Abstract
Swietenia macrophylla (SM) is a medicinally important plant found in tropical and subtropical regions of the world. The ethyl acetate fraction of the seeds of S. macrophylla (SMEAF) is reported to exhibit potent anticancer, antitumor, anti-inflammatory and antifeedant activities. Till date, there have been no studies reported on the acute oral toxicity profile of the ethyl acetate fraction of the seeds of SM. The objective of the present study was to determine the acute toxicity of SMEAF and evaluate the in-vitro neuroprotective activity of SMEAF using primary neuronal cell cultures. In acute oral toxicity study, the SMEAF did not produce any lethal signs of morbidity and mortality. Histo-pathological findings, support the safety of SMEAF, as there were no significant changes observed in any of the parameters studied. Based on the results obtained in MTT assay, we infer that SMEAF has a significant neuroprotective effect, as it increased the cell viability and exhibited protection to the neuronal cells against TBHP induced oxidative stress. Thus, SMEAF can be suggested for use in the development of herbal drug formulations with neuroprotective potential.
Keywords: Swietenia macrophylla, Ethyl acetate extract, Neuroprotection, Oxidative stress, Acute oral toxicity
1. Introduction
Medicines from natural origin have been used as a source of remedy for the prevention, cure and treatment of different ailments in Traditional Systems of Medicine (TSMs) (Rates, 2001). Therapeutically important plants in TSMs have been extensively explored recently, for their benefits and various applications in herbal health supplements (Borris, 1996, Gurib-Fakim, 2006, Katiyar et al., 2012). The World Health Organization – Traditional medicine strategy published recently states that traditional and Complementary medicines have gained a growing economic importance worldwide (Anon., 2013).
Swietenia macrophylla plant is an important medicinal plant indigenous to tropical and subtropical regions of the world, and it belongs to Meliaceae family. It is considered as one of the functional foods in Malaysia. Literature survey reveals publications that report the presence of limonoids, swietenioides, methyl angolensate and diacetyl swietenioides (Goh et al., 2010, Goh and Kadir, 2011, Mootoo et al., 1999). Traditionally, the seeds have been used as anti-hypertensive, anti-diabetic and anti-inflammatory agents (King et al., 2013). Commercially, the fruits have been used in the formulation of health products for blood circulation (Eid and El-Enshasy, 2013). S. macrophylla has also been reported for its hypolipidemic, anti-infective, anticancer, antimicrobial, and antidiarrheal effect (Goh et al., 2010, Kalpana and Pugalendi, 2011). In a recent study, the ethanolic extract of S. macrophylla fruits has been reported to possess potential neuropharmacological benefits with positive antinociceptive effect (Das, 2009). The objective of the present study was to investigate the acute oral toxicity profile of ethyl acetate fraction from S. macrophylla seed extract (SMEAF) in murine models and evaluate the neuroprotective potential of SMEAF using primary neuronal culture. The acute toxicity study was carried out in mice, as per the standard protocol of the Organization for Economic Cooperation and Development (OECD) guidelines.
2. Materials and methods
2.1. Plant material and preparation of crude extracts
The dried seeds of the S. macrophylla (500 g) were procured from the local market and authenticated, and a voucher specimen (No. KLU046901) was deposited in the Herbarium of Institute of Biological Sciences, Faculty of Science, University of Malaya, Malaysia.
The crude extract was prepared, after soaking 500 g of ground sample in ethanol for 72 h with occasional stirring at room temperature. The extraction solvent was then decanted and concentrated using a rotary vacuum evaporator at 40 °C. The obtained extract was further extracted with n-hexane repeatedly. The obtained n-hexane extracts were combined, dried with anhydrous sodium sulfate, filtered and concentrated with a rotary vacuum evaporator. The hexane insoluble residue was then subjected to solvent–solvent extraction and partitioning using ethyl acetate and water in the ratio of 1:1. Both layers were separated, filtered and evaporated to dryness to obtain ethyl acetate and aqueous fractions. The dried ethyl acetate fraction was dissolved in DMSO and used for acute oral toxicity and neuroprotection studies (Supriady et al., 2015).
2.2. Animals
Toxicity study was conducted on 12 healthy male Balb/c mice, with body weights ranging between 18 and 22 g, with age of about 7 weeks. Animals were obtained from the animal facility of Monash University Malaysia and maintained under standard husbandry conditions such as temperature 25 ± 2 °C, relative humidity of 50 ± 10%, 12 h light and dark cycle, stress free environment and provided with standard diet and sanitary conditions. All the experimental procedures related to the animal study, were approved by Monash University Animal Ethical Committee (MARP/2015/040) and were conducted following the standard protocol for animal use and care.
BALB/c mice were selected for this study, as several studies have reported this strain as a good strain for toxicity evaluation. Also, the merits of using BALB/c mice in toxicity studies include their attributes, like low incidence of mammary tumors, longer life spans, resistance to development of atherosclerosis due to dietary factors and good responses in the production of monoclonal antibodies (Heshu et al., 2014, Potter, 1985).
2.3. Acute oral toxicity study and LD50 determination
The OECD guideline number 425 up and down procedure is used to minimize the number of animals required for acute oral toxicity testing. In the present study, OECD guideline no. 425 was employed for the determination of LD50 value of SMEAF and changes in general behavior were evaluated according to the Irwin test (Roux et al., 2004). The mice were distributed into two groups, SMEAF and control (OECD, 1994). Animals were fasted overnight before the experiment, and were administered with a single dose of SMEAF (2000 mg/kg)/vehicle and observed for various behaviors described in Irwin test. Special attention was given to first 4 h, followed by 24 h monitoring for any signs of morbidity and mortality. Based on the results of the short-profile study of the extract, the dose of the next group of animals was determined as per the OECD guideline no. 425. The LD50 of the test extract was calculated using AOT 425 software provided by the Environmental Protection Agency (EPA), USA.
2.3.1. Clinical observations, body weight, water and food consumption
After the administration of a single dose of SMEAF, mice were observed initially for 4 h for any abnormal clinical signs and once daily for 14 days. The parameters were recorded for both, the treatment and control groups (Roux et al., 2004).
All the mice were observed and recorded daily for changes in posture, skin, fur, eyes, behavior, morbidity and mortality as per Irwin’s test. On the first day before treatment, the body weight of animals was measured and recorded, followed by daily observations and recording thereafter, prior to necropsy. Food and water consumption was measured and recorded on a daily basis throughout the duration and prior to necropsy.
2.3.2. Hematological and biochemical studies
Hematological and biochemical studies were performed by using the standard method as described by Jothy et al. (2011). On the 15th day, animals were anesthetized to collect blood samples for hematological and biochemical analysis. No mortality was recorded as all the animals from both the groups survived till the end of the study period. Mice were fasted overnight prior to blood collection. Before carrying out necropsy procedure, all the mice were weighed and euthanized by CO2 asphyxia. Immediately after euthanization, 500 μL of blood sample was collected from each animal through cardiac puncture into both EDTA – containing and non-heparinized tubes for hematological and biochemical analyses, respectively.
The hematology studies were carried out for complete blood count (CBC) which includes hemoglobin levels (HGB), hematocrit levels (HCT), mean cell hemoglobin levels (MCH), mean cell hemoglobin concentration (MCHC), mean corpuscular volume (MCV), red blood cell count (RBC), white blood cell count (WBC), and blood platelet count (PLT). It also includes a differential count i.e.; neutrophil (N), lymphocyte (L), monocyte (M), eosinophil (E), basophil (B), and immature granulocyte (G).
The biochemical measurements included liver and kidney function tests. Liver function test involved estimation of total protein (TP), albumin (ALB), globulin (GLOB), total bilirubin (TB), conjugated bilirubin (CB), alkaline phosphatase (ALK), G-glutamyl transferase (GT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST).
2.3.3. Necropsy and histopathology
After the collection of the blood for hematology and biochemistry studies, the animals were decapitated and vital organs were excised and weighed. The vital organs include brain, heart, lungs, kidney, lever, spleen and testes.
All the seven vital organs collected, were transferred to 10% formalin for fixation and then embedded into paraffin to prepare paraffin blocks and sectioned with a semi automatic rotary microtome. Tissue sections of thickness 5 μm of major isolated organs were taken, later stained with hematoxylin and eosin (H and E) and observed under an optical microscope (Olympus, Tokyo, Japan).
2.4. Neuroprotection studies
2.4.1. Primary neuronal culture
Primary neuronal culture for cortex neurons was obtained from rat pups (day 3–5) using a standard protocol with minor modifications of the method described in Pacifici and Peruzzi (2012). Briefly, the brains were dissected out and kept in ice cold Krebs buffer until the separation of cortex region. Krebs buffer was comprised of NaCl, 126; CaCl2 2.5; KCL 2.5; NaHCO3 25; MgCl2·6H2O, 1.2; NaH2PO4·2H2O 1.2; and Glucose 11, in milli Molar concentrations. Cortex regions were then incubated for 15 min in trypsin (0.25%) at 37 °C followed by washing twice with Krebs buffer solution at 37 °C. Each washing was carried out for 5 min. Finally, the cell pellet was suspended in small quantity of Krebs buffer with 0.1% DNase. The cell number in the filtrate was determined by using hemocytometer and cells were plated with culture media in 12 well culture plates (2 mL/well), which were precoated with poly-D-lysine. Neuron cells were plated at a density of 0.5 × 106 cells/mL (50% density) and maintained at 37 °C in a CO2 incubator for 7–8 days for the development of the cells. Every third day, culture was changed to serum-free Neurobasal media containing 1 × B27, 0.5 mM L-glutamine, 0.01 μg/mL streptomycin, and 100 U/mL penicillin.
2.4.2. MTT assay
MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) assay was performed by using the standard method as described by Shin et al. with minor modification (Shin et al., 2009). In brief, confluent cortex primary neuronal cells were seeded into the sterile flat bottom 96-well plate and allowed to adhere for 24 h. Cells were treated with 1 μg/mL, 10 μg/mL, and 30 μg/mL SMEAF for 2 h prior to TBHP (tert-Butyl hydroperoxide) exposure (0.001 mM) for 24 h. Twenty microliters of MTT solution was added to each well and the plate was further incubated at 37 °C in CO2 for 4 h. After 4 h of incubation, the solution was removed and 100 μL of DMSO (Dimethyl sulfoxide) was added to each well. The absorbance was measured spectrophotometrically using a microplate reader and percentage viability was calculated.
2.5. Statistical analysis
All the data were analyzed using Statistical Package for Social Sciences (SPSS) version 22.0. Data are represented as Mean ± SEM. Statistical analyses were performed using t-test and one-way ANOVA, and p values less than 5% were considered statistically significant (p < 0.05).
3. Results
3.1. Acute oral toxicity and LD50 determination
There were no mortalities in the present study. At termination, all the animals (6/6; 100%) survived at 2000 mg/kg dose including vehicle control (6/6, 100%). The LD50 value for SMEAF was calculated to be >2000 mg/kg.
3.2. Clinical observations, body weight, water and food consumption
In this study, SMEAF 2000 mg/kg treatment did not exhibit any abnormal clinical signs on the general behavioral pattern of mice. No toxic signs and symptoms or mortality was observed in any of the treated animals at 4, 24 h and up to 14 days after treatment.
Mice in both, vehicle treated and SMEAF treated groups were found to be normal and no significant changes were displayed in behavior, skin effect, hair loss, breathing, and postural abnormalities. Among the SMEAF treated group, after 2 h a slightly higher defecation was observed. This defecation, continued till 4 h and then was discontinued. The probable reason for defecation in animals could be the stress and fear of receiving the oral administration of the extract.
The data for body weight of the animals are shown in Fig. 1. No significant changes were observed in the body weight of the mice administered with SMEAF as compared to the control group. All the mice showed a normal increment in the body weight in both control and SMEAF treated groups. The difference for the first 3 days is observed to have a wide range in the representative graph. However, the values of the mean body weights were found to be 19 and 20 g, for control and SMEA extract groups, respectively. This difference was found to be statistically insignificant.
Figure 1.
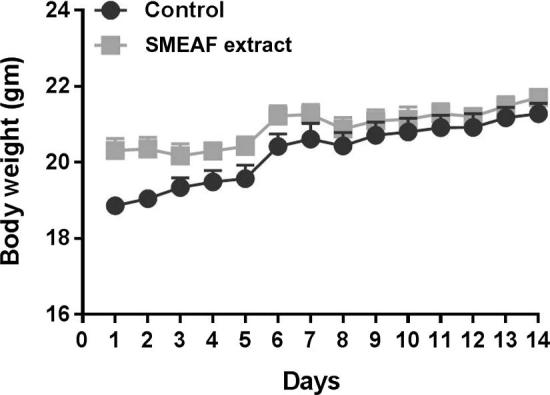
Body weights of mice during 14 days of study (n = 6). Data are expressed as mean ± SEM and analyzed by t-test (p < 0.05) as compared to that of the control group.
The results for food and water intake, between extract treated mice and control mice groups are shown in Figure 2, Figure 3 respectively. No significant changes in food and water intake were observed between both the groups throughout the experiment period, which indicates that SMEAF has no toxic effects. The difference in the food and water intake was found to be significant on 6th and 7th day only. This trend was not observed again during the study. The probable reason for such observation could possibly be environmental factors. The impact of environmental factors such as light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats is well reported (Castelhano-Carlos and Baumans, 2009).
Figure 2.
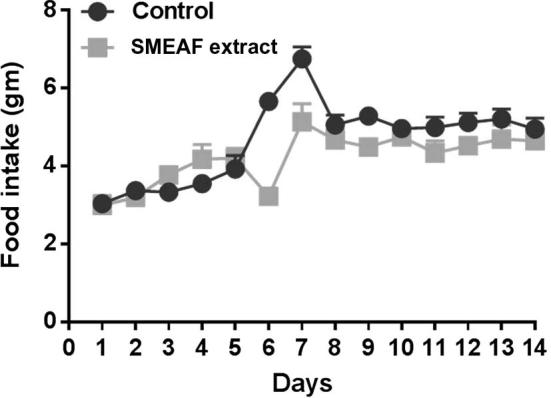
Food intake of mice during 14 days of study (n = 6). Data are expressed as mean ± SEM and analyzed by t-test (p < 0.05) as compared to that of the control group.
Figure 3.
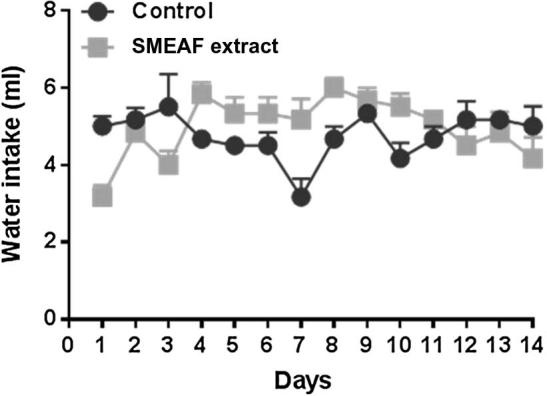
Water intake of mice during 14 days of study (n = 6). Data are expressed as mean ± SEM and analyzed by t-test (p < 0.05) as compared to that of the control group.
3.3. Macroscopic observations and weight of the organs
The vital organs were grossly observed for their appearance, size and color and compared between control and SMEAF treated mice. No significant changes were observed in the absolute and relative organ weight of seven principle organs in SMEAF group when compared to the vehicle control. The major organ weights are shown in Fig. 4. There were no signs of the toxicity and no significant differences in the appearance; size and color of the organs of the SMEAF treated mice as compared to the control mice. Liver weights of SMEA treated group appeared to be higher compared to the control group. One of the considerable reasons for this is the difference in the body weights of the animals. When liver weight was checked with respect to body weight of the animals, they were found to be in equal ratio. In order to confirm whether this liver weight is because of liver toxicity (fatty liver), histopathological studies were conducted, and liver sections were found to exhibit normal tissue characteristics.
Figure 4.
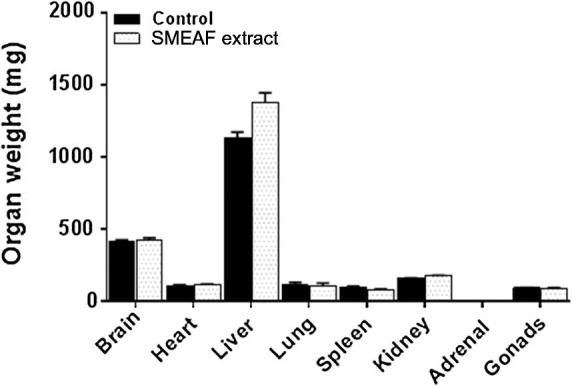
Organ weight of mice after 14 days of study (n = 6). Data are expressed as mean ± SEM and analyzed by t-test (p < 0.05) as compared to that of the control group.
3.4. Hematology and blood biochemistry
There were no significant differences in the complete blood count and differential count of cells between SMEAF treated and control groups. Hematology results are shown in Figure 7, Figure 8. No changes were observed in parameters pertaining to liver and kidney functions between animals belonging to SMEAF and the control group. Results for serum biochemistry, are shown in Table 1, Table 2.
Figure 7.
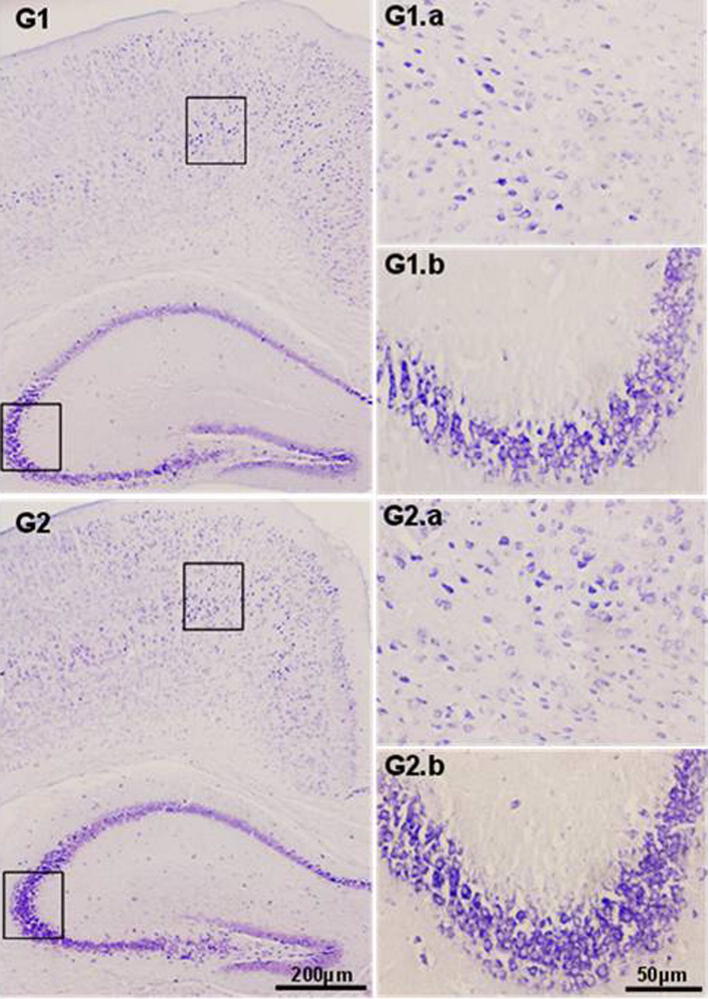
Histological examination of brain sections of experimental animals at 4× and 20× magnification. G1 represents sections of control group animals and G2 represents sections of SMEAF treated animals. (a represents cortex and b represents hippocampus CA3 region.)
Figure 8.
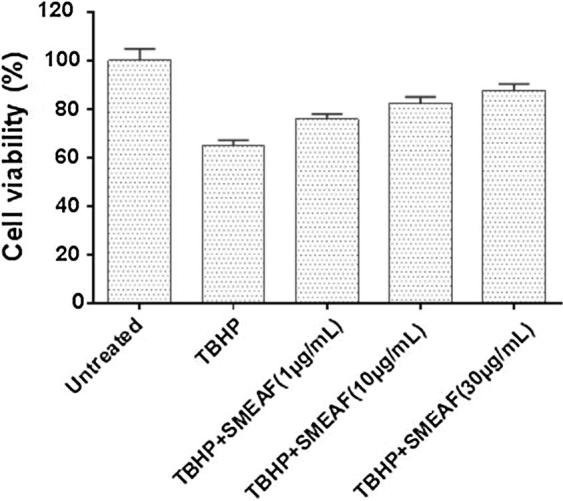
Effect of SMEAF on TBHP treated cell viability in primary neuronal culture (n = 6) at 1 μg/mL, 10 μg/mL and 30 μg/mL concentrations. Data are expressed as Mean ± SEM and analyzed by ANOVA, followed by Dunnet’s test (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001) for each concentration with respect to the TBHP treated group.
Table 1.
Hematology values of mice treated with control and SMEAF 2000 mg/kg (means ± SEM).
| Control | SMEAF treated | |
|---|---|---|
| Complete blood count (CBC) | ||
| HGB (g/L) | 163.50 ± 0.96 | 163.75 ± 2.14 |
| HCT (L/L) | 0.57 ± 0.01 | 0.56 ± 0.01 |
| RBC (1012/L) | 10.44 ± 0.13 | 9.77 ± 0.25 |
| MCV (fl) | 53.50 ± 0.87 | 50.50 ± 1.32 |
| MCH (pg) | 15.48 ± 0.13 | 15.75 ± 0.10 |
| MCHC (g/L) | 293.00 ± 1.08 | 290.00 ± 2.74 |
| RDW (%) | 20.93 ± 0.19 | 19.50 ± 0.15 |
| WBC (109/L) | 9.30 ± 0.40 | 6.08 ± 0.14 |
| Platelet (109/L) | 1063.00 ± 61.98 | 963.50 ± 13.07 |
| Differential count | ||
| Neutrophil (109/L) | 0.91 ± 0.16 | 0.55 ± 0.02 |
| Lymphocyte (109/L) | 8.15 ± 0.72 | 5.41 ± 0.21 |
| Monocyte (109/L) | 0.07 ± 0.02 | 0.01 ± 0.00 |
| Eosinophil (109/L) | 0.01 ± 0.00 | 0.00 ± 0.00 |
| Basophil (109/L) | 0.03 ± 0.01 | 0.02 ± 0.00 |
| Immature granulocyte (109/L) | 0.00 ± 0.00 | 0.00 ± 0.00 |
n = 6, analyzed by t-test, significantly different from the control group: ∗P ⩽ 0.05.
Table 2.
Biochemical parameters of mice treated with control and SMEAF 2000 mg/kg (means ± SEM).
| Control | SMEAF treated | |
|---|---|---|
| Renal function test | ||
| Sodium (mmol/L) | 150.25 ± 0.25 | 146.67 ± 0.33 |
| Potassium (mmol/L) | 6.48 ± 0.30 | 7.13 ± 0.30 |
| Chloride (mmol/L) | 113.00 ± 0.91 | 111.67 ± 1.20 |
| Carbon dioxide (mmol/L) | 22.25 ± 1.03 | 21.33 ± 1.20 |
| Anion gap (mmol/L) | 21.50 ± 0.29 | 21.33 ± 2.03 |
| Urea (mmol/L) | 8.18 ± 0.30 | 7.60 ± 0.67 |
| Creatinine (umol/L) | 18.75 ± 0.48 | 19.67 ± 0.88 |
| Liver function test | ||
| Total protein (g/L) | 51.25 ± 0.75 | 51.00 ± 1.16 |
| Albumin (g/L) | 32.25 ± 0.48 | 32.00 ± 0.58 |
| Globulin (g/L) | 19.00 ± 0.41 | 19.00 ± 0.58 |
| Total bilirubin (umol/L) | 2.00 ± 0.00 | 2.00 ± 0.00 |
| Conjugated bilirubin (umol/L) | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Alkaline phosphatase (U/L) | 159.75 ± 15.05 | 152.67 ± 4.92 |
| Alanine aminotransferase (U/L) | 41.25 ± 2.69 | 61.00 ± 17.06 |
| AST (U/L) | 152.50 ± 29.69 | 230.33 ± 73.35 |
| G-glutamyl transferase (U/L) | 2.00 ± 0.41 | 2.67 ± 0.88 |
n = 6, analyzed by t-test, significantly different from the control group: ∗P ⩽ 0.05.
3.5. Necropsy and histopathological analysis
There were no significant changes on the gross morphology of seven principal organs of animals treated with SMEAF, compared to the control group animals. The obtained results are shown in Table 3.
Table 3.
Necropsy findings after oral treatment of SMEAF 2000 mg/kg.
| Organs/groups | Condition | Control (vehicle)a | SMEAF 2000 mg/kga |
|---|---|---|---|
| Brain | Normal | 6/6 | 6/6 |
| Abnormality detected | 0/6 | 0/6 | |
| Heart | Normal | 6/6 | 6/6 |
| Abnormality detected | 0/6 | 0/6 | |
| Lungs | Normal | 6/6 | 6/6 |
| Abnormality detected | 0/6 | 0/6 | |
| Kidney | Normal | 6/6 | 6/6 |
| Abnormality detected | 0/6 | 0/6 | |
| Liver | Normal | 6/6 | 6/6 |
| Abnormality detected | 0/6 | 0/6 | |
| Spleen | Normal | 6/6 | 6/6 |
| Abnormality detected | 0/6 | 0/6 | |
| Testes | Normal | 6/6 | 6/6 |
| Abnormality detected | 0/6 | 0/6 | |
The values stated are indicated for 6 mice per group, as observed animals/total observed animals.
In histopathological analysis, autopsy at the end of the study revealed no apparent changes in all major organs of the animals belonging to both the control and treated groups. The microscopic structure of the organs (illustrated in Figure 5, Figure 6, Figure 7) indicates no significant structural differences between the control and SMEAF groups. When viewed under the light microscope using various magnification powers, no alteration in the cell structure was observed in all the organs from both the groups. The structure of cells was found to be identical in the organs of the extract treated animals when compared with organs of control group animals.
Figure 5.
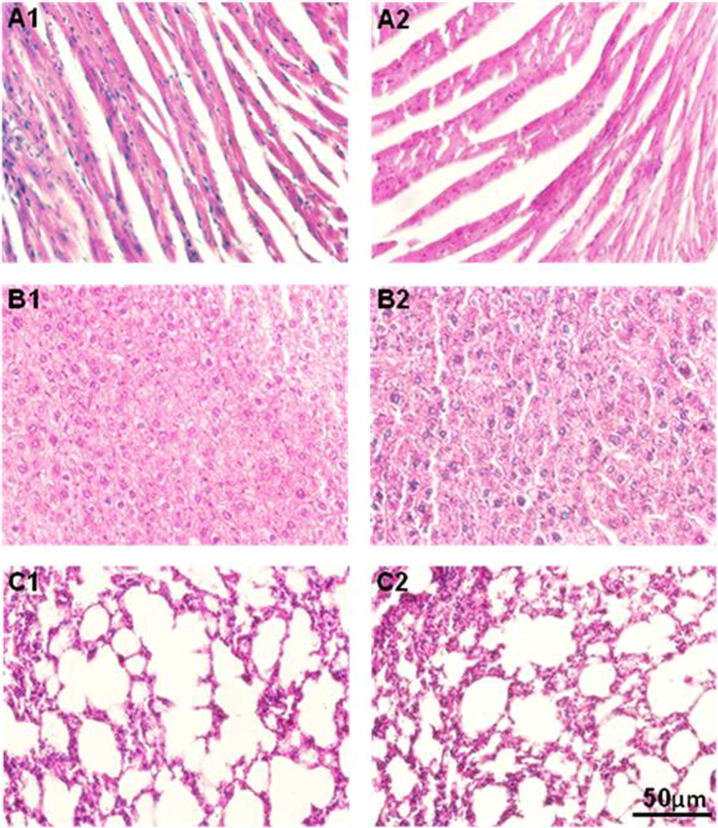
Histopathological observations of heart, liver and lungs of experimental animals: A1 and A2 represent sections of heart of control group and SMEAF treated animals. No change in nucleus of myocyte, myocardium, and blood vessels was observed; B1 and B2 represent sections of liver of control group and SMEAF treated animals. No structural changes in hepatocytes, portal triad and central vein between the two study groups were observed. C1 and C2 represent sections of lungs of control group and SMEAF treated animals. No structural changes in pulmonary vessel, bronchiole, and alveoli in both the treated and control groups were observed.
Figure 6.
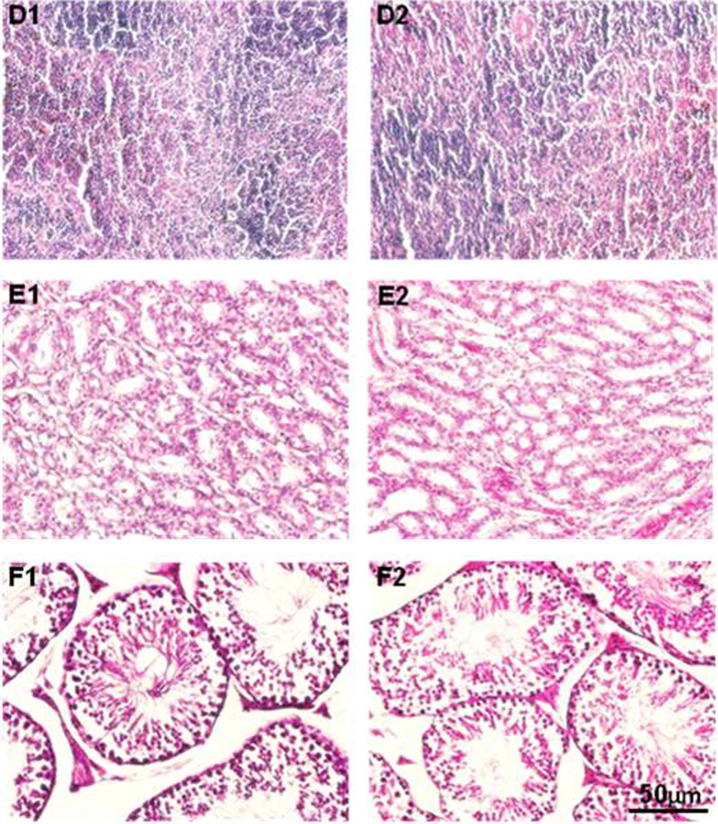
Histopathological observations of spleen, kidneys and testes of experimental animals: D1 and D2 represent spleen of control and SMEAF treatment groups. E1 and E2 represent kidneys of control and SMEAF treatment groups. F1 and F2 represent testes of control and SMEAF treatment groups. No distinct structural differences were observed in the structure of the cells in both the treated and control groups.
3.6. Neuroprotective effects of SMEAF against TBHP induced oxidative stress
In the present study, the neuroprotective effect of SMEAF was examined with the TBHP-induced oxidative stress model using primary cortex neuronal cell. The neuroprotective effect of SMEAF was evaluated by MTT assay. The results (as observed in Fig. 8) indicate that, increasing concentration of SMEAF resulted in a substantial improvement in viability of cells when compared with TBHP treated cells, up to 30 μg/mL concentration. The results obtained, thus provide evidence of SMEAF exhibiting significant neuronal protective effect against the damage caused by oxidation.
4. Discussion
Since past few decades, phytochemical compounds have been extensively used for therapeutic applications due to their benefits and because they are considered to have minimal, or no side effects (Abdel-Rahman et al., 2012, Brinker, 1983). However, it is of utmost importance to evaluate the toxic effects of the plant materials, extracts and phytoconstituents before being administered for systemic use. Thus, there is a need to establish the safety profiles of the plant based extracts and compounds, considering their possible short term and long term adverse effects (Sarma et al., 2008). In general, acute toxicity studies provide valuable information about the lethal dose and safe dose range of the plant extracts. These studies provide valuable information for further evaluation of pharmacological properties of traditional plant based medicines (Hasani-Ranjbar et al., 2010, Palombo, 2011).
The in-vitro assay was performed to determine the potential for possible neuroprotection of SMEAF which can serve as a basis for future in-vivo pharmacological studies (Fakeye et al., 2009). The present study demonstrated the efficacy of selected extracts against cellular toxicity and the viability of primary neuronal cells against TBHP induced oxidative stress.
The current study established the acute toxicity effect of SMEAF in male Balb–c mice, which is known to be a better animal model compared to rats for prediction of human LD50 dose (Walum et al., 1995).
In toxicity studies, any untoward clinical symptoms in mice indicate the toxicity of the organs in the treated groups (Eaton and Klaassen, 1996). Mice orally administrated with SMEAF 2000 mg/kg showed no clinical signs of distress, and there were no noticeable symptoms of toxicity or mortality during 14 days of the study period. All the animals followed normal weight and behavior pattern with no significant changes between the groups. Also, no change was observed in physical appearance features such as eyes, fur and skin which were found to be normal while there was a normal increase in the body weight of the animals (Fig. 2). This indicates that, the administration of the SMEAF extract has no adverse effects on the growth of the animals (Jothy et al., 2011, Stevens and Mylecraine, 1994). The food and water intake was also not affected by the administration of SMEAF. Thus we can infer that, the extract did not induce appetite suppression, which is an indication of normal carbohydrate, protein or fat metabolism (Jothy et al., 2011, Stevens and Mylecraine, 1994, Klassen, 2013).
Significant loss in body weight of animals up to 10% of initial body weight, with the administration of extract is considered as toxic for its use. Also, organ weight is a more specific index of physiological and pathological status of animals (Auletta, 1995, Raza et al., 2002). In our study, no changes were noticed in gross observations of the primary organs from both the groups.
Our results support the basis of safety of SMEAF extract, with LD50 value greater than 2000 mg/kg (Dybing et al., 2002). According to the chemical labeling and classification of acute oral toxicity guidelines prescribed by OECD, the SMEAF falls under the lowest toxicity class (LD50 > 2000 mg/kg). The extracts with LD50 values higher than 2000 mg/kg via oral route are considered safe and practically non-toxic (Roopashree, 2009, Balijepalli et al., 2015). The histological examination is the basis for establishing treatment related pathological changes in cell structures of organs (Balijepalli et al., 2015). In this study, histological examination has revealed, no structural changes in the cell structure of the organs of experimental animals. The liver is considered as a primary target organ for acute toxicity due to the first step in first pass metabolism (Co-operation and Development, 1995). Examination of liver tissues has shown normal hepatocytes and no changes in the structure of the cells between groups (Fig. 5B1 and B2). No inflammatory changes, necrosis, fibrosis or local fatty degeneration was observed in the liver (Salawu et al., 2009).
Microscopic study of the heart and spleen (Fig. 5A1 and A2; and Fig. 6D1 and D2) of the animals treated with SMEAF showed no changes in the organ tissue compared to the control group. The spleen tissues from both the groups were normal, with well labeled white and red pulp. The hematopoietic system is very sensitive to the effect of toxic compounds and it serves as an important factor to determine toxicity in animals (Akanmu et al., 2004). At the end of study period, the hematological parameters were similar for both the groups except for the WBC count. The values for the WBC count of the control and SMEAF treated were 9.30 ± 0.40 and 6.08 ± 0.40 respectively, and they were still found to be within the reference range. In the differential count, low levels of neutrophils, lymphocytes and monocytes counts were observed in both the control and treatment groups (Diana et al., 2003). The results also indicate that, SMEAF extract did not alter serum levels of ALP, and TBIL.
5. Conclusion
The in-vitro data suggest, SMEAF has significant protective effects against TBHP induced oxidative stress and it possesses neuroprotective activity. The results obtained in our study demonstrate that, SMEAF does not produce any in-vivo toxicity at the dose of 2000 mg/kg, thus demonstrating its potential safety in systemic use. However, further explorative studies are required for evaluation of its chronic toxicity and pharmacological benefits, in order to be considered safe and beneficial for therapeutic use.
Acknowledgment
We are grateful to the eScienceFund of Ministry of Science, Technology and Innovation (MOSTI), Malaysia, for providing financial support to GBH (02-02-10-SF0215).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Leonard Williams, Email: llw@ncat.edu.
Mohd Farooq Shaikh, Email: farooq.shaikh@monash.edu.
References
- Abdel-Rahman A. The safety and regulation of natural products used as foods and food ingredients. Toxicol. Sci. 2012;123:333–348. doi: 10.1093/toxsci/kfr198. [DOI] [PubMed] [Google Scholar]
- Akanmu M. Toxicity potentials of Cassia fistula fruits as laxative with reference to Senna. Afr. J. Biomed. Res. 2004;7:23–26. [Google Scholar]
- Anon. World Health Organization; 2013. WHO Traditional Medicine Strategy: 2014–2023. [Google Scholar]
- Auletta C.S. CRC Press; London: 1995. Acute, Subchronic, and Chronic Toxicology. [Google Scholar]
- Balijepalli M.K. Acute oral toxicity studies of Swietenia macrophylla seeds in Sprague Dawley rats. Pharmacog. Res. 2015;7:29–38. doi: 10.4103/0974-8490.147197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borris R.P. Natural products research: perspectives from a major pharmaceutical company. J. Ethnopharmacol. 1996;51:29–38. doi: 10.1016/0378-8741(95)01347-4. [DOI] [PubMed] [Google Scholar]
- Brinker F. National College of Naturopathic Medicine; Oregon: 1983. An introduction to the toxicology of common botanical medicines. 1. [Google Scholar]
- Castelhano-Carlos M.J., Baumans V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab. Anim. 2009;43:311–327. doi: 10.1258/la.2009.0080098. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development, 1995. Guideline for the Testing of Chemicals: Repeated Dose 28-day Oral Toxicity Study in Rodents. 407, OECD, Paris.
- Das A. Anti-nociceptive activity of the fruits of Swietenia macrophylla King. J. Pharm. Res. 2009;2:1367–1369. [Google Scholar]
- Diana C.D., Jessica L.B., Elahe T.C. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC. Clin. Pathol. 2003;3:1–6. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybing E. Hazard characterization of chemicals in food and diet: Dose response, mechanisms and explanation issues. Food Chem. Toxicol. 2002;40:237–282. doi: 10.1016/s0278-6915(01)00115-6. [DOI] [PubMed] [Google Scholar]
- Eaton D.L., Klaassen C.D. Casarett and Doull’s Toxicology: The Basic Science of Poisons. vol. 5. John Wiley & Sons; 1996. Principles of Toxicology; p. 13. [Google Scholar]
- Eid A.M., El-Enshasy H.A. A review on the phytopharmacological effect of Swietenia macrophylla. Int. J. Pharm. Pharm. Sci. 2013;5:47–53. [Google Scholar]
- Fakeye T.O. S. Toxic effects of oral administration of extracts of dried calyx of Hibiscus sabdariffa Linn. (Malvaceae) Phytother. Res. 2009;23:412–416. doi: 10.1002/ptr.2644. [DOI] [PubMed] [Google Scholar]
- Goh B.H., Kadir A. In vitro cytotoxic potential of Swietenia macrophylla King seeds against human carcinoma cell lines. J. Med. Plant Res. 2011;5:1395–1404. [Google Scholar]
- Goh B.H. Swietenolide diacetate from the seeds of Swietenia macrophylla. Acta Crystall. Sect. E: Struct. Rep. Online. 2010;66 doi: 10.1107/S1600536810017733. o1396-o1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: tradition of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Hasani-Ranjbar S. A systemic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J. Gastroenterol. 2010;15:3073. doi: 10.3748/wjg.15.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshu S.R., Abdullah R., Hemn H.O. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. BioMed. Res. Int. 2014:1–15. doi: 10.1155/2014/563930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothy S.K. Acute oral toxicity of methanolic seeds extract of Cassia fistula in mice. Molecules. 2011;16:5268–5282. doi: 10.3390/molecules16065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpana K., Pugalendi K.V. Antioxidative and hypolipidemic efficacy of alcoholic seeds extract of Swietenia macrophylla in streptozotocin diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2011;22:11–21. doi: 10.1515/jbcpp.2011.001. [DOI] [PubMed] [Google Scholar]
- Katiyar C. Drug discovery from the plant sources: an integrated approach. AYU. 2012;33:10. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S.M. Review on biological activities and phytochemical of Swietenia macrophylla King. Molecules. 2013;18:10465–10483. doi: 10.3390/molecules180910465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen C.D. vol. 1236. McGraw-Hill; New York: 2013. (Casarett and Doull’s Toxicology: The Basic Science of Poisons). [Google Scholar]
- Mootoo B.S. Limonoids from Swietenia macrophylla and S. aubrevilleana. J. Nat. Prod. 1999;62:1514–1517. doi: 10.1021/np990199x. [DOI] [PubMed] [Google Scholar]
- OECD, 1994. OECD Guidelines for the Testing of Chemicals: Organization for Economic Cooperation and Development.
- Pacifici M., Peruzzi F. Isolation and culture of rat embryonic neural cells: a quick protocol. J. Visual. Exp. 2012;63:e3634. doi: 10.3791/3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid. Complim. Alter. Med. 2011:1–15. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. vol. 122. Springer-Verlag; New York: 1985. History of the BALB/c family. (The BALB/c Mouse: Genetics and Immunology, Current Topics in Microbiology and Immunology). [Google Scholar]
- Rates S.M.K. Plants as source of drugs. Toxicon. 2001;39:603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Raza M. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver, and kidney of Swiss albino mice. Sci. Pharmaceut. 2002;70:135–145. [Google Scholar]
- Roopashree T. Acute oral toxicity studies of antipsoriatic herbal mixture comprising of aqueous extract of calendula officinalis, Momordica charantia, Cassia tora and Azadirachta indica seeds oil. Thai J. Pharm. Sci. 2009;33:74–83. [Google Scholar]
- Roux S., Sable E., Porsolt R.D. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr. Prot. Pharmacol. 2004;10:10. doi: 10.1002/0471141755.ph1010s27. [DOI] [PubMed] [Google Scholar]
- Salawu O.A. Acute and sub-acute toxicological evaluation of the methanolic stem bark extract of Crossopteryx febrifuga in rats. Afr. J. Pharm. Pharmacol. 2009;3:621–626. [Google Scholar]
- Sarma D.N. Safety of green tea extracts. Drug Saf. 2008;31:469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- Shin Y.J. Protective effects of clusterin on oxidative stress-induced cell death of human corneal endothelial cells. Mol. Vision. 2009;15:2789. [PMC free article] [PubMed] [Google Scholar]
- Stevens K., Mylecraine L. CRC Press; USA: 1994. Issues in chronic toxicology. (Principles and Methods in Toxicology). [Google Scholar]
- Supriady H. SMEAF attenuates the production of pro-inflammatory mediators through the inactivation of Akt-dependent NF-κB, p38 and ERK1/2 pathways in LPS-stimulated BV-2 microglial cells. J. Funct. Foods. 2015;17:434–448. [Google Scholar]
- Walum E. The MEIC program and its implications for the prediction of acute human systemic toxicity. Alternat. Methods Toxicol. 1995;11:275–282. [Google Scholar]