Abstract
Tacrolimus is a potent immunosuppressant; however, it suffers from several problems such as poor water solubility (4–12 μg/mL), low and variable oral bioavailability in patients, and narrow therapeutic window that could not be solved by the currently available i.v. formulation (Prograf®). Moreover, Prograf® contains HCO-60 (PEGylated castor oil) as a surfactant, which is reported to cause several side effects including hypersensitivity reactions. Therefore, the aim of the present study was to investigate the potential of PEO-b-PCL polymeric micelles as alternative vehicles for the solubilization and delivery of tacrolimus. Four PEO-b-PCL block copolymers, with different molecular weights of PCL, were synthesized by ring opening polymerization of ε-caprolactone using methoxy polyethylene oxide (5,000 g mol−1) as initiator and stannous octoate as catalyst. Synthesized copolymers were characterized for their average molecular weights and polydispersity index by 1H NMR and gel permeation chromatography (GPC), respectively. Drug-free micelles of PEO-b-PCL were prepared through a co-solvent evaporation method using acetone as the organic co-solvent. Tacrolimus-loaded micelles were prepared using the same method with different initial amounts of drug. Prepared micelles were characterized for their mean diameter size and polydispersity of the micellar population by dynamic light scattering, and an HPLC assay was used to determine the encapsulation efficiency of tacrolimus. The average molecular weights of the synthesized copolymers were in the range of 8,400–28,000 with narrow distributions (PDI = 1.06–1.11). The copolymers were designated according to the degree of polymerization of ε-caprolactone, namely PEO114-b-PCL30, PEO114-b-PCL60, PEO114-b-PCL120, and PEO114-b-PCL200. All the prepared micelles were having diameters sizes less than 100 nm with narrow distributions. The highest drug solubilization was achieved with PEO114-b-PCL120, where the aqueous solubility of tacrolimus exceeded 300 μg/mL. Our results show a potential for PEO-b-PCL micelles as solubilizing vehicles for the delivery of tacrolimus.
Keywords: Block copolymer, PEO-b-PCL, Polymeric micelles, Tacrolimus, Drug delivery
1. Introduction
Tacrolimus is a 23-membered macrolide isolated from Streptomyces tsukubaensis. It is a potent immunosuppressive agent used clinically to reduce the risk of organ rejection in postoperative transplant patients (Rath, 2013). It binds to an immunophilin, FK506 binding protein 12 and creates a new complex (Halloran, 1996). This new complex inhibits the calcineurin phosphatase which prevents the translocation of activated T-cell transcription factor and promotes proliferation of helper T-cells which results in suppression of immune response associated with tissue or organ transplant (Thomson et al., 1995).
Tacrolimus is a BCS class II with low solubility and high permeability drug. Although it is a potent immunosuppressant, however, it suffers from several problems such as poor water solubility (4–12 μg/mL), low oral bioavailability, narrow therapeutic window, and high intra- and inter-subject variability (Venkataramanan et al., 1995). Low oral bioavailability of tacrolimus is due to various factors such as low solubility, extensive first pass metabolism in liver and gut, P-glycoprotein mediated drug efflux, influence of food intake and concomitant medication (Venkataramanan et al., 1995, Tamura et al., 2002). Moreover, the currently available i.v. formulation of tacrolimus (Prograf®) contains HCO-60 (PEGylated castor oil) as a surfactant, which is reported to cause several side effects including hypersensitivity reactions (Nicolai and Bunyavanich, 2012, Jang et al., 2015, Hisatomi et al., 1993).
Different pharmaceutical approaches such as prodrugs, cyclodextrin intrusion complexes, liposomes, nano lipid particles, pH sensitive microspheres, and self microemulsifying drug delivery system were applied to overcome the problems associated with tacrolimus delivery (Patel et al., 2012). All of these approaches were aimed to increase the water solubility of tacrolimus in order to achieve proper absorption from the gastrointestinal tract or to minimize the pre-systemic metabolism by cytochrome P450 and inhibition of P-glycoprotein mediated efflux.
In the present study, PEO-b-PCL polymeric micelles were used as vehicles to overcome the solubility problem of tacrolimus, and to serve as safer alternative to the HCO-60-based formulation. Polymeric micelles are self-assemblies of synthetic amphiphilic molecules in which hydrophobic ends make the core of micelles and hydrophilic ends make the shell. Polymeric micelles are generally made up of diblock or multiblock copolymers where individual block polymers/unimers are attached to each other with non-covalent interaction. The core of a micelle is hydrophobic and it helps in solubilization of hydrophobic drugs, while the shell is hydrophilic and helps in suspending the micelles in aqueous media. The advantages of PEO-b-poly(ester) micelles are biocompatibility, enhanced drug solubility, controlled release, and increased blood circulation time (Aliabadi and Lavasanifar, 2006).
Methoxy poly(ethylene oxide) (PEO) and poly(ε-caprolactone) (PCL) were selected to synthesize diblock copolymer for micelle preparation. PCL is a synthetic, lipophilic, semi-crystalline, and biodegradable polymer (Dash and Konkimalla, 2012). It forms the core of micelles and helps in solubilization of lipophilic drugs. PEO is a hydrophilic polymer that makes up the shell of micelles. It also adds the stealth property to the micelles, which helps in evading the reticulo-endothelial system (RES) and improving the blood compatibility of micelles (Lin et al., 2005, Otsuka et al., 2003). PEO-b-PCL diblock copolymers with different molecular weights of the core-forming block were synthesized by controlled ring opening polymerization of ε-caprolactone. A series of four polymers were synthesized and characterized by nuclear magnetic resonance (NMR) spectroscopy and gel permeation chromatography (GPC). Micelles were prepared using these four diblock copolymers with different drug to polymer ratios. The prepared micelles were characterized in terms of drug loading, encapsulation efficiency, diameter size, and polydispersity index.
2. Materials and methods
2.1. Materials
Tacrolimus was extracted from expired Prograf® 5 mg capsules (Batch # 7241), as previously described (Binkhathlan et al., 2015). Methoxy PEO (Mn 5,000), stannous octoate (∼95%), ε-caprolactone (97%), and THF (HPLC grade) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Acetonitrile (HPLC grade) was supplied by Fisher Scientific Co. (Leicestershire LE/15 RG, UK). Deionized water was prepared in-house using Millipore system.
2.2. Methods
2.2.1. Synthesis of PEO-b-PCL block copolymers
PEO-b-PCL block copolymers were synthesized by ring opening polymerization of ε-caprolactone using methoxy PEO (Mn 5,000) as an initiator and stannous octoate as a catalyst, as previously reported (Aliabadi et al., 2005). Briefly, methoxy PEO, ε-caprolactone and stannous octoate were added to a previously flamed ampoule, nitrogen purged, and then sealed under vacuum. The reaction proceeded at 140 °C for 4 h. Different ε-caprolactone to methoxy PEO feed ratios were used to synthesize PEO-b-PCL block copolymers with varying degrees of ε-caprolactone polymerization.
2.2.2. Characterization of the synthesized copolymers
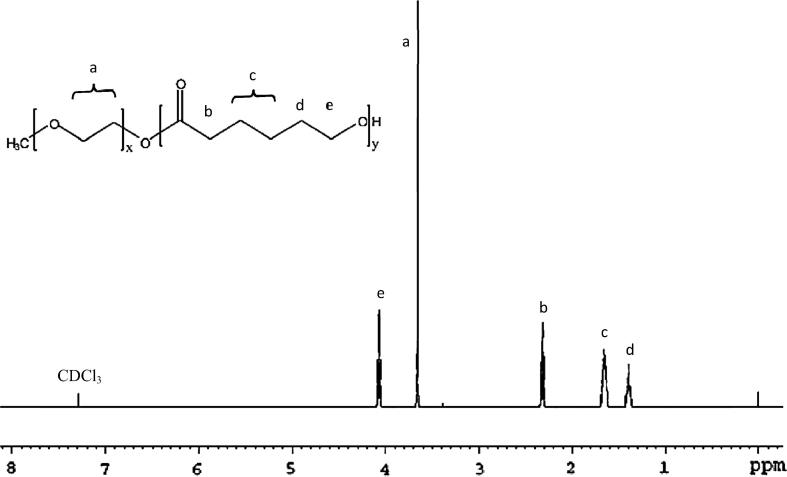
1H NMR spectrum of PEO-b-PCL in CDCl3 at 500 MHz (Bruker Ultra shield 500.133 MHz spectrometer) was used to determine the number averaged molecular weight of the block copolymers. The degree of polymerization of ɛ-caprolactone was estimated by comparing the peak intensity of PEO (–O–CH2–CH2; δ = 3.65 ppm) to that of PCL (–O–CH2; δ = 4.075 ppm). The number-averaged molecular weights, weight-averaged molecular weights and molecular weight distributions of the synthesized copolymers were determined by gel permeation chromatography (Viscotek TDA 305-040 Triple Detector Array, Viscotek Corp., Houston, TX, USA). Samples (100 μL from 15 mg/mL polymer stock solutions in THF) were injected into an 8.0 × 300 mm Viscotek T6000M column (Viscotek Corp., Houston, TX, USA) with guard column (Viscotek TGuard, Org Guard Col 10 × 4.6 mm, No. C14J0020). The mobile phase was THF delivered at a flow rate of 1 mL/min. The calibration curve was established with polystyrene standards (molecular weight range: 1,570–46,500 Da).
2.2.3. Preparation of micelles
Tacrolimus-loaded PEO-b-PCL micelles were prepared using co-solvent evaporation method. Briefly, predetermined amounts of the drug and the polymer in milligrams were dissolved in 1 mL acetone using three different drug-to-polymer ratios, namely 1:10, 2:10 and 3:10 w/w. This mixture was added in a dropwise manner to 6 mL water under continuous stirring and left for 4 h. The remaining acetone was removed by evaporation at room temperature under vacuum. After evaporation of acetone the colloidal solution was centrifuged at a speed of 13,000 rpm (15,890g) for 5 min. The supernatant was taken and characterized for micellar size, polydispersity, drug loading and encapsulation efficiency. Drug-free micelles were prepared in an identical manner without adding the drug.
2.2.4. Determination of particle size and polydispersity of micelles
Mean diameter and polydispersity of both drug-free and tacrolimus-loaded micelles in aqueous media were measured by dynamic light scattering (Zetasizer Nano ZS, Malvern Instrument Ltd., UK). The concentration of block copolymers was 10 mg/mL. All measurements were performed at 25 °C temperature and the results were reported as the mean ± standard deviation (SD) of three independent samples.
2.2.5. Determination of drug loading and encapsulation efficiency
100 μl of supernatant was diluted with 1 mL of acetonitrile and vortexed vigorously. 200 μl of the resulting mixture was further diluted 10 times using acetonitrile and vortexed again. The amount of tacrolimus in final mixture was determined using reversed phase high performance liquid chromatography (HPLC). The HPLC system (Waters™ 1500 series controller, USA) was equipped with wavelength detector (Waters™ 2489 a Dual™ Absorbance detector, USA), pump (Waters™ 1525 a Binary pump, USA), and an automated sampling system (Waters™ 2707 Plus Autosampler, USA). The HPLC system was monitored by “Breeze (Waters™)” software. Tacrolimus was analyzed by injecting 100 μL to a C18 column (Macherey-Nagel, 4.6 × 150 mm, 10 μm particle size). Acetonitrile and milli Q water (pH adjusted to 3 by orthophosphoric acid) at a ratio of 75:25 was used as a mobile phase. Flow rate of the mobile phase was 1 mL/min and detection was carried out at 215 nm. Column temperature was kept at 60 °C. Each sample was assayed for at least three times. Drug loading and encapsulation efficiency of drug loaded micelles were calculated using the following equations
2.2.6. Determination of the morphology of the assembled structure
Morphology of the assembled structures in the present study was characterized by transmission electron microscopy (TEM). An aqueous droplet of micellar solution (20 μL) was placed on a copper-coated grid (Ted Pella, Inc, USA). The grid was held horizontally for 20 s to allow the colloidal aggregates to settle. A drop of 2% solution of phosphotungstic acid (PTA) in PBS (pH = 7.0) was then added to provide the negative stain. After 1 min, the excess fluid was removed by a strip of filter paper [26]. The samples were then allowed to dry at room temperature and loaded into a JEOL JEM-1400 Transmission electron microscope (JAPAN) operating at an acceleration voltage of 120 kV. Images were recorded with a Gatan Ultrascan high-resolution digital camera and processed with JADAS (The JEOL Automated data acquisition system) software.
2.2.7. Statistical analyses
All data are reported as mean ± SD, unless otherwise indicated. Differences between the mean values were compared using analysis of variance (ANOVA) followed by post-hoc analyses using Tukey-Kramer multiple comparisons test. The level of significance was set at α = 0.05.
3. Results and discussion
Nanomedicine is an emerging field with a great potential to improve the safety and efficacy of drugs that are currently in the market or under development. Problems such as low aqueous solubility, limited oral absorption or bioavailability, poor pharmacokinetic profile, intolerable toxicity were solved by applying nano delivery systems (Farokhzad and Langer, 2009, Haley and Frenkel, 2008). Methoxy poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) copolymers are among the nanomaterials extensively used for drug delivery applications (Gou et al., 2011).
There are only few papers that reported the use PEO-b-PCL or PCL-b-PEO-b-PCL as delivery system for tacrolimus. Although some of these reports showed tacrolimus-loaded micelles with good properties, e.g. high encapsulation efficiency and a diameter size of less than 100 nm, the majority of these formulations used PEO with a molecular weight of 2000 Daltons (Allen et al., 1998, Wang et al., 2011, Wang et al., 2013). There are several studies that investigated the optimum molecular weight of PEO that provides the optimum “stealth” properties i.e. lowest protein (opsonins) adsorption and longer circulation time (Lin et al., 2005, Alexis et al., 2008, Fang et al., 2006). The results of these studies concluded that, by holding other factors constant, 5000 (degree of polymerization ∼114) was found to be the optimum molecular weight of PEO for development of drug delivery systems (Lin et al., 2005, Alexis et al., 2008, Fang et al., 2006). And, indeed, it is the most widely used in development of drug-loaded polymeric micelles. Therefore, the aim of the current study was to investigate the potential of PEO114-b-PCL polymeric micelles as vehicles for the solubilization and delivery of tacrolimus.
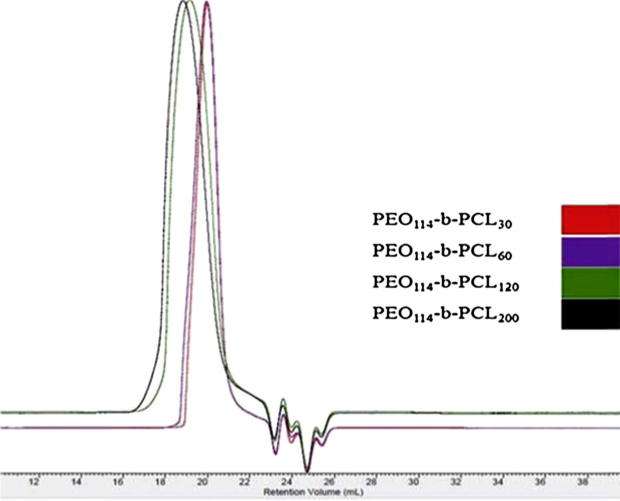
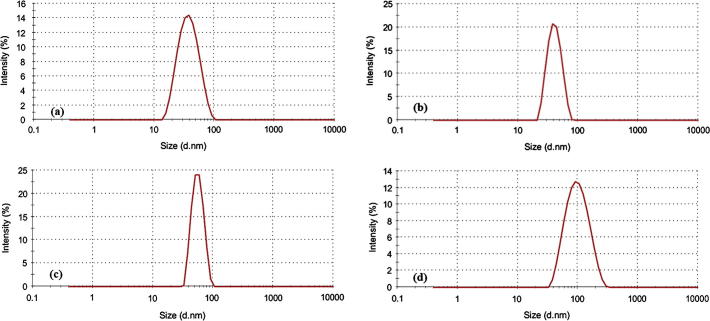
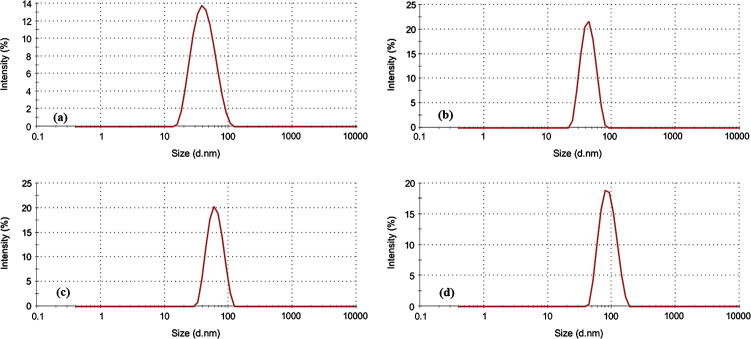
Here, we successfully synthesized PEO114-b-PCL with four different molecular weights of PCL. On the basis of the degree of polymerization of ε-caprolactone the synthesized polymers were designated as PCL30, PCL60, PCL120, and PCL200 as determined by 1H NMR (Table 1). The 1H NMR spectra did not show any traces of unreacted monomer (Fig. 1), and all the synthesized copolymers eluted as single peaks as shown in GPC chromatograms (Fig. 2). Table 1 shows a good agreement between the theoretical and the molecular weights calculated by 1H NMR and GPC. Diameter size of drug-free micelles was in the range of 38.09 ± 3.89 nm to 91.39 ± 6.76 nm (Fig. 3; Table 2). Micellar diameter of all tacrolimus-loaded micelles was in the range of 37.15 ± 4.10 nm to 86.99 ± 6.00 nm (Fig. 4; Table 2). Out of the four copolymers, the smallest micelle size was observed with PCL30 while PCL200 produced the largest. Moreover, the mean diameter size of all the prepared micelles was less than 100 nm.
Table 1.
Characteristics of the synthesized PEO-b-PCL block copolymers and drug-free micelles.
| Block copolymera | Theoretical MW (g/mol) | Mn (g/mol)b | Mn (g/mol)c | PDId | Size of micelles (nm)e | Polydispersitye |
|---|---|---|---|---|---|---|
| PEO114-b-PCL30 | 8,400 | 8,400 | 11,100 | 1.11 | 38.09 ± 3.89 | 0.15 ± 0.06 |
| PEO114-b-PCL60 | 11,800 | 11,800 | 12,300 | 1.14 | 39.35 ± 2.21 | 0.09 ± 0.01 |
| PEO114-b-PCL120 | 18,800 | 18,800 | 25,600 | 1.09 | 55.13 ± 2.70 | 0.06 ± 0.05 |
| PEO114-b-PCL200 | 28,000 | 28,200 | 35,000 | 1.06 | 91.39 ± 6.76 | 0.18 ± 0.06 |
Data are presented as mean ± SD (n = 3).
The number shown as a subscript indicates the polymerization degree of each block determined by 1H NMR.
Number-average molecular weight measured by 1H NMR.
Number-average molecular weight measured by GPC using PS standards.
Polydispersity index (Mw/Mn) determined by GPC.
Average diameter (Zave) and polydispersity were estimated by the DLS technique.
Figure 1.
A representative 1H NMR spectrum of PEO114-b-PCL120. The degree of polymerization of ɛ-caprolactone was estimated by comparing the peak intensity of PEO (–O–CH2–CH2; δ = 3.65 ppm) to that of PCL (–O–CH2; δ = 4.075 ppm).
Figure 2.
GPC chromatograms of synthesized PEO-b-PCL copolymers. Samples (100 μL from 15 mg/mL polymer stock solutions in THF) were injected into an 8.0 × 300 mm Viscotek T6000 M column (Viscotek Corp., Houston, TX, USA) with guard column. The mobile phase was THF delivered at a flow rate of 1 mL/min.
Figure 3.
Representative size distribution profiles of drug-free PEO-b-PCL micelles by dynamic light scattering (Zetasizer Nano ZS, Malvern Instrument Ltd., UK) for the following: (a) PCL30, (b) PCL60, (c) PCL120, and (d) PCL200. The concentration of block copolymers was 10 mg/mL.
Table 2.
Particle size, polydispersity, drug loading and encapsulation efficiency of tacrolimus in different micelles.
| Block copolymer | Drug: polymer ratio (w/w) | Drug loading (%)a | Encapsulation efficiency (%)a | Diameter of micelles (nm)b | Polydispersityb |
|---|---|---|---|---|---|
| PEO114-b-PCL30 | 1:10 | 1.70 ± 0.36 | 18.62 ± 3.92 | 41.22 ± 4.83 | 0.29 ± 0.12 |
| 2:10 | 1.26 ± 0.28 | 7.58 ± 1.69 | 37.15 ± 4.10 | 0.25 ± 0.07 | |
| 3:10 | 1.28 ± 0.44 | 5.56 ± 1.89 | 39.26 ± 2.00 | 0.31 ± 0.11 | |
| PEO114-b-PCL60 | 1:10 | 2.17 ± 0.37 | 27.85 ± 4.45 | 42.83 ± 1.88 | 0.14 ± 0.10 |
| 2:10 | 1.55 ± 0.54 | 7.25 ± 1.74 | 40.41 ± 1.88 | 0.13 ± 0.01 | |
| 3:10 | 1.66 ± 0.41 | 7.19 ± 1.79 | 45.19 ± 2.86 | 0.24 ± 0.08 | |
| PEO114-b-PCL120 | 1:10 | 2.82 ± 0.12 | 31.22 ± 1.57 | 54.73 ± 3.02 | 0.09 ± 0.05 |
| 2:10 | 1.92 ± 0.63 | 11.52 ± 3.80 | 58.01 ± 3.14 | 0.12 ± 0.03 | |
| 3:10 | 1.60 ± 0.20 | 6.94 ± 0.85 | 58.28 ± 1.14 | 0.10 ± 0.04 | |
| PEO114-b-PCL200 | 1:10 | 2.50 ± 0.41 | 21.41 ± 5.97 | 86.99 ± 6.00 | 0.14 ± 0.07 |
| 2:10 | 1.49 ± 0.29 | 8.91 ± 1.73 | 84.41 ± 4.40 | 0.10 ± 0.02 | |
| 3:10 | 1.75 ± 0.52 | 7.59 ± 2.24 | 83.83 ± 1.48 | 0.11 ± 0.02 | |
Data are presented as mean ± SD (n = 3).
The amount of drug in each formulation was determined by an HPLC assay.
Average diameter (Zave) and polydispersity were estimated by the DLS technique.
Figure 4.
Representative size distribution profiles of tacrolimus-loaded PEO-b-PCL micelles by dynamic light scattering (Zetasizer Nano ZS, Malvern Instrument Ltd., UK) for the following: (a) PCL30, (b) PCL60, (c) PCL120, and (d) PCL200. The concentration of block copolymers was 10 mg/mL.
Comparison of drug-free micelles prepared from different copolymers showed a significant (p < 0.05) increase in particle size with the increase in the PCL chain length from 60 to 120, and from 120 to 200. Similar effects were observed with drug-loaded micelles. Drug loading did not significantly alter the size of the micelles.
The morphology of the prepared tacrolimus-loaded micelles was studied by TEM, and it revealed that the micelles had spherical shape (Fig. 5). This is consistent with previous work performed on PEO-b-PCL micelles using the same preparation method as well as the same PEO/PCL ratios (Aliabadi et al., 2007).
Figure 5.
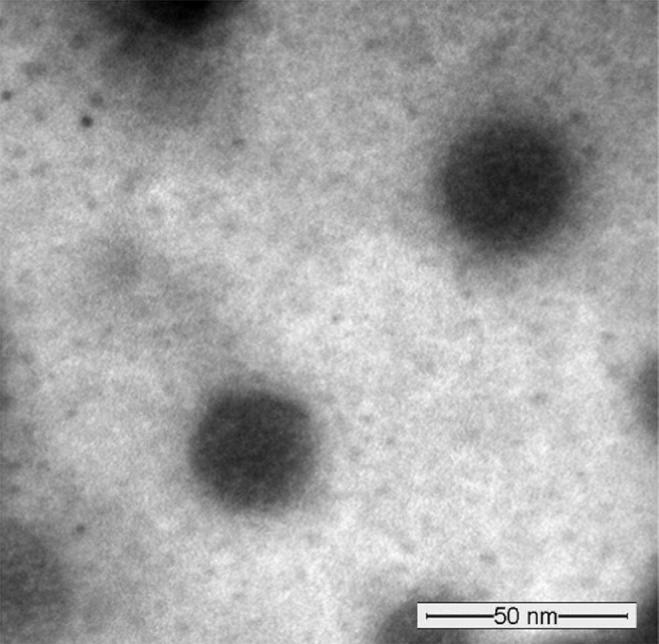
A representative TEM image obtained from tacrolimus-loaded PEO114-b-PCL120 micelles (at 1:10 drug-to-polymer ratio) using JEOL JEM-1400 Transmission electron microscope (JAPAN) operating at an acceleration voltage of 120 kV.
It is assumed that increasing the PCL chain length will increase the size of core and allow higher encapsulation of hydrophobic drug tacrolimus. Although the size of micelles prepared with PCL200 was highest, the encapsulation efficiency was comparatively lower than the micelles prepared with PCL120. This further confirms the encapsulation and drug loading are not only dependent upon the PCL chain lengths but also on the interaction of the copolymer with drug.
Results of drug loading and encapsulation efficiencies of different formulations are shown in Table 2. As the PCL chain length increased from 30 to 120 both drug loading and encapsulation efficiency increased. Further increase in PCL chain length to 200 decreased both drug loading and encapsulation efficiency. Moreover, in all four copolymers, increasing the drug to polymer ratio resulted in a decrease in both drug loading and encapsulation efficiency. However, the statistically significant (p < 0.05) difference in drug loading among all formulations was only seen between PCL30 and PCL120 at 1:10 drug:polymer ratio. Formulation prepared with PCL120 showed the highest drug loading and encapsulation efficiency, 2.82 ± 0.12% and 31.22 ± 1.57% respectively. This translates to an increase in aqueous solubility of tacrolimus from less than 12 μg/mL to 312.2 μg/mL i.e. a more than 25-fold increase in aqueous solubility of tacrolimus. This concentration is relevant to the one used clinically for intravenous administration of tacrolimus. Because as per the manufacturer instructions, Prograf® (5 mg/mL concentrate for solution for infusion) should be diluted with a suitable vehicle to reach a concentration in the range of 0.004–0.02 mg/mL prior to use. Based on the significant body of literature that supports the biocompatibility and safety of PEO-b-PCL copolymers (Bulcao et al., 2013, Fang et al., 2009, Gong et al., 2009, Kim et al., 2003), the use of PEO-b-PCL micellar formulation of tacrolimus would provide a clear advantage as being an “HCO-60-free formulation”.
4. Conclusion
PEO-b-PCL block copolymers with different molecular weights of the core-forming block were successfully synthesized, characterized and further formulated as micelles carrying tacrolimus as payload. All of the prepared micellar formulations were able to enhance the aqueous solubility of tacrolimus. Our results showed a potential for PEO-b-PCL micelles as solubilizing vehicles for the delivery of tacrolimus. Formulation prepared with PEO114-b-PCL120 at a 1:10 w/w drug polymer ratio has shown good particle size with best drug loading and encapsulation efficiency. Further studies including in vivo pharmacokinetic, efficacy, and toxicity are underway in order to establish the suitability of PEO-b-PCL micelles as a delivery system for tacrolimus.
Acknowledgment
The authors are grateful to the College of Pharmacy Research Centre and the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alexis F. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliabadi H.M., Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006;3:139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- Aliabadi H.M. Micelles of methoxy poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine A. J. Controll. Release: Official J. Controll. Release Soc. 2005;104:301–311. doi: 10.1016/j.jconrel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Aliabadi H.M. Encapsulation of hydrophobic drugs in polymeric micelles through co-solvent evaporation: the effect of solvent composition on micellar properties and drug loading. Int. J. Pharm. 2007;329:158–165. doi: 10.1016/j.ijpharm.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Allen C. Polycaprolactone-b-poly(ethylene oxide) block copolymer micelles as a novel drug delivery vehicle for neurotrophic agents FK506 and L-685,818. Bioconjug. Chem. 1998;9:564–572. doi: 10.1021/bc9702157. [DOI] [PubMed] [Google Scholar]
- Binkhathlan Z. Reutilization of tacrolimus extracted from expired Prograf(R) capsules: physical, Chemical, and pharmacological assessment. AAPS PharmSciTech. 2015 doi: 10.1208/s12249-015-0433-7. [DOI] [PubMed] [Google Scholar]
- Bulcao R.P. Acute and subchronic toxicity evaluation of poly(epsilon-caprolactone) lipid-core nanocapsules in rats. Toxicol. Sci.: Off. J. Soc. Toxicol. 2013;132:162–176. doi: 10.1093/toxsci/kfs334. [DOI] [PubMed] [Google Scholar]
- Dash T.K., Konkimalla V.B. Poly-small je, Ukrainian-caprolactone based formulations for drug delivery and tissue engineering: a review. J. Controll. Release: Official J. Controll. Release Soc. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- Fang C. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci.: Official J. Eur. Federation Pharm. Sci. 2006;27:27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Fang F. Acute toxicity evaluation of in situ gel-forming controlled drug delivery system based on biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) copolymer. Biomed Mater. 2009;4:025002. doi: 10.1088/1748-6041/4/2/025002. [DOI] [PubMed] [Google Scholar]
- Farokhzad O.C., Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- Gong C.Y. Acute toxicity evaluation of biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PEG-PCL-PEG hydrogel. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91:26–36. doi: 10.1002/jbm.b.31370. [DOI] [PubMed] [Google Scholar]
- Gou M. PCL/PEG copolymeric nanoparticles: potential nanoplatforms for anticancer agent delivery. Curr. Drug Targets. 2011;12:1131–1150. doi: 10.2174/138945011795906642. [DOI] [PubMed] [Google Scholar]
- Haley B., Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Halloran P.F. Molecular mechanisms of new immunosuppressants. Clin. Transplant. 1996;10:118–123. [PubMed] [Google Scholar]
- Hisatomi A. Toxicity of polyoxyethylene hydrogenated castor oil 60 (HCO-60) in experimental animals. J. Toxicol. Sci. 1993;18(Suppl 3):1–9. doi: 10.2131/jts.18.supplementiii_1. [DOI] [PubMed] [Google Scholar]
- Jang H.J. Safety Evaluation of Polyethylene Glycol (PEG) compounds for cosmetic use. Toxicol. Res. 2015;31:105–136. doi: 10.5487/TR.2015.31.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y. Toxic characteristics of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) nanospheres; in vitro and in vivo studies in the normal mice. Biomaterials. 2003;24:55–63. doi: 10.1016/s0142-9612(02)00248-x. [DOI] [PubMed] [Google Scholar]
- Lin W.J. Comparison of two pegylated copolymeric micelles and their potential as drug carriers. Drug Deliv. 2005;12:223–227. doi: 10.1080/10717540590952672. [DOI] [PubMed] [Google Scholar]
- Nicolai S., Bunyavanich S. Hypersensitivity reaction to intravenous but not oral tacrolimus. Transplantation. 2012;94:e61–e63. doi: 10.1097/TP.0b013e31826e5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka H. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- Patel P. Formulation strategies for drug delivery of tacrolimus: an overview. Int. J. Pharm. Investig. 2012;2:169–175. doi: 10.4103/2230-973X.106981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath T. Tacrolimus in transplant rejection. Expert Opin. Pharmacother. 2013;14:115–122. doi: 10.1517/14656566.2013.751374. [DOI] [PubMed] [Google Scholar]
- Tamura S. Tacrolimus is a class II low-solubility high-permeability drug: the effect of P-glycoprotein efflux on regional permeability of tacrolimus in rats. J. Pharm. Sci. 2002;91:719–729. doi: 10.1002/jps.10041. [DOI] [PubMed] [Google Scholar]
- Thomson A.W. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther. Drug Monit. 1995;17:584–591. doi: 10.1097/00007691-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R. Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 1995;29:404–430. doi: 10.2165/00003088-199529060-00003. [DOI] [PubMed] [Google Scholar]
- Wang Y. Preparation of Tacrolimus loaded micelles based on poly(varepsilon-caprolactone)-poly(ethylene glycol)-poly(varepsilon-caprolactone) Int. J. Pharm. 2011;407:184–189. doi: 10.1016/j.ijpharm.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Wang Y. Micelles of methoxy poly(ethylene glycol)-poly(epsilon-caprolactone) as a novel drug delivery vehicle for tacrolimus. J. Biomed. Nanotechnol. 2013;9:147–157. doi: 10.1166/jbn.2013.1489. [DOI] [PubMed] [Google Scholar]