Abstract
Quality of pharmaceutical product largely depends upon the environment controls during its storage and handling. Each pharmaceutical product should be handled and stored under specified storage condition labelled on product information data sheet or product pack. Hence the temperature excursions during receipt of raw materials, manufacturing of pharmaceutical products and distribution should be managed during entire product life cycle with holistic approach. The research is based on primary data and exploratory study through literature review. The temperature excursion may be observed during transportation of raw materials manufacturing as well as distribution of pharmaceutical products, which have potential to deteriorate the product quality. Temperature excursion in pharmaceutical industry should be recorded and reported to the manufacturer for further investigation and risk analysis. The concept of temperature excursions, its reasons, consequences and handling mechanism should be well understood to ensure the concerted efforts under the aegis of Quality Management System. Based on the reasons and consequences of temperature excursions during pharmaceutical operations, a system based quality management has been envisaged through this study. The concept and procedure to handle temperature excursion have evolved after this study which shall be useful to pharmaceutical industry as well as to medicine distributors and consumers.
Keywords: GMP, Temperature excursions management, Formulation, Quality by Design, Degradation products
1. Introduction
The pharmaceutical product quality largely depends upon the storage environmental conditions. Natural reasons or human negligence could create uncalled-for situation causing temperature excursions. The most important environmental parameter having significant potential to impact quality of pharmaceutical product is temperature. If the temperature excursions are not handled systematically, there shall be an adverse impact on product quality.
There is a growing need to manage the environment excursions during pharmaceutical operations and its impacts on quality of products. In an era of Quality by Design (QbD) for pharmaceutical products, the attention is paid towards inbuilt quality instead of inspected quantity (Roy et al., 2012). As manufacturers have extensive knowledge about critical product and process parameters and quality attributes, the impact assessment has to be extended to temperature excursions. The temperature and relative humidity (RH) beyond limit shall lead to product degradation rate and microbial growth. This concept is the theoretical basis for the pharmaceutical guidelines that provide recommendations for long-term, intermediate, and accelerated storage conditions and for establishing shelf life periods or expiry dates of products (Scrivens, 2012).
Pharmaceutical regulatory bodies expect strict adherence of Good Manufacturing Practices (GMP) and Good Distribution Practices (GDP) during plant manufacturing and product distribution processes. GMP and GDP are deemed as synonyms of Quality System in pharmaceutical business. Since temperature excursions are observed during raw material receipt, manufacturing operation and distribution of pharmaceutical products, there is a need of holistic approach of quality system which shall be based on both GMP and GDP.
1.1. Research methodology
The following instruments have been used to generate data for the study:
-
(a)
A survey has been conducted amongst pharmaceutical professionals to understand their experience regarding environmental condition during pharmaceutical manufacturing and that during distribution process.
-
(b)
The guidance papers issued by drug regulatory agencies and related literature and scientific search engines such as Google were searched for pharmaceutical supply chain risk management studies in English language. Searching through databases was done with different keywords: supply chain risk, Good Distribution Practices, Quality Risk Management, and pharmaceutical. Searching in each database was adapted to databases characteristics and additionally pharmaceutical risk. The result studies and meeting abstracts were screened at 4 steps and exclusion process was based on consensus of both the authors.
1.2. Data and analysis
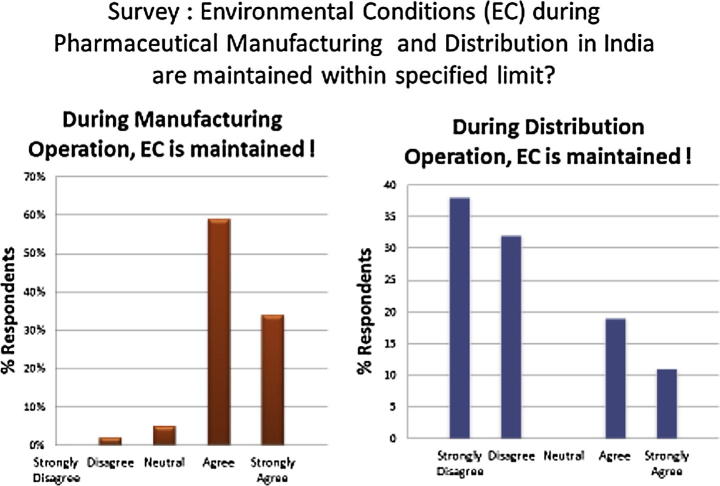
The research survey study amongst pharmaceutical professionals in India reveals that the records of environment condition (EC) monitoring during manufacturing and distribution operations follow a contrast trend (refer Chart 1). The survey alludes that deployment and monitoring of data logger results during manufacturing as per GMP are in place, whereas that during distribution operation is not so methodological.
Chart 1.
Trend of Environment Condition (EC) monitoring practices during manufacturing and distribution operations.
As a part of Quality Management System (QMS), pharmaceutical industry has identified a number of core quality elements which are followed at manufacturing site level. Few of such core elements of plant quality system may be listed as,
-
a.
Documents and Record Control: There shall be good documentation practices in organization to ensure the document and online records are adequately maintained.
-
b.
Deviation Control: Incidences leading to departure from documented and approved instructions shall be recorded and evaluated for potential impact on product quality.
-
c.
Change Control: The changes to an approved design, equipment or system in pharmaceutical facility shall be adequately reviewed and validated.
-
d.
Validation Master Plan: The validation master plan shall exhibit management philosophy, strategy and commitment of organization towards validations of processes and qualification of equipment.
-
e.
Quality Risk Management: Quality risk to product shall be identified and evaluation shall be made to estimate the severity, occurrence and detectability. A robust quality risk management.
-
f.
Training and Awareness: The organization shall develop and implement robust training programme for personnel engaged in GMP operations.
-
g.
Market Complaint Handling system: There shall be a documented procedure to receive, log and investigate each market complaint to further facilitate necessary corrective and preventive action.
-
h.
Recall Management, etc.: There shall be documented procedure to handle the recall or market returned goods.
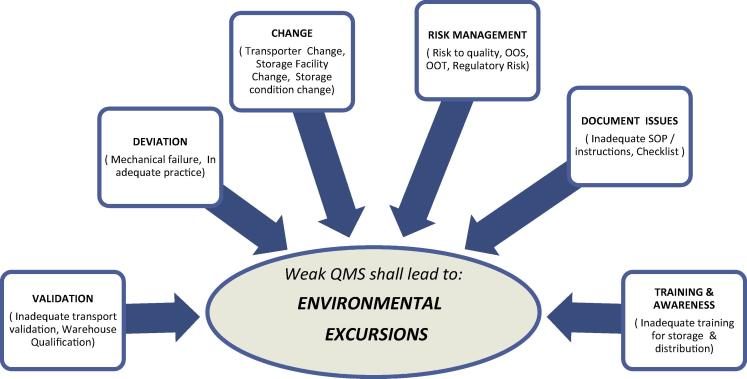
It is observed that inadequate QMS components have direct impact on consistency of storage conditions with respect to temperature and humidity. The temperature excursion is a common notion which signified the general environmental excursions (see Fig. 1).
Figure 1.
Effect of inadequate Quality System element can cause Environmental Excursions (EC).
A research survey amongst pharmaceutical professionals finds that the core quality assurance aspects are followed by each manufacturing site as a part of cGMP but during distribution practices (out of plant) the above quality elements are not followed meticulously. The environmental excursion management is interlinked with Product Complaint Management, Quality Risk Management, Deviation Management and Change Control Management, sometimes as a cause or else as an effect.
2. Discussions – facets of temperature excursion
Quality and environmental excursions are two important aspects of pharmaceutical operational excellence. An integrated approach for managing the quality system should include the temperature excursion management. The overall temperature excursion management can be laid down in following steps.
2.1. Understanding temperature excursion
The environmental condition during pharmaceutical business is defined by temperature (T) and relative humidity (RH). The temperature and relative humidity are used and monitored during manufacturing processes particularly when the product intermediates are exposed to environment. During packaged conditions temperature is measured, monitored and controlled. Any spike in measured value of T and RH shall be construed as environmental excursion. During manufacturing the temperature is always maintained below 27 °C, and whenever any deviation from this limit is observed, the case is thoroughly investigated and impact on product quality is mitigated.
According to European Compliance Academy, a temperature excursion is the deviation from the labelled storage condition of a product for any duration whether during transportation or distribution. Studies indicate that if there is exposure of product or intermediate beyond specified environmental limits for substantial time, there shall be generation of impurities as result of product degradation. Such degradation products are not only regarded as undesired but also shall have adverse reaction to the patient’s health.
The temperature excursion phenomena are also applicable during manufacturing and transportation of active pharmaceutical ingredients (API) prior to receipt at pharmaceutical manufacturing site. Deviation against storage condition can lead to significant qualitative change in API, such as degradation, decomposition, polymerization and impurity level increase.
2.2. Storage temperature and humidity limits
Specified directions are stated in some monographs with respect to temperature and humidity at which official articles shall be stored and distributed (including the shipment of articles to the customer), when stability data indicate that storage and distribution at a lower or higher temperature and humidity produce undesired results.
Cold chain products are those products which have to be necessarily stored under cold condition (refer Table 1). For products from class of cold chain, the storage condition is maintained at 2–8 °C. Similarly the relative humidity shall be maintained below 60% and 40% depending upon the hygroscopic nature of product.
Table 1.
Typical storage conditions.
| Label | Temperature details as per USP |
|---|---|
| Freezer | Indicates a place in which the temperature is maintained thermostatistically between −25 and −10 °C |
| Cold | Any temperature not exceeding 8 °C. The “refrigerator” is a cold place in which the temperature is maintained between 2 and 8 °C |
| Cool | Any temperature between 8 and 15 °C |
| Control room temperature | A temperature maintained between 20 and 25 °C, that results in a mean kinetic temperature (MKT) calculated not to be more than 25 °C and that allows EXCURSIONS between 15 and 30 °C |
United States Pharmacopoeia (USP) has described the different labelling terminologies related to temperature conditions. The general chapter of USP clarified various storage labelling conditions.
2.3. Measurement devices
Temperature and Humidity measuring devices (popularly known as data loggers) are available in market, which log the temperature at a defined (preset) interval that can be downloaded in computer system for review, evaluation, investigation and record. Periodic verification of calibration status of temperature data loggers and upgradation of software is the prerequisite of uninterrupted and accurate information about product storage condition.
Calibration and periodic verification of measurement device are keys of correct recording. In addition to that periodic cleaning of measuring device and appropriate precautions should be taken to avoid blockage or damage of sensor. It is recommended to use a temperature measurement reference instrument which is of higher accuracy than the device to be checked. The temperature and relative humidity sensor should be placed on the hottest spot, concluded after temperature mapping of the area.
2.4. Locations where environmental or temperature excursions may take place
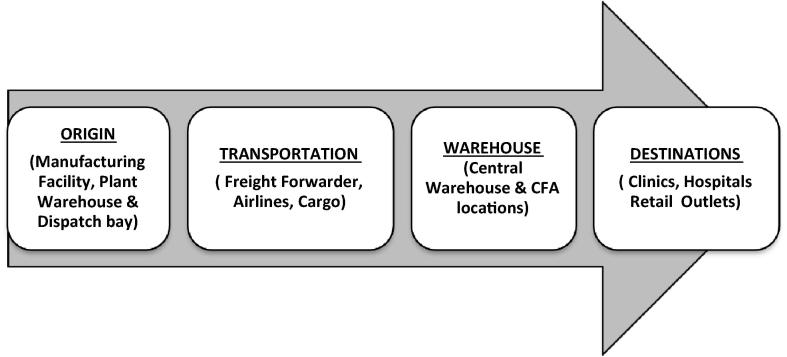
To manage the temperature excursion related issue, it is important to know the places where temperature excursion can occur. The deviations can be observed against the temperature limits not only at manufacturing sites or during transportation or distribution rather, it can be caused at the end of business i.e., retail outlets and drug shops (see Fig. 2).
Figure 2.
Locations wherein excursions can occur.
2.5. Reason of temperature excursion
In pharmaceutical manufacturing plant and storage area, the specified temperature is maintained with help of air handling units (AHU). The design and capacity of AHU are selected on the basis of volume of work area or manufacturing cubical.
2.5.1. Temperature excursions in manufacturing area caused due to following reasons (not limited to)
-
a.
Inadequate number of air handling unit (AHU) installed which are actually required to maintain the desired temperature conditions inside manufacturing shop floor.
-
b.
Leakage or rupture from air duct, resulting in insufficient cooling effect.
-
c.
Mechanical failure in air handling units (AHU), such as breakdown.
-
d.
Power failure making the AHU operation defunct.
-
e.
Lack of quality system and weak discipline of Good Manufacturing Practices (GMP) on production shopfloor.
-
f.
General awareness about the consequences of not maintaining temperature within limit and undue keenness to prevent power consumption.
-
g.
Extreme weather changes and obsolete contingency plan to handle unprecedented temperature fluctuations.
2.5.2. During transportation of pharmaceutical product, following reasons (not limited to) cause temperature excursion
-
a.
Unexpected delay in transportation due to which the temperature control cannot be maintained effectively.
-
b.
Product pallets are kept in hot zones of airport or shipping yards.
-
c.
Reefer containers or refrigerated control vans are not deployed for transportation.
-
d.
The transport agency fails to maintain the planned transport condition.
-
e.
Higher cost to maintain temperature within limits and other business issues.
-
f.
Power failure due to short longevity of power bank during longer travel time.
-
g.
Good Distribution Practices (GDP) understanding amongst supply chain personnel about the adverse impact on product quality.
2.6. Consequences of temperature excursion
The storage condition for product is assigned on the basis of scientific studies to avoid deterioration during product life cycle. If the temperature excursion is not taken due care the following negative impacts are commonly noticed:
-
a.
Loss of assay.
-
b.
Increase of impurity.
-
c.
Separation of layers of liquid products.
-
d.
Change in dissolution pattern of solid dosage.
-
e.
Discolouration of products.
Achieving the stability by design for solid dosage pharmaceutical products shall require establishment of limits for storage temperature (T) and relative humidity (RH). Within these prescribed temperature (T) and relative humidity (RH) limit, the kinetics of degradation of a pharmaceutical product can be estimated using and extended Arrhenius model (Porter, 2013).
An excursion can have a significant impact on quality of products, which can be investigated as a part of therapeutic drug properties. As a consequence of excursion, the following impact can be observed (see Fig. 3).
Figure 3.
Impact on quality and therapeutic drug properties due to temperature.
2.7. Review of sensitivity of drug products towards temperature excursions
To control temperature excursions is often complicated because there is no way to predict the condition that the product will be exposed to. The management of temperature excursion becomes more important particularly for the drug products, which are sensitive towards temperature conditions. Due care against potential excursions is mandatory during the course of drug manufacturing as well as during distribution process.
2.7.1. Product sensitive towards higher temperature
Drug product sensitive to higher temperature excursions can depreciate the intended action by receiving the thermal energy. This may instigate,
-
•
Transformation of degraded ingredients because of oxidation, hydrolysis, decomposition, polymerization. As longer as the time of exposure of product to unspecified temperature, higher would be the impact on quality.
-
•
Modification of the drug dissolution pattern (either higher or lower) in case of solid dosage drug product.
-
•
Separation of emulsions.
2.7.2. Product sensitive towards lower temperature
The adverse impact on product quality is not observed only due to exposure to higher temperature. Drug product sensitive to lower temperature can also depreciate the intended action by losing their therapeutic characters. This may cause,
-
•
Lattice positions dislocation due to extremely lower temperature.
-
•
Change in property of biological products.
2.7.3. Cold chain products
A deficiency in monitoring and maintenance system usually affects the products’ therapeutic properties and causes quality risks such as lack of effect, intoxications. In case of cold chain products the challenge of temperature excursions is bigger, because there is a task to preserve the adequate storage and temperature conditions throughout the product life cycle. Quality Assurance personnel must make sure that conditions of storage are observed at any time during manufacturing, transport and distribution.
2.7.4. In case of lyophilized drug products
Lyophilization process is commonly used for pharmaceuticals/biopharmaceuticals to improve the stability and shelf life. A disruption of the initial freezing rate due to temperature excursion can potentially lead to incomplete crystallization of crystalline excipients or heterogeneous moisture distribution in the lyophilized products. Temperature excursion during drying can lead to collapse or there could be melt back related negative impact on product quality.
3. Result – solution strategy and model for temperature excursion management
3.1. Solution strategy against excursions
In view of significant impact on product quality due to environmental excursions, the pharmaceutical industry should establish a documented programme for ‘Temperature Excursion Management’. As a part of this programme, the storage condition database and standard operating procedure (SOP) are required. The details of strategy are as under.
3.1.1. Development of storage condition database
To handle the environment excursion, the strategic planning, effective packaging and well documented procedure are recommended. Development of storage database of pharmaceutical product is useful to assign the standard storage condition.
The storage conditions suitable for product are assigned through the following information:
-
a.
At the product development stage, the semi finished product and final pharmaceutical product dosage are subjected to challenging conditions (i.e., forced conditions) to observe the potential impact on quality attributes.
-
b.
Hold time studies are carried out to establish the database for allowable time period at specified storage condition without impacting quality. The quality attributes of product intermediates include chemical, microbiological and pharmacological determinants at various time points of stability.
-
c.
Studies of the long term (i.e., real time) and accelerated condition stability study data of each product formula form an assurance for the storage condition that shall be suitable to product safety. The real time stability data are generated in laboratory to assess the change in quality attributes of product throughout the expiration date. The accelerated stability data are generated to evaluate the impact on quality of product under slightly stressed environmental condition.
A freeze/thaw study for multiple cycles should be conducted to specify the effect of freezing, if any, and the subsequent thawing. Samples from different layers (top, middle and bottom) of container are drawn for analysis at the end of the cycle (see Fig. 4).
-
a.
Know your formula.
-
b.
Carryout Quality Risk Analysis.
-
c.
Establish Stability Study Protocol.
-
d.
Evaluate Stability Study Data.
-
e.
Improve Product Formula.
-
f.
Redefine Product Formula.
Figure 4.
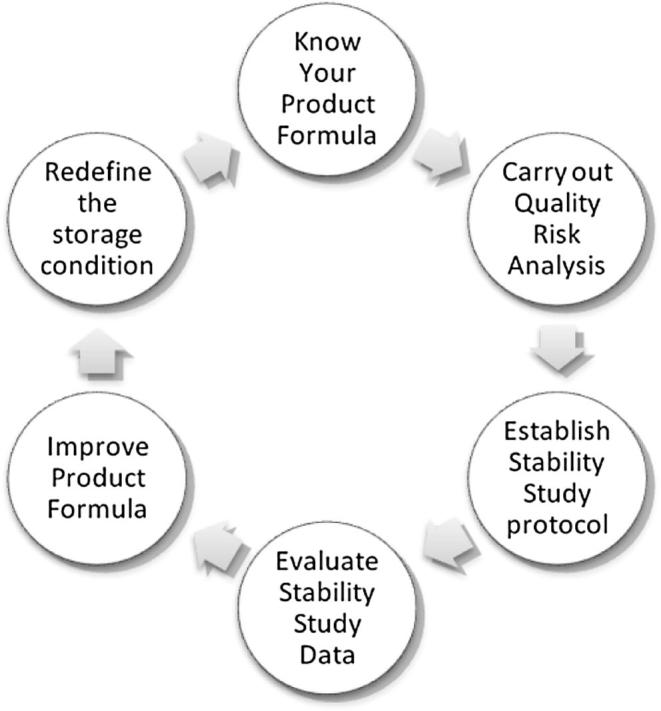
Temperature Management and Control Cycle.
3.1.2. Thermal packing during transportations
Packaging configuration should include the provision of thermal packing and display of appropriate storage condition. Caution notes to avoid storage outside the specified conditions shall help supply chain personnel to protect the product quality.
The storage condition should be effectively displayed on the packaging of pharmaceutical product. The packaging configuration card must contain the details of data loggers.
3.1.3. Ten step procedure to deal with temperature excursions
The temperature excursion has regulatory implications as well as business impact due to impact on quality.
A standard operating procedure (SOP) compromising of following steps should be established and adherence should be ensured through adequate training to concerned personnel:
-
i.
The provision of technical agreement should be laid down in SOP. Technical agreement between manufacturer and distributor should clearly assign the responsibility of notifying the details of environmental excursions during transit, storage and distribution process. A contract is a written agreement between two or more interested parties which creates obligations that are enforceable by law. Before services are provided by a vendor, a contract must be put in place and executed by the parties.
- A typical contract agreement should be executed between the following:
-
–Active Pharmaceutical Ingredient (API) supplier – Pharmaceutical product manufacturer.
-
–Active Pharmaceutical Ingredient (API) supplier – transporter, cargo.
-
–Pharmaceutical product manufacturer – transporter, cargo, etc.
-
–
-
ii.
Product characteristics database should be accessible to all concerned personnel for ready reference. Such database should clearly mention the storage instruction and precautions prominently highlighted on packs of product. Ideally larger display of storage condition on shipment pack shall alert the distribution agencies to avoid potential excursions.
-
iii.An operational checklist on the basis of Integrated Quality Management System (QMS) approach should include Qualification status of
-
(a)manufacturing facility with special attention on environmental controls,
-
(b)transport media (motor van, shipping containers, air cargo, etc.),
-
(c)replenishment and handling devices,
-
(d)storage warehouse in loaded conditions,
-
(e)distribution centres, and
-
(f)drug seller’s outlet and pharmacy.
-
(a)
-
iv.
Standard Operating Procedure (SOP) should be in place for each critical steps of manufacturing and distribution that may have trigger temperature excursions. Adequate training (in the form of personalized, awareness or document read) should be imparted to all operation executives. The key personnel across the pharmaceutical business should be aware about the SOP on investigation, handling and managing the temperature excursions.
-
v.
Records related to temperature excursions and duration should be regularly reviewed and approved to ascertain whether an excursion may have occurred and a systematic investigation should be performed. This should be formally signed off physically or electronically.
-
vi.
Investigation report against temperature excursions and duration should be notified immediately to the responsible person (as per the technical agreement) or manufacturer in a timely fashion.
-
vii.
Product quality risk analysis should be carried out against each case of temperature excursions. As a part of risk evaluation, the specimen complaint sample and control sample (retained by manufacturer) may be simultaneously analysed by using the validated analytical method. Due focus should be there to estimate the loss of assay as well as increase in impurity in sample impacted due to temperature excursion.
-
viii.
The stability data should be available with manufacturer against each excursion to evaluate and justify that there is no impact on product quality due to the excursions. The stability study under accelerated condition and freeze–thaw study are the relevant to evaluate the impact on product quality in a scientific manner.
An accelerated stability study programme in line with ICH:Q1 guidelines should be carried out.
A typical freeze–thaw study comprises of estimating the quality impact due to storage of product at extremely low and high temperatures, such as −20 and +50 °C for a duration up to 12 days in multiple cycles depending upon the proposed route, time and length of travel (Adadevoh, 2002, GCC Guidelines, 2007).
-
ix.
The statistics of temperature excursion cases should be evaluated periodically by quality professionals and in case of recurring observation of excursions are noticed from a particular facility, that particular mode or facility should be subjected to requalification.
-
x.
Appropriate corrective actions should be taken to avoid the recurrence of temperature excursions, if any. Modify the storage and transportation conditions on the basis of quality risk management programme.
4. Conclusion
Temperature excursion is a general term that represents the environmental excursions. There is a need of holistic approach to handle the temperature excursions starting from raw material manufacturing site to medicine retailers shop to protect quality of product. The temperature excursion at any stage of pharmaceutical business operation must be reported as soon as possible and investigated appropriately. The consequences of deviation against temperature and humidity limits should be studied appropriately by quality assurance personnel. The risk of temperature excursions cannot be ruled out, but it can be minimized through effective system. Alternative is to use thermal resistant packaging and stringent control measures during transit and shipment, to avert the undesired quality impact on pharmaceutical product. The systematic approach to handle the issues related temperature excursions becomes inevitable for pharmaceutical manufacturers.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adadevoh, K., 2002. Short-term, Freeze Thaw and Shipping Studies. ARMWG RFP11 Report.
- The GCC Guidelines for Stability Testing of Drug Substances and Pharmaceutical Products, 2007, Edition Two (1428 H – 2007G).
- Porter William R. Degradation of pharmaceutical solids accelerated by changes in both relative humidity and temperature and combined storage temperature and storage relative humidity (T × h) design space for solid products. J. Valid. Technol. 2013;19(2) [Google Scholar]
- Roy Souvik, Ruitberg Chiristian, Sethuraman Ananth. Troubleshooting during the manufacturing of lyophilized drug product – being prepared for the unexpected. Am. Pharm. Rev. 2012 [Google Scholar]
- Scrivens G. Mean kinetic relative humidity: A new concept for assessing the impact of variable relative humidity on pharmaceuticals. Pharm. Technol. 2012;36(11) [Google Scholar]