Abstract
Leishmaniasis is a worldwide health problem, highly endemic in developing countries. Moreover, the severe side effects and the reported drug resistance make it an urgent need to search for effective drugs that can replace or supplement those currently used. In a research program designed to investigate the antileishmanial activity of plants collected from the Egyptian flora, twenty extracts from fifteen plants growing in Egypt have been investigated for in vitro leishmanicidal activity against Leishmania donovani promastigotes. Among the tested extracts, the methanol extract of Euphorbia peplus aerial parts exhibited a significant antileishmanial activity as it produced 100% inhibition of growth with activity similar to amphotericin B. The total extract was subjected to liquid-liquid fractionation using solvents of different polarities, followed by testing the antileishmanial activity of the successive fractions. Phytochemical exploration of the active n-hexane fraction (which produced 75% inhibition of growth) led to isolation of four compounds: simiarenol (1), 1-hexacosanol (2), β-sitosterol (3), and β-sitosterol-3-O-glucoside (4) from the biologically active sub-fractions. Structure elucidation was aided by 1D and 2D NMR techniques. In conclusion, E. peplus plant has many non-polar secondary metabolites that can be used as drug leads for treatment of leishmaniasis.
Keywords: Euphorbia peplus, Euphorbiaceae, Leishmanicidal activity, NMR
1. Introduction
Leishmaniasis is a vector-borne disease which is transmitted by sandflies. It is caused by about 20 different species of the genus Leishmania (Habtemariam, 2003). This disease has a wide range of clinical symptoms that ranges from self-healing ulcers, which is called cutaneous leishmaniasis, to progressive nasopharyngeal infections (mucocutaneous leishmaniasis) and fatal disseminating visceral leishmaniasis (Ahua et al., 2007). Due to the occurrence of visceral leishmaniasis as an opportunistic infection in HIV-infected patients, the expansion of the AIDS pandemic makes the emergence of leishmaniasis/HIV co-infection a serious problem (Rocha et al., 2005). Since long time attention was paid toward the promising potential of medicinal plants for treatment of prevailing ailments. Moreover, the high cost and limited availability of effective pharmaceutical products suggest the use of native plants for symptomatic treatment of leishmaniasis in areas where it is endemic. In traditional medicine, the treatment of this disease usually consists of oral administration of crude plant extracts for visceral leishmaniasis, and topical preparations for the treatment of skin infections (Chan-Bacab and Peña-Rodríguez, 2001).
The genus Euphorbia comprises the largest among genera of the family Euphorbiaceae. It includes about 1600 known species (Ali et al., 2013), ranging from annuals to trees; all contain latex and have unique flower structure. Some of the reported folk medicinal uses of Euphorbia include treatment of skin diseases, gonorrhea, migraines, intestinal parasites, and warts (Jassbi, 2006). In Iran some species are used as purgative (Upadhyay et al., 1976). In addition, different species of Euphorbia contain macrocyclic diterpenoids with antibacterial, anticancer, PGE2-inhibitory, anti-HIV, and analgesic activity (Jassbi, 2006). Euphorbia peplus L. is originally native to Europe and North Africa (Zhi-Qin et al., 2010). The plant has a milky sap that is used in traditional medicine for treatment of non-melanoma skin cancer; the active compounds have been determined to be diterpene esters (Ramsay et al., 2011). In our search for leishmanicidal secondary metabolites, twenty extracts from fifteen plants growing in Egypt have been investigated for in vitro leishmanicidal activity against Leishmania donovani promastigotes. Among the tested extracts, the methanol extract of E. peplus aerial part exhibited a powerful leishmanicidal activity. Moreover, biologically-guided isolation of four compounds from the aerial parts of E. peplus is presented.
2. Materials and methods
2.1. General experimental
1D and 2D NMR spectra were recorded on a Bruker Avance III 400 MHz with BBFO Smart Probe and Bruker 400 MHz AEON Nitrogen-Free Magnet (Bruker AG, Switzerland) using the chemical shift of CDCl3 solvent peak at 7.24 (s) ppm in 1H and 77.2 (t) ppm in 13C NMR as an internal reference standard. Data were analyzed using Topspin 3.1 Software. LC-ESIMS was obtained using a Bruker Bio Apex FT-MS in ESI mode.
2.2. Material for chromatography
Thin layer chromatography (TLC), pre-coated silica gel 60 F254 plates (Fisher Scientific, Suwanee, GA) for TLC; developing system: n-hexane-EtOAc (8:2 and 7:3) and visualization using 10% H2SO4 in MeOH. Column chromatography (CC) was performed with silica gel (230–400 mesh) and Sephadex LH-20 (Pharmacia Biotech, Uppsala).
2.3. Plant materials
All plant materials were collected in Egypt and were identified by Dr. M. Elgebaly, Faculty of Science, Cairo University. Botanical names and plant parts are listed in Table 1. Voucher samples were deposited in the Pharmacognosy Department, Faculty of Pharmacy, Beni-Suef University, with voucher numbers listed in Table 1. E. peplus L. was collected on March, 2013, from El-Nil public garden, Beni-Suef, Egypt.
Table 1.
Plant list and their primary antileishmanial screening result.
| Family and plant name (voucher no.) | Part used | % of inhibition |
|---|---|---|
| Acanthaceae | ||
| Adhatoda vasica (BUPD-45) | Leaf | 45 |
| Stem | 14 | |
| Aizoaceae | ||
| Mesembryanthemum crystallinum (BUPD-22) | Aerial part | 0 |
| Mesembryanthemum forsskaolii (BUPD-21) | Aerial part | 14 |
| Aizoon canariensis (BUPD-46) | Aerial part | 17 |
| Trianthemum portulacastrum (BUPD-47) | Aerial part | 20 |
| Asteraceae | ||
| Tagetes patula (BUPD-48) | Leaf | 12 |
| Stem | 10 | |
| Flower | 15 | |
| Brassicaceae | ||
| Brassica rapa rapa (BUPD-49) | Aerial part | 8 |
| Chenopodiaceae | ||
| Anabasis setifera (BUPD-50) | Aerial part | 5 |
| Atriplex lindleyi (BUPD-51) | Aerial part | 6 |
| Euphorbiaceae | ||
| Euphorbia helioscopia (BUPD-52) | Aerial part | 48 |
| Euphorbia peplus (BUPD-53) | Aerial part | 100 |
| Gramineae | ||
| Sorghum bicolor (BUPD-54) | Seedlings | 19 |
| Solanaceae | ||
| Solanum nigrum (BUPD-55) | Leaf | 14 |
| Stem | 14 | |
| Flower | 18 | |
| Zygophyllaceae | ||
| Zygophyllum coccineum (BUPD-56) | Aerial part | 14 |
| Zygophyllum decumbens (BUPD-57) | Aerial part | 6 |
2.4. Extraction
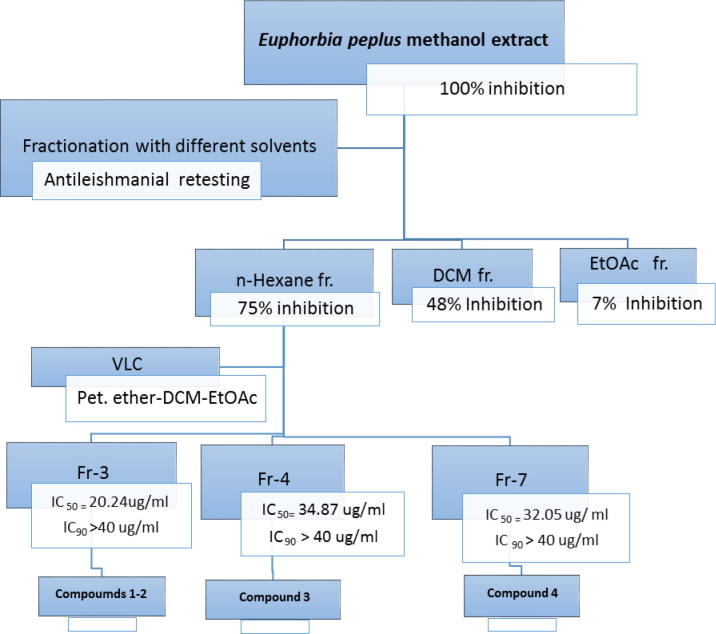
The collected samples (200 g each) were dried under controlled temperature not exceeding 45 °C, pulverized and then extracted with 80% methanol (300 ml × 3) by percolation. The extracts were then dried under reduced pressure at temperature not exceeding 45 °C. Preliminary testing of the prepared extracts used for antileishmanial activity against the protozoan L. donovani (National Center for Natural Products Research NCNPR, University of Mississippi, Oxford, MS, USA), revealed significant activity for the alcohol extract of E. peplus (Family Euphorbiaceae). The air-dried aerial part of E. peplus (1 kg) was pulverized using a laboratory mill and extracted with 80% methanol (5L × 5). The methanol was removed by vacuum distillation to give (100 g) residue. Successive fractionation using solvents of different polarities such as n-hexane, DCM and EtOAc was done, then the fractions were subjected to antileishmanial screening. The n-hexane fraction was the most active (Fig. 1).
Figure 1.
Scheme for biologically-guided isolation of secondary metabolites from Euphorbia peplus aerial parts.
2.5. Chromatographic isolation
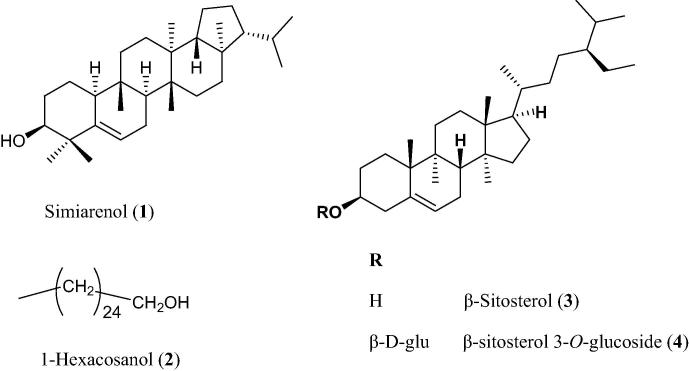
Hexane fraction (25 g) was subjected to CC over silica gel for column (200 g, 4 cm × 65 cm) using gradient elution starting with pet. ether-DCM till 100% DCM then adding EtOAc in 5% increments. Seven collective fractions were obtained after TLC monitoring. Column subfractions were subjected to primary antileishmanial screening, and three fractions were found active as shown in Fig. 1. Fraction 3 (eluted with 50% DCM in pet. ether) was rechromatographed over silica gel eluted with pet. ether-EtOAc in 5% increments to get compound 1 (25 mg) and another sub-fraction that was rechromatographed over Sephadex-LH-20 eluted with DCM-MeOH (80:20) to get compound 2 (63 mg). Fraction 4 (eluted with 75% DCM in pet. ether) was rechromatographed using silica gel column and isocratic elution with 10% EtOAc in pet. ether to get compound 3 (12 mg). Fraction 7 (eluted with 100% EtOAc) was rechromatographed on silica gel column eluted with DCM-MeOH in a gradient fashion to give compound 4 (18 mg) (see Fig. 2).
Figure 2.
Compounds isolated from Euphorbia peplus aerial part.
2.6. Characterization of the isolated compounds
Compound (1): Simiarenol: ESIMS showed [M+1]+ at m/z 427.1743 indicating a molecular formula of C30H50O. 1H NMR (400 MHz, CDCl3): δ 5.64 (d, J = 5.0 Hz, 1H), 3.50 (m, 1H), 2.18–1.18 (m, 24H), 1.16 (s, 3H), 1.07 (s, 3H), 1.03 (s, 3H), 0.95 (s, 3H), 0.92 (s, 3H), 0.91 (overlapped d, 3H), 0.85 (overlapped d, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 142.0, 122.0, 76.4, 60.1, 51.8, 50.9, 50.3, 44.3, 42.8, 40.8, 39.3, 38.6, 35.4, 34.8, 34.2, 30.8, 29.1, 29.0, 28.3, 27.8, 25.5, 24.1, 22.9, 21.9, 19.9, 18.1, 17.9, 16.1, 15.8, 15.0. NMR data were consistent with the literature (Duarte, 2008).
Compound (2): 1-Hexacosanol: ESIMS showed [M+1]+ at m/z 383.2057 indicating a molecular formula of C26H54O. 13C NMR (CDCl3, 100 MHz): δC 63.1 (CH2-OH), 32.7 (CH2), 31.9 (CH2), 29.4 (CH2), 29.3 (CH2), 29.2 (CH2), 25.4 (CH2), 22.7 (CH2), 14.2 (CH3) indicating unsaturated primary fatty alcohol. 13C NMR data were consistent with fatty alcohols (Osborne and Stevens, 1996).
Compound (3): β-sitosterol: ESIMS showed [M+1]+ at m/z 415.2953 indicating a molecular formula C29H50O. 13C NMR (100 MHz, CDCl3) δ 140.77, 121.72, 71.81, 56.78, 56.06, 50.14, 45.84, 42.33, 42.31, 39.78, 37.26, 36.51, 36.15, 33.95, 31.91, 31.67, 29.16, 28.25, 26.08, 24.31, 23.07, 21.09, 19.82, 19.40, 19.04, 18.79, 11.99, 11.86. Data were consistent with previously published data (Kongduang et al., 2008).
Compound (4): β-sitosterol-3-O-glucoside: ESIMS spectrum showed an M+ at m/z 576.4 and for the aglycon β-sitosterol at m/z 414.4. CO-TLC with authentic sample using CHCl3-MeOH (8:2) confirmed the identification of compound 4 as β-sitosterol-3-O-glucoside.
2.7. Assay for leishmanicidal activity
Antileishmanial activity was tested in vitro on a culture of L. donovani promastigotes. In a 96-well micro plate assay appropriately diluted extracts were added to the leishmania promastigotes culture (2 × 106 cells mL−1). The plates were incubated at 26 °C for 72 h and growth of Leishmania promastigotes was determined by use of the Alamar blue assay (Moawad et al., 2013). Standard fluorescence was measured by a Fluostar Galaxy plate reader (excitation wavelength: 544 nm; emission wavelength: 590 nm). Pentamidine (IC50 1.01 and IC90 2.03 μg/mL) and amphotericin B (IC50 0.47 and IC90 0.65) were used as the drug controls. Percent growth was calculated and plotted against the tested concentrations to determine the IC50 and IC90 values.
3. Results and discussion
Fifteen plants related to the families Acanthaceae, Aizoaceae, Asteraceae, Brassicaceae, Chenopodiaceae, Euphorbiaceae, Gramineae, Solanaceae and Zygophyllaceae (Table 1) were tested for leishmanicidal activity against L. donovani promastigotes. Among the tested extracts, the methanolic extract of E. peplus showed 100% growth inhibition with activity similar to amphotericin B. The methanolic extract was fractionated using n-hexane, DCM, and EtOAc and these fractions were subjected to rescreening as L. Donovani promastigotes growth inhibitor. The n-hexane fraction showed 75% inhibition (Fig. 1). Hexane fraction was subjected to VLC fractionation and the sub-fractions were screened for antileishmanial activity. Three fractions were found active (IC50 = 20.24, 34.87, 32.05 μg/ml) and the other fractions showed activity at >40 μg/ml and considered inactive. The active fractions were analyzed using HPLC/HRMS in addition to chromatographic isolation of four known compounds elucidated as simiarenol (1), 1-hexacosanol (2), β-sitosterol (3), and β-sitosterol-3-O-glucoside (4).
The total methanolic extract showed 100% inhibition of growth with activity similar to amphotericin B. Upon fractionation, the n-hexane fraction showed 75% inhibition and then the n-hexane subfractions showed activity with IC50 = 20.24, 34.87, 32.05 μg/ml and IC90 > 40 μg/ml compared to pentamidine (IC50 = 1.01 and IC90 = 2.03 μg/ml) and amphotericin B (IC50 = 0.47 and IC90 = 0.65 μg/ml) which indicated synergistic effect of secondary metabolites in E. peplus total methanolic extract with special concentration in the n-hexane fraction.
Although the isolated compounds were previously reported in the title plant, the result of their presence in the active antileishmanial fractions adds to their biologic importance. Compound 1 was assigned to be 5-adianene-3-β-ol which is commonly named as simiarenol. Simiarenol was first isolated from Rhododendron simiarum (Aplin et al., 1966). It was previously isolated from Euphorbia lagascae and Euphorbia tuckeyana (Duarte, 2008) as well as detected in the epicuticular leaf waxes from Euphorbia characias, Euphorbia nicaeensis, E. peplus (Hemmers et al., 1988) and Euphorbia lathyris (Hemmers et al., 1989).
Compound 2 was a saturated fatty alcohol (1-hexacosanol). Fatty acids and alcohols exhibit antimicrobial effects as they can prevent the growth of or directly kill bacteria, fungi and other microbes by affecting multiple cellular targets, including the cell membrane (Desbois, 2012).
The inhibiting activity of some triterpenes and sterols for promastigotes and intracellular amastigotes of Leishmania amanuensis was previously reported but β-sitosterol was inactive against this species (Torres-Santos et al., 2004), and we reported here the activity of β-sitosterol (3) and its 3-O-glucoside derivative (4) toward L. donovani promastigotes.
In conclusion, many of non-polar components in E. peplus exhibited synergistic effect to produce leishmanicidal activity against L. donovani promastigotes. So far, it is the first report of these compounds to such activity that may be helpful in future to include the n-hexane extract in food supplements for treatment of different leishmaniasis caused by such pathogen.
Acknowledgments
The authors thank Dr. Mohamed Radwan and Marsha Wright for the antimicrobial screening which is supported by the NIH, NIAID, Division of AIDS, Grant No. AI 27094 and the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009, University of Mississippi, Oxford, MS, USA.
This study was funded by a grant from Research Funding Unit, Beni-Suef University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahua K.M., Ioset J.R., Ioset K.N., Diallo D., Mauël J., Hostettmann K. Antileishmanial activities associated with plants used in the Malian traditional medicine. J. Ethnopharmacol. 2007;110:99–104. doi: 10.1016/j.jep.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Ali A.a., Sayed H.M., Ibrahim S.R.M., Zaher A.M. Anti-inflammatory activities of Euphorbia peplus L. Phytopharmacology. 2013;4:69–80. [Google Scholar]
- Aplin R.T., Arthur H.R., Hui W.H. The structure of the triterpene simiarenol (a E:B-friedo-hop-5-ene) from the Hong Kong species of Rhododendron simiarum. J. Chem. Soc. C Org. 1966;1:1251–1256. [Google Scholar]
- Chan-Bacab M.J., Peña-Rodríguez L.M. Plant natural products with leishmanicidal activity. Nat. Prod. Rep. 2001;18:674–688. doi: 10.1039/b100455g. [DOI] [PubMed] [Google Scholar]
- Desbois A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Antiinfect. Drug Discov. 2012;7:111–122. doi: 10.2174/157489112801619728. [DOI] [PubMed] [Google Scholar]
- Duarte N. Universidade de Lisboa Faculdade de Farmácia; 2008. Structural Characterization and Biological Activities of Terpenic and Phenolic Compounds Isolated from Euphorbia lagascae and Euphorbia tuckeyana. PhD Thesis. [Google Scholar]
- Habtemariam S. In vitro antileishmanial effects of anti bacterial diterpenes from two Ethiopian Premna species: P. schimperi and P. oligotricha. BMC Pharmacol. 2003;3:1–6. doi: 10.1186/1471-2210-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmers H., Gülz P.G., Marner F.J. Triterpenoids in epicuticular waxes of three European Euphorbia species. Z. Naturforsch. 1988;43c:799–805. [Google Scholar]
- Hemmers H., Gülz P.G., Marner F.J. Pentacyclic triterpenoids in epicuticular waxes from Euphorbia lathyris L., Euphorbiaceae. Z. Naturforsch. 1989;44c:193–201. [Google Scholar]
- Jassbi A.R. Chemistry and biological activity of secondary metabolites in Euphorbia from Iran. Phytochemistry. 2006;67:1977–1984. doi: 10.1016/j.phytochem.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Kongduang D., Wungsintaweekul J., De-eknamkul W. Biosynthesis of b-sitosterol and stigmasterol proceeds exclusively via the mevalonate pathway in cell suspension cultures of Croton stellatopilosus. Tetrahedron Lett. 2008;49:4067–4072. [Google Scholar]
- Moawad A., Hetta M., Zjawiony J.K., Ferreira D., Hifnawy M. Two new dihydroamentoflavone glycosides from Cycas revoluta. Nat. Prod. Res. 2013:1–7. doi: 10.1080/14786419.2013.832675. [DOI] [PubMed] [Google Scholar]
- Osborne R., Stevens J.F. Epicuticular waxes and glaucousness of Encephalartos leaves. Phytochemistry. 1996;42:1335–1339. [Google Scholar]
- Ramsay J.R., Suhrbier a., Aylward J.H., Ogbourne S., Cozzi S.-J., Poulsen M.G., Baumann K.C., Welburn P., Redlich G.L., Parsons P.G. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br. J. Dermatol. 2011;164:633–636. doi: 10.1111/j.1365-2133.2010.10184.x. [DOI] [PubMed] [Google Scholar]
- Rocha L.G., Almeida J.R.G.S., Macêdo R.O., Barbosa-Filho J.M. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Torres-Santos E.C., Lopes D., Oliveira R.R., Carauta J.P.P., Falcao C.A.B., Kaplan M.A.C., Rossi-Bergmann B. Antileishmanial activity of isolated triterpenoids from Pourouma guianensis. Phytomedicine. 2004;11:114–120. doi: 10.1078/0944-7113-00381. [DOI] [PubMed] [Google Scholar]
- Upadhyay R.R., Zarintan M.H., Ansarin M. Irritant constituents of Iranian plants. Ingenol from Euphorbia seguieriana. Planta Med. 1976;30:196–197. doi: 10.1055/s-0028-1097717. [DOI] [PubMed] [Google Scholar]
- Zhi-Qin S., Shu-Zhen M., Ying-Tong D., Xiao-Jiang H. A new jatrophane diterpenoid from Euphorbia peplus. Chin. J. Nat. Med. 2010;8:81–83. [Google Scholar]