Abstract
The rotavirus-induced diarrhea of human and animal neonates is a major public health concern worldwide. Until recently, no effective therapy is available to specifically inactivate the rotavirion particles within the gut. Passive immunotherapy by oral administration of chicken egg yolk antibody (IgY) has emerged of late as a fresh alternative strategy to control infectious diseases of the alimentary tract and has been applied in the treatment of diarrhea due to rotavirus infection. The purpose of this concise review is to evaluate evidence on the properties and performance of anti-rotavirus immunoglobulin Y (IgY) for prevention and treatment of rotavirus diarrhea in human and animal neonates. A survey of relevant anti-rotavirus IgY basic studies and clinical trials among neonatal animals (since 1994-2015) and humans (since 1982-2015) have been reviewed and briefly summarized. Our analysis of a number of rotavirus investigations involving animal and human clinical trials revealed that anti-rotavirus IgY significantly reduced the severity of clinical manifestation of diarrhea among IgY-treated subjects relative to a corresponding control or placebo group. The accumulated information as a whole depicts oral IgY to be a safe and efficacious option for treatment of rotavirus diarrhea in neonates. There is however a clear need for more randomized, placebo controlled and double-blind trials with bigger sample size to further solidify and confirm claims of efficacy and safety in controlling diarrhea caused by rotavirus infection especially among human infants with health issues such as low birth weights or compromised immunity in whom it is most needed.
Keywords: Rotavirus, diarrhea disease, neonates, oral passive immunotherapy, IgY
Introduction
Globally diarrhea is considered as one of the leading contributor to neonatal death in human and animal (Dhama et al., 2009). Among infants and young children less than five years old, rotavirus comprises the most important single etiologic agent of severe diarrhea world-wide. Thus far, no specific and reliable anti-rotavirus therapy addressing this pediatric scourge is currently available. Starting in the early 1990s, some investigators in our group introduced the anti-rotavirus IgY for oral passive immunization among young children and animals as a specific treatment modality for rotavirus diarrhea with a potential for both prophylactic and therapeutic applications. Since then, the efficacy of IgY has been demonstrated in the treatment and prevention of infectious diseases of the skin (Acne) (Selven et al., 2012), oral cavity (candidiasis, dental caries, periodontitis,) (Ibrahim et al., 2008; Nguyen et al., 2011; Yokoyama et al., 2007), stomach (gastritis) (Suzuki et al., 2004) intestine (celiac disease, cholera, diarrhea) (Gujrat et al., 2012; Hirai et al., 2010; Rahman et al., 2012), metabolic syndrome (Hirose et al., 2013), cystic fibrosis (Nilsson et al., 2008) and others (environmental care e.g., Norovirus, Influenza, House dust mite etc., snake venom,) (Dai et al., 2012; Lee et al., 2014; Lee et al., 2015; Nguyen et al., 2010). The aim of this review is to summarize the highlights of cumulated information on anti-rotavirus IgY with focus on in vitro investigations, clinical trials in human and animal subjects as well as the potential direction and future needs in antirotavirus IgY clinical research.
Intrinsic Properties of IgY and Its Application to Heterologous Hosts
IgY is the major serum immunoglobulin in bird, reptile, which is also found in high concentrations in chicken egg yolk . IgY showed Y shaped structure having two antigen binding sites with two heavy (H) and two light (L) chains like to mammalian IgG. Molecular weight (~180 kDa) of IgY is heavier compared with mammalian IgG (~160 kDa). The antibody fragment domain without hinge region in the IgY structure, gives IgY less flexibility to bind with a broad range of antigenic epitopes and gives a relatively strong affinity and avidity to specific antigens.
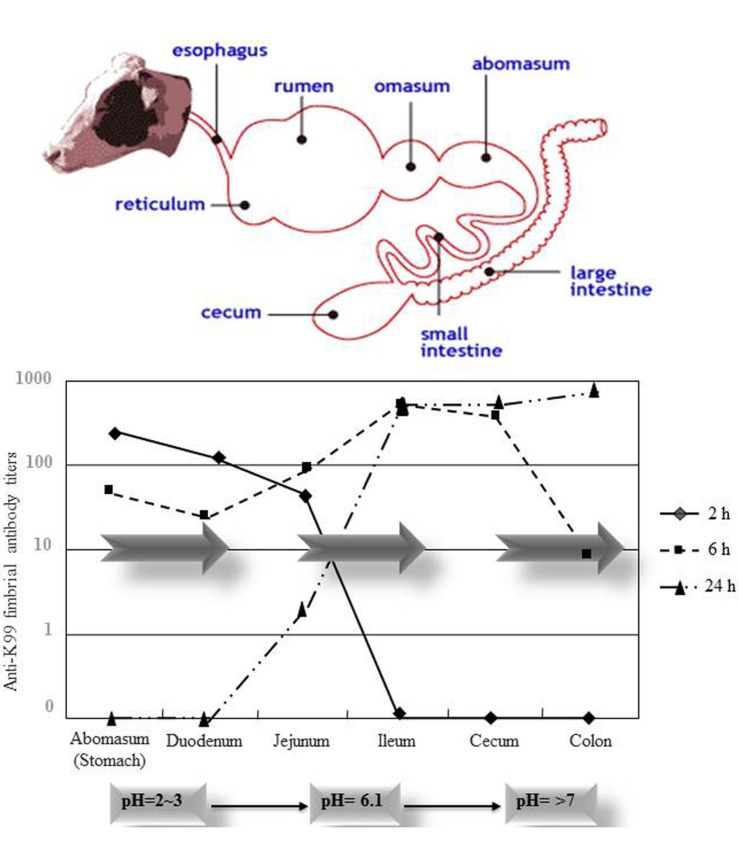
Proteinaceous nature of IgY make it physicochemically sensitive to heat, pressure, pH and pepsin. This properties of IgY pose real challenges to its oral application for different gastrointestinal disorders. To overcome these problems, several studies have been conducted in the last decade. Heat, pH and pressure stabilities of anti-Helicobacter pylori IgY were evaluated by Shin et al. (2002). IgY is stable at temperatures ranging between 30°C and at 70°C, at pH 3.5-11.0 and to pressure up to 2942000 mmHg. Inclusion of stabilizing agents (e.g., glycerol or glycine, sucrose, maltose) conferred comparatively more protection against high pressure, acid pH and thermal denaturation of IgY. Longterm storage (20 years) of IgY is reported without any remarkable loss of antibody titer at +4°C (Kollberg et al., 2015). IgY shown resistant against trypsin and chymotrypsin inactivation and retained 40% immunologic activity after 8 h of digestion in vitro in the presence of these pancreatic enzymes (Hatta et al., 1993). While it may be degraded by pepsin under acidic conditions, encapsulated IgY were shown to be more resistant both to pepsin and gastric conditions (Li et al., 2009). In particular, our group has investigated the in vivo passage and the efficacy of IgY in the gastrointestinal tract of pigs (Yokoyama et al., 1993) and calves (Ikemori et al., 1996). Results showed that IgY was transported as functional antibodies from the stomach to the small intestine of calves while retaining much of their original biological activity (Fig. 1). These properties of IgY have paved the way for its utilization as an anti-infectious agent within the mammalian gut. As such, IgY has been customized to serve as immunologic sentinels against an array of harmful alimentary pathogens including viruses, fungi and bacteria (Müller et al., 2004; Rahman et al., 2013a).
Fig. 1. In vivo passage of IgY in the gastrointestinal tract of calves. Anti-K99 fimbriae antibody titers of IgY in the gastrointestinal tract of calves after 2, 6 and 24 h post administration (Data from Ikemori et al., 1996).
The Modus Operandi Underlying Anti-Rotavirus IgY Efficacy
Over 650 studies on IgY have been published and indexed in PubMed portal between 1980 to first quarter of 2016 showing the effectiveness of IgY in enhancing the immune system. Eggs contain IgY in amounts sufficient to protect the developing avian embryo against the common infectious diseases of birds. By tweaking the bird’s immune system, IgY can be tailor-made to protect hosts other than birds against infectious microorganisms proper to the heterologous host with a high degree of accuracy at the molecular level. Within a heterologous gut such as those of mammals, IgY molecules are not subject to immune rejection by the host since they are outside the circulatory system. Even when they are detected by dendritic cells along the gut wall, they are recognized as normal food protein or glycoprotein materials and not as pathogen-associated molecular patterns. IgY is particularly useful for those who do not respond adequately to vaccines, such as immunocompromised patients, or when vaccines do not share enough commonality with the antigenic epitopes of microorganisms circulating within a given epidemiological region in which case a specifically tailored IgY would be extremely useful. When IgY is used to target an infectious diarrhea-causing microorganism within the intestinal tract for host protection, some mechanisms of action is proposed: (1) microbial adhesion inhibition to cell surfaces (Rahman et al., 2013b), (2) viral colonization suppression by preventing intercellular spread thereby tamping down the overall severity of enteritis, (3) microbial (virus, bacterial, fungal) agglutination acting as a “biological glue” with resulting microbial immobilization thereby facilitating their being flushed down the gut, (4) enzyme activity inhibition, and (5) toxin activity neutralization (Arimatsu et al.,2014; Neri et al., 2012).
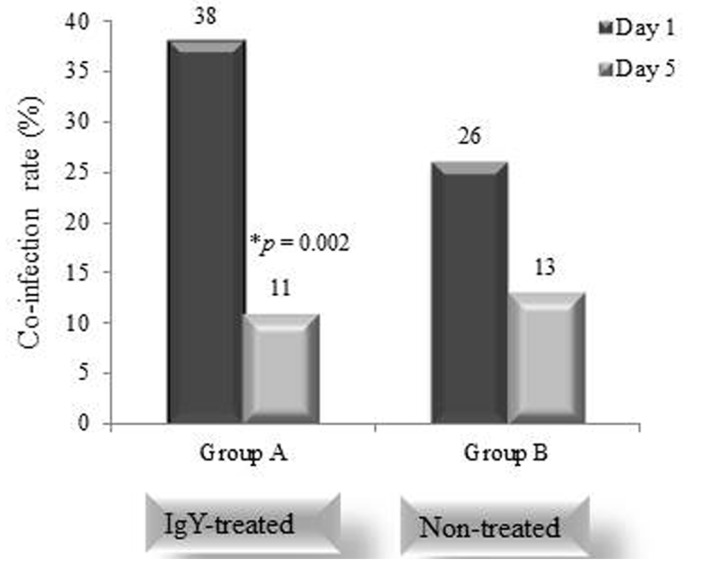
In the case of anti-rotavirus IgY, we postulate that it specifically neutralizes the infectivity of the pathogenic virion by blocking its entry to host enterocyte preventing initial infection and suppression or minimization of intestinal colonization or cell-to-cell spread of infection resulting in down-modulated clinical symptoms in rotavirus-induced enteritis. Likewise, some of the rotavirus NSP4, a nonstructural protein synthesized by rotavirus and which is considered a viral enterotoxin (Parr et al., 2006) is neutralized by IgY since the production process of the IgY used in this study includes not only the whole virion particle but also the original culture media where the NSP4 is secreted by infected tissue culture cells. Non-dividing mature enterocytes near the tips of the villi is the proper site of rotaviruses replication (Robert et al., 2004) and the preliminary diarrheal process is initiated in the small intestine when rotavirus binds to and infects enterocytes. This leads to a chain of steps of molecular reaction eventually shown diarrheal malabsorption through the reduction of enterocytes absorption capacity, reduction of Na+-solute cotransporters, and reduction of digestive enzymes (disaccharidases, peptidases, and other enzymes) expression on the enterocytes apical microvilli surface (Robert et al., 2004). The resulting accumulation of unabsorbed nutrients and water in the gut may give rise to proliferation of opportunistic or pathogenic bacteria that feed on these unabsorbed nutrients potentially leading to more severe diarrhea. Moreover, inflammation oriented antimicrobials secretion allowing pathogens to occupy the commensal niche by altering the composition of the gut microbiota, (Baumler et al., 2016; Bhavnani et al., 2012; Raffatellu et al., 2010). Thus, the suppression of rotavirus replication and spread of infection or colonization along the intestinal tract by anti-rotavirus IgY will indirectly lead to reduction in co-infection by bacterial pathogens. An interesting yet unpublished experience by our group showed that among a population of rotavirus-confirmed pediatric patients below five years of age in a Myanmar hospital, about a quarter of them have accompanying bacterial infection. Using anti-rotavirus IgY as an intervention tool to mitigate symptoms of rotavirus diarrhea, we observed a statistically significant drop in co-infection rate with diarrheacausing bacteria among IgY-treated subjects (Group A) relative to non-treated subjects (Group B) (Fig. 2) even when they have not been treated with antimicrobials. Inasmuch as rotavirus infects the enterocytes found at the tips of small intestinal villi which are involved mainly in absorption of nutrients, the structural and functional disruption of enterocytes by infecting rotavirus would inevitably result in malabsorption at the cellular level and diarrhea at the clinical level. Malabsorption favors bacterial proliferation that tips the balance in favor of the co-infecting microorganisms which are otherwise commensalistic or held in check by innate host resistance mechanisms under normal gut conditions. Rotavirus opens the door as it were to co-infecting bacteria which tend to proliferate more rapidly on account of the more nutritious gut milieu which in turn sets the stage toward development of enteritis. Upon oral administration of anti-rotavirus IgY, the neutralization of infectious virions most likely preserves and protects the enterocytes resulting in prevention of fluid imbalance and restoration or rebalancing of the normal intestinal microflora, a condition that does not favor opportunistic or naturally harmful gut microorganisms.
Fig. 2. Bacterial analysis of stool samples was performed on all patients in groups A and B on days 1 and 5 during the treatment. The co-infection rate in the group A decreased significantly than the Group B. Significant differences compared with Day 1 and 5 (*p≤0.05) in Group A (Data from Clinical trial in Myanmar 2015, Unpublished).
Advantages of IgY
Many investigators recognized the advantages of chicken IgY application in terms of its merits when compared to antibiotics, vaccines and oral immunotherapy. Some advantages of IgY relative to antibiotics (Rahman et al., 2013a) include the following: 1) It is natural, 2) It is safe and is not absorbed into the body circulation (no toxic tissue residues), 3) It avoids environmental contamination with synthetic chemical drugs, 4) It does not induce specific pathogenic microorganisms resistance since it is directed to multi epitopics antigenic targets which need multiple genes for their synthesis. 5) It is highly specific in its reactivity and controls only targeted pathogens without affecting normal bacterial flora, 6) It has a potentially broad spectrum of specificity when customized against viruses, bacteria or fungi, and 7) It does not induce adverse side effects unlike synthetic drugs.
Compared to vaccination, IgY based passive immunotherapy has advantages due to its: 1) local and quick action, 2) higher specific activity, 3) applicability to a different age of patients from infants to adults including low birth weight or immunodeficient patients or women during pregnancy, 4) nontoxicity as a normal part of the human Diet, 5) long shelf life even when kept in powder form with minimal moisture at room or ambient temperature without the need for cold chain, and, 6) being cheaper and faster to produce than vaccines.
IgY is attractive for oral immunotherapy due to its several properties which include: 1. Collection from poultry is animal-friendly without pain or stress, 2. Higher binding avidity to antigens compared to mammalian IgG and reacts more avidly to the same antigens when tested in competition assays (Ikemori et al., 1993) 3. Industrial scale production is possible and at low cost relative to vaccine production, 4. Long shelf life, 5. Absence of drug residues in meat and compliance with legal requirements in many countries that prohibit antibiotics as growth promoter for poultry and livestock, 6. Being a natural food ingredient, IgY has no attendant risk of side effects when taken orally except in cases of egg allergy especially when using non-purified IgY containing egg white and perhaps other non-globulin yolk materials.
IgY against Rotavirus Diarrhea in Animals
Animal rotaviruses are considered potential reservoirs for genetic exchange with human rotaviruses. Epidemiologically, rotavirus is the most important single causative agent of neonatal diarrhea among animals worldwide (Dhama et al., 2009). There are many evidences that show animal rotaviruses can infect humans, either via direct or indirect transmission of the virus. Throughout history, humans and animals have lived together closely within the same environment and have cross-infected each other as a consequence of this ecological contingency. It is not farfetched therefore to consider promotion of animal health as indirectly promoting human health. This holds true for prevention and control of animal rotaviruses. IgY has been a subject of recent studies as an alternative approach to prevent or control diarrhea in animals caused by rotavirus and other diarrhea-causing enteric pathogens. This is also in light of recent trends in legal restrictions in many countries regarding the use of antimicrobials as growth promoter for livestock and poultry. The therapeutic value and safety of using oral anti-rotavirus IgY in animals is now well-established after extensive studies over the past decades (Vega et al., 2015). Efficacy of IgY in controlling and preventing diarrhea (specially rotaviral diarrhea) in domestic animals from 1994 to 2015 has been critically reviewed by Diraviyam et al., 2014. The authors pooled accumulated data from 49 studies of 4 different animal species (piglets, mice, poultry and calves) that revealed that IgY significantly reduced the risk of diarrhea in treatment groups when compared to corresponding placebo groups. This general observation based on data from 49 studies supports the view that IgY is a useful tool for prophylaxis and treatment of diarrhea in animals.
Clinical Trials of Anti-Rotavirus IgY in Young Children
There were very few clinical studies done so far on antirotavirus IgY involving young children and studies discussed below are in our view the ones worth noting. A published study by Barnes et al., 1982, reported that no significant difference observed in the rates of rotavirus infection between test versus placebo groups of hospitalized low birth weight babies, but found no adverse effects after administration of oral immunoglobulin preparations. The reviewers concluded that future well-designed neonatal trials must be conducted using the newer preparations of anti-rotaviral immunoglobulins (colostrum, egg yolk immunoglobulins).
In 2001, Sarker et al. investigated the efficacy of IgY in the treatment of acute viral gastroenteritis in infants and children in a randomized double blind clinical trial. In this study, the researchers evaluated the therapeutic efficacy of human rotavirus specific egg yolk immunoglobulins among rotavirus diarrhea positive children. In this trial, hyperimmune egg yolk was administered to the children without using any carrier. Researchers observed a modest improvement of diarrhea in IgY treated group in the form of stool volume reduction and earlier clearance of rotavirus shedding from stool in children indicating a potential role of chicken IgY in the management of this infection. In a 2011 retrospective study, importance of the oral administration of anti-rotaviral immunoglobulin preparations for boosting the local immunity has been recognized as a potentially useful strategy in treating rotaviral infections especially in low birth weight babies (Pammi et al., 2011). However, no randomized controlled trials to assess the effecacy or safety of oral IgY preparations for the treatment of rotavirus diarrhea in hospitalized low birth weight infants have been reported at that time.
In 2012, Rahman and colleagues published a randomized, double-blind, placebo-controlled trial of rotavirus-specific IgYs report, for rotavirus-associated diarrhea among hospitalized pediatric patients at Central Myanmar (Rahman et al., 2012). In this clinical trial, anti-rotavirus IgY was orally administered to hospitalized children using a flavored disaccharide (maltitol) as main carrier. Comparing 26 patients given anti-rotavirus IgY against 26 placebo patients given the carrier alone, IgY shortened the mean duration of intravenous fluid administration by 3 d, dramatically reduced the daily frequency of viral shedding as well as stopped viral shedding in stool in 3 d (IgY group) vs 8 d (placebo), shortened the mean duration of diarrhea from 7.7 d for placebo to 5.6 d for IgY group, reduced the mean intake of oral rehydration fluid volume from 919.1 mL (placebo) to 699.3 mL (IgY group), reduced the intravenous fluid administration mean duration from 8 d (placebo group) to 5 d (IgY group), and shortened the viral shedding mean duration in stool from the 1st day of admission from 4.2 d (placebo group) to 3 d (IgY group). These differences between the placebo and IgY treatment groups were found to be statistically significant. Neutralizing rotavirus infectivity in acute enteritis and reducing the duration of viral shedding by specific IgY may potentially prevent further spread of infection in a community or hospital setting, which is very important from the public health standpoint. It is worth noting that IgY influenced the above clinical parameters despite the presence of co-infecting enteric pathogens in 92% of the subjects indicating an important role played by IgY as an adjunct to supportive therapy for infant rotavirus diarrhea.
The observed clinical efficacy of anti-rotavirus IgY in the study by Rahman et al., 2012, also highlights the contribution of rotavirus as a pivotal virulence factor in determining the course of infection during mixed infection with other non-cholera enteric pathogens. In a recent study conducted by our group (Hlaing et al.,Unpublished) using infant milk formula (supplied by Lotte Food Co., Ltd., Korea) as carrier for anti-rotavirus IgY with the same range of genotypic cross-reactivity as the 2012 study by Rahman et al., the most frequent co-infection combinations were rotavirus-E. coli. The co-infection rate in IgY treated group of 47 rotavirus-confirmed hospitalized children decreased significantly (p=0.002) (Fig. 2) compared to a placebo group of 47 children being observed in the same hospital. It is known that rotavirus and other pathogenic bacteria indirectly promote each other’s growth in the gut and may result in more severe diarrhea. Thus, the reduction of rotavirus load in the gut due to oral IgY administration may allow patients to clear other pathogenic bacteria more effectively (Hlaing et al.,Unpublished). In the Myanmar study in 2012 by Rahman et al. and our 2015 study (Hlaing et al.,Unpublished) the IgY formulation used that were prepared using the same specially selected reassortant serotypes which shown strong reactivity against all major infant rotavirus serotypes. Moreover, this anti-rotavirus IgY was found to be safe to administer inasmuch as adverse effects and unusual responses were not seen in any of the participants in both trials.
To gain maximum benefits from anti-rotavirus IgY, focus should be made not only on treatment of patients with prior infection but on protection from possible rotavirus infection (prophylaxis) as in the case of seasonally acquired rotavirus infection in highly endemic areas. Ideally, a randomized, controlled community-based study is needed to explore this pre-emptive approach using antirotavirus IgY to verify its safety, efficacy and economic feasibility compared to the new commercially available rotavirus vaccine that incidentally comes at prohibitive costs.
From functional Food ao Active Pharmaceutical Ingredient
The results of two clinical trials in Myanmar reported in 2012 by Rahman et al., and our 2015 unpublished data indicate that the use of oral dosage form of anti-rotavirus IgY in a child-friendly carrier (such as infant milk formula or maltitol) is a promising, safe and effective adjunct to management of acute diarrhea in pediatric patients. The infant milk formula is particularly attractive as it addresses the nutritional as well as therapeutic needs of rotavirus-infected children in a user-friendly manner. For diarrhea patients below five years of age and who are lactose tolerant, the infant milk formula made available in powder form comes out as an ideal vehicle for oral IgY delivery. It must be noted however that lozenges, tablets or capsules for older children may also be used as oral delivery systems as had been done for some IgY products with various target infections now being sold in the Japanese and East Asian markets (Table 1; Fig. 3). While all these products are currently classified as food supplements, the forward trend is toward pharmaceuticalization of IgY which may only be a few years away into the future. The re-classification of IgY as drug would provide a fresh breath of air in the field of therapeutics where the dearth in novel synthetic antimicrobials has cast a pall of uncertainty to the healthcare industry and medicine end-users especially now that we are facing increasingly resistant populations of pathogenic microorganisms.
Table 1. IgY supplemented food products available commercially in global biohealth market.
| Product name | Product type | Country | Sales start |
|---|---|---|---|
|
Drinking Yoghurt | Korea | 2001.5 |
| Capsule | Japan | 2001.10 | |
| Shell egg | Japan | 2003.4 | |
| Tablet | Japan | 2003.10 | |
| Regular Yoghurt | Japan | 2004.7 | |
| Drinking Yoghurt | Taiwan | 2004.8 | |
| Tablet | Japan | 2005.6 | |
| Tablet | Japan | 2005.6 | |
| Drinking Yoghurt | Japan | 2010.7 | |
| Sachet | Vietnam | 2015.2 | |
|
Lozenge | Japan | 2005.9 |
| Drinking Yoghurt | Korea | 2006.9 | |
| Tablet | Japan | 2007.5 | |
| Lozenge | America | 2009.8 | |
| Lozenge | Japan | 2010.5 | |
| Lozenge | Vietnam | 2013.11 | |
|
Lozenge | Japan | 2005.9 |
| Lozenge | America | 2009.8 | |
| Lozenge | Vietnam | 2013.11 | |
|
Dental gel | Japan | 2012.5 |
|
AC Filter | Japan | 2003.10 |
| Mask | Japan | 2005.10 | |
| Mask | Japan | 2006.10 | |
| Tablet | Japan | 2008.12 | |
| Lozenge | Vietnam | 2013.10 | |
|
Baby milk | Korea | 2009.6 |
| Baby milk | Korea | 2014.9 | |
|
Baby milk | Korea | 2014.9 |
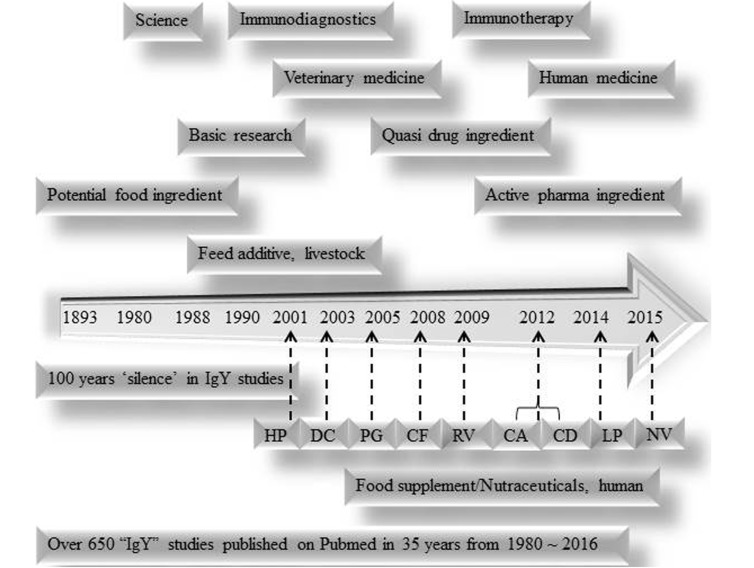
Fig. 3. Future direction of IgY research: The timeline shows 100 years of progression from feed/food additive or health supplement toward nutraceutical and active pharmaceutical ingredient status.
HP = Anti-Helicobacter pylori IgY for gastritis (2001); DC = Anti-Streptococcus mutans IgY for dental caries (2003); PG = Anti-Porphyromonas gingivalis IgY for periodontitis (2005); CF = Anti-Pseudomonas aeruginosa IgY for cystis fibrosis (2008); RV = Anti-Rotavirus IgY for rotaviral diarrhea (2009); CA = Anti-Candida albicans IgY for candidiasis (2012); CD = Anti-Gliadin IgY for celiac diseases (2012); LP = Anti-Lipase IgY for metabolic syndrome (2014); NV = Anti-Norovirus IgY for Norovirus infection (2015).
Future Prospects and Imperatives
The egg-derived IgY is a natural food ingredient that has gained attention through recent studies designed to explore new methods for alimentary tract disease treatment and control as a way to supplant synthetic antibiotics or antimicrobials that are increasingly associated with downside issues such as adverse reactions and microbial resistance. With the recent findings by some workers in our group on the development of IgY against Vibrio cholera (Hirai et al., 2010) and Shiga-toxin (Neri et al., 2012) the stage is set toward combined use of IgYs against cholera, shiga toxin and rotavirus for control of pediatric diarrhea as a natural, resistance-free and safe treatment for pediatric diarrheal diseases in the foreseeable future. Commercial infant milk formulas would be better off if they come prefortified with anti-rotavirus IgY as well as IgY tailor-made against other pathogens of pediatric concerns such as Salmonella spp. or even Cronobacter sakazakii which is an occasional contaminant of powdered infant milk formula. On the clinical side, there is a need not only for more clinical studies with bigger sample size but also a need to focus on the use of anti-rotavirus IgY on young or very young children with health issues such as low birth weight or immunodeficiency in whom this IgY is sorely needed as a potential means to significantly improve their quality of life.
Conclusion
The accumulated information as a whole depicts oral IgY to be a safe and efficacious option for treatment of rotavirus diarrhea in neonates. There is however a clear need for more randomized, placebo controlled and double-blind trials with bigger sample sizes to further solidify and confirm claims of efficacy and safety in controlling diarrhea caused by rotavirus infection especially among human infants with health issues such as low birth weights or compromised immunity in whom it is most needed. The data gap is perceptible not only on the therapeutic side but also on the equally important preventive side of IgY application which is best addressed through community-based clinical studies.
Acknowledgments
We thank all members for their time and commitment to the project and all individuals who participated in the study. We are grateful to the Department of Medical Research, Yangon, for expert input and for supporting the study implementation.
Footnotes
Funding Clinical trial in Myanmar (2015) is a joint collaborative study. This study was co-funded by Lotte Food Co. Ltd, Korea and Immunology Research Institute in Gifu, Japan.
Competing Interests The authors declare that they have no competing interests.
References
- 1.Arimitsu H., Sasaki K., Kohda T., Shimizu T., Tsuji T. Evaluation of Shiga toxin 2e-specific chicken egg yolk immunoglobulin: Production and neutralization activity. Microbiol. Immunol. 2014;58:643–648. doi: 10.1111/1348-0421.12197. [DOI] [PubMed] [Google Scholar]
- 2.Barnes G. L., Hewson P. H., Mclellan J. A., Doyle L. W., Knoches A. M. L., Kitchen W. H. A randomised trial of oral gammaglobulin in low-birth-weight infants infected with rotavirus. The Lancet. 1982;319:1371–1373. doi: 10.1016/S0140-6736(82)92496-5. [DOI] [PubMed] [Google Scholar]
- 3.Bäumler A. J., Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhavnani D., Goldstick J. E., Cevallos W., Trueba G., Eisenberg J. N. S. Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: Evidence from a community-based study in Northwestern Ecuador. Am. J. Epidemiol. 2012;176:387–395. doi: 10.1093/aje/kws220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai Y. C., Wang Y. Y., Zhang X. F., Tan M., Xia M., Wu X. B., Jiang X., Nie J. Evaluation of anti-norovirus IgY from egg yolk of chickens immunized with norovirus P particles. J. Virol. Methods. 2012;186:126–131. doi: 10.1016/j.jviromet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhama K., Chauhan R. S., Mahendran M., Malik S. V. S. Rotavirus diarrhea in bovines and other domesticated animals. Vet. Res. Comm. 2009;33:1–23. doi: 10.1007/s11259-008-9070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diraviyam T., Zhao B., Wang Y., Schade R., Michael A., Zhang X. Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: A systematic review and meta-analysis. PLoS ONE. 2014;9:e97716. doi: 10.1371/journal.pone.0097716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gujral N., Löbenberg R., Suresh M., Sunwoo H. In vitro and in vivo binding activity of chicken egg yolk immunoglobulin Y (IgY) against gliadin in food matrix. J. Agric. Food Chem. 2012;60:3166–3172. doi: 10.1021/jf205319s. [DOI] [PubMed] [Google Scholar]
- 9.Hatta H., Tsuda K., Akachi S., Kim M., Yamamoto T., Ebina T. Oral passive immunization effect of antihuman rotavirus IgY and its behavior against proteolytic enzymes. Biosci. Biotechnol. Biochem. 1993;57:1077–1081. doi: 10.1271/bbb.57.1077. [DOI] [PubMed] [Google Scholar]
- 10.Hirai K., Arimitsu H., Umeda K., Yokota K., Shen L., Ayada K., Kodama Y., Tsuji T., Hirai Y., Oguma K. Passive oral immunization by egg yolk immunoglobulin (IgY) to Vibrio cholerae effectively prevents cholera. Acta Med. Okayama. 2010;64:163–170. doi: 10.18926/AMO/40008. [DOI] [PubMed] [Google Scholar]
- 11.Hirose M., Ando T., Shofiqur R., Umeda K., Kodama Y., Nguyen S. V., Goto T., Shimada M., Nagaoka S. Anti-obesity activity of hen egg anti-lipase immunoglobulin yolk, a novel pancreatic lipase inhibitor. Nutr. Metab. (Lond). 2013;10:70. doi: 10.1186/1743-7075-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hlaing M. T., Theingi W. M., Mo Mo W., Kyaw Z. T., Rahman S., Umeda K., Nguyen V. S., Icatlo F. C. Jr., Moriguchi K. H., Taniguchi K., Tsuji T., Oguma K. Immunoglobulin Y-fortified infant milk formula as adjunct to treatment of diarrhea among rotavirus-infected hospitalized children in Myanmar: a randomized, double-blind, placebo-controlled trial. 2015. Unpublished data.
- 13.Ibrahim El-S. M., Rahman A. K. M. S., Isoda R., Umeda K., van Sa N., Kodama Y. In vitro and in vivo effectiveness of egg yolk antibody against Candida albicans (anti-CA IgY) Vaccine. 2008;26:2073–2080. doi: 10.1016/j.vaccine.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Ikemori Y, Peralta R.C., Kuroki M., Yokoyama H., Kodama Y. Avidity of chicken yolk antibodies to enterotoxigenic Escherichia coli fimbriae. Poult Sci. 1993;72:2361–2365. doi: 10.3382/ps.0722361. [DOI] [PubMed] [Google Scholar]
- 15.Ikemori Y., Ohta M., Umeda K., Peralta R. C., Kuroki M., Yokoyama H., Kodama Y. Passage of chicken egg yolk antibody treated with hydroxypropyl methylcellulose phthalate in the gastrointestinal tract of calves. J. Vet. Med. Sci. 1996;58:365–367. doi: 10.1292/jvms.58.365. [DOI] [PubMed] [Google Scholar]
- 16.Kollberg H. Avian antibodies (IgY) to fight antibiotic resistance. Clin. Microbiol. 2015;4(2):1000194. [Google Scholar]
- 17.Lee C. H., Lee Y. C., Liang M. H., Leu S. J., Lin L. T., Chiang J. R., Yang Y. Y. Antibodies against venom of the snake Deinagkistrodon acutus. Appl. Environ. Microbiol. 2015;82:71–80. doi: 10.1128/AEM.02608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K. E., Han B. K., Han J. Y., Hong J. Y., Kim M. N., Heo W. I., Sohn M. H., Kim K. W., Kim K. E. Production of egg yolk antibodies specific to house dust mite proteins. Yonsei Med. J. 2014;55:999–1004. doi: 10.3349/ymj.2014.55.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X. Y., Jin L. J., Uzonna J. E., Li S. Y., Liu J. J., Li H. Q., Lu Y. N., Zhen Y. H., Xu Y. P. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY): In vivo evaluation in a pig model of enteric colibacillosis. Vet. Immunol. Immunopathol. 2009;129:132–136. doi: 10.1016/j.vetimm.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Müller S., Schubert A., Zajac J., Dyck T., Oelkrug C. IgY antibodies in human nutrition for disease prevention. Nutr. J. 2015;14:109. doi: 10.1186/s12937-015-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neri P., Tokoro S., Sugiyama T., Umeda K., Shimizu T., Tsuji T., Kodama Y., Mori H. Recombinant Shiga toxin B subunit can induce neutralizing immunoglobulin Y antibody. Biol. Pharm. Bull. 2012;35:917–923. doi: 10.1248/bpb.35.917. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson E., Larsson A., Olesen H.V., Wejåker P. E., Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol. 2008;43:892–899. doi: 10.1002/ppul.20875. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen H. H., Tumpey T. M., Park H. J., Byun Y. H., Tran L. D., Nguyen V. D., Kilgore P. E., Czerkinsky C., Katz J. M., Seong B. L., Song J. M., Kim Y. B., Do H. T., Nguyen T., Nguyen C. V. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS ONE. 2010;5:e10152. doi: 10.1371/journal.pone.0010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen S. V., Icatlo F. C., Jr., Nakano T., Isogai E., Hirose K., Mizugai H., Kobayashi-Sakamoto M., Isogai H., Chiba I. Anti cell-associated glucosyltransferase immunoglobulin Y suppression of salivary mutans streptococci in healthy young adults. J. Ame. Dent. Assoc. 2011;142:943–949. doi: 10.14219/jada.archive.2011.0301. [DOI] [PubMed] [Google Scholar]
- 25.Pammi M., Haque K. N. Oral immunoglobulin for the treatment of rotavirus diarrhea in low birth weight infants. Cochrane Database Syst. Rev. 2011;5 doi: 10.1002/14651858.CD003742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parr R. D., Storey S. M., Mitchell D. M., McIntosh A. L., Zhou M., Mir K. D., Ball J. M. The rotavirus enterotoxin NSP4 directly interacts with the caveolar structural protein caveolin-1. J. Virol. 2006;80:2842–2854. doi: 10.1128/JVI.80.6.2842-2854.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffatellu M., Baumler A. J. Salmonella's iron armor for battling the host and its microbiota. Gut Microbes. 2010;1:70–72. doi: 10.4161/gmic.1.1.10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman S., Higo-Moriguchi K., Htun K. W., Taniguchi K., Icatlo F. C., Jr., Tsuji T., Kodama Y., van Nguyen S., Umeda K., Oo H. N., Myint Y. Y., Htut T., Myint S. S., Thura K., Thu H. M., Fatmawati N. N., Oguma K. Randomized placebo-controlled clinical trial of immunoglobulin Y as adjunct to standard supportive therapy for rotavirus-associated diarrhea among pediatric patients. Vaccine. 2012;30:4661–4669. doi: 10.1016/j.vaccine.2012.04.091. [DOI] [PubMed] [Google Scholar]
- 29.Rahman S., Nguyen S., Icatlo F., Umeda K., Kodama Y. Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Hum. Vaccines Immunotherapeutics. 2013a;9:1039–1048. doi: 10.4161/hv.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman S., Umeda K., Icatlo F. C., Jr., Lee K. W., Kim S. H., Choi H. J., Han B. K., Park Y. S., Sa N. V. In vitro cytoprotective effect of infant milk formula fortified with human rotavirus-specific hyperimmune yolk immunoglobulins (IgY) Food Sci. Biotechnol. 2013b;22:1699–1705. doi: 10.1007/s10068-013-0269-4. [DOI] [Google Scholar]
- 31.Robert F. R. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarker S. A., Casswall T. H., Juneja L. R., Hoq E., Hossain I., Fuchs G. J., Hammarström L. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J. Pediatr. Gastroenterol. Nutr. 2001;32:19–25. doi: 10.1097/00005176-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Selvan K., Sentila R., Michael A. Generation and characterization of chicken egg yolk antibodies against Propionibacterium acnes for the prevention of acne Vulgaris. Ind. J. Dermatol. 2012;57:15–19. doi: 10.4103/0019-5154.92669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin J. H., Yang M., Nam S.W., Kim J. T., Myung N. H., Bang W.G., Roe I. H. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin. Diagn. Lab. Immunol. 2002;9:1061–1066. doi: 10.1128/CDLI.9.5.1061-1066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H., Nomura S., Masaoka T., Goshima H., Kamata N., Kodama Y, Ishii H., Kitajima M., Nomoto K., Hibi T. Effect of dietary anti-Helicobacter pylori-urease immunoglobulin Y on Helicobacter pylori infection. Aliment Pharmacol. Therapeut. 2004;20:185–192. doi: 10.1111/j.1365-2036.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 36.Vega C., Bok M., Saif L., Fernandez F., Parreño V. Egg yolk IgY antibodies: A therapeutic intervention against group A rotavirus in calves. Res. Vet. Sci. 2015;103:1–10. doi: 10.1016/j.rvsc.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokoyama H., Peralta R. C., Sendo S., Ikemori Y., Kodama Y. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing. Am. J. Vet. Res. 1993;54:867–872. [PubMed] [Google Scholar]
- 38.Yokoyama K., Sugano N., Shimada T., Shofiqur R. A., Ibrahim El-S. M., Isoda R., Umeda K., van Sa N., Kodama Y., Ito K. Effects of egg yolk antibody against Porphyromonas gingivalis gingipains in periodontitis patients. J. Oral Sci. 2007;49:201–206. doi: 10.2334/josnusd.49.201. [DOI] [PubMed] [Google Scholar]