ABSTRACT
The glycoprotein complex (GPC) of arenaviruses, composed of stable signal peptide, GP1, and GP2, is the only antigen correlated with antibody-mediated neutralization. However, despite strong cross-reactivity of convalescent antisera between related arenavirus species, weak or no cross-neutralization occurs. Two closely related clade B viruses, Machupo virus (MACV) and Junín virus (JUNV), have nearly identical overall GPC architecture and share a host receptor, transferrin receptor 1 (TfR1). Given structural and functional similarities of the GP1 receptor binding site (RBS) of these viruses and the recent demonstration that the RBS is an important target for neutralizing antibodies, it is not clear how these viruses avoid cross-neutralization. To address this, MACV/JUNV chimeric GPCs were assessed for interaction with a group of α-JUNV GPC monoclonal antibodies (MAbs) and mouse antisera against JUNV or MACV GPC. All six MAbs targeted GP1, with those that neutralized JUNV GPC-pseudovirions competing with each other for RBS binding. However, these MAbs were unable to bind to a chimeric GPC composed of JUNV GP1 containing a small disulfide bonded loop (loop 10) unique to MACV GPC, suggesting that this loop may block MAbs interaction with the GP1 RBS. Consistent with this loop causing interference, mouse anti-JUNV GPC antisera that solely neutralized pseudovirions bearing autologous GP1 provided enhanced neutralization of MACV GPC when this loop was removed. Our studies provide evidence that loop 10, which is unique to MACV GP1, is an important impediment to binding of neutralizing antibodies and contributes to the poor cross-neutralization of α-JUNV antisera against MACV.
IMPORTANCE Multiple New World arenaviruses can cause severe disease in humans, and some geographic overlap exists among these viruses. A vaccine that protects against a broad range of New World arenaviruses is desirable for purposes of simplicity, cost, and broad protection against multiple National Institute of Allergy and Infectious Disease-assigned category A priority pathogens. In this study, we sought to better understand how closely related arenaviruses elude cross-species neutralization by investigating the structural bases of antibody binding and avoidance. In our studies, we found that neutralizing antibodies against two New World arenaviruses, Machupo virus (MACV) and Junín virus (JUNV), bound to the envelope glycoprotein 1 (GP1) with JUNV monoclonal antibodies targeting the receptor binding site (RBS). We further show that altered structures surrounding the RBS pocket in MACV GP1 impede access of JUNV-elicited antibodies.
KEYWORDS: GPC, arenavirus, clade B viruses, glycoprotein, Junín virus, Machupo virus, neutralizing antibodies, New World arenavirus
INTRODUCTION
Members of the Mammarenavirus genus within Arenaviridae represent a large group of enveloped viruses broadly divided into two major groups: Old World (OW) and New World (NW) (1, 2). Of the 30 known members, a number have been described to infect humans and some can cause severe cases of hemorrhagic fevers (3). Mammarenaviruses (henceforth, arenaviruses) are small ambisense RNA viruses that encode only four proteins, one of which embeds in the virion envelope, the glycoprotein complex (GPC; reviewed in reference 4). The precursor glycoprotein is translated as a single polypeptide that is proteolytically cleaved into its three noncovalently associated subunits that compose the GPC: stable signal peptide (SSP), the receptor binding domain (GP1), and the membrane-traversing fusion domain (GP2). The arenavirus GPC is unique among enveloped viruses in being the only viral glycoprotein described to retain the signal peptide as part of the infectious, virion-associated glycoprotein complex that forms heterotrimers, rather than heterodimers (4). The role of viral glycoproteins of all enveloped viruses is 2-fold. First is to bind to a host receptor, be it on the exterior of the host cell and/or within the vesicular network, and second, to facilitate fusion with a host membrane to allow the release of the genome into the cytoplasm for viral genome expression and replication. Arenavirus GP1 provides the former function, whereas GP2 provides the latter.
The GPC is similar to other enveloped virus glycoproteins in being a crucial target of the humoral response during infection (5–9). In the early years of investigating the antigenic relationships among both NW and OW arenaviruses, one clear consistency with antisera was that complement fixation tests (CF) and indirect immunofluorescence assays (IFA) revealed strong cross-reactivity, whereas virus neutralization tests and plaque reduction assays revealed strong species specificity (2, 7, 9–22). Studies comparing the antibodies that react in CF and IFA to those effective in neutralization assays determined that the majority of the highly cross-reactive CF and IFA-directed antibodies were against the internal nucleoprotein of the virus, while the minimally cross-reactive GPC-specific antibodies were the mediators of neutralization (7, 9, 23, 24).
Two of the most closely related viruses within clade B of the NW group, Machupo virus (MACV; Carvallo strain) and Junín virus (JUNV; recXJ13 strain), share 69.0% identity and 79.4% similarity in amino acid sequence within the GPC. Although sequence conservation is considerably stronger in the GP2 than the GP1 (GP2, 86.3% identical and 95.7% similar; GP1, 46.3% identical and 58.7% similar), crystal structures of both MACV GP1 (25) and JUNV GP1 (26) have been published within the last several years, and superimposition of the two structures demonstrates remarkable structural homology. Together, this provides strong evidence that the full glycoprotein complex structures must be extremely similar between the two viruses. For all arenaviruses in this subgroup where a cellular receptor has been identified, these viruses utilize the same host receptor, transferrin receptor 1 (TfR1) (27). Hence, in addition to having similar overall architecture, the functionally sensitive receptor binding sites (RBS) of these two viruses must also be sufficiently similar for this critical interaction. This inference is supported by the comparative analysis of both GP1 crystal structures (26).
Because host receptor engagement is required for infection, it is a step commonly targeted by neutralizing antibodies. Studies investigating relationships between neutralizing antibodies and respective viral glycoproteins have revealed that potently neutralizing antibodies sometimes function as host receptor mimics. This has been shown previously with influenza (28, 29), HIV-1 (30, 31), poliovirus (32), and most recently, JUNV (26). With such strong structural and functional homology between MACV and JUNV GPCs, even within the RBS, it was unclear how these two viruses broadly avoid cross-neutralization.
In the following study, we sought to characterize epitopes of multiple monoclonal antibodies (MAbs) and polyclonal antisera to investigate structural distinctions within the GPC that prevent cross-neutralization of these two closely related viruses. To facilitate these studies, the well-established vesicular stomatitis virus (VSV) pseudovirus system was used in which the native glycoprotein gene, G, has been replaced with that of an enhanced green fluorescent protein reporter gene in the VSV genome (VSVΔG-eGFP) and arenavirus GPCs were supplied in trans in the producer cells (33). Previous work indicates that this pseudovirus platform faithfully reflects the sensitivity of antibody-mediated neutralization against arenavirus GPCs (34, 35). Through our examination, we found that GP1 was the target of all MAbs assessed, as well as neutralizing antisera. We also demonstrated that MACV GPC obscures its RBS accessibility with adjacent structural modifications to evade interaction with neutralizing α-JUNV antibodies.
RESULTS
α-JUNV GPC MAbs bind to and inhibit JUNV, but not MACV, pseudovirions.
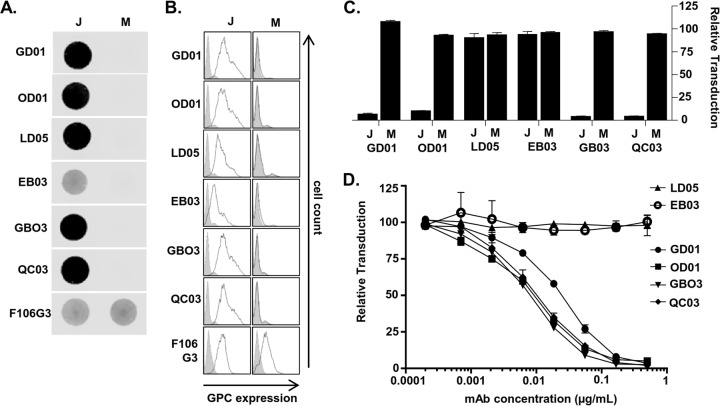
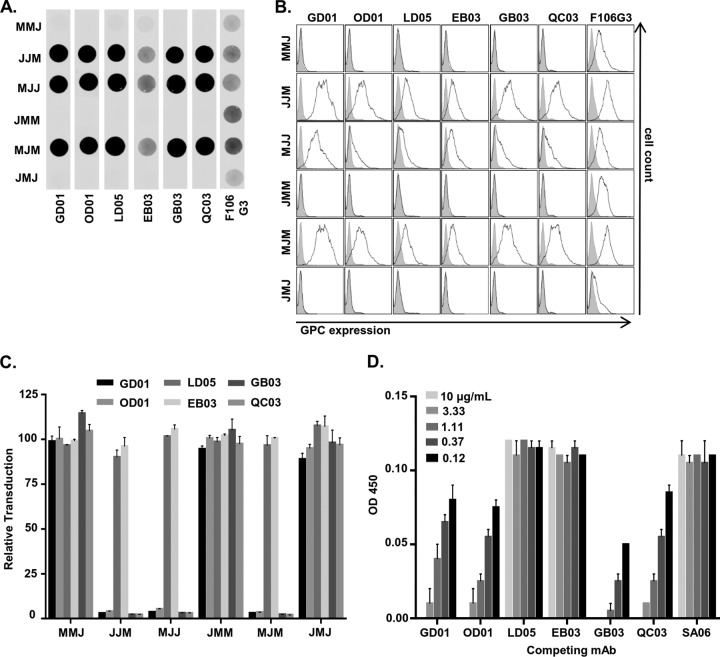
To explore how MACV and JUNV GPCs interact with antibodies, we initially turned to a series of anti-JUNV GPC MAbs available through the Biodefense and Emerging Infections Research Resources Repository (BEI Resources). Of the six GPC-specific MAbs (GD01-AG02 [GD01], OD01-AA09 [OD01], LD05-BF09 [LD05], EB03-AB11 [EB03], GB03-BE08 [GB03], and QC03-BF11 [QC03]), four neutralize JUNV infection (GD01, OD01, GB03, and QC03) (36). None, however, were reported to exhibit cross-reactivity to other NW arenaviruses, including MACV, in indirect fluorescence assays (36). Three of these neutralizing MAbs were demonstrated recently to protect guinea pigs to various degrees against lethal JUNV challenge (37), providing an additional rationale for identifying the location of epitopes on JUNV GPC. To confirm their specificity to JUNV GPC, we used nondenaturing dot blots of purified MACV-VSV or JUNV-VSV pseudovirions. VSV pseudovirions bearing equivalent amounts of either JUNV or MACV GPC, as assessed by anti-MACV GP2 MAb F106G3, were bound directly to nitrocellulose. F106G3 has previously been shown to be cross-reactive with JUNV GP2 (38). These blots were also incubated with each of the six α-JUNV MAbs. All six α-JUNV MAbs only recognized JUNV-VSV (Fig. 1A). Similarly, these same MAbs only bound to JUNV GPC in flow cytometric analysis of surface staining of 293T cells transfected with a JUNV or MACV GPC expression plasmid (Fig. 1B). In contrast, F106G3 detected roughly equivalent amounts of JUNV and MACV GP2 on the surfaces of GPC-transfected cells. In line with the inability of the JUNV GPC MAbs to detect MACV GPC, none of the JUNV-VSV-neutralizing MAbs cross-neutralized MACV-VSV, but the four MAbs previously described to neutralize JUNV infection did so in our experiments as well (Fig. 1C).
FIG 1.
α-JUNV GPC MAbs bind to and inhibit JUNV but not MACV pseudovirions. (A) Dot blot analysis of nondenatured JUNV-VSV (J)- or MACV-VSV (M)-associated GPCs. Pseudoviruses were incubated with lysis buffer prior to direct application to nitrocellulose. Blots were incubated with either a 1:500 dilution of each α-JUNV GPC MAb or a 1:20 dilution each α-MACV GP2 MAb. (B) Cell surface expression analysis of JUNV (J)- or MACV-GPC (M)-expressing 293T cells. Cells were transfected with GPC-expressing plasmids at 48 h prior to staining with a 1:50 dilution of α-JUNV MAb or a 1.5:2 dilution of α-MACV GP2 MAb. Filled histograms represent cells transfected with empty vector; unfilled histograms represent cells transfected with the respective GPC plasmids. (C) Neutralization assays were performed to assess the capacity of each α-JUNV MAb to inhibit either JUNV-VSV (J) or MACV-VSV (M) transduction. Pseudoviruses were incubated with MAb at 0.5 μg/ml prior to the application to Vero cells. Transduction is represented as the percentage of GFP-positive cells relative to cells incubated with nonspecific MAb, as assessed by flow cytometry. Bars are representative of two or three independent experiments with the indicated standard errors of the mean (SEM). (D) Neutralization assay assessing the capacity of each α-JUNV MAb to inhibit JUNV-VSV transduction in a dose-dependent manner. Pseudovirions were incubated in serial dilutions of MAb prior to application to Vero cells. Transduction is represented as described for the previous panel. Data points are representative of two independent experiments with the indicated SEM.
To compare the ability of each of the α-JUNV MAbs to neutralize JUNV-VSV, we generated dose-response inhibition curves. All four neutralizing MAbs potently inhibited virus transduction with three having nearly identical strengths of inhibition and GD01 having a slightly weaker effect (Fig. 1D and Table 1). Our findings with the JUNV recXJ13 strain GPC are similar to those in another report assessing neutralization by these MAbs against live Candid#1 strain of JUNV, except OD01, in that study, was the weakest MAb (37). Even at high concentrations, no neutralization was detected by LD05 or EB03.
TABLE 1.
α-JUNV GPC MAb concentrations providing 50% inhibition of JUNV/VSV transduction
| MAb against JUNV GPC | IC50 (ng/ml) |
|---|---|
| GD01 | 23.9 |
| OD01 | 8.3 |
| LD05 | NIa |
| EB03 | NI |
| GB03 | 7.7 |
| QC03 | 10.4 |
NI, no inhibition detected.
Use of MACV/JUNV chimeras to map neutralizing epitopes.
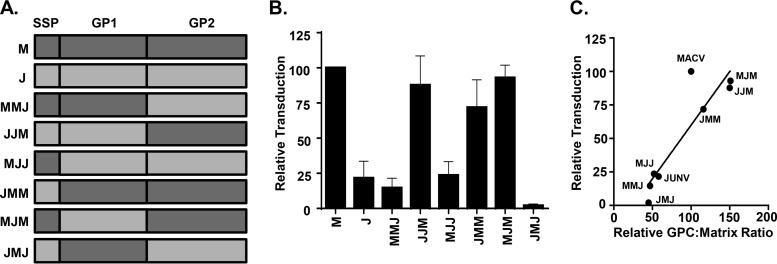
Multiple lines of evidence indicate that at least some of these MAbs bind to JUNV GP1. York et al. used LD05 to pull down GP1 from JUNV GPC transfected cell lysates and supernatants (39). Further, GD01 MAb was recently shown to bind to JUNV GP1 RBS residues, and QC03 competes with GD01 for GP1 binding (26). However, the epitopes targeted by LD05, OD01, EB03, and GB03 have not been identified. To confirm and extend others' observations that each of these MAbs is GP1-specific, we constructed a series of chimeric GPC structures in which the SSP, GP1, or GP2 segments of JUNV GPC (recXJ13) were mix-matched with those of MACV (Carvallo) into six M/J combinations (Fig. 2A). We were only interested in assessing MAb binding to glycoprotein complexes that transduced cells since the transduction capability provides evidence of functional competence and therefore, structural relevance, of GPC. Thus, we first assessed the transduction efficiency of these novel M/J chimera-pseudotyped VSVΔG-eGFP particles. All M/J chimeras were capable of producing transduction-competent virus, albeit to widely varying capacities with those virions bearing the GP2 from JUNV having markedly lower transduction levels than those bearing the MACV GP2 (Fig. 2B). To explore whether transduction efficiency was altered by GPC incorporation onto pseudovirions, equal amounts of pseudovirions were analyzed for both VSV matrix and GP2 using MAbs 23H12 and F106G3, respectively. Once the GPC/matrix ratio for each chimera was established, that ratio was correlated with the transduction efficiency of the pseudovirions. We found that the ratio was lower in virions containing JUNV GP2, and this correlated with lower transduction levels (Fig. 2C), suggesting that the amount of GPC on the pseudovirion likely influences transduction. Nonetheless, all chimeric pseudovirions had demonstrable levels of transduction, indicating that the chimeric GPCs were able to bind to cellular receptors and facilitate membrane fusion events.
FIG 2.
Characterization of MACV/JUNV GPC chimera pseudoviruses. (A) Diagram of M/J chimeric GPC assembly and nomenclature. (B) Analysis of transduction competence among various M/J chimera-VSV pseudovirions. Pseudovirion preparations were normalized to MACV-VSV (M) for matrix expression by dot blot analysis and applied to Vero cells in duplicate. Transduction is represented as the percentage of cells GFP-positive relative to MACV-VSV, as assessed by flow cytometry. Bars are representative of three independent pseudovirion preparations with the indicated SEM. (C) Correlative analysis of the GPC/matrix ratio as a predictor of transduction competence. Quantitation of GPC or VSV matrix was determined by densitometry analysis of dot blots. GPC/VSV matrix ratios were calculated as the GP2 (F106G3) signal intensity divided by the matched VSV matrix (23H12) signal intensity averaged across three dilutions. Analysis and representation of transduction competence was performed as described for panel B. Data points represent the GPC/VSV matrix ratios and the percentage of GFP-positive cells (transduction) relative to those of WT MACV GPC (WT = 100).
M/J chimera GPC-VSVs equalized by GP2 expression were then assessed for their ability to bind to each of the MAbs in nondenaturing dot blots (Fig. 3A). The expression of M/J chimera GPC-transfected cell surface staining was also assessed (Fig. 3B). We found in both assessments that only M/J chimeras that contained a JUNV GP1 were recognized by any of the MAbs. From these data, we concluded that all six anti-JUNV GPC MAbs bind to the GP1 domain of the GPC. This conclusion was further validated by neutralization assays in which only M/J chimeric pseudovirions that contain a JUNV GP1 were susceptible to transduction inhibition by the four neutralizing MAbs (Fig. 3C).
FIG 3.
JUNV GP1 is the target of both neutralizing and nonneutralizing MAbs. (A) Dot blot analysis of nondenatured M/J chimera-VSV-associated GPCs. Pseudoviruses were incubated with lysis buffer prior to direct application to nitrocellulose. Blots were incubated with either a 1:500 dilution of each α-JUNV GPC MAb or a 1:20 dilution of each α-MACV GP2 MAb. (B) Cell surface expression analysis of M/J chimera-expressing 293T cells. Cells were transfected with GPC-containing plasmids 48 h prior to staining with a 1:50 dilution of each α-JUNV GPC MAb or a 1.5:2 dilution of α-MACV GP2 MAb. Filled histograms represent cells transfected with empty vector; unfilled histograms represent cells transfected with the respective GPC plasmids. (C) Neutralization assays were performed to assess the capacity of each α-JUNV GPC MAb to inhibit M/J chimera-VSV transduction. Pseudovirions were incubated with each MAb at 0.5 μg/ml prior to application to Vero cells. Transduction is represented as the percentage of GFP-positive cells relative to cells incubated with nonspecific MAb, as assessed by flow cytometry. Bars are representative of two or three independent experiments with the indicated SEM. The results shown in panels A to C were determined contemporaneously with the findings depicted in Fig. 1A to C, and the relevant positive control results are also shown in those panels. (D) An ELISA assessing the capacity of each α-JUNV GPC MAb to compete with MAb GD01 for the receptor binding site of JUNV GP1 was performed. JUNV-VSV-coated ELISA plate wells were incubated with 0.5 μg of biotinylated GD01/ml mixed with decreasing concentrations of nonbiotinylated forms of each of the six JUNV GP1-specific MAbs or a nonspecific control (SA06) (concentrations were as indicated in the inset key). Plates were developed in TMB after incubation with a 1:4,000 dilution of HRP-conjugated streptavidin, and the results are presented as units of optical density at 450 nm. Bars are representative of two independent experiments with the indicated SEM.
Next, we investigated whether multiple neutralizing epitopes are targeted or if the four neutralizing MAbs are functionally redundant. Because others have shown that MAb GD01 successfully neutralizes infection by engaging in the same molecular interactions required for binding with TfR1 (26), we performed a competition enzyme-linked immunosorbent assay (ELISA) in which JUNV-VSV-coated wells were incubated with a mixture of biotinylated GD01 and one of each of the six α-JUNV MAbs or a control MAb, SA06-AF08 (SA06) (Fig. 3D). The results demonstrated that all four neutralizing MAbs competed for GD01 binding, providing evidence that all target the same or nearby residues within the RBS. In contrast, the two nonneutralizing MAbs competed as poorly as the irrelevant MAb that served as the negative control. Our findings, in conjunction with those previously reported (26), highlight that the JUNV GP1 RBS is a critical target for this panel of neutralizing MAbs and that the nonneutralizing MAbs, while binding to GP1 epitopes, do not compete for GD01-specific epitopes within the RBS or block the ability of other MAbs to interact with this epitope.
We also utilized our M/J chimeras to determine whether the neutralizing capabilities of polyclonal antisera also mapped to GP1. We theorized that neutralizing antisera might target both GP1 and GP2 epitopes since the mechanics of fusion of other enveloped viruses can be targeted by neutralizing antibodies (40, 41), and both GP1 and GP2 domains of NW arenaviruses are readily recognized by DNA-vaccination-elicited antisera (35).
Murine anti-MACV GPC and anti-JUNV GPC antisera were generated by vaccination with MACV GPC or JUNV GPC bearing VSV pseudovirions. We found that a prime/boost strategy using the VSVΔG-eGFP pseudovirus platform elicited a polyclonal antibody response to the MACV and JUNV GPCs, and we assessed the ability of antisera from vaccinated mice to neutralize either wild-type or M/J chimera pseudovirions. As expected, both MACV-VSV and JUNV-VSV vaccinations led to the production of strong self-neutralizing, but weak cross-neutralizing, antisera (Fig. 4, top panel).
FIG 4.
Neutralizing mouse antiserum targets autologous GP1. Pooled sera from female IFNAR−/− BALB/c mice (n ≥ 3) vaccinated with JUNV-VSV, MACV-VSV, or PBS (α-JUNV, α-MACV, or normal mouse serum [NMS], respectively) were evaluated for neutralizing activity against MACV-VSV (M) or JUNV-VSV (J) (top), M/J chimera-VSV with JUNV GP1 (middle), and M/J chimera-VSV with MACV GP1 (bottom). Pseudovirions were incubated with antisera or PBS prior to application to Vero cells (dilution factors were as indicated in the inset key). Transduction is represented as the percentage of GFP-positive cells relative to cells receiving PBS treatment, as assessed by flow cytometry. Bars are representative of two or three independent experiments with the indicated SEM.
Antisera most robustly neutralized M/J chimeras that contained the autologous GP1, supporting previous conjecture that neutralizing antibodies from both MACV- and JUNV-specific antisera target the GP1 domain (Fig. 4, middle and bottom panels). Therefore, despite the functional importance of GP2, GP1 appears to be the primary, if not the exclusive, target of neutralizing antibodies elicited in mice.
Interestingly, we consistently observed that high concentrations of anti-MACV antisera provide modest, but detectable inhibition of JUNV transduction. Similarly, these antisera also provided some inhibition of chimeras bearing JUNV GP1. This suggests that MACV antisera contain antibodies that are likely targeting some epitopes that are available on both prefusion GPCs, whereas JUNV antisera do not.
Region 4 within GP1 plays a critical role in RBS accessibility for α-JUNV neutralizing antibodies.
Although neutralizing antibody binding to the JUNV or MACV GP1 could have been reasonably anticipated, it remains unclear how MACV GP1 avoids neutralization from the four neutralizing MAbs that target the RBS and α-JUNV antisera. Both viruses intimately engage with TfR1 using a clade-wide conserved pocket (27) and the contact residue comparisons between MACV GP1-TfR1 and JUNV GP1-GD01 were used to explain the mechanism of inhibition by MAb GD01 (26). Although sequence differences between MACV and JUNV GP1 RBS may account for the inability of GD01 or the other RBS-targeting MAbs to cross-react, it is also possible that unique structural accessibility to the MACV RBS pocket, rather than the pocket itself, controls whether antibodies bind to the native GPC structure.
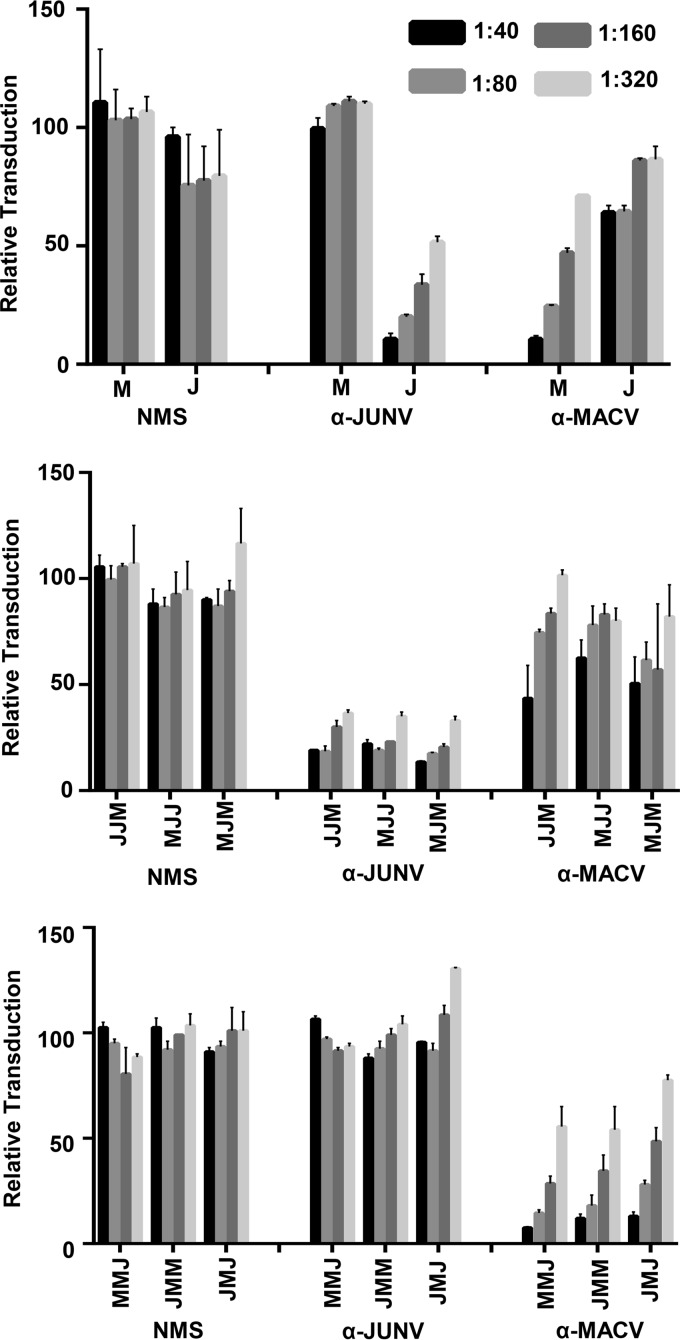
To learn more about which regions of GP1 in addition to the RBS influence engagement of the MAbs, the MJM chimera characterized above was further dissected using a strategy previously used on other arenavirus GP1s (42). A universal point of conservation among all arenavirus GP1s is a series of four cysteine residues that engage in disulfide bonds likely critical to the formation of the globular structure (Fig. 5A). Using these cysteines as points of transition, the GP1 was divided into five regions in which JUNV (recXJ13) fragments were sequentially replaced with MACV (Carvallo) (Fig. 5B). Only the MJM-4 chimera was capable of producing transduction-competent pseudovirions (data not shown), but parallel chimeric GPC pseudovirus studies substituting equivalent regions from the vaccine strain of JUNV, Candid#1, were more successful. The Candid#1 GP1 sequence differs from the recXJ13 strain by only three amino acid residues located in GP1 region 4 (T168A, E186G, and S206P) (Fig. 5A), none of which reportedly contribute to the attenuation of the strain (43). Further, and most relevant to this study, Candid#1 GP1 is readily recognized by all six MAbs and neutralized by all four neutralizing antibodies (data not shown). Use of the Candid#1 sequence allowed for the successful production of MJM-1 and MJM-5 in addition to MJM-4 pseudovirions, although still neither MJM-2 nor MJM-3 pseudovirions (Fig. 5C).
FIG 5.
Region 4 within GP1 plays a critical role in RBS accessibility for α-JUNV neutralizing antibodies. (A) MACV (Carvallo; M) or JUNV (Candid#1; J) GP1 amino acid sequences aligned according to conserved cysteine residues. The three amino acid residues that differ in the Candid#1 strain from the recXJ13 strain of JUNV are underlined. Conserved cysteines are indicated by gaps in region labels, and partners in disulfide bonds for all cysteine residues are indicated by connecting lines. The five regions exchanged to form the various intraGP1 MJM chimeras are indicated by the labeled gray bars above and below the sequences. Circles marking specific residues within the sequences are presented as indicated in Fig. S2A in a study by Mahmutovic et al. (28). White circle indicates JUNV GP1 residue predicted to only contact TfR1, gray circles indicate JUNV GP1 residues only contacted by GD01, and black circles indicate JUNV GP1 residues that are both predicted to contact TfR1 and interact with GD01. (B) Diagram of MJM intraGP1 chimera assembly and nomenclature. (C) Analysis of transduction competence among various MJM intraGP1 chimera-VSV pseudovirions. Pseudovirion preparations were normalized to MJM-VSV for VSV matrix expression by dot blot analysis and applied to Vero cells in duplicate. Transduction is represented as the percentage of GFP-positive cells relative to MJM-VSV, as assessed by flow cytometry. Bars are representative of three independent pseudovirion preps with the indicated SEM. (D) Cell surface expression analysis of MJM intraGP1 chimera-expressing 293T cells. Cells were transfected with GPC-containing plasmids 48 h prior to staining with a 1:50 dilution of each α-JUNV GPC MAb or a 1.5:2 dilution of F106G3. Filled histograms represent cells transfected with empty vector; unfilled histograms represent cells transfected with respective GPC plasmids. (E) Dot blot analysis of nondenatured MJM intraGP1 chimera-VSV-associated GPCs. Pseudoviruses were incubated with lysis buffer prior to direct application to nitrocellulose. Blots were incubated with either a 1:500 dilution of each α-JUNV MAb or a 1:20 dilution of F106G3. (F) Neutralization assays assessing the capacity of each α-JUNV MAb to inhibit MJM intraGP1 chimera-VSV transduction. Pseudovirions were incubated with each MAb at 0.5 μg/ml prior to application to Vero cells. Transduction is represented as the percentage of GFP-positive cells relative to cells incubated with nonspecific MAb, as assessed by flow cytometry. Bars are representative of two or three independent experiments with the indicated SEM. (G) Neutralization assays assessing the capacity of polyclonal antisera (α-JUNV, α-MACV, or NMS) to inhibit the transduction of each MJM intraGP1 chimera-VSV. Pseudovirions were incubated with decreasing concentrations of each antiserum type or PBS prior to application to Vero cells (dilution factors were as indicated in the inset key). Transduction is represented as the percent of GFP-positive cells relative to cells receiving PBS treatment, as assessed by flow cytometry. Bars are representative of two or three independent experiments with the indicated SEM.
Using the three functional chimeric constructs, we investigated whether these region swaps affected MAb binding by performing the same series of assays used with the M/J chimeras above. Cell surface staining with MAbs LD05, EB03, or F106G3 indicated that low, but consistently detectable levels of all three chimeric GPCs are expressed on the surface of transfected 293T cells (Fig. 5D). Further, dot blots with these MAbs show that all three chimeric GPCs incorporate onto VSV pseudovirions (Fig. 5E). In a similar manner, MJM-1 and MJM-5 on the cell surface and in dot blots were also detected by the neutralizing MAbs GD01, OD01, GB03, and QC03. However, the neutralizing MAbs were unable (or very poorly able in the case of OD01 in the dot blot) to detect MJM-4 in these assays. Consistent with the inability of these neutralizing MAbs to bind to MJM-4, their capacity to inhibit MJM-4-VSV transduction was also lost (Fig. 5F). This effect extended to α-JUNV polyclonal antisera, since the capacity of α-JUNV antisera to neutralize MJM-4-VSV was considerably reduced compared to MJM-1 or MJM-5 (Fig. 5G). Thus, when region 4 of JUNV is replaced with MACV sequence, the ability of the neutralizing antibodies to bind to GP1 and mediate neutralization is reduced or abolished. Certainly, one possible explanation for this observation is that neutralizing MAbs are interacting with residues within region 4. However, crystal structure analysis demonstrates that this is not the case, at least not for GD01, an antibody with which all three other neutralizing MAbs compete (26) (Fig. 3D). Studies with both MACV (25) and JUNV (26) identify that TfR1 and GD01, respectively, interact with residues within region 2 almost exclusively. Two additional direct interactions with GD01 were reported in the JUNV GP1 N terminus of region 4, but both residues are conserved between MACV and JUNV sequences and thus were not altered by the region swap.
Using the rationale that a more compact molecule might be able to access the JUNV GP1 RBS that is present in MJM-4, F(ab′)2 fragments of our neutralizing MAbs were also tested for their ability to neutralize. F(ab′)2 fragments were selected for these studies rather than smaller Fab fragments to maintain high-affinity interactions that might be required for mediating neutralization. No better neutralization of MJM-4-VSV by the F(ab′)2 fragments was observed than the full-length antibodies (data not shown). The loss of MAb binding to MJM-4-VSV despite the retention of the JUNV RBS helps to unveil a potential explanation for the lack of cross-neutralization against MACV: structurally unique elements exist within this region of MACV that significantly restricts RBS accessibility for antibodies without preventing host receptor engagement.
Loop 10 within region 4 of MACV GP1 contributes to the RBS inaccessibility.
Upon closer examination of the sequences within region 4, one prominent difference stood out between the two species. An extra disulfide-bonded loop exists in MACV that is not present in JUNV (Fig. 5A) or other clade B viruses that are known to utilize TfR1 as a receptor (Fig. 6B). This loop has been noted previously and termed loop 10 (25). Interestingly, loop 10 narrows the opening of the RBS of MACV (Fig. 6A), although no contact points were reported to exist between residues within loop 10 and TfR1 (25). To determine whether this loop alone prevents α-JUNV GPC antibody access to the MACV RBS, we attempted to produce two additional GPC constructs: one in which loop 10 was removed from MACV GPC (residues C220-G230 were removed; “M-loop10”), and one in which loop 10 was inserted into JUNV GPC (MACV C220-G230 were inserted between JUNV P219 and T220; “J+loop10”). Only the M-loop10 construct produced transduction-competent pseudovirions and could be further assessed (Fig. 6C). Although loop 10 has been predicted to prevent GD01 interaction in MACV (26), its removal alone was not sufficient to allow binding of any of the neutralizing α-JUNV MAbs to native MACV GPC in a dot blot (Fig. 6D), nor does it allow MAb-mediated neutralization of MACV-VSV (data not shown). The F(ab′)2 fragments of the neutralizing MAbs that were described above were also tested for their ability to neutralize M-loop10. However, we found no better neutralization by the F(ab′)2 fragments than the full-length antibodies (data not shown). Therefore, additional changes such as specific residue changes in the RBS likely would be required to allow these α-JUNV MAbs to bind to MACV GPC. Surprisingly, given our findings with the MAbs, the loss of loop 10 was sufficient to induce notable cross-neutralization of M-loop10 by the α-JUNV polyclonal antisera (Fig. 6E), with both JUNV and MACV antisera inhibiting M-loop10 in a dose-dependent manner. Thus, our findings indicate that loop 10 appears to be an important impediment to JUNV antisera interactions with MACV GPC.
FIG 6.
Loop 10 within region 4 of MACV GP1 contributes to the RBS inaccessibility. (A) Structural alignment of MACV-GP1 (black, PDB 3KAS) and JUNV-GP1 (white, PDB 5EN2) crystal structure cartoons with critical residues of TfR1 represented as white sticks (left). TfR1 residues are included to show the general location of receptor binding pocket. Emphasis drawn to highlight the distinction of loop 10 in MACV GP1 as it relates to the openness of the aforementioned pocket and the disulfide bond is indicated by lines (right; figures created with PyMOL software). (B) Glycoprotein precursor (GPC) protein sequences were obtained for each virus species from the National Center for Biotechnology Information (NCBI) and were Clustal Omega aligned with Megalign Pro software (DNASTAR, Lasergene). NCBI numbers: Junín (NP_899218.1), Machupo (AIG51558.1), Tacaribe (NP_694849.1), Sabia (YP_089665.1), Chapare (YP_001816782.1), Amapari (YP_001649208.1), and Guanarito (AAN09938.1). (C) Analysis of transduction competence of M-loop10-VSV pseudovirions. Pseudovirion preps were normalized to MACV-VSV for matrix expression by dot blot analysis and applied to Vero cells in duplicate. Transduction is represented as the percentage of GFP-positive cells relative to MACV-VSV, as assessed by flow cytometry. Bars are representative of three independent pseudovirion preparations with the indicated SEM. (D) Dot blot analysis of nondenatured MACV-, JUNV-, or M-loop10-VSV-associated GPCs. Pseudoviruses were incubated with lysis buffer prior to direct application to nitrocellulose. Blots were incubated with either a 1:500 dilution of each α-JUNV GPC MAb or a 1:20 dilution of each α-MACV GP2 MAb. (E) A neutralization assay assessing the capacity of polyclonal antisera (α-JUNV, α-MACV, or NMS) to inhibit transduction of M-loop10-VSV was performed. Pseudovirions were incubated with decreasing concentrations of antisera or PBS prior to application to Vero cells (dilution factors were as indicated in the inset key). Transduction is represented as the percentage of GFP-positive cells relative to cells receiving PBS treatment, as assessed by flow cytometry. Bars are representative of two independent experiments with the indicated SEM.
DISCUSSION
Although it has been appreciated for a number of years that there is poor antibody cross-neutralization between closely related NW arenaviruses, specific GPC regions important for the immunogenic specificity are not well studied. Using novel JUNV and MACV chimeric GPCs, we mapped elements within the GPCs of MACV and JUNV that are important for neutralization and identify other GPC sequences that prevent cross-neutralization. Six anti-JUNV GPC MAbs that recognize the native conformation of JUNV GPC, but do not bind to MACV GPC (36), were studied. We extended previous observations that all six bind to GP1. The neutralizing capabilities of JUNV and MACV GPC antisera raised in mice also mapped to the GP1. A previous crystallographic study visualized one of the four neutralizing MAbs, GD01, bound to soluble JUNV GP1, identifying residues within the JUNV GP1 RBS that bound (26). Here, we found that all four neutralizing MAbs compete with GD01, indicating that these MAb all target the same or overlapping epitopes within JUNV GP1 RBS. In contrast, the two nonneutralizing MAbs did not compete for binding with the neutralizing MAbs. The fact that the neutralizing MAbs against JUNV GPC target the same or similar epitopes within the RBS suggests that the epitopes within JUNV GPC that lead to neutralization may be quite restricted.
Neutralization by antibody targeting of the RBS of these NW arenaviruses is similar to that observed with some other enveloped viruses such as the human coronavirus, SARS (44), whereas these findings stand in contrast to the filovirus Ebola virus, where neutralizing MAbs do not bind within the RBS but instead target GP1/GP2 interactions involved in fusion events (45, 46). Earlier studies with OW arenaviruses identified GP1 as the target of all neutralizing MAbs against these viruses (9, 47, 48). Recent work by Mahmutovic et al. (26) and results described in the present study extended this notion to NW arenaviruses.
Three of the neutralizing MAbs studied here—GB03, 0D01, and GD01—have been assessed for their efficacy as therapeutic strategies against lethal JUNV challenge (37). Interestingly, these authors found combination therapy with all three MAbs to be less effective than GB03 alone. As we demonstrated, since all three MAbs compete for the same or overlapping epitopes within the RBS, binding of the more poorly neutralizing GD01 is likely to compete for RBS binding with the more effective GB03 MAb.
Through these studies, we also identified a region within MACV GP1 that limits antibody access to the RBS. α-JUNV antisera proved ineffective at neutralizing wild-type MACV-VSV, but when a small loop (loop 10) that is unique to MACV GP1 was deleted, α-JUNV antisera was able to neutralize MACV GPC pseudovirion transduction as effectively as α-MACV antisera. Conversely, insertion of region 4 of MACV GPC that contains loop 10 into JUNV GP1 (MJM-4) reduced the ability of both JUNV antisera and all four neutralizing α-JUNV MAbs to bind to JUNV GP1 and inhibit pseudovirion transduction. Thus, we propose that loop 10 within MACV GPC serves to restrict antibody access to the RBS, thereby preventing antibody binding and subsequent neutralization. Loop 10 had been previously predicted to restrict access of GD01 to the RBS (26), and our study extends that restriction to other antibodies that neutralize JUNV by binding to GP1 RBS. Interestingly, the C terminus of region 4, within which loop 10 resides, has been previously implicated by Martin et al. as relevant to host receptor engagement, in that swaps of this region between other NW arenaviruses impacted TfR1-dependent transduction (42). With the new understanding stemming from structural studies of the NW arenavirus GP1s that the RBS resides within region 2 (25, 26), it is likely that region 4 of other NW arenaviruses may also interfere with availability of the RBS. To explore whether more compact versions of the neutralizing α-JUNV GPC MAbs were effective at neutralizing MACV GPC lacking loop 10, we tested the ability of F(ab′)2 fragments generated from the MAbs but found that they were also unable to neutralize pseudovirions bearing MACV GPC lacking loop 10. These findings suggest that, in addition to loop 10, other MACV GP1 constraints prevent the binding of these MAbs. Constraints that should be evaluated in future studies are likely to include residue differences that are found in the GP1 RBS and region 4 of JUNV and MACV GPC. In addition, the generation of Fab fragments could help determine whether these additional constraints are due fully to limited RBS access or to sequence differences in yet unidentified contact residues important for binding. Mahmutovic et al. noted in their studies that the CDR H3 region within the Fab domain of GD01 was a likely source of interference with loop 10 (26).
Another notable finding from these studies was an apparent one-way relationship between MACV- and JUNV GPC-elicited antisera. Although α-JUNV antisera were unable to neutralize MACV-VSV, α-MACV antisera had low, but detectable, neutralizing activity against JUNV-VSV and many of chimeric GPC pseudovirions that contained the JUNV GP1. This observation aligns with the differing levels of accessibility between these two viruses' RBSs. Neutralizing antibodies that are generated against MACV GPC must successfully circumvent loop 10 in MACV. Therefore, these antibodies would be predicted to access the more open RBS of JUNV. In contrast, α-JUNV antibodies are generated against a more open RBS were not able to cross-neutralize constructs having more restricted access to the RBS. Hence, a MACV-derived vaccine construct may have greater potential for eliciting broadly specific antibodies.
As a rule, arenavirus GP2 antibodies are often strongly cross-reactive but not neutralizing (9, 36, 49). This observation aligns with the pairing of very high sequence homology among arenavirus GP2 domains and the poor cross-neutralization of polyclonal antisera. GP1, in contrast, elicits antibodies with low cross-recognition but strong autologous neutralization. This is also consistent with the pairing of lower sequence identity and homology among arenavirus GP1 domains and poor cross-neutralization of polyclonal antisera. The only neutralizing GP2-specific MAb that, to our knowledge, has ever been characterized (F100G5) (38), was elicited in mice vaccinated with purified and chemically denatured GP2 ectodomains. Because the F100G5 epitope is only accessible during the process of fusion (38), it would exclusively bind to GP2 within endosomes of virus-infected cells. Although delivery of such a MAb to the endosomal compartment of infected cells would likely prevent NW arenavirus fusion events and thus serve as an effective therapy, such an antibody would not be anticipated to neutralize extracellular virions nor contribute to the in vivo antibody repertoire important for mediating neutralization.
A number of our chimeric GPCs expressed and transduced very poorly or not at all, whereas other closely related ones were functional. This was likely due to the structural incompatibility of some MACV/JUNV regions. As a consequence, we only assessed chimeric constructs that retained transduction competency since this indicated that the constructs retained the ability to successfully engage with host receptor, TfR1, and facilitate membrane fusion. In the first set of M/J chimeras, in which the three major protein domains (SSP, GP1, and GP2) were mix-matched, there was a clear pattern in that any M/J chimera that contained a JUNV GP2 had significantly lower GPC incorporation and transduction potential than the other M/J chimeras. The cause of this reduced incorporation remains unknown and mapping of JUNV GP2 sequences that reduce GPC incorporation onto virions would provide insights into this. The production of transduction-competent M/J chimera-VSVs became more unpredictable as complex intra-GP1 MJM chimeras were produced. One study that initially performed intercysteine segment swaps of NW arenavirus GP1s also reported similar unpredictability (42). A better understanding of the constraints that reduced GPC functionality of some of chimeric constructs could be gained from future detailed structural analysis.
MATERIALS AND METHODS
Cells, plasmids, pseudoviruses, antisera, MAbs, and F(ab′)2 fragments.
Vero cells (ATCC-CCL-81) and 293T cells (ATCC-CRL-3216) were each maintained in Dulbecco modified Eagle medium supplemented with 5 to 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (pen/strep) in a 37°C incubator with 5% CO2. MACV (Carvallo) and JUNV (Candid#1; both kindly provided by Susan Ross, University of Illinois at Chicago) and JUNV (recXJ13; kindly provided by Jay Hooper, USAMRIID) GPC cDNA sequences were cloned into pcDNA3.1(−) plasmids. Various chimeric forms of these viral glycoprotein complexes were created using Gibson Assembly master mix (New England BioLabs) before transformation into DH5α Escherichia coli cells. All constructs were confirmed by Sanger sequencing.
Pseudovirions were produced by polyethylenimine (PEI) transfection of 293T cells with a viral glycoprotein-expressing plasmid 18 to 24 h prior to transduction with Lassa virus GPC-pseudotyped VSVΔG-eGFP at a multiplicity of infection of ∼1. At 3 h postransduction, the cells were washed once with phosphate-buffered saline (PBS) and replenished with fresh media supplemented with 1.5% FBS and 1% pen/strep. Supernatant pseudovirus was collected 24 h after a PBS wash, filtered through a 0.45-μm-pore size syringe filter, and either frozen at −80°C in aliquots or concentrated and purified through ultracentrifugation with a 20% sucrose cushion at 90,000 × g for 2 h at 4°C and resuspended in PBS. Virus stocks were distributed into aliquots and stored at −80°C. In cases of ultracentrifugation, supernatant pseudovirus was collected both 24 and 48 h after a PBS washing step.
For the production of α-GPC antisera, pseudovirus stocks containing the specified GPC were concentrated and purified through ultracentrifugation. Stocks were further treated with Detoxi-Gel endotoxin removing resin (Thermo Scientific) according to the manufacturer's instructions prior to freezing. For vaccination, female IFNAR−/− BALB/c mice between 6 and 8 weeks of age were intramuscularly injected with 108 transducing units of MACV GPC- or JUNV GPC-pseudotyped VSVΔG-eGFP or PBS in a total volume of 100 μl. At 2 weeks after priming, mice were boosted with the same treatment. At 3 weeks postboost, the mice were CO2 anesthetized, and blood was collected via postmortem cardiac puncture. Blood was allowed to clot for 30 min, and sera were separated using BD Microtainer tubes with serum separators (Becton Dickinson, catalog no. 365956) according to the manufacturer's instructions. Sera from multiple mice was combined, divided into aliquots, and stored at −80°C; in-use aliquots were stored at 4°C.
All α-JUNV MAbs (GD01-AG02, 0.99 mg/ml; OD01-AA09, 1.00 mg/ml; LD05-BF09, 0.99 mg/ml; EB03-AB11, 0.96 mg/ml; GB03-BE08, 0.99 mg/ml; QC03-BF11, 0.84 mg/ml) and the α-JUNV nucleoprotein MAb (SA06-AF08, 1.10 mg/ml) were developed by others (36) and obtained through the National Institute of Allergy and Infectious Diseases-established organization, BEI Resources. F106G3 is an α-MACV GP2 MAb developed by others (38) and was produced in-house through maintenance of the hybridoma (kindly provided by Grant McClarty, Public Health Agency of Canada). Hybridoma cells were kept in Dulbecco modified Eagle medium supplemented with 15% FBS, 1% pen/strep, 1% nonessential amino acids, and 1% glutamate. Spent medium was regularly collected, filtered through a 0.45-μm-pore size syringe filter, and stored at −80°C. Large batches were later concentrated through an Amicon Ultra 50-ml centrifugal filter with a 100-kDa cutoff (Sigma) and stored at 4°C. The α-VSV-matrix hybridoma, 23H12, was purchased through Kerafast and produced in-house through similar means as described for F106G3. F(ab′)2 fragments of GD01, OD01, GB03, and QC03 were produced using an F(ab′)2 Micro preparation kit (Pierce, catalog no. 44688) according to the manufacturer's instructions. Briefly, each MAb was incubated with immobilized pepsin protease to selectively digest whole MAbs into F(ab′)2 fragments that retain antigen binding activity.
Immunostaining assays (dot blots and cell surface expression analysis).
To normalize pseudovirus preparations for VSV-matrix or arenavirus-GP2 expression, viruses were incubated in 5× lysis buffer (PBS containing 0.125% NP-40) prior to serial dilution in PBS. All dilutions were then directly applied to a nitrocellulose membrane via a dot blot apparatus (Minifold I 96-well system; Cole-Parmer; Vernon Hills, IL). Blots were blocked for 1 h at room temperature, incubated with primary antibody for either 2 h at room temperature or overnight at 4°C, and incubated with IRDye800CW or IRDye680RD goat anti-mouse secondary (LI-COR) at 1:5,000 for 1 h at room temperature. Blots were then visualized with a LI-COR Odyssey CLx Imaging System, and fluorescence measurements were used to calculate volume adjustments for normalization. For dot blot studies evaluating epitope availability of the chimeric GPCs, pseudovirions were normalized for GP2 quantity using the MAb F106G3, and a single concentration of each pseudovirus was assessed.
For surface staining experiments, 293T cells were plated overnight prior to PEI transfection with plasmids containing respective GPC constructs. The medium was replaced at 24 h posttransfection, and cells were lifted from their wells with 5 mM EDTA in PBS at 48 h posttransfection. Lifted cells were transferred into a 96-well round-bottom plate at ∼200,000 cells per well. Cells were washed with 5% FBS in PBS three times prior to incubation with primary antibodies for 1 h on ice. Primary-stained cells were washed three times and incubated with Cy5-conjugated donkey anti-mouse IgG(H+L) secondary (Jackson ImmunoResearch) at 1:1,000 for 15 min on ice. Secondary-stained cells were washed three times and subsequently analyzed via flow cytometry using a FACSCalibur (Becton Dickinson). Data were analyzed with single cell analysis software (FlowJo).
Transduction assays.
Transduction competency of each GPC chimera was compared to the respective parental construct. For instance, chimeras that contained with the SSP-GP1-GP2 domain exchanges between JUNV and MACV were compared to the wild-type JUNV and MACV constructs. Vero cells were plated at 50,000 cells per well in 48-well tissue culture plates overnight, and a transduction curve was produced for the parental GPC-bearing pseudovirus in order to establish the volume required to achieve ∼20% transduction. All pseudoviruses of a given batch were then normalized to the parental pseudovirus for VSV Matrix expression via dot blot analysis, applied to Vero cells in triplicate, and assessed for GFP expression 18 to 24 h later by flow cytometry.
Neutralization assays.
For neutralization assays involving purified MAbs or F(ab′)2 fragments, pseudoviruses were normalized to achieve ∼30% transduction and were aliquoted into Eppendorf tubes. Each MAb was added to a final concentration of 0.5 μg/ml (unless otherwise specified) in a total volume of 250 μl. Eppendorf tubes containing media, pseudovirus, and MAb were vortexed and incubated at 37°C for 30 min prior to application of mixture to Vero cells in 48-well tissue culture plates as described above for transduction assays. For neutralization assays involving mouse antisera, respective antiserum samples were diluted 10× in PBS, followed by three subsequent 2× dilutions. Pseudoviruses were normalized by transduction competence and were diluted in media according to transduction normalization to a final volume of 45 μl per replicate. A 15-μl portion of antiserum dilution was added to each 45-μl aliquot of pseudovirus to achieve 1:40, 1:80, 1:160, and 1:320 final antiserum dilutions. Eppendorf tubes containing media, pseudovirus, and antisera were vortexed and incubated at 37°C. After 30 min of incubation, 200 μl of medium was added to each replicate prior to application of entire volume to Vero cells in the 48-well format described above for the transduction assays. In all neutralization assays, Vero cells were assessed for GFP expression at 18 to 24 h postransduction by flow cytometry.
ELISAs.
To detect competition between mouse MAbs, JUNV-VSV pseudovirions were immobilized overnight at 1.25 μg/well in bicarbonate coating buffer (14 mM Na2CO3, 35 mM NaHCO3; pH 9.6) at 4°C on 96-well Nunc MaxiSorp plates (Thermo Fisher Scientific). The following day, the plates were washed three times with wash buffer (PBS containing 0.15% [vol/vol] Tween 20) and blocked with 200 μl of blocking buffer (PBS containing 2% bovine serum albumin [wt/vol]) for 1 h at 37°C. The plates were washed four times with wash buffer. GD01 was biotinylated with EZ-Link NHS-biotin (catalog no. 20217) according to the manufacturer's protocol (Thermo Fisher Scientific) and, at a constant concentration of 0.5 μg/ml, was mixed with an nonbiotinylated competitor MAb at multiple concentrations (0.12 to 10 μg/ml). Next, 100-μl portions of MAb mixtures were added in quadruplicate and allowed to bind for 3 to 4 h at 37°C. Plates were washed five times with wash buffer and incubated in 100 μl of horseradish peroxidase (HRP)-conjugated streptavidin (1:4,000 dilution; Thermo Fisher Scientific) for 1 h at 37°C. The plates were then washed and developed in 50 μl of TMB (3,3′,5,5′-tetramethylbenzidine) substrate in the dark at room temperature for 30 min. The reaction was terminated with 2 M H2SO4, and absorbance measurements were read at 450 nm on Synergy H1 hybrid microtiter plate (BioTek, USA).
ACKNOWLEDGMENTS
The study was supported by a developmental award to W.M. from a National Institutes of Health (NIH) Regional Center of Excellence grant (U54 AI1057156 [David Walker, principal investigator]) and by NIH grant R01 AI077519 (to W.M.). R.B.B. was supported by NIH training grant T32 AI007533.
REFERENCES
- 1.Irwin NR, Bayerlova M, Missa O, Martinkova N. 2012. Complex patterns of host switching in New World arenaviruses. Mol Ecol 21:4137–4150. doi: 10.1111/j.1365-294X.2012.05663.x. [DOI] [PubMed] [Google Scholar]
- 2.Wulff H, Lange JV, Webb PA. 1978. Interrelationships among arenaviruses measured by indirect immunofluorescence. Intervirology 9:344–350. doi: 10.1159/000148956. [DOI] [PubMed] [Google Scholar]
- 3.Radoshitzky SR, Bao Y, Buchmeier MJ, Charrel RN, Clawson AN, Clegg CS, DeRisi JL, Emonet S, Gonzalez JP, Kuhn JH, Lukashevich IS, Peters CJ, Romanowski V, Salvato MS, Stenglein MD, de la Torre JC. 2015. Past, present, and future of arenavirus taxonomy. Arch Virol 160:1851–1874. doi: 10.1007/s00705-015-2418-y. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Zhou Z, Zhang L, Wang S, Xiao G. 2016. Structure-function relationship of the mammarenavirus envelope glycoprotein. Virol Sin 31:380–394. doi: 10.1007/s12250-016-3815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casals J, Buckley SM, Cedeno R. 1975. Antigenic properties of the arenaviruses. Bull World Health Organ 52:421–427. [PMC free article] [PubMed] [Google Scholar]
- 6.Gschwender H, Lehmann-Grube F. 1973. Antigenic properties of the LCM virus: virion and complement-fixing antigen, p 25–35. In Lymphocytic choriomeningitis virus and other arenaviruses. Springer, Berlin, Germany. [Google Scholar]
- 7.Buchmeier MJ, Gee SR, Rawls WE. 1977. Antigens of Pichinde virus. I. Relationship of soluble antigens derived from infected BHK-21 cells to the structural components of the virion. J Virol 22:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cresta B, Padula P, de Martinez Segovia M. 1980. Biological properties of Junin virus proteins. I. Identification of the immunogenic glycoprotein. Intervirology 13:284–288. [DOI] [PubMed] [Google Scholar]
- 9.Buchmeier MJ, Lewicki HA, Tomori O, Oldstone MBA. 1981. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: Generation, characterization, and cross-reactivity with other arenaviruses. Virology 113:73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- 10.Bruno-Lobo GG, Bruno-Lobo M, Johnson KM, Webb PA, de Paola D. 1968. Pathogenesis of Junin virus infection in the infant hamster. An Microbiol 15:11–33. [PubMed] [Google Scholar]
- 11.Rowe WP, Pugh WE, Webb PA, Peters CJ. 1970. Serological relationship of the Tacaribe complex of viruses to lymphocytic choriomeningitis virus. J Virol 5:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KM, Webb PA, Justines G. 1973. Biology of TaCaribe-complex viruses, p 241–258. In Lehmann-Grube F. (ed), Lymphocytic choriomeningitis virus and other arenaviruses: symposium held at the Heinrich-Pette-Institut für Experimentelle Virologie und Immunologie, Universität Hamburg, October 16–18 Springer, Berlin, Germany. [Google Scholar]
- 13.Casals J. 1975. Arenaviruses. Yale J Biol Med 48:115–140. [PMC free article] [PubMed] [Google Scholar]
- 14.Calisher CH, Tzianabos T, Lord RD, Coleman PH. 1970. Tamiami virus, a new member of the TaCaribe group. Am J Trop Med Hyg 19:520–526. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KM, Halstead SB, Cohen SN. 1967. Hemorrhagic fevers of Southeast Asia and South America: a comparative appraisal. Prog Med Virol 9:105–158. [PubMed] [Google Scholar]
- 16.Berge TO. 1975. International catalogue of arboviruses. U.S. Department of Health, Education, and Welfare, Washington, DC. [Google Scholar]
- 17.Trapido H, Sanmartin C. 1971. Pichinde virus, a new virus of the Tacaribe group from Colombia. Am J Trop Med Hyg 20:631–641. [PubMed] [Google Scholar]
- 18.Buckley SM, Casals J. 1970. Lassa fever, a new virus disease of man from West Africa. III. Isolation and characterization of the virus. Am J Trop Med Hyg 19:680–691. [DOI] [PubMed] [Google Scholar]
- 19.Dutko F, Pfau C. 1978. Arenavirus defective interfering particles mask the cell-killing potential of standard virus. J Gen Virol 38:195–208. doi: 10.1099/0022-1317-38-2-195. [DOI] [PubMed] [Google Scholar]
- 20.Monath TP. 1973. Lassa fever. Trop Doctor 3:155–161. doi: 10.1177/004947557300300404. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta S, Rawls WE. 1979. Pseudotypes of vesicular stomatitis virus and Pichinde virus. J Gen Virol 42:141–148. doi: 10.1099/0022-1317-42-1-141. [DOI] [PubMed] [Google Scholar]
- 22.Howard CR, Lewicki H, Allison L, Salter M, Buchmeier MJ. 1985. Properties and characterization of monoclonal antibodies to Tacaribe virus. J Gen Virol 66(Pt 7):1383–1395. doi: 10.1099/0022-1317-66-7-1383. [DOI] [PubMed] [Google Scholar]
- 23.Smadel JE, Wall MJ. 1940. A soluble antigen of lymphocytic choriomeningitis. II. Independence of anti-soluble substance antibodies and neutralizing antibodies, and the role of soluble antigen and inactive virus in immunity to infection. J Exp Med 72:389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutter G, Gschwender H. 1973. Antigenic alteration of cells in vitro infected with LCM virus, p 51–59. In Lymphocytic choriomeningitis virus and other arenaviruses. Springer, Berlin, Germany. [Google Scholar]
- 25.Abraham J, Corbett KD, Farzan M, Choe H, Harrison SC. 2010. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat Struct Mol Biol 17:438–444. doi: 10.1038/nsmb.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmutovic S, Clark L, Levis SC, Briggiler AM, Enria DA, Harrison SC, Abraham J. 2015. Molecular basis for antibody-mediated neutralization of New World hemorrhagic fever mammarenaviruses. Cell Host Microbe 18:705–713. doi: 10.1016/j.chom.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, Farzan M, Choe H. 2007. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 446:92–96. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt AG, Therkelsen MD, Stewart S, Kepler TB, Liao HX, Moody MA, Haynes BF, Harrison SC. 2015. Viral receptor-binding site antibodies with diverse germline origins. Cell 161:1026–1034. doi: 10.1016/j.cell.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu R, Krause JC, McBride R, Paulson JC, Crowe JE Jr, Wilson IA. 2013. A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nat Struct Mol Biol 20:363–370. doi: 10.1038/nsmb.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Fischer ER, Kouiavskaia D, Hansen BT, Ludtke SJ, Bidzhieva B, Makiya M, Agulto L, Purcell RH, Chumakov K. 2013. Cross-neutralizing human anti-poliovirus antibodies bind the recognition site for cellular receptor. Proc Natl Acad Sci U S A 110:20242–20247. doi: 10.1073/pnas.1320041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, Kawaoka Y. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci U S A 94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iha K. 2013. Pseudotyped vesicular stomatitis virus for analysis of entry of arenaviruses and its application to serodiagnosis of Argentine hemorrhagic fever. PhD dissertation. University of Tokyo, Tokyo, Japan. [Google Scholar]
- 35.Golden JW, Maes P, Kwilas SA, Ballantyne J, Hooper JW. 2016. Glycoprotein-specific antibodies produced by DNA vaccination protect guinea pigs from lethal Argentine and Venezuelan hemorrhagic fever. J Virol 90:3515–3529. doi: 10.1128/JVI.02969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez A, Pifat DY, Kenyon RH, Peters CJ, McCormick JB, Kiley MP. 1989. Junin virus monoclonal antibodies: characterization and cross-reactivity with other arenaviruses. J Gen Virol 70:1125–1132. doi: 10.1099/0022-1317-70-5-1125. [DOI] [PubMed] [Google Scholar]
- 37.Zeitlin L, Geisbert JB, Deer DJ, Fenton KA, Bohorov O, Bohorova N, Goodman C, Kim D, Hiatt A, Pauly MH, Velasco J, Whaley KJ, Altmann F, Gruber C, Steinkellner H, Honko AN, Kuehne AI, Aman MJ, Sahandi S, Enterlein S, Zhan X, Enria D, Geisbert TW. 2016. Monoclonal antibody therapy for Junin virus infection. Proc Natl Acad Sci U S A 113:4458–4463. doi: 10.1073/pnas.1600996113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.York J, Berry JD, Stroher U, Li Q, Feldmann H, Lu M, Trahey M, Nunberg JH. 2010. An antibody directed against the fusion peptide of Junin virus envelope glycoprotein GPC inhibits pH-induced membrane fusion. J Virol 84:6119–6129. doi: 10.1128/JVI.02700-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.York J, Romanowski V, Lu M, Nunberg JH. 2004. The signal peptide of the Junin arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J Virol 78:10783–10792. doi: 10.1128/JVI.78.19.10783-10792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, Tauer C, Berger R, Barrett N, Jungbauer A, Katinger H. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 41.Gorny MK, Zolla-Pazner S. 2000. Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41. J Virol 74:6186–6192. doi: 10.1128/JVI.74.13.6186-6192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin VK, Droniou-Bonzom ME, Reignier T, Oldenburg JE, Cox AU, Cannon PM. 2010. Investigation of clade B New World arenavirus tropism by using chimeric GP1 proteins. J Virol 84:1176–1182. doi: 10.1128/JVI.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albarino CG, Bird BH, Chakrabarti AK, Dodd KA, Flint M, Bergeron E, White DM, Nichol ST. 2011. The major determinant of attenuation in mice of the Candid1 vaccine for Argentine hemorrhagic fever is located in the G2 glycoprotein transmembrane domain. J Virol 85:10404–10408. doi: 10.1128/JVI.00856-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coughlin MM, Prabhakar BS. 2012. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev Med Virol 22:2–17. doi: 10.1002/rmv.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dias JM, Kuehne AI, Abelson DM, Bale S, Wong AC, Halfmann P, Muhammad MA, Fusco ML, Zak SE, Kang E, Kawaoka Y, Chandran K, Dye JM, Saphire EO. 2011. A shared structural solution for neutralizing ebolaviruses. Nat Struct Mol Biol 18:1424–1427. doi: 10.1038/nsmb.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bale S, Dias J, Fusco M, Hashiguchi T, Wong A, Liu T, Keuhne A, Li S, Woods V, Chandran K. 2012. Structural basis for differential neutralization of ebolaviruses. Viruses 4:447–470. doi: 10.3390/v4040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruns M, Cihak J, Muller G, Lehmann-Grube F. 1983. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology 130:247–251. [DOI] [PubMed] [Google Scholar]
- 48.Parekh BS, Buchmeier MJ. 1986. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology 153:168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- 49.Ruo S, Mitchell S, Kiley M, Roumillat L, Fisher-Hoch S, McCormick J. 1991. Antigenic relatedness between arenaviruses defined at the epitope level by monoclonal antibodies. J Gen Virol 72:549–555. doi: 10.1099/0022-1317-72-3-549. [DOI] [PubMed] [Google Scholar]