∗ production significance is shown as baseline versus other time-points; global significance of time is p < 0.01, and time ∗ production is p = 0.592.
| Eprex® (IU/mL) | r-EPO (IU/mL) | Time (p) | Time ∗ production (p) | |
|---|---|---|---|---|
| Baseline | 46399.7 ± 6474 | 42910.3 ± 1501 | ||
| 60 days | 42458.5 ± 821 | 48064.6 ± 327 | 0.736 | 0.343 |
| 90 days | 34033.5 ± 2488 | 30223.5 ± 4088 | 0.007 | 0.933 |
| 120 days | 33130.7 ± 2984 | 36625.5 ± 5031 | 0.092 | 0.717 |
Significant values are shown in bold characters (p < 0.05).
Figure 1.
r-EPO = repackaged Erythropoietin.
Interaction between time and production method was overall non-significant (p = 0.592), as well as the single contrasts between baseline and the different time-points (Table 1, Fig. 1).
During the clinical trial, baseline serum EPO was 15.1 ± 2 mIU/mL in the EPO group and 12.9 ± 1 in the placebo group (p = 0.398). Forty-eight hours after drug administration, serum EPO was 1489.5 ± 100 mIU/mL in r-EPO group, and 12.6 ± 1 in the placebo group (p < 0.001 for the interaction of time and treatment).
Repackaging-to-administration time (RtA) was calculated for all patients receiving r-EPO. This was 49.2 ± 23.5 days (range 1–104). We then used RtA as a covariate in the serum EPO GLM-RM. This did not produce significant results, indicating that RtA did not influence serum EPO levels.
4. Discussion
When we started our trial, no stability tests were available for repackaged EPO. As for pharmacopeia rules, the expiration date was set at 30 days after repackaging procedures (AAVV, 2008). Unfortunately, microbiological tests take up to 14 days, thus reducing the window of use of every preparation to 14 days.
We demonstrate that repackaging of EPO does not alter its concentration if compared to the original product (Eprex®). This is true both for repackaging procedures and for the stability in polypropylene tubes. We considered a time frame up to 120 days; therefore, longer-term stability cannot be guaranteed. Nevertheless, considering that the normal shelf life of original and generic EPO is 24 months, and that preparation and microbiological tests may take up to 2 weeks, the remaining three-and-a-half months are an acceptable window of use for any clinical activity.
Similar results were recently reported for different injectable drugs. Stability studies have been performed after repackaging and storage of bevacizumab in polypropylene syringes (Paul et al., 2012a). Bevacizumab was stable in its primary, secondary and tertiary structure for up to 90 days. Similarly Rituximab and Trastuzumab were found to be stable up to 180 days after repackaging in polyolefin infusion bags (Paul et al., 2012b, Paul et al., 2013).
We found higher EPO content than declared at baseline. This is not surprising as it is similar to previous reported data on Eprex® (Park et al., 2009, Brinks et al., 2011). Unfortunately, after 4 months of 4 °C storage, EPO concentration was −24% than baseline in the Eprex® vials, and −17% in the r-EPO vials. This is unexpected, and may not be attributed to inadequate storage or transportation, as temperature logging was regularly performed. End of shelf-life use can also be out ruled as it was at least 18 months before expiration for all batches of Eprex®. To our knowledge no studies have yet addressed this point, and future real-life studies may be more informative.
Administration of r-EPO to patients increased serum EPO concentration to a mean value of 1.5 IU/mL at 48 h after administration of 1200 IU/kg subcutaneously. This is a similar value to that previously reported in pharmacokinetics studies (Ramakrishnan et al., 2004, Krzyzanski et al., 2005), and to a previously reported trial with Eprex® in FRDA patients (Saccà et al., 2011).
5. Conclusions
Repackaging of EPO in polypropylene syringes is possible and does not alter the stability of the protein. This is confirmed after in vivo injection during a clinical trial. The expiration date of r-EPO can be extended up to 4 months after repackaging, if allowed by the original expiration date.
Acknowledgments
This study was supported from the Keith Michael Andrus Award form the “Friedreich’ Ataxia Research Alliance (FARA)” to FS and AF, form a grant from the “Associazione Italiana per la lotta alle Sindromi Atassiche (AISA)” to FS, and an unrestricted grant from AISA sez. Campania to FS. We are grateful to all patients and their families for taking part in the trial.
Footnotes
Peer review under responsibility of King Saud University.
References
- AAVV, 2008. Preparazioni parenterali. In: Farmacopea ufficiale della Repubblica Italiana. Istituto Poligrafico e Zecca dello Stato 2008, section 0520. ISBN 8824028845 ISBN-13 9788824028844.
- Boesch S., Sturm B., Hering S., Goldenberg H., Poewe W. Friedreich’s ataxia: clinical pilot trial with recombinant human erythropoietin. Ann. Neurol. 2007;62(5):521–524. doi: 10.1002/ana.21177. Nov, PubMed PMID: 17702040. [DOI] [PubMed] [Google Scholar]
- Boesch S., Sturm B., Hering S., Scheiber-Mojdehkar B., Steinkellner H. Neurological effects of recombinant human erythropoietin in Friedreich’s ataxia: a clinical pilot trial. Move. Disord. 2008;15(23):1940–1944. doi: 10.1002/mds.22294. Oct. [DOI] [PubMed] [Google Scholar]
- Boesch S., Nachbauer W., Mariotti C., Sacca F., Filla A., Klockgether T., Klopstock T., Schöls L., Jacobi H., Büchner B., vom Hagen J.M., Nanetti L., Manicom K. Safety and tolerability of carbamylated erythropoietin in Friedreich’s ataxia. Move. Disord. 2014;29:935–939. doi: 10.1002/mds.25836. [DOI] [PubMed] [Google Scholar]
- Brinks V., Hawe A., Basmeleh A.H., Joachin-Rodriguez L., Haselberg R., Somsen G.W., Jiskoot W., Schellekens H. Quality of original and biosimilar epoetin products. Pharm. Res. 2011;28:386–393. doi: 10.1007/s11095-010-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli L., Ricci C., Egan C.G. Epoetin beta for the treatment of chemotherapy-induced anemia: an update. OncoTargets Ther. 2015;8:583–591. doi: 10.2147/OTT.S77497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol. Rev. 1992;72:434–449. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Krzyzanski W., Jusko W.J., Wacholtz M.C., Minton N., Cheung W.K. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoieitn after multiple subcutaneous doses in healthy subjects. Eur. J. Pharm. Sci. 2005;26:295–306. doi: 10.1016/j.ejps.2005.06.010. [DOI] [PubMed] [Google Scholar]
- McGirr A., Pavenski K., Sharma B., Cusimano M.D. Blood conservation in neurosurgery: erythropoietin and autologous donation. Can. J. Neurol. Sci. 2014;41:583–589. doi: 10.1017/cjn.2014.14. [DOI] [PubMed] [Google Scholar]
- Park S.S., Park J., Ko J., Chen L., Meriage D., Crouse-Zeineddini J., Wong W., Kerwin B.A. J. Pharm. Sci. 2009;98(5):1688–1699. doi: 10.1002/jps.21546. Biochemical assessment of erythropoietin products from Asia versus US Epoetin alfa manufactured by Amgen, May. [DOI] [PubMed] [Google Scholar]
- Paul M., Viellard V., Roumi E., Cauvin A., Despiau M.C., Laurent M., Astier A. Long-term stability of Bavacizumab repackaged in 1 mL polypropylene syringes for intravitreal administraiton. Ann. Pharm. Fran. 2012;70:139–154. doi: 10.1016/j.pharma.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Paul M., Vieillard V., Jaccoulet E., Astier A. Long-term stability of diluted solutions of the monoclonal antibody rituximab. Int. J. Pharm. 2012;436:282–290. doi: 10.1016/j.ijpharm.2012.06.063. [DOI] [PubMed] [Google Scholar]
- Paul M., Vieillard V., Da Silva Lemos R., Escalup L., Astier A. Long-term physico-chemical stability of diluted trastuzumab. Int. J. Pharm. 2013;448:101–104. doi: 10.1016/j.ijpharm.2013.02.039. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan R., Cheung W.K., Wacholtz M.C., Minton N., Jusko W.J. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J. Clin. Pharmacol. 2004;44:991–1002. doi: 10.1177/0091270004268411. [DOI] [PubMed] [Google Scholar]
- Saccà F., Piro R., De Michele G., Acquaviva F., Antenora A. Epoetin alfa increases frataxin production in Friedreich’s ataxia without affecting hematocrit. Move. Disord. 2011;26(4):739–742. doi: 10.1002/mds.23435. Mar. [DOI] [PubMed] [Google Scholar]
- Saccà F., Puorro G., Marsili A., Antenora A., Pane C. Long-term effect of epoetin alfa on clinical and biochemical markers in friedreich ataxia. Move. Disord. 2016 doi: 10.1002/mds.26552. EPub 16 Feb 2016. [DOI] [PubMed] [Google Scholar]