Abstract
Heat treatment of milk aims to inhibit the growth of microbes, extend the shelf-life of products and improve the quality of the products. Heat treatment also leads to denaturation of whey protein and the formation of whey protein-casein polymer, which has negative effects on milk product. Hence the milk heat treatment conditions should be controlled in milk processing. In this study, the denaturation degree of whey protein and the combination degree of whey protein and casein when undergoing heat treatment were also determined by using the Native-PAGE and SDS-PAGE analysis. The results showed that the denaturation degree of whey protein and the combination degree of whey protein with casein extended with the increase of the heat-treated temperature and time. The effects of the heat-treated temperature and heat-treated time on the denaturation degree of whey protein and on the combination degree of whey protein and casein were well described using the quadratic regression equation. The analysis strategy used in this study reveals an intuitive and effective measure of the denaturation degree of whey protein, and the changes of milk protein under different heat treatment conditions efficiently and accurately in the dairy industry. It can be of great significance for dairy product proteins following processing treatments applied for dairy product manufacturing.
Keywords: whey protein, casein, heat treatment, thermal denaturation
Introduction
Heat treatment is essential to dairy processing by inhibiting microbial growth in milk, extending product shelf-life and improving product quality. Heat treatment, depending on the processing conditions, can result in irreversible changes in milk protein structure. When milk is heated at temperatures range from 70 to 100°C, the whey protein, which mainly includes α-lactalbumin (α-La) and β-lactoglobulin (β-Lg), can denature due to heat treatment, while the structure of the casein micelle is not obviously changed (Vasbinder and de Kruif, 2003). Following conformation changes, thiol of β-Lg exposed. the thiol group of β-Lg will be exposed. Disulfide associated with other active thiol group will be generated, which can lead to the denaturation irreversibly (Morand et al., 2011). As for α-La, although polymerization reaction can’t occur with α-La, in consequence of the absence of free thiol group, the disulfide bonds inside will also lead it to denature due to thiol-disulfide bond exchange reaction (Mulvihill and Donovan, 1987). In this reaction, β-Lg polymerise with κ-casein and αs2-casein (Vasbinder et al., 2003). The effectiveness of protein denaturation and aggregation is controlled by the heating conditions and the chemical environment with the heating temperature and pH being probably the most important factors in determining the rate and extent of protein denaturation and the degree of the subsequent interaction of the whey proteins with the casein micelles.
In this study, the rate of milk protein denaturation with different heat treatment conditions was examined. The relationship between the denaturation degree of milk protein and heating conditions was established through quadratic regression equation.
Materials and Methods
Sample of heat treatment
Raw milk was collected from Dalian Sanhuan dairy Co., Ltd. and kept at low temperature to inhibit bacterial activity. A portion of raw milk was heated in a water bath range from 65°C to 100°C every 10°C for 10 min, 20 min and 30 min, in order to investigate the effects of different temperature on the degree of denaturation of whey protein. On the other hand, another sector of raw milk was heated respectively at 75°C, 85°C and 95°C range from 5 min to 30 min every 5 min, for explaining the effects of different heat-treated time on the degree of denaturation of whey protein. The milk was subsequently allowed to cool to ambient temperature. All samples were freshly prepared and kept at 4°C until analyses.
Determination of protein content
The protein content of samples, donated by Cp, treated under different temperature was determined by using UV Spectrophotometry (Cao and Huang, 2005). In detail, the samples were added into the 1 mL centrifuge tube, which were skimmed at 10000 rpm at 4°C for 25 min using a centrifuge (Sorvall Legend Micro17, Thermo Fisher Scientific, USA). The supernatant was diluted 10 times, the absorbance of the supernatant under 260 nm and 280 nm was measured by UV spectrophotometer (TU-1900, Beijing Pgeneral Co., Ltd., China). Cp was calculated by the followi
Cp was calculated by the following formula:
| (1) |
Denaturation degree of heat-treated whey protein
The denaturation degree of the heat-treated whey protein was detected by Native-PAGE on 15% separation gel and 5% spacer gel according to Lucey (1998). The supernatant samples were subsequently maxed with the sample buffer without 2-mercaptoethanol and loaded onto gels together with a set of molecular weight standards. An aliquot of each of these diluted subsamples was mixed with a stacking gel mixture and immediately placed in the appropriate loading well of the Native-PAGE gels and allowed to set. The vertical electrophoresis unit (AE8135, ATTO, Japan) was used in conjunction with the power supply. The gel was stained with the 0.23% solution of Coomassie Blue R-250 for 90 min, and destained in the methanol/acetic acid solution (5% methanol, 7% acetic acid). Destained gels have been scanned using electrophoresis gel imaging system (Gel Doc™ XR+, Bio-Rad, USA), and analyzed with Image Lab 3.0 software.
The ratio of denatured whey proteins was calculated as follows:
| (2) |
where S is the ratio of denatured whey protein (%); W1 is the relative amount of whey protein in the supernatant fluid of raw milk; W2 is the relative amount of whey protein in the supernatant fluid of heat treatment milk.
Degree of the combination of whey protein and casein
SDS-PAGE (Jang and Swaisgood, 1990; Vasbinder and Fred, 2004) was commonly used to determine degree of the combination of whey protein and casein in the present paper. A further set of each subsample (1 mL) was mixed with 20 μL of 2-mercaptoethanol and heated at 94°C for 4 min to reduce the disulfide bonds. These subsamples were then mixed with SDS sample buffer and loaded onto SDS gels together with a set of molecular weight standards. The preparation and running of the gels, and their staining and destaining, photography, and quantitative scanning, followed the procedures described by Mahmoudi et al. (2007).
The degree of the combination of whey protein and casein was calculated as follows:
| (3) |
where C is the whey protein and casein combination degree (%); W1 is the relative amount of whey protein in the supernatant fluid of raw milk; W2 is the relative amount of whey protein in the supernatant fluid of heat treatment milk.
Statistical analysis
All experiments were performed in triplicate. The obtained data was reported as mean values. Multivariate regression statistical analysis was conducted using Origin 8.5. The significance of the differences between the mean values was determined with F- test (p<0.05) using IBM SPSS version 20.0.
Results and Discussion
Influence of different heat-treated temperature on the denaturation degree of whey protein
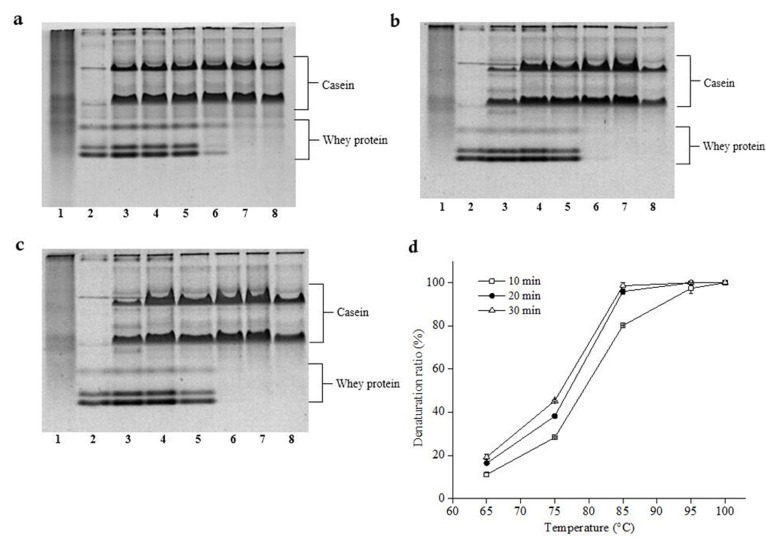
Whey protein, with poor thermal stability in raw milk, could denature under heat treatment with different denaturation degrees. By comparing the relative amounts of nature whey protein in unheated milk with that of the milk heat-treated from 65°C to 100°C every 10°C for 10 min, 20 min and 30 min respectively, the denaturation degree of whey protein could be determined using Native-PAGE. The denaturation degree of milk protein at different heat-treated conditions was shown in Fig. 1. Compared with the untreated raw milk, lower intensity of the band corresponding to β-Lg and the markedly lower intensity of the band corresponding to α-La in the severely heated samples. The difference between untreated and heat-treated milk confirmed that whey protein had different denaturation degrees under different heat-treated conditions: With the increase of the heat treatment temperature, whey protein bands almost disappeared when the temperature especially higher than 85°C (Lane 4, 5, and 6), which showed that almost all of the whey protein denatured, the denaturation degree of the whey protein under 65°C (Lane 2) was minimum, showing whey protein was hardly denatured. Moreover, there was a greater difference between the initial rates of the native protein and the heated protein. The result of the ratio of denatured whey protein during heat treatment (Fig. 1) indicated that as heattreated temperature rise, the increase rate of whey protein denaturation degree were essentially absent in the PAGE patterns of the severely heat-treated samples. The denaturation degree of whey protein increased from 28.34% to 45.37% as the heat treatment time extended from 65°C to 85°C for 10 min. Almost all whey protein denatured at 95°C for 10 min.
Fig. 1. Native-PAGE and ratio of denatured whey proteins in different temperature of heat-treated milk.
a, b and c: Native-PAGE of whey proteins of heat-treated milk for 10 min, 20 min and 30 min respectively. lane 1: casein, lane 2: whey protein, lane 3: raw milk, lanes 4 to 8: 65°C, 75°C, 85°C, 95°C and 100°C. d: Denaturation ratio. Whey proteins of heat-treated (10 min (white squares), 20 min (black circles), 30 min (white triangles)).
Whey proteins (α-La and β-Lg) or whey protein with casein could be related by hydrophobic and/or hydrogen bonding between proteins. With the increase of extent of heat treatment, β-Lg form small polymer (Mahmoudi et al., 2007) and it gradually formed bigger β-Lg polymer. When the temperature rised further, β-Lg spiral structure was going to disappear, the embedding within the natural structure of hydrophobic side chain groups exposed (Vasbinder and Fred, 2004).
The denaturation degree of whey protein had a quadratic regression relationship with heat treatment temperature:
Y = −360.97576 + 7.50609 T − 0.02854 T2 (10 min);
Y = −586.84195 + 13.46156 T − 0.06562 T2 (20 min);
Y = −639.09182 + 14.97326 T − 0.07562 T2 (30 min).
where Y is the denaturation degree of whey protein; T is the heat-treated temperature.
Influence of different heat-treated time on the denaturation degree of whey protein
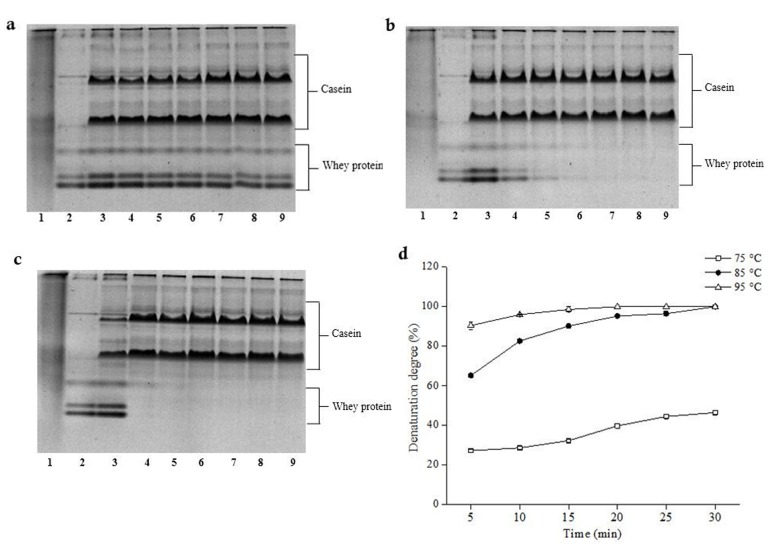
Under different heat treatment conditions, the ratio of denatured whey proteins was studied. Native-PAGE electrophoresis of milk heat-treated at different time under the same temperature was shown in Fig. 2. It illustrated that whey protein bands of milk changed from deep to shallow under different temperatures. This result showed that whey protein denatured differently at different temperatures. In addition, under 85°C and 95°C, whey protein denatured completely. Whey protein bands of milk treated at 75°C were relatively deep, showing a little denatruation degree of whey protein; under 85°C (10 min) (Lane 3) and 95°C (5 min) (Lane 2), whey protein bands almost disappeared, suggesting that whey protein denatured completely. The denaturation degree of whey protein increased from 27.31% to 46.50% gradually at 75°C from as time goes on; denaturation degree of whey protein increased from 65.30% to 100% at 85°C in the course of time (Fig. 2). These results showed that whey protein could denature under certain heat treatment. Vasbinder et al. (2003) reported that the denaturation of whey proteins existed in two forms: soluble protein aggregates and on the surface of the casein micelle aggregation. Thus, heat treatment for long time could make the hydrophobic groups in the protein interaction enhanced, which may further promote the combination of β-Lg with κ-casein (Patel et al., 2006).
Fig. 2. Native-PAGE and ratio of denatured whey proteins at different time in heat-treated milk.
a, b and c Native-PAGE of whey proteins of heat-treated milk for 75°C, 85°C, 95°C respectively. lane 1: casein, lane 2: whey protein, lane 3: raw milk, lanes 4 to 8: 5 min, 10 min, 15 min, 20 min, 25 min, 30 min. d Denaturation ratio. Whey proteins of heat-treated (75°C (white squares), 85°C (black circles), 95°C (white triangles)).
The relationship between denaturation degree of whey protein with the heat-treated time at different temperatures was established as follows:
75°C: Y = 22.597 + 0.69199 t − 0.00488 t2;
85°C: Y = 50.563 + 3.62422 t − 0.06764 t2;
95°C: Y = 85.092 + 1.30131 t − 0.02726 t2.
where Y is the denaturation degree of whey protein; t is the heat-treated time.
Effects of different heat-treated temperature on the combination degree of whey protein and casein
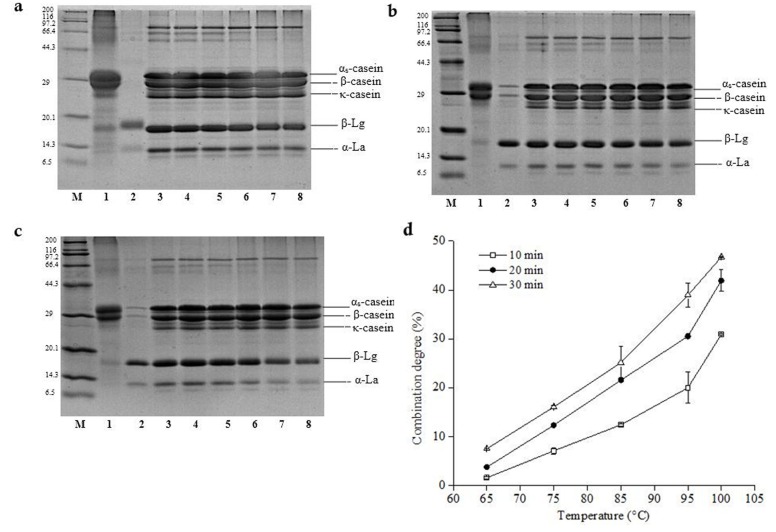
According to the report of Singh et al. (1996), some denatured whey protein would combine with casein in milk under heat treatment due to the poor thermal stability of whey protein. The polymer precipitation could be obtained by high-speed centrifugation. Therefore, the combination degree of whey protein and casein in milk could be accurately determined by comparing the contents of whey protein in centrifugal supernatant of unheated and heat-treated milk using SDS-PAGE. As shown in Fig. 3, changes of whey protein (α-La and β-Lg) in heat treated milk were most obviously. Comparing with raw milk untreated, the content of total whey protein was decreased in supernatant fluid by heat-treated. Whey protein bands at 65°C (Lane 2) was the darkest, which was closed to the bands of raw milk (Lane 1); with the increase of heat-treated temperature, the whey protein bands became shallow gradually. Under the same temperature, whey protein bands in milk were the darkest when heat-treated for 10 min; the whey protein bands when heat-treated for 30 min were the shallowest, indicating that whey protein denaturation degree was higher when heat-treated for 30 min. In addition, the combination degree of whey protein with casein varied at different heat-treated conditions (Fig. 3). With the increase of heat-treated temperature, the combination degree of whey protein with casein increased obviously from 7.56% under 65°C to 16.09% under 75°C, up to maximum 46.74% at 100°C. The results were similar to the findings of Guyomarch et al. (2003). Heat treatment over 60°C caused whey protein denaturation, leading to the formation of whey protein polymer by itself or combined with casein. The further research confirmed that heat treatment at higher temperature could promote the combination of β-Lg with κ-casein. Although casein is relatively stable, whey proteins (α-La and β-Lg) could combine with casein to generate stable polymer by disulfide bonds, leading to the size change of the casein micelle (Mckinnon et al., 2009).
Fig. 3. SDS-PAGE and combination degree of whey protein and casein in different temperature of heat-treated milk.
a, b and c SDS-PAGE of whey proteins of heat-treated milk for 10 min, 20 min and 30 min respectively. lane M: Marker, lane 1: casein, lane 2: whey protein, lane 3: raw milk, lanes 4 to 8: 65°C, 75°C, 85°C, 95°C and 100°C. d Combination degree of whey protein and casein (10 min (white squares), 20 min (black circles), 30 min (white triangles)).
The relationship between the combination degree of whey protein with casein under different temperatures was established as follows:
Y = 66.3476 − 2.12271 T + 0.017519 T2 (10 min);
Y = 20.00307 − 1.065 T + 0.01267 T2 (20 min);
Y = 27.32839 − 1.21571 T + 0.01408 T2 (30 min).
where Y is the combination degree of whey protein with casein; T is the heat-treated temperature.
Effects of different heat-treatment time on the combination degree of whey protein and casein
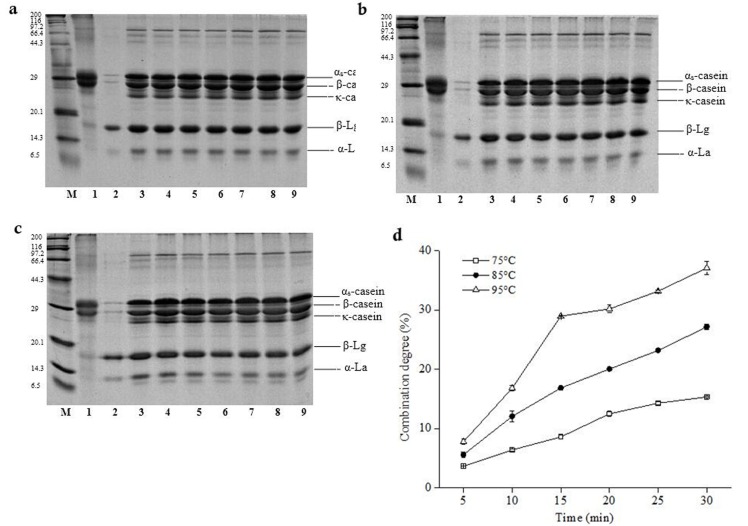
The milk protein in supernatants heat-treated for different time were analyzed by SDS-PAGE. The results showed that the bands of α-La and β-Lg in heat treated milk turned from deep to shallow (Fig. 4). As the heat treated-time prolonged, α-La stripes almost disappeared especially at 95°C. It implied that nearly all of α-La combined with casein. However, the bands of β-Lg were relatively deep at 75°C. This represented that the combination degree of whey protein with casein was low. Pesic et al. (2012) pointed out that the reduction of whey protein in supernatant fluid and the different degrees of whey protein combined with casein during heat treatment. The combination degree of whey protein with casein increased with the prolonged heat-treated time (Fig. 4). The variation trend of combination degree of whey protein with casein was almost same in heat treated milk at 75°C, 85°C and 95°C for 5 min. It further illustrated that the combination degree of whey protein with casein were affected by the synergy of temperature and time (Smits and van Brouwershaven, 1980). Jovanovic et al. (2007) pointed out that in the process of heating of milk, whey protein change through the thiol and disulfide bond in intermolecular disulfide bond exchange. Furthermore, disulfide bonds, which formed between casein and whey protein or whey proteins when heat treated, played a key role in the aggregation of milk system in the heat-treatment (Waungana et al., 1996).
Fig. 4. SDS-PAGE and combination degree of whey protein and casein in different temperature of heat-treated milk.
a, b and c SDS-PAGE of whey proteins of heat-treated milk for 75°C, 85°C, 95°C respectively. lane M: Marker, lane 1: casein, lane 2: whey protein, lane 3: raw milk, lanes 4 to 9: 5 min, 10 min, 15 min, 20 min, 25 min, 30 min. d Combination degree of whey protein and casein (75°C (white squares), 85°C (black circles), 95°C (white triangles)).
The relationship between the combination degree of whey protein with casein and the heated-treated time at different temperatures was established as follows:
75°C: Y = −0.129 + 0.74262 t − 0.00718 t2;
85°C: Y = −0.125 + 1.30239 t − 0.01364 t2;
95°C: Y = −4.386 + 2.6827 t − 0.0445 t2.
where Y is the combination degree of whey protein and casein; t is the heat-treated time.
Conclusion
The results indicated that heat-treatment temperature and time have significant effects on the whey protein denaturation and the combination of whey protein and casein. With the increase of temperature and time of heat treatment, both the denaturation degree of whey protein and the combination degree of whey protein and casein increased. The denaturation and the combination degree was found to have the quadratic regression relationship with the heat-treated temperature and time. The equations proposed in this study can be used to figure the detailed relationship between the denaturation degree of milk protein and heat treatment conditions. It is essential for dairy industry to choose suitable heat treatment conditions to improve the stability of the milk protein and reduce nutrition loss.
Acknowledgments
This work was financially supported by Liaoning Province Natural Science Foundation Projects (201602053), National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2013BAD18B04), National Natural Science Foundation Projects (31501513), Key University Science and Technology Platform of Liaoning Province (No. 2011-191), and National Engineering Research Center of Seafood (2012FU125X03).
References
- 1.Cao Y., Huang B. S. Total protein content of Pinellia with ultraviolet absorption. Hubei J. Tradit. Chinese Med. 2005;27:48–50. [Google Scholar]
- 2.Guyomarch F., Law A., Dalgleish D. Formation of soluble and micelle-bound aggregates in heated milk. J. Agric. Food Chem. 2003;51:4652–4660. doi: 10.1021/jf0211783. [DOI] [PubMed] [Google Scholar]
- 3.Jang H. D., Swaisgood H. E. Disulfide bond formation between thermally denatured β-lactoblobulin and κ-casein in casein micelles. J. Dairy Sci. 1990;73:900–904. doi: 10.3168/jds.S0022-0302(90)78746-2. [DOI] [Google Scholar]
- 4.Jovanovic S., Barac M., Macej O. SDS-PAGE analysis of soluble proteins in reconstituted milk exposed to different heat treatments. Sensors. 2007;7:371–383. doi: 10.3390/s7030371. [DOI] [Google Scholar]
- 5.Lucey A. Effect of interactions between denatured whey proteins and casein micelles on the formation and rheological properties of acid skim milk gels. J. Dairy Res. 1998;65:555–567. doi: 10.1017/S0022029998003057. [DOI] [Google Scholar]
- 6.Mahmoudi N., Mehalebi S., Nicolai T., Durand D., Riaublanc A. Light-scattering study of the structure of aggregates and gels formed by heat-denatured whey protein isolate and β-lactoglobulin at neutral pH. J. Agr. Food Chem. 2007;55:3104–3111. doi: 10.1021/jf063029g. [DOI] [PubMed] [Google Scholar]
- 7.Mckinnon I. R., Yap S. E., Augustin M. A., Memar Y. Diffusing-wave spectroscopy investigation of heated reconstituted skim milks containing calcium chloride. Food Hydrocolloid. 2009;23:1127–1133. doi: 10.1016/j.foodhyd.2008.08.009. [DOI] [Google Scholar]
- 8.Morand M., Guyomarch F., Pezennec S. On how κ-casein affects the interactions between the heat-induced whey protein/κ-casein complexes and the casein micelles during the acid gelation of skim milk. Int. Dairy J. 2011;21:670–678. doi: 10.1016/j.idairyj.2011.01.012. [DOI] [Google Scholar]
- 9.Mulvihill D. M., Donovan M. Whey proteins and their thermal denaturation - A review. Irish J. Food Sci. Tech. 1987;11:43–75. [Google Scholar]
- 10.Patel H. A., Singh H., Anema S. G., Creamer L. K. Effects of heat and high hydrostatic pressure treatments on disulfide bonding interchanges among the proteins in skim milk. J. Agric. Food Chem. 2006;54:3409–3420. doi: 10.1021/jf052834c. [DOI] [PubMed] [Google Scholar]
- 11.Pesic M. B., Barac M. B., Stanojevic S. Heat induced casein-whey protein interactions at natur pH of milk: A comparison between caprine and bovine milk. Small Ruminant Res. 2012;108:77–86. doi: 10.1016/j.smallrumres.2012.06.013. [DOI] [Google Scholar]
- 12.Singh H., Roberts M. S., Munro P. A. Acid-induced dissociation of casein micelles in milk: Effects of heat treatment. J. Dairy Sci. 1996;79:1340–1346. doi: 10.3168/jds.S0022-0302(96)76490-1. [DOI] [Google Scholar]
- 13.Smits P., van Brouwershaven J. H. Heat-induced association of β-lactoglobulin and casein micelles. J. Dairy Res. 1980;47:313–325. doi: 10.1017/S0022029900021208. [DOI] [PubMed] [Google Scholar]
- 14.Vasbinder A. J., Alting A. C., de Kruif K. G. Quantification of heat-induced casein-whey protein interactions in milk and its relation to gelation kinetics. Colloids and Surfaces B: Biointerfaces. 2003;31:115–123. doi: 10.1016/S0927-7765(03)00048-1. [DOI] [Google Scholar]
- 15.Vasbinder A. J., de Kruif K. G. Casein-whey protein interactions in heated milk: The influence of pH. Int. Dairy J. 2003;13:669–677. doi: 10.1016/S0958-6946(03)00120-1. [DOI] [Google Scholar]
- 16.Vasbinder A. J., Fred V. D. Gelation of casein-whey protein mixtures. J. Dairy Sci. 2004;87:1167–1176. doi: 10.3168/jds.S0022-0302(04)73265-8. [DOI] [PubMed] [Google Scholar]
- 17.Waungana A., Singh H., Bennett R. J. Influence of denaturation and aggregation of β-lactoglobulin on rennet coagulation properties of skim milk and ultrafiltered milk. Food Res. Int. 1996;29:715–721. doi: 10.1016/S0963-9969(97)00011-2. [DOI] [Google Scholar]