Abstract
In this study, we aimed to improve the anticancer effect of 5-FU on human colon cancer cell lines by incorporating in poly(d,l lactic-co-glycolic acid) (PLGA) nanoparticles (NPs). The 5-FU-PLGA NPs were prepared by nanoprecipitation technique. Prepared NPs were moderately dispersed with an average diameter of 133 ± 25.19 nm. Scanning Electron Microscope (SEM) images revealed spherical structures with subtle surface irregularity. Free 5-FU dose–response curves were constructed (12.5–2000 μM) using MTT assay on HCT 116 and HT-29 cell lines for 1, 3, and 5 days. The calculated IC50 on HCT 116 were 185 μM after 1 day, 11.3 μM after 3 days, and 1.48 μM after 5 days. On HT-29, IC50 was only reached after 5 days of 5-FU treatment (11.25 μM). The HCT 116 viability following treatment with 100 μM 5-FU in free or NPs forms for 3 days was 38.8% and 18.6%, respectively. Similarly, when 250 μM was applied, HCT 116 viability was 17.03% and 14.6% after treatment with free and NPs forms of 5-FU, respectively. Moreover, HT-29 cell viability after 250 μM 5-FU treatment in free or NPs forms was 55.45% and 34.01%, respectively. We also noticed that HCT 116 cells were more sensitive to 5-FU-PLGA NPs as compared to HT-29 cells. Overall, our data indicate that 5-FU activity is time dependent and the prolonged effects created by PLGA NPs may contribute, at least in part, to the noticed enhancement of the anticancer activity of 5-FU drug.
Keywords: 5-Fluorouracil, Nanoparticles, Colon cancer, PLGA, Drug delivery
1. Introduction
Colorectal cancer (CRC) has been considered a major health burden and a leading factor causing mortality and morbidity worldwide (World Health Organization, 2002). Conventional CRC chemotherapy provides marginal improvement to patients (American Cancer Society, 2006). In some cases, chemotherapy has failed to make significant impact on the prognosis of disease due to poor bioavailability, poor tissue selectivity/specificity, and in vivo degradation, which can lead to serious side effects (Arias, 2008, Nair et al., 2011). Moreover, chemotherapeutics are usually administered in high doses, which increase the risk of side effects and drug resistance (Duran et al., 2008, Wong et al., 2007, Zamboni et al., 2012). Thus far, many potent chemotherapeutics have been developed and extensively studied over the last few decades. However, insufficient and nonspecific drug delivery remains a major problem that adversely affects chemotherapeutic anticancer drug efficacy (Gottesman, 2002).
5-Fluorouracil (5-FU) is one of the earliest and still most commonly used anticancer drugs. Nonetheless, despite its potency in treating cancers, its clinical applications are limited due to its short half-life, disease resistance, and severe side effects such as myelosuppression, dermatitis, cardiotoxicity, neurotoxicity, nausea, vomiting and gastrointestinal, which is associated with its high non-specific in vivo distribution (Blanke et al., 1999, Cai et al., 2006, Cao and Rustum, 2000, Di Paolo et al., 2001, Fata et al., 1999, Schmoll et al., 1999, van Kuilenburg et al., 2000). Therefore, it is imperative to develop a strategy that overcomes the limitations as well as further improvements in the anticancer response of 5-FU. In this study, we have utilized polylactic-co-glycolic acid (PLGA) nanoparticles (NPs) to improve the anticancer activity of 5-FU in human colon cancer cells.
PLGA is a synthetic copolymer approved by the US FDA for human use (Jain, 2000). It has been extensively studied as a carrier for a wide range of drugs including chemotherapeutics (Lee et al., 2004). In addition to its biodegradability, PLGA is biocompatible, mechanically strong, soluble in a wide range of organic solvents, and can be easily processed and fabricated in various forms and sizes (Avgoustakis, 2004). In aqueous media, PLGA is hydrolyzed into lactic and glycolic acids that get consumed in the citric acid cycle and subsequently eliminated as carbon dioxide and water (Jalil and Nixon, 1990). As a drug carrier, the most widely used PLGA copolymer is PLGA 50:50 owing to its fastest degradation rate (Park, 1995).
The aim of the current study was to improve the anticancer effect of 5-FU on human colon cancer cells with the help of PLGA NPs. For this purpose, 5-FU was loaded on PLGA (50:50) NPs. In most of the previously published studies, PLGA NPs were prepared by double-emulsion solvent evaporation method (Li et al., 2008, Parikh et al., 2003, Hu et al., 2013, Lin et al., 2012, Nair et al., 2011). In this study, we have explored nanoprecipitation technique for loading of 5-FU on PLGA NPs. The 5-FU-PLGA NPs complex was further characterized for size, shape, surface charge and size distribution. Thereafter, we have studied the anticancer effects of 5-FU loaded PLGA NPs on two types of human colon cancer cell lines HCT 116 and HT-29. We observed that 5-FU-PLGA NPs showed higher cytotoxicity to both types of colon cancer cell line as compared to free 5-FU.
2. Materials and methods
2.1. Chemicals and reagents
5-FU, PLGA (50:50 MW 40,000–75,000), poloxamer 188 NF (Pluronic® F-68 NF, MW 8350), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, MW 414) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Human colon cancer cell lines HCT 116 and HT-29 were bought from the European Collection of Cell Cultures (ECACC) (Salisbury, UK). Trypsin-EDTA (0.25%), McCoy’s 5a, Fetal Bovine Serum (FBS), Penicillin–Streptomycin–Glutamine (100×), and Gentamicin (10 mg/mL) were obtained from Life Technologies through Salehiya EstPO (Riyadh, Saudi Arabia).
2.2. Preparation of 5-FU-PLGA nanoparticles
5-FU-PLGA NPs were prepared by nanoprecipitation technique (Barichello et al., 1999). Briefly, 100 mg of PLGA was dissolved in 10 mL of acetone. This solution was added dropwise within 45 s to a stirring solution of 20 mL distilled water containing 5 mg 5-FU and 200 mg poloxamer 188 and kept on stirring at 1000 rpm overnight. Then, the formed suspension was washed three times with double-deionized water at 4 °C at 35,000g for 15 min (Optima MAX-XP Benchtop Ultracentrifuge, Brea, CA, USA) and then freeze-dried (LABCONCO FreeZone 4.5 Liter Benchtop Freeze Dry System, Kansas City, MO, USA) for 3 days to remove water.
2.3. Characterization of 5-FU-PLGA nanoparticles
2.3.1. Particle size and zeta-potential
The effective diameter and polydispersity index along with zeta potential were determined by dynamic light scattering using Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). In brief, lyophilized 5-FU and 5-FU-PLGA NPs were re-suspended in 1.5 mL distilled water and poured in a quartz cuvette. Analysis was performed at ambient temperature with an angle of detection of 90°. Zeta potential was measured by laser micro-electrophoresis. Similarly, NPs were re-suspended in 0.5 mL distilled water and added to a folded capillary cell. The Analysis was performed at ambient temperature with an applied voltage of 50 V and an applied voltage offset of −0.92 V. Each value represents the average of at least five measurements ± standard deviation (SD).
2.3.2. Surface morphology
Size and surface morphology of dry powder of 5-FU-PLGA NPs were examined under the scanning electron microscope SEM EVO LS10 (Carl-Zeiss, Cambridge, UK). Particles were mounted on double-sided adhesive carbon tape (SPI Supplies, West Chester, USA) and coated under high-vacuum evaporator with gold in a Q 150R sputter coater unit (Quorum Technologies Ltd., East Sussex, UK) in an argon atmosphere at 20 mA for 120 s. The coated samples were scanned, and photomicrographs were taken at an acceleration voltage of 1–10 kV.
2.3.3. Estimation of 5-FU content on PLGA nanoparticles
5-FU content in 5-FU-PLGA NPs complex was analyzed by high performance liquid chromatography (HPLC). The HPLC system consisted of an Agilent model connected to HP instruments (Agilent Technologies, Hewlett-Packard-Strasse 8, Waldbronn, Germany), which contains Agilent Technologies 1200 G1329A ALS autosampler, HP 1100 G1322A degasser, HP 1100 G1312A bin pump, HP 1100 G1315A diode array detector, and Agilent ChemStation for LC and LC/MS systems software. Quantification was achieved by isocratic elution as described previously (Sun et al., 2007). Mobile phase consisted of phosphate buffer 40 mM (5.44 g Potassium dihydrogen orthophosphate (MW 136.09) in 1 L of distilled water and adjusted to pH 7.2 by 10% (w/v) sodium hydroxide) and delivered at a flow rate of 1 mL/min at ambient temperature. C18 guard column (ODS-Hypersil, 5 μm, 20 × 2.1 mm) and C18 analytical column (Waters® μBondapak C18, 125 Å, 10 μm, 3.9 mm × 150 mm) were used. UV detection was performed at 260 nm and the injection volume was 90 μL. For standard curve preparation, serial dilution of 5-FU stock solution was performed to obtain 100, 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.78 μg/mL.
To assess 5-FU content on PLGA NP, 1 mL was taken from the decanted supernatant and 5-FU concentration was determined by HPLC after plotting a standard curve. Indirect determination of the entrapment efficiency was attained using the following equation:
For direct determination of entrapment efficiency, 5 mg of 5-FU NPs was first dissolved in 50 μL of chloroform. Thereafter, chloroform was evaporated at room temperature and then 500 μL of distilled water was added to the precipitant and mixed by vigorous vortexing for 30 min to dissolve the precipitated 5-FU but not PLGA. After that, the formed suspension was centrifuged by Spectrafuge™ 24D centrifuge (Labnet International, Inc., Edison, NJ, USA) at 5000 rpm for 3 min at 25 °C. The 5-FU content was determined by HPLC after plotting a standard curve and the entrapment efficiency was determined by the following equation:
The method of extracting 5-FU from PLGA NPs was validated by the following spike study: 5 mg of PLGA NPs was spiked with 0.5 mL of 5-FU solution containing 0.25 mg drug and gently vortexed for 5 s. The sample was kept at room temperature for 6 h until completely dried. Then 50 μL of chloroform was used to completely dissolve the NPs and then was left for 4 h to evaporate. Thereafter, 0.5 mL of water was mixed by vigorous vortexing for 30 min. After that, the formed suspension was centrifuged by Spectrafuge™ 24D centrifuge (Labnet International, Inc., Edison, NJ, USA) for 3 min at 5000 rpm at 25 °C. The 5-FU content was determined by HPLC after plotting a standard curve. The recovery was around 99%.
Percentage of drug loading (DL%) was also calculated using the following equation:
2.3.4. Cumulative release in vitro of 5-FU from PLGA nanoparticles
Aliquots of 5 mg/mL of the suspended 5-FU-PLGA NPs in PBS were placed in a shaking water bath at 37 °C for 45 days. At designated time intervals, a set of triplicate samples was removed, and the supernatant was separated from the particles by centrifugation. Determination of 5-FU concentrations was conducted by HPLC as mentioned earlier.
2.4. Cell culture and nanoparticles treatment
Human colon cancer cell lines, HCT 116 and HT-29 were used to assess the cytotoxicity of 5-FU and 5FU-PLGA NPs. Each cell line was propagated in the designated medium and passaged according to ECACC recommendation. Cells were cultured in McCoy’s 5a medium supplemented with 10% FBS, 1% penicillin-streptomycin and 1% gentamicin at 5% CO2 and 37 °C. At 85% confluence, cells were harvested using 0.25% trypsin and were sub-cultured into 25 cm2 flasks or 48-well plates according to selection of experiments. Cells were allowed to attach the surface for 24 h prior to NPs exposure.
Dry powder of 5-FU and 5-FU-PLGA NPs was suspended in cell culture medium at a concentration of 100 mM and diluted to appropriate concentrations according to selection of experiments. The dilutions of NPs were then sonicated using a sonicator bath at room temperature for 15 min at 40 W to avoid NPs agglomeration prior to cell exposure. Cells not exposed to NPs served as controls in each experiment.
2.5. Cytotoxicity assay
Cytotoxicity of 5-FU and 5-FU-PLGA NPs in HCT 116 and HT-29 cells was examined by MTT cell proliferation assay. This assay assesses the mitochondrial function by measuring ability of viable cells to reduce MTT into blue formazon product. In brief, 7 × 104 cells/well were seeded in 48-well plates and exposed to different concentrations of NPs for 24, 72 and 120 h. At the end of the exposure time, culture medium was removed from each well to avoid interference of NPs and replaced with new medium containing MTT solution in an amount equal to 10% of culture volume and incubated in CO2 incubator (SANYO Electric Biomedical Co., Ltd., Osaka, Japan) for 2–4 h at 37 °C until a purple-colored formazan product developed. The formed purple formazan crystals were solubilized by the addition of equal volume of isopropanol to each well and gentle shaking for 30 min. Color intensity was then measured by a microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) at 570 nm and percentage cell viability was calculated relative to untreated cells group. The results represent mean ± SD of at least three replicates.
2.6. Statistical analysis
Statistical analysis of obtained results was done by one-way analysis of variance (ANOVA) using IBM SPSS statistics 22.0 software. P < 0.05 was taken as a criterion for a statistically significant difference.
3. Results and discussion
This work aims to improve single-dose effect of 5-FU anticancer drug on human colon cancer cells. For this purpose, 5-FU was entrapped in PLGA NPs and treated to HCT 116 and HT-29 cell lines in order to assess its in vitro cytotoxicity.
3.1. Characterization of 5-FU-PLGA nanoparticles
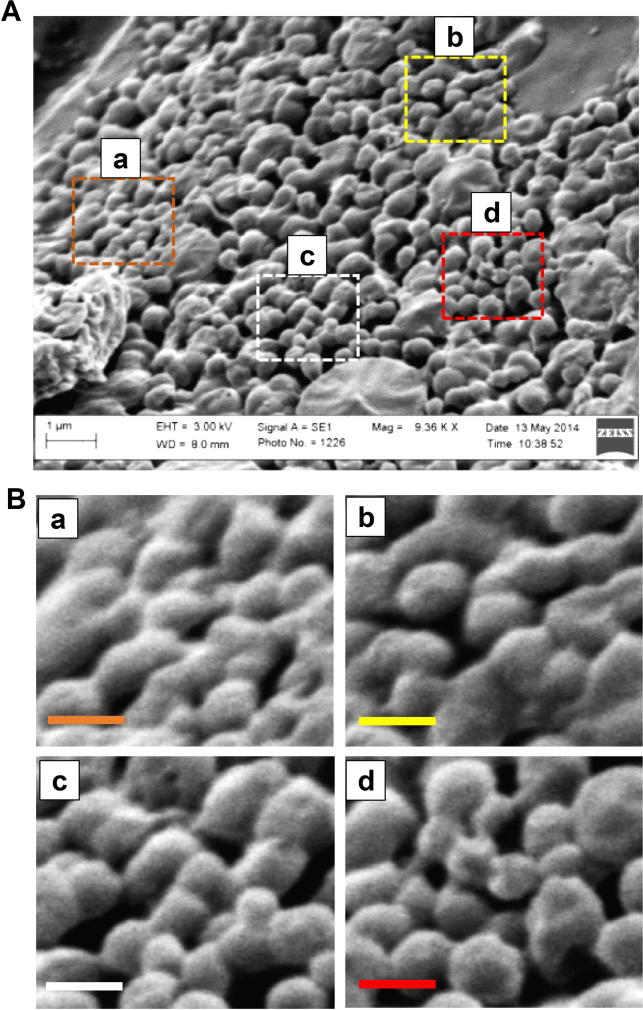
The 5-FU-loaded PLGA NPs showed a mean particle diameter of 133 ± 25.19 nm. When compared to the mean diameter of empty PLGA NPs (117.9 ± 21.53 nm), the size of 5-FU-PLGA NPs was slightly increased. Although no statistical significance was determined when both means were compared, the observed increment in the mean particle diameter of the 5-FU-loaded PLGA NPs can be attributed to successful entrapment of the drug. These observations are consistent with the work of Kumar and his colleagues, who used double-emulsion solvent evaporation technique to encapsulate 5-FU (Nair et al., 2011). The authors reported 135 nm mean diameter of empty PLGA NPs and 150 nm after 5-FU encapsulation. Our SEM images revealed spherical structures of 5-FU-PLGA NPs with slight surface irregularity (Fig.1A). During nano-precipitation, hydrophobic polymers are driven to self-assemble into spherical or irregular nanoparticles as a result of the quick transfer from good-solvent to poor-solvent conditions (Hornig et al., 2009). Since this method is not emulsion-based, the relatively quick precipitation of PLGA contributed to the irregularity of the formed spherical shape. Nonetheless, 5-FU NPs were produced with measured polydispersity index of 0.352 indicating moderate dispersity, which is inferred by the SEM image (Fig.1B).
Figure 1.
Scanning Electron Microscope (SEM) image of 5-FU loaded on PLGA NPs. (A) Low magnification image and (B) high magnification image (scale bar 300 nm).
3.2. Determination of 5-FU content
HPLC assay, reported in (Alanazi et al., 2009) was utilized to quantify 5-FU entrapment within PLGA NPs (direct method) as well as un-entrapped 5-FU recovered in the supernatant after PLGA NPs preparation (indirect method). 5-FU content was calculated from standard curve. Direct and indirect assessments were consistent and showed EE% of ∼40% and DL% of ∼7%. This entrapped amount, although can be possibly increased, was found sufficient to exert cytotoxic action. This can be attributed to the choice of PLGA NPs preparation technique. We chose nanoprecipitation because of the physicochemical properties of 5-FU. Owing to its solubility profile (sparingly soluble) as well as low affinity to PLGA, nanoprecipitation was thought to improve 5-FU EE% in PLGA NPs. Very few reports have explored this technique for 5-FU loading in PLGA NPs (Karmi et al., 2011, Ashwanikumar et al., 2014).
3.3. In vitro release study
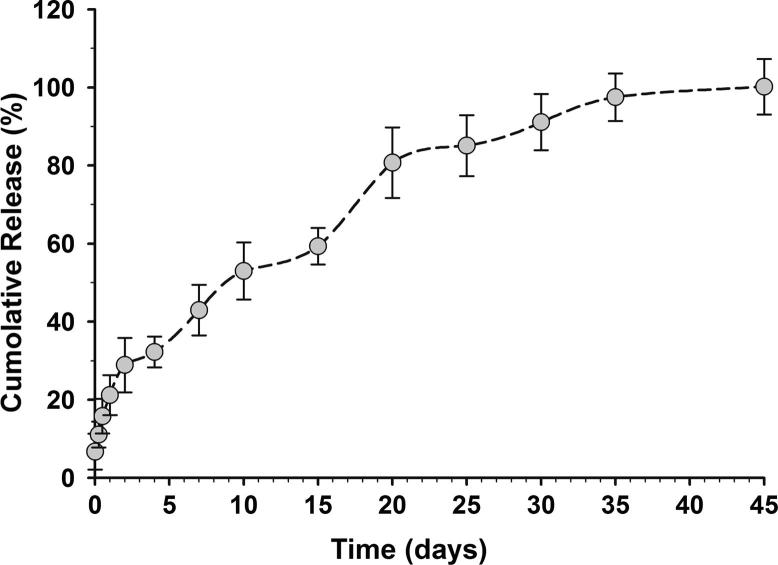
The cumulative release of 5-FU was following classical triphasic release profile of PLGA NPs (Fig. 2). Initial burst release was documented reaching ∼12% in the first 6 h, ∼16% after 12 h, and ∼22% after 24 h. Thereafter, ∼60% of 5-FU was sustain-released over the next two weeks. Another climb in 5-FU level was noticed as a result of polymer degradation reaching ∼80% by day 20 of the study. The rest 20% was then slowly released over the next two weeks. The prolonged release of 5-FU, 45 days, is primarily due to the high molecular weight of PLGA used in this formulation (Shive and Anderson, 1997). However, it is worth noting that PLGA NPs degradation pattern may remarkably vary following cellular uptake and, hence, 5-FU release and pharmacological onset.
Figure 2.
Cumulative in vitro release of 5-FU from PLGA NPs. In vitro release profile of 5-FU in PBS detected over 45 days. Data represented are mean ± SD of three identical experiments made in three replicate.
3.4. Determination of cytotoxicity of 5-FU and 5-FU-PLGA nanoparticles
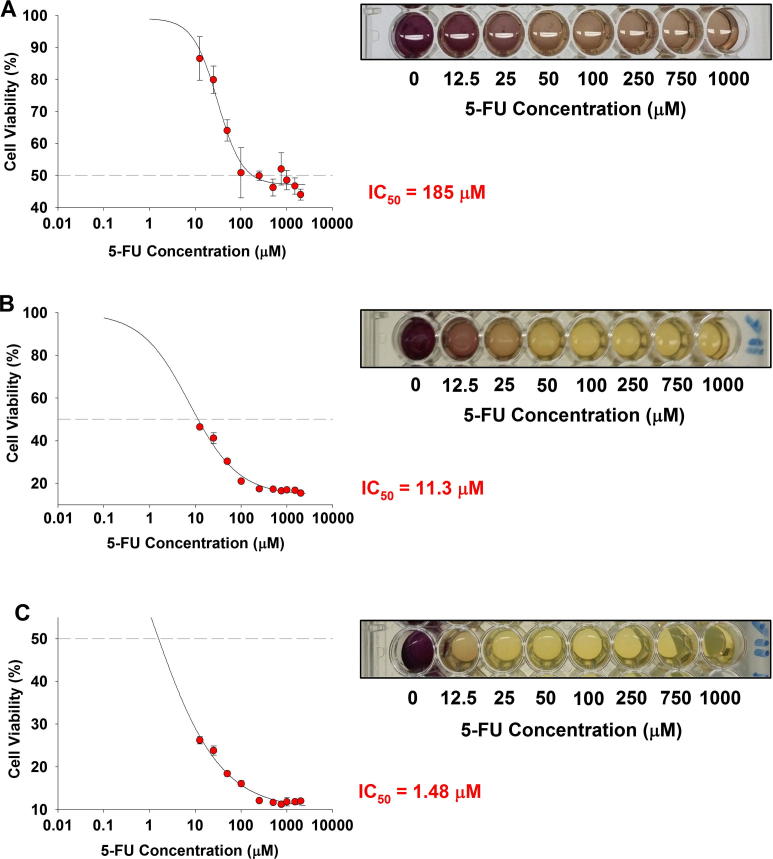
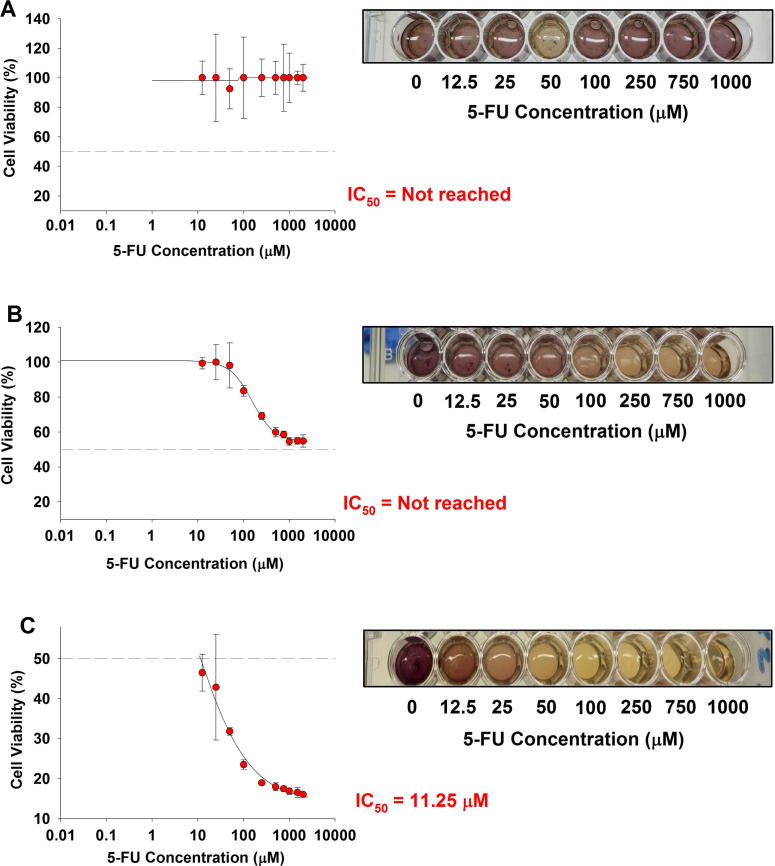
We evaluated the cytotoxic ability of free 5-FU and 5-FU-PLGA NPs against two human colon cancer cell lines (HCT 116 and HT-29). These cell lines were chosen based on their p53-expression profile: wild type in the case of HCT 116 and mutant in HT-29. Dose–response curves were constructed for free 5-FU in concentration range (12.5–2000 μM) against relative cell viability percentage of HCT 116 cells (Fig. 3) and HT-29 cells (Fig. 4) and for 1, 3, and 5 days. Results showed that both types of colon cells responded to the cytotoxic effect of free 5-FU in dose-dependent and time-dependent manner. In spite of the observed dose dependency, the time factor demonstrated more influence on 5-FU activity (Table 1). The calculated IC50 of 5-FU after 1 day exposure to HCT 116 was around 185 μM (Fig.3A). A remarkably lower concentration, 11.3 μM, was needed to reduce cell viability to 50% after 3 days of single-dose exposure (Fig.3B). Furthermore, approximately 1.48 μM of 5-FU was sufficient to reduce 50% cell viability after 5 days of exposure (Fig.3C). Similarly, the time-dependent response was more palpable in the case of HT-29 than mere increment of 5-FU concentration. While no IC50 was reached after 1 day or 3 days of 5-FU treatment at any concentration (Fig.3A and B), the cytotoxic effect was profound (IC50 equals 11.25 μM) after 5 days of initial treatment (Fig.4C).
Figure 3.
Cytotoxicity of 5-FU in HCT 116 cells. Cells were exposed to different concentrations of 5-FU for different time intervals. At the end of the exposure, MTT cell viability was determined as described in materials and methods. (A) 1-day exposure, (B) 3-day exposure and (C) 5-day exposure time. Data represented are mean ± SD of three identical experiments made in three replicate.
Figure 4.
Cytotoxicity of 5-FU in HT-29 cells. Cells were exposed to different concentrations of 5-FU for different time intervals. At the end of the exposure, MTT cell viability was determined as described in materials and methods. (A) 1-day exposure, (B) 3-day exposure and (C) 5-day exposure time. Data represented are mean ± SD of three identical experiments made in three replicate.
Table 1.
IC50 values of 5-FU for colon cancer cells.
| Colon cancer cell line | IC50 values (μM) |
||
|---|---|---|---|
| 1 day | 3 day | 5 day | |
| HCT 116 | 185 | 13.5 | 1.48 |
| HT-29 | Not reached | Not reached | 11.25 |
These observations indicate that the cytotoxic effect of 5-FU is more dependent on prolonged exposure to cells rather than availability of higher concentration with short cell exposure. Recently, Han and colleagues (Sui et al., 2014) reported that autophagy is activated in a time-dependent manner in 5-FU-treated HCT 116 and HT-29 cells. Moreover, the aberrant expression of p-53 is thought to abrogate 5-FU ability to induce p53-dependent cell growth arrest and apoptosis. This was supported by the fact that HCT 116 p53−/− cells were less responsive than their p53+/+ counterparts (Sui et al., 2014). Hence, p53 deletion or mutation can induce resistance to 5-FU (Subbarayan et al., 2010, Huang et al., 2009, Sun et al., 2007). Interestingly, the IC50 calculated after 5 days of treatment in case of HT-29 cells (11.25 μM) was approximately similar to that of HCT 116 after 3 days of 5-FU treatment (11.3 μM). Given that HT-29 cells are less sensitive to 5-FU treatment, this further indicates that prolonged 5-FU exposure enhances the desired cytotoxicity. Therefore, we next explored whether PLGA NPs can enhance the cytotoxic effect of 5-FU.
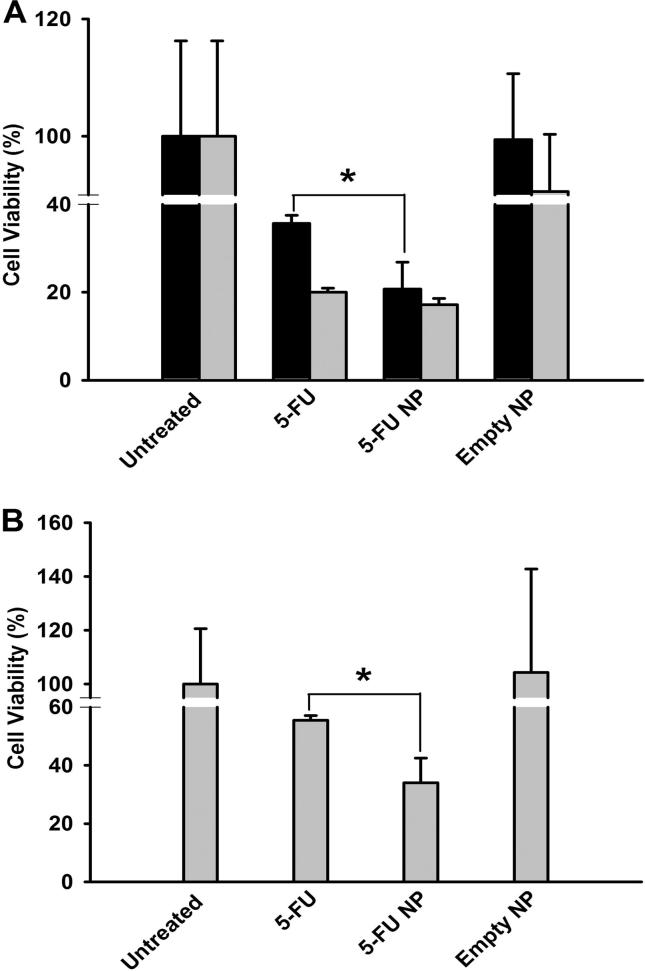
HCT 116 or HT-29 cells were incubated with 5-FU, 5-FU-PLGA NPs, or empty PLGA NPs for 3 days. Thereafter, cell viability was determined by MTT assay. As shown in Fig.5A, applying 100 μM 5-FU in free or NPs forms on HCT 116 cells resulted in significant inhibition of cell viability reaching 38.8% and 18.6%, respectively. Similarly, when 250 μM was applied, both free and NPs forms of 5-FU demonstrated significant reduction in cell viability with marginal advantage of 5-FU-PLGA NPs (14.6%) over the free 5-FU (17.03%). However, no statistical significance was noticed at this concentration. A slight reduction in cell viability was also recorded with empty PLGA NPs (91.3%) when used in an amount equivalent to the amount containing 250 μM 5-FU. Nonetheless, this reduction in cell viability was not statistically significant when compared to untreated cells.
Figure 5.
Cytotoxicity of 5-FU and 5-FU-PLGA NPs in HCT 116 and HT-29 cells. Cells were exposed to 100 μM (black bars) or 250 μM (gray bars) of 5-FU and 5-FU-PLGA NPs. At the end of the exposure, MTT cell viability was determined as described in materials and methods. (A) HCT 116 cells and (B) HT-29 cells. Data represented are mean ± SD of three identical experiments made in three replicate. *Significant difference between 5-FU and 5-FU-PLGA NPs exposure (p < 0.05 for each).
In HT-29 cells, only 250 μM concentration was used in the cytotoxicity assay. The recorded cell viability with 5-FU NPs was 34.01%, while the cell viability recorded with free 5-FU was 55.45% (Fig.5B). Beside the significant advantage of 5-FU NPs, it was not possible for us to reduce HT-29 cell viability with 5-FU below 50% except when formulated in PLGA NPs. This observation suggests that the PLGA NPs not only retained but also improved the single-dose effect of 5-FU on human colon cancer cell lines. Our results are consistent with others work where superiority of 5-FU-loaded NPs has been reported in different cancer cell lines (Wang et al., 2015, Ali et al., 2011, Nair et al., 2011). We attribute this to the prolonged drug exposure provided by the PLGA system as well as possible evasion of inactivation mechanisms such as intracellular degradation. This is still to be investigated. We did not investigate the cytotoxicity of 5-FU-PLGA NPs on non-cancerous cell lines. We will explore the mechanisms of anticancer activity of 5-FU-PLGA NPs and their biocompatibility to non-cancerous cells in future studies.
4. Conclusions
We have made progress toward developing PLGA NPs for 5-FU delivery by nano-precipitation technique. Our data showed that 5-FU cytotoxicity to colon cell lines is likely to be more influenced by the treatment time rather than dose escalation. We also demonstrated that PLGA-based NPs can aid in improving the anticancer activity of 5-FU and reducing the exposure time needed to reach similar effect with free 5-FU. Furthermore, the superiority of 5-FU-PLGA NPs over free 5-FU was consistent even with a more resistant cell line. Nevertheless, further mechanistic investigations are still required to understand the enhancing effect of PLGA NPs on 5-FU anticancer activity.
Acknowledgment
This project was funded by the Research Groups Program (Research Group number RG-1436-027), Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alanazi F.K., Yassin A.E., El-Badry M., Mowafy H.A., Alsarra I.A. Validated high-performance liquid chromatographic technique for determination of 5-fluorouracil: applications to stability studies and simulated colonic media. J. Chromatogr. Sci. 2009;47:558–563. doi: 10.1093/chromsci/47.7.558. [DOI] [PubMed] [Google Scholar]
- Ali I., Rahis Uddin K. Salim, Rather M.A., Wani W.A., Haque A. Advances in nano drugs for cancer chemotherapy. Curr. Cancer Drug Targets. 2011;11:135–146. doi: 10.2174/156800911794328493. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . American Cancer Society; 2006. American Cancer Society’s Complete Guide to Colorectal Caner. [Google Scholar]
- Arias J.L. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules. 2008;13:2340–2369. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwanikumar N., Kumar N.A., Nair S.A., Kumar G.S. Dual drug delivery of 5-fluorouracil (5-FU) and methotrexate (MTX) through random copolymeric nanomicelles of PLGA and polyethylenimine demonstrating enhanced cell uptake and cytotoxicity. Colloids Surf. B: Biointerfaces. 2014;122:520–528. doi: 10.1016/j.colsurfb.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Avgoustakis K. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Curr. Drug Deliv. 2004;1:321–333. doi: 10.2174/1567201043334605. [DOI] [PubMed] [Google Scholar]
- Barichello J.M., Morishita M., Takayama K., Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev. Ind. Pharm. 1999;25:471–476. doi: 10.1081/ddc-100102197. [DOI] [PubMed] [Google Scholar]
- Blanke C.D., Teng M., Choy H. The role of UFT in combined-modality therapy. Oncology (Williston Park) 1999;13:47–54. [PubMed] [Google Scholar]
- Cai C., Zhou K., Wu Y., Wu L. Enhanced liver targeting of 5-fluorouracil using galactosylated human serum albumin as a carrier molecule. J. Drug Target. 2006;14:55–61. doi: 10.1080/10611860600613324. [DOI] [PubMed] [Google Scholar]
- Cao S., Rustum Y.M. Synergistic antitumor activity of irinotecan in combination with 5-fluorouracil in rats bearing advanced colorectal cancer: role of drug sequence and dose. Cancer Res. 2000;60:3717–3721. [PubMed] [Google Scholar]
- Di Paolo A., Danesi R., Falcone A., Cionini L., Vannozzi F., Masi G., Allegrini G., Mini E., Bocci G., Conte P.F., Del Tacca M. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann. Oncol. 2001;12:1301–1306. doi: 10.1023/a:1012294617392. [DOI] [PubMed] [Google Scholar]
- Duran J.D., Arias J.L., Gallardo V., Delgado A.V. Magnetic colloids as drug vehicles. J. Pharm. Sci. 2008;97:2948–2983. doi: 10.1002/jps.21249. [DOI] [PubMed] [Google Scholar]
- Fata F., Ron I.G., Kemeny N., O’Reilly E., Klimstra D., Kelsen D.P. 5-Fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer. 1999;86:1129–1134. doi: 10.1002/(sici)1097-0142(19991001)86:7<1129::aid-cncr5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Gottesman M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Hornig Stephanie, Heinze Thomas, Becer C. Remzi, Schubert Ulrich S. Synthetic polymeric nanoparticles by nanoprecipitation. J. Mater. Chem. 2009;19:3838–3840. [Google Scholar]
- Hu J., Wei J., Liu W., Chen Y. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a drug delivery system by emulsion electrospinning. J. Biomater. Sci. Polym. Ed. 2013;24:972–985. doi: 10.1080/09205063.2012.728193. [DOI] [PubMed] [Google Scholar]
- Huang C., Zhang X.M., Tavaluc R.T., Hart L.S., Dicker D.T., Wang W., El-Deiry W.S. The combination of 5-fluorouracil plus p53 pathway restoration is associated with depletion of p53-deficient or mutant p53-expressing putative colon cancer stem cells. Cancer Biol. Ther. 2009;8:2186–2193. doi: 10.4161/cbt.8.22.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Jalil R., Nixon J.R. Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: problems associated with preparative techniques and release properties. J. Microencapsul. 1990;7:297–325. doi: 10.3109/02652049009021842. [DOI] [PubMed] [Google Scholar]
- Karmi A., Husseini G.A., Faroun M., Sowwan M. Multifunctional nanovehicles for combined 5-fluorouracil and gold nanoparticles based on the nanoprecipitation method. J. Nanosci. Nanotechnol. 2011;11:4675–4683. doi: 10.1166/jnn.2011.4156. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Chae G.S., Kim M.S., Cho S.H., Lee H.B., Khang G. Degradation behaviour in vitro for poly(d,l-lactide-co-glycolide) as drug carrier. Biomed. Mater. Eng. 2004;14:185–192. [PubMed] [Google Scholar]
- Li X., Xu Y., Chen G., Wei P., Ping Q. PLGA nanoparticles for the oral delivery of 5-Fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studies. Drug Dev. Ind. Pharm. 2008;34:107–115. doi: 10.1080/03639040701484593. [DOI] [PubMed] [Google Scholar]
- Lin Y., Li Y., Ooi C.P. 5-Fluorouracil encapsulated HA/PLGA composite microspheres for cancer therapy. J. Mater. Sci. – Mater. Med. 2012;23:2453–2460. doi: 10.1007/s10856-012-4723-2. [DOI] [PubMed] [Google Scholar]
- Nair K.L., Jagadeeshan S., Nair S.A., Kumar G.S. Biological evaluation of 5-fluorouracil nanoparticles for cancer chemotherapy and its dependence on the carrier, PLGA. Int. J. Nanomed. 2011;6:1685–1697. doi: 10.2147/IJN.S20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh R.H., Parikh J.R., Dubey R.R., Soni H.N., Kapadia K.N. Poly(d,l-lactide-co-glycolide) microspheres containing 5-fluorouracil: optimization of process parameters. AAPS PharmSciTech. 2003;4:E13. doi: 10.1208/pt040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- Schmoll H.J., Buchele T., Grothey A., Dempke W. Where do we stand with 5-fluorouracil? Semin. Oncol. 1999;26:589–605. [PubMed] [Google Scholar]
- Shive M.S., Anderson J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Subbarayan P.R., Sarkar M., Nelson G., Benitez E., Singhal S., Ardalan B. Chronic exposure of colorectal cancer cells in culture to fluoropyrimidine analogs induces thymidylate synthase and suppresses p53. A molecular explanation for the mechanism of 5-FU resistance. Anticancer Res. 2010;30:1149–1156. [PubMed] [Google Scholar]
- Sui X., Kong N., Wang X., Fang Y., Hu X., Xu Y., Chen W., Wang K., Li D., Jin W., Lou F., Zheng Y., Hu H., Gong L., Zhou X., Pan H., Han W. JNK confers 5-fluorouracil resistance in p53-deficient and mutant p53-expressing colon cancer cells by inducing survival autophagy. Sci. Rep. 2014;4:4694. doi: 10.1038/srep04694. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun X.X., Dai M.S., Lu H. 5-Fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J. Biol. Chem. 2007;282:8052–8059. doi: 10.1074/jbc.M610621200. [DOI] [PubMed] [Google Scholar]
- van Kuilenburg A.B., Haasjes J., Richel D.J., Zoetekouw L., Van Lenthe H., De Abreu R.A., Maring J.G., Vreken P., van Gennip A.H. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin. Cancer Res. 2000;6:4705–4712. [PubMed] [Google Scholar]
- Wang Y., Li P., Chen L., Gao W., Zeng F., Kong L.X. Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles. Drug Deliv. 2015;22:191–198. doi: 10.3109/10717544.2013.875603. [DOI] [PubMed] [Google Scholar]
- Wong H.L., Bendayan R., Rauth A.M., Li Y., Wu X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2002. Cancer Incidence in Five Continents. In: The World Health Organization and The International Agency for Research on Cancer, Lyon.
- Zamboni W.C., Torchilin V., Patri A.K., Hrkach J., Stern S., Lee R., Nel A., Panaro N.J., Grodzinski P. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin. Cancer Res. 2012;18:3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]