Abstract
The aim of this study was the assessment of the physicochemical stability of d-α-tocopherol formulated in medium chain triglyceride nanoemulsions, stabilized with Tween®80 and Lipoid®S75 as surfactant and co-surfactant, respectively. d-α-tocopherol was selected as active ingredient because of its well-recognized interesting anti-oxidant properties (such as radical scavenger) for food and pharmaceutical industries. A series of nanoemulsions of mean droplet size below 90 nm (polydispersity index < 0.15) have been produced by high-pressure homogenization, and their surface electrical charge (zeta potential), pH, surface tension, osmolarity, and rheological behavior, were characterized as a function of the d-α-tocopherol loading. In vitro studies in Caco-2 cell lines confirmed the safety profile of the developed nanoemulsions with percentage of cell viability above 90% for all formulations.
Keywords: d-α-tocopherol, Nanoemulsions, High-pressure homogenization, Rheology, Osmolarity, Surface tension, Caco-2-cells, Cytotoxicity
1. Introduction
The increase of reactive oxygen species (ROS, known as free radicals) levels in living organisms, as a result of metabolism, environmental exposure and aging, can ultimately contribute to cell death induced by several harmful events on cell structures, such as DNA, RNA, proteins and lipids (Hosain et al., 2016, Nakazawa et al., 2016). The use of anti-oxidants to protect cells from excess of ROS and to delay the cells aging and death is, therefore, a regular practice.
Tocopherols are a family of natural and synthetic compounds, being d-α-tocopherol or vitamin E the most popular member, and are preferentially absorbed and accumulated in humans (Brigelius-Flohé and Traber, 1999). These molecules contain two main structural elements, the chromanol head (benzodihydropyran containing an alcohol group), and the phytyl tail consisting of repeats of saturated isoprenoid units. d-α-tocopherol has antioxidant function, by scavenging peroxyl radicals, and is able to protect the lipids, present in the fat phase of foodstuff, as well as those in membrane of living cells, from auto-oxidation (Atkinson and Traber, 2007). However, like other lipophilic compounds, d-α-tocopherol is poorly soluble in water and is highly sensitive to various environmental factors, such as light, oxygen, alkali and temperature (Zigoneanu et al., 2008).
In order to improve its biological stability during manufacturing and storage, d-α-tocopherol has been incorporated in nanocarriers. Several studies report the use of different types of nanocarriers to promote the antioxidant activity in foodstuff, as well as to preserve its nutritional value. Examples are liposomes (Khanniri et al., 2016), solid lipid nanoparticles and nanostructured lipid carriers (Hentschel et al., 2008, Souto et al., 2005), and micro/nanoemulsions (Kumar et al., 2016, Zheng et al., 2016).
The advantages of loading d-α-tocopherol in o/w nanoemulsions (NEs) mainly rely on the improvement of the chemical stability of the vitamin (avoiding auto-oxidation phenomenon), with potential to enhance its oral bioavailability and anti-oxidant properties when formulated in foodstuff and in pharmaceutical products. Being highly lipophilic, d-α-tocopherol is an excellent candidate to be loaded into NEs. These nanocarriers are usually generated within a size range in the nanometric scale, typically 20–300 nm, being transparent and kinetically stable (Clares et al., 2014, Souto et al., 2011). Transparency of the NEs is attributed to their very small size and narrow polydispersity. NEs have the advantage of being composed of physiological and biodegradable lipids, therefore, minimizing the toxicological events. Previous works have shown that the incorporation of d-α-tocopherol into NEs has not only promoted the protection of d-α-tocopherol against degradation, but also enhanced its bioavailability (Alayoubi et al., 2014). The small size of the emulsified nanodroplets offers them inherent stability against aggregation, flocculation, creaming, and sedimentation.
The purpose of this work was to assess the physical stability of d-α-tocopherol formulated in NEs composed of medium chain triglycerides, stabilized with soya lecithin in the inner oil phase and with polysorbate 80 in the outer aqueous phase. The mean droplet size, zeta potential, surface tension, osmolarity and rheological behavior, have been monitored in d-α-tocopherol-free and d-α-tocopherol-loaded NEs. Cell viability studies have also been carried out to assess the cytotoxic profile of the developed NEs in Caco-2 cell lines, before and after d-α-tocopherol loading.
2. Materials and methods
2.1. Materials
Miglyol 812 N (medium chain triglycerides) was supplied from Sasol GmbH (Hamburg, Germany), and soya lecithin hydrogenated (Lipoid®S75) was obtained from Lipoid GmbH (Ludwigshafen am Rhein, Germany). Polysorbate 80 (Tween®80), penicillin, streptomycin, l-glutamine, Hank’s balanced salt solution (HBSS), and Alamar blue® were obtained from Invitrogen (Alfagene Portugal), and d-α-tocopherol was obtained from Sigma-Aldrich (St. Louis, MO, USA). Ultra-purified water was obtained from Milli® Q Plus system, home supplied.
2.2. Methods
2.2.1. Production of nanoemulsions
For the production of the inner oil phase, a mixture of d-α-tocopherol, Miglyol 812 and soybean lecithin was firstly prepared by stirring it for 5 min at 30 °C until a homogeneous solution has been obtained. The oil phase was dispersed in a water surfactant solution composed of Polysorbate 80 (Tween®80) 1.5% (m/V) under high shear homogenization (Ultra-Turrax®, T25, IKA, Germany) for 1 min to obtain a coarse emulsion. This emulsion was then processed in the high pressure homogenizer (EmulsiFlex®-C3, Avestin), in the continuous mode at 1000 bar and at room temperature.
2.2.2. Mean droplet size, pH and surface electrical charge
The mean droplet size was determined by photon correlation spectroscopy (PCS) with a Zetasizer Nano ZS (Malvern Instruments Ltd., UK). PCS yields the mean droplet size (z-Ave) and the polydispersity index (PI) as a measure of the width of the size distribution. For the determination of the surface electrical charge, the zeta potential (ZP) was measured in the samples previously diluted with double-distilled water adjusted to a conductivity of 50 μS/cm with a solution of 0.9% (m/V) NaCl. The pH was measured using a microprocessor-based pH and temperature bench meters (Hanna Instruments, Romania) with the glass electrode HI 1330. The pH of the nanoemulsions was in the range of 5.5–6.0 and the field strength was 20 V/cm, and the ZP analyzed by laser Doppler anemometry using a Zetasizer Nano ZS (Malvern Instruments Ltd., UK).
2.2.3. Rheological measurements
The rheological analysis of the developed nanoemulsions was performed using the rheometer Rheo Stress RS 100 (Haake Instruments Karlsruhe, Germany) equipped with a cone-and-plate test geometry (plate diameter 20 mm, cone angle 4°). A volume of 2.5 mL of each nanoemulsion was tested at a controlled temperature of 22 ± 0.1 °C under shear rate control conditions within the range 1–50 s−1. Rheograms of apparent viscosity were recorded against shear rate, and the data were fitted to Power Law (or Ostwald) model, according to the following equation: , where stands for the shear stress (Pa), for the shear rate (s−1) and K is the consistency index parameter that gives an idea of the viscosity of the fluid. The flow behavior index is represented by n (n < 1, Pseudoplastic behavior; n = 1, Newtonian behavior; n > 1, Dilatant behavior).
2.2.4. Surface tension and osmolarity
To determine the surface tension, a volume of 20 ml of sample was placed in a thermostatically controlled glass at 22 ± 0.1 °C, and the measurements were done in a Krüss Interfacial Tensiometer K10 PST (Krüss, Germany) equipped with a platinum ring. A Semi-Micro Osmometer K-7400 (Knauer, Berlin, Germany) was used to determine the osmolarity by placing a volume of 150 μL of sample in a glass capillary. For both analyses, measurements were carried out in triplicate.
2.2.5. Cell viability studies
Caco-2 cells, a human colorectal adenocarcinoma cell line, obtained from Cell Line Services, AG (Germany), were used as cell model to perform the cytotoxicity assay. Caco-2 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (V/V) fetal bovine serum (FBS), antibiotics (100 U/mL of penicillin and 100 μg/mL of streptomycin) and 1 mM l-glutamine in a controlled humidity atmosphere of 5% CO2/95% air, at 37 °C, as previously described (Andreani et al., 2014, Severino et al., 2014). The cytotoxicity of NEs was evaluated applying the Alamar blue (AB) reduction method, for the comparison of the proliferation rate and viability of non-exposed Caco-2 cells (control, 0 μg/mL) with exposed Caco-2 cells, to appropriate NEs’ concentrations during pre-determined time intervals. Briefly, cells were detached from the culture flaks with trypsin, counted and seeded into 96-well microplates at a density of 5 × 104 cells/mL (100 μL/well). NEs, diluted in FBS-free culture media to various concentrations, from 25 to 400 μg/mL (25, 50, 100, 200, 400 μg/mL), were added to the cells, 24 h after seeding and after removal of culture medium. Microplates were placed in the incubator, and cells were exposed for 48 h. After exposure, the media containing the NEs (and the control) was removed and replaced by FBS-free medium supplemented with 10% (V/V) of AB. The absorbance readings occurred about 4 h after AB addition, at 570 and 620 nm using a Multiskan EX microplate reader (MTX Labsystems, USA). The percentage of AB reduction was calculated as previously described by us (Andreani et al., 2014). For the statistical analysis of data, a one-way analysis of variance (ANOVA) test was applied. Results correspond to the mean of three independent experiments (n = 3) ± SD. Cell viability results were normalized to the control (untreated cells) and are expressed as % of control. A p-value < 0.05 was considered statistically significant.
3. Results and discussion
The first step of this work was the production and characterization of d-α-tocopherol-free and d-α-tocopherol-loaded nanoemulsions (using different combinations with medium chain triglycerides). The composition of the developed NEs is shown in Table 1.
Table 1.
Composition of the developed nanoemulsions (%, m/V).
| Nanoemulsion | d-α-tocopherol (%) | Miglyol®812 (%) | Lipoid®S75 (%) | Tween®80 (%) | Water ad. (%) |
|---|---|---|---|---|---|
| NE | – | 5 | 0.5 | 1.5 | 100 |
| NEαT1 | 1 | 4 | 0.5 | 1.5 | 100 |
| NEαT2 | 2 | 3 | 0.5 | 1.5 | 100 |
| NEαT3 | 3 | 2 | 0.5 | 1.5 | 100 |
| NEαT4 | 4 | 1 | 0.5 | 1.5 | 100 |
| NEαT5 | 5 | – | 0.5 | 1.5 | 100 |
NE, nanoemulsion; αT, d-α-tocopherol; 1-5, percentage of d-α-tocopherol; ad., adjusted to.
Preliminary screening using different proportions of d-α-tocopherol and Miglyol®812 has been carried out using a suitable combination of Tween®80 (1.5% w/V) and Lipoid®S75 (0.5% w/V) as surfactant and co-surfactant, respectively, to optimize the nanoemulsion composition with respect to its physical stability. Tween®80 is a non-ionic surfactant commonly used in food and pharmaceutical industries. Lipoid®S75 is a soybean lecithin with 70% phosphatidylcholine, selected for its compatibility with the inner oil phase (i.e. d-α-tocopherol and/or Miglyol®812). Nanoemulsions were characterized by determining the mean diameter of the oil droplets (Z-Ave with the corresponding polydispersity index, PI), the surface electrical charge (ZP, zeta potential, in mV), pH, surface tension (σ, in mN/m), and osmolarity (Osm/L). The physicochemical properties of the developed NE formulations are summarized in Table 2.
Table 2.
Physicochemical properties of the freshly prepared nanoemulsions.
| Nanoemulsion | Z-Ave (nm) | PI | ZP (mV) | pH | Σ (mN/m) | Oms/L |
|---|---|---|---|---|---|---|
| NE | 65.2 ± 2.1 | 0.04 ± 0.00 | −17.68 ± 0.02 | 5.1 ± 0.2 | 24.3 ± 1.8 | 0.362 ± 0.053 |
| NEαT1 | 69.9 ± 0.5 | 0.11 ± 0.03 | −18.03 ± 0.11 | 5.2 ± 0.5 | 22.1 ± 2.8 | 0.356 ± 0.006 |
| NEαT2 | 73.8 ± 1.3 | 0.10 ± 0.03 | −16.09 ± 0.04 | 5.3 ± 0.1 | 22.0 ± 3.1 | 0.298 ± 0.017 |
| NEαT3 | 72.6 ± 0.6 | 0.09 ± 0.01 | −16.11 ± 0.10 | 5.5 ± 0.3 | 22.3 ± 1.5 | 0.239 ± 0.013 |
| NEαT4 | 89.1 ± 1.2 | 0.05 ± 0.02 | −15.04 ± 0.13 | 6.1 ± 0.2 | 20.8 ± 1.9 | 0.219 ± 0.022 |
| NEαT5 | 89.5 ± 0.7 | 0.08 ± 0.04 | −12.01 ± 0.13 | 6.4 ± 0.0 | 20.1 ± 1.4 | 0.264 ± 0.041 |
The mean droplet size diameter was recorded at values below 90 nm (PI < 0.15) for all nanoemulsions, a result that contributed to the transparency of the formulations and for their kinetic stability. An increase of the mean size was recorded when increasing the load of d-α-tocopherol. A similar trend was recorded for the ZP, surface tension and osmolarity values. All nanoemulsions showed pH between 4.9 and 6.4. The increase of the pH values with the increased loading of d-α-tocopherol has been attributed to the anti-oxidant properties of the vitamin E, which limits the formation of free fatty acids commonly observed in phospholipids-composed formulations. In aqueous dispersions, phospholipids may undergo hydrolysis/lipid peroxidation generating free fatty acids. In addition, NEs are unbuffered; therefore, if fatty acid molecules are generated within the dispersion, the pH values will decrease. The presence of the anti-oxidant d-α-tocopherol lowers the risk of lipid peroxidation, and contributes to the enhanced physicochemical stability of the nanoemulsions. These results are in agreement with the values recorded for the ZP, which translate the decreased concentration of ionic species.
While the decreased tendency was observed for the ZP values with the increased loading of d-α-tocopherol, the relatively low ZP values obtained were attributed to the presence of the hydrophilic chains of polysorbate surrounding the oil droplets. The slightly negative ZP values were due to the charge of lecithin present at the droplets interface. The higher concentrated d-α-tocopherol NE kept the nanosize range, and slightly decreased the absolute ZP values, and results that are in agreement with those observed for the pH. Similarly, the low values of surface tension of the particles were attributed to the surfactant composition, which lowers the oil/water surface tension. Formulations with lower surface tension values offer, in general, good spreading properties. Table 1 shows the decrease of the surface tension with the increase of the concentration of d-α-tocopherol in NE from 24.3 ± 1.8 mN/m down to 20.1 ± 1.4 mN/m, followed by the decrease of the osmolarity (0.264 ± 0.041 Oms/L for NEαT5).
The most frequent factor contributing to the instability of nanoemulsions is known to be the risk of Ostwald ripening, which can be determined applying the Lifshitz-Slyozov and Wagner (LSW) equation as follows (Lifshitz and Slezov, 1961, Wagner, 1961):
where ω stands for the rate of change of the cube of the number average radius, D for the diffusion coefficient of the dispersed oil phase in the aqueous phase, γ for the interfacial tension between the inner oil and outer aqueous phases. The c∞ is the bulk solubility of the oil in the aqueous phase, ρ is the oil density, and k is the LSW theoretical constant. This equation describes the reaction-controlled coarsening phenomena of spherical particles in suspension as a function of their surface-area density, and is known as the classical theory of particle coarsening. This differential equation describes coarsening of particles in a solution, assuming a low volume fraction of particles. Interesting behavior was also recorded by the rheological studies of the nanoemulsions (Table 3). The maximum viscosity values were obtained within the range of 3.49 and 6.46 mPa s, with the consistency index increasing from 0.0022 to 0.0031 Pa sn. Although low viscous dispersions were obtained, the n values were recorded below 1, which demonstrate the pseudoplastic behavior of the nanoemulsions. As expected, the increased the d-α-tocopherol concentration the increased the nanoemulsions viscosity. While both oils have similar density at 20 °C (0.95 g/cm3), as bulk materials d-α-tocopherol shows a higher viscosity (dynamic, 4200 mPa s) than Miglyol®812 (dynamic, 30 mPa s) at 20 °C (material safety data sheet from supplier). The lowest surface tension recorded for NEαT5 did not compromise its pseudoplastic behavior, i.e. the formulations kept their spreading properties for topical administration.
Table 3.
Rheological properties of the developed nanoemulsions.
| Nanoemulsion | Maximum viscosity (mPa s) | Flow behavior index (n) | Consistency index K (Pa sn) |
|---|---|---|---|
| NE | 3.49 | 0.9751 | 0.0022 |
| NEαT1 | 4.07 | 0.9699 | 0.0025 |
| NEαT2 | 4.82 | 0.9613 | 0.0027 |
| NEαT3 | 5.12 | 0.9511 | 0.0028 |
| NEαT4 | 5.64 | 0.9427 | 0.0029 |
| NEαT5 | 6.46 | 0.9258 | 0.0031 |
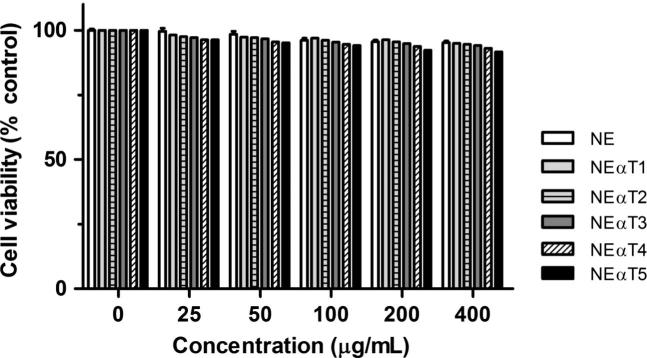
The toxicity of the different nanoemulsions was studied using the Alamar Blue reduction assay in Caco-2 cell line. The results obtained for the cell viability were compared to those of non-exposed cells (control) and are given in terms of percentage of control (Fig. 1).
Figure 1.
Viability of Caco-2 cells after 48 h of exposure to different concentrations of nanoemulsions (25, 50, 100, 200 and 400 μg/mL). Cell viability is expressed as the percentage of control (non-exposed cells), as the average (±SD) of 3 different experiments.
From the obtained results, the developed nanoemulsions revealed no toxicity (Fig. 1), comparing to non-exposed cells (0 μg/mL). As several reports state that cell viability is above 70% of the control, our results translate the presence of a safe material (Doktorovova et al., 2016, Doktorovova et al., 2014). In comparison with cells exposed to empty nanoemulsions (NE, lighter gray bars), no statistical significant differences were observed after 48 h of exposure to the 5 different concentrations of all tested formulations (p > 0.05). All nanoemulsions depicted a slightly negative surface charge, and it is known that negatively charged particles exhibit no (or very little) toxicity on biological membranes, in comparison with positively charged particles (Doktorovova et al., 2014, Souto et al., 2011). It is interesting also to realize that although cell viability was on average above 90%, increasing the d-α-tocopherol concentration in the NE decreased the cell viability in about 8.5%, in comparison with the control (non-exposed cells). While the presence of the anti-oxidant d-α-tocopherol lowers the risk of lipid peroxidation (and increases pH, Table 2), contributing to the enhanced physicochemical stability of the nanoemulsions, these results were attributed to both the increased viscosity and the increased particle size with the increased loading of d-α-tocopherol.
4. Conclusions
Vitamin E (d-α-tocopherol) has been successfully loaded in NE, either alone or in combination with medium chain triglycerides. Small-sized nanoemulsions (<90 nm) and narrow size range (<0.15) have been produced by high pressure homogenization. The nanoemulsions showed pseudoplastic behavior, with osmolarity compatible with biological fluids. The cell viability was on average above 90%, which anticipates the biocompatibility of these systems to be used as drug delivery systems for in vivo administration.
Acknowledgements
The authors would like to thank the financial support received from Portuguese Science and Technology Foundation (FCT) and from European Funds (PRODER/COMPETE) under the projects UID/AGR/04033/2013 and POCI-01-0145-FEDER-006958. This work was also financed through the projects UID/QUI/50006/2013 and M-ERA-NET/0004/2015-PAIRED, receiving financial support from FCT/MEC through national funds, and co-financed by FEDER, under the Partnership Agreement PT2020.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alayoubi A., Ayoub N.M., Malaviya A., Sylvester P.W., Nazzal S. Entrapment into nanoemulsions potentiates the anticancer activity of tocotrienols against the highly malignant (+SA) mouse mammary epithelial cells. J. Nanosci. Nanotechnol. 2014;14(5):4002–4005. doi: 10.1166/jnn.2014.8843. [DOI] [PubMed] [Google Scholar]
- Andreani T., Kiill C.P., de Souza A.L., Fangueiro J.F., Fernandes L., Doktorovova S., Santos D.L., Garcia M.L., Gremiao M.P., Souto E.B., Silva A.M. Surface engineering of silica nanoparticles for oral insulin delivery: characterization and cell toxicity studies. Colloids Surf. B Biointerf. 2014;123:916–923. doi: 10.1016/j.colsurfb.2014.10.047. [DOI] [PubMed] [Google Scholar]
- Atkinson J., Traber M.G. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43(1):4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohé R., Traber M.G. Vitamin E: function and metabolism. FASEB J. 1999;13(10):1145–1155. [PubMed] [Google Scholar]
- Clares B., Calpena A.C., Parra A., Abrego G., Alvarado H., Fangueiro J.F., Souto E.B. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: effect on skin permeation. Int. J. Pharm. 2014;473(1–2):591–598. doi: 10.1016/j.ijpharm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Doktorovova S., Kovačević A.B., Garcia M.L., Souto E.B. Pre-clinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016 doi: 10.1016/j.ejpb.2016.08.001. (in press) [DOI] [PubMed] [Google Scholar]
- Doktorovova S., Souto E.B., Silva A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers – a systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014;87(1):1–18. doi: 10.1016/j.ejpb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Hentschel A., Gramdorf S., Muller R.H., Kurz T. Beta-carotene-loaded nanostructured lipid carriers. J. Food Sci. 2008;73(2):N1–N6. doi: 10.1111/j.1750-3841.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- Hosain M.Z., Mori T., Kishimura A., Katayama Y. Synergy between phenotypic modulation and ROS neutralization in reduction of inflammatory response of hypoxic microglia by using phosphatidylserine and antioxidant containing liposomes. J. Biomater. Sci. Polym. Ed. 2016;27(3):290–302. doi: 10.1080/09205063.2015.1125565. [DOI] [PubMed] [Google Scholar]
- Khanniri E., Bagheripoor-Fallah N., Sohrabvandi S., Mortazavian A.M., Khosravi-Darani K., Mohammad R. Application of liposomes in some dairy products. Crit. Rev. Food Sci. Nutr. 2016;56(3):484–493. doi: 10.1080/10408398.2013.779571. [DOI] [PubMed] [Google Scholar]
- Kumar S., Rao R., Kumar A., Mahant S., Nanda S. Novel carriers for coenzyme Q10delivery. Curr. Drug Deliv. 2016 doi: 10.2174/1567201813666160104130631. [DOI] [PubMed] [Google Scholar]
- Lifshitz I.M., Slezov V.V. Kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. B Solids. 1961;19:35–50. [Google Scholar]
- Nakazawa T., Miyanoki Y., Urano Y., Uehara M., Saito Y., Noguchi N. Effect of vitamin E on 24(S)-hydroxycholesterol-induced necroptosis-like cell death and apoptosis. J. Steroid Biochem. Mol. Biol. 2016 doi: 10.1016/j.jsbmb.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Severino P., Andreani T., Jager A., Chaud M.V., Santana M.H., Silva A.M., Souto E.B. Solid lipid nanoparticles for hydrophilic biotech drugs: optimization and cell viability studies (Caco-2 & HEPG-2 cell lines) Eur. J. Med. Chem. 2014;81:28–34. doi: 10.1016/j.ejmech.2014.04.084. [DOI] [PubMed] [Google Scholar]
- Souto E.B., Gohla S.H., Muller R.H. Rheology of nanostructured lipid carriers (NLC) suspended in a viscoelastic medium. Pharmazie. 2005;60(9):671–673. [PubMed] [Google Scholar]
- Souto E.B., Nayak A.P., Murthy R.S. Lipid nanoemulsions for anti-cancer drug therapy. Pharmazie. 2011;66(7):473–478. [PubMed] [Google Scholar]
- Wagner C. Teorie der Alterung von Niederschlägen durch Umlösen. Zeitugen Elektrochemie. 1961;65:581–591. [Google Scholar]
- Zheng N., Gao Y., Ji H., Wu L., Qi X., Liu X., Tang J. Vitamin E derivative-based multifunctional nanoemulsions for overcoming multidrug resistance in cancer. J. Drug Target. 2016:1–7. doi: 10.3109/1061186X.2015.1135335. [DOI] [PubMed] [Google Scholar]
- Zigoneanu I.G., Williams L., Xu Z., Sabliov C.M. Determination of antioxidant components in rice bran oil extracted by microwave-assisted method. Bioresour. Technol. 2008;99(11):4910–4918. doi: 10.1016/j.biortech.2007.09.067. [DOI] [PubMed] [Google Scholar]