ABSTRACT
Influenza is a zoonotic disease that poses severe threats to public health and the global economy. Reemerging influenza pandemics highlight the demand for universal influenza vaccines. We developed a novel virus platform using extracellular domain IV of the matrix 2 protein (M2e), AdC68-F3M2e, by introducing three conserved M2e epitopes into the HI loop of the chimpanzee adenovirus (AdV) fiber protein. The M2e epitopes were expressed sufficiently on the AdV virion surface without affecting fiber trimerization. Additionally, one recombinant adenovirus, AdC68-F3M2e(H1-H5-H7), induced robust M2e-specific antibody responses in BALB/c mice after two sequential vaccinations and conferred efficient protection against homologous and heterologous influenza virus (IV) challenges. We found that the use of AdV with tandem M2e epitopes in fiber is a potential strategy for influenza prevention.
IMPORTANCE Influenza epidemics and pandemics severely threaten public health. Universal influenza vaccines have increasingly attracted interest in recent years. Here, we describe a new strategy that incorporates triple M2e epitopes into the fiber protein of chimpanzee adenovirus 68. We optimized the process of inserting foreign genes into the AdC68 structural protein by one-step isothermal assembly and demonstrated that this 225-bp HI loop insertion could be well tolerated. Furthermore, two doses of adjuvant-free fiber-modified AdC68 could confer sufficient protection against homologous and heterologous influenza virus infections in mice. Our results show that AdC68-F3M2e could be pursued as a novel universal influenza vaccine.
KEYWORDS: universal influenza vaccine, M2e, adenovirus, HI loop, immune responses
INTRODUCTION
Influenza virus (IV) is a continuously evolving respiratory pathogen that places enormous burdens on health care systems (1). The most cost-effective way to prevent IV is vaccination (2). The efficacy of commonly used trivalent inactivated split vaccines (TIV) largely depends on the accuracy of the prediction of epidemic strains in the upcoming flu season (3). In 2009, the novel H1N1 strain, which had antigenically shifted from the contemporary seasonal strain, swept across the globe. Studies revealed that little or even no cross-reactive antibody against H1N1pdm09 was induced by seasonal vaccines (4). In 2014 to 2015, >80% of circulating H3N2 viruses in the United States antigenically mismatched the recommended vaccine strains. The estimated vaccine effectiveness (VE) against H3N2 was ∼18% in that flu season, while the VE of antigen-matched influenza B viruses was 45% (5). Recurrent human infection with avian influenza viruses poses additional challenges to vaccine development (6). The intrinsic limitations of TIV motivate the development of universal influenza vaccines.
One basic strategy in developing universal IV vaccines is to screen for conserved epitopes that would serve as immunogens. A total of 17 of 24 amino acids of extracellular domain IV of the matrix 2 protein (M2e) are 94% identical among different subtypes, making it an ideal candidate vaccine antigen (7). M2e forms tetramers on the influenza virion surface which act as ion channels (8). The small size of the protein, as well as its rarity on the virion surface, contributes to the poor immunogenicity of M2e. During natural infection, the human body barely generates M2e-specific antibodies (9). Hence, one of the main difficulties in developing M2e-based influenza vaccines is improving immunogenicity. Previous studies adopted various approaches to enhance M2e potency, such as coupling M2e to different chemical or genetic vectors (10–16). These conjugates conferred to mice complete or partial protection against IV challenges. However, some fusion constructs were supplemented with experimental adjuvants, including Freund's adjuvant (11, 14) and monophosphoryl lipid A (15), which induced severe side effects and were not clinically suitable. Thus, universal influenza vaccines free of strong adjuvants are in urgent demand.
In this study, we established a delivery system by inserting M2e peptides into the adenovirus (AdV) fiber protein. The AdV vector presented a tandem repeat of M2e (derived from H1N1, H5N1, and H7N2) on the virion surface. These AdVs could induce potent anti-M2e immune responses by the prime-boost strategy. More importantly, intramuscular immunization with one adjuvant-free fiber-modified AdV conferred complete protection against homologous H1N1 challenge and led to 80% and 75% survival in BALB/c mice infected with heterosubtypic H9N2 and H5N1, respectively.
RESULTS
Construction of fiber-modified AdV molecular clones.
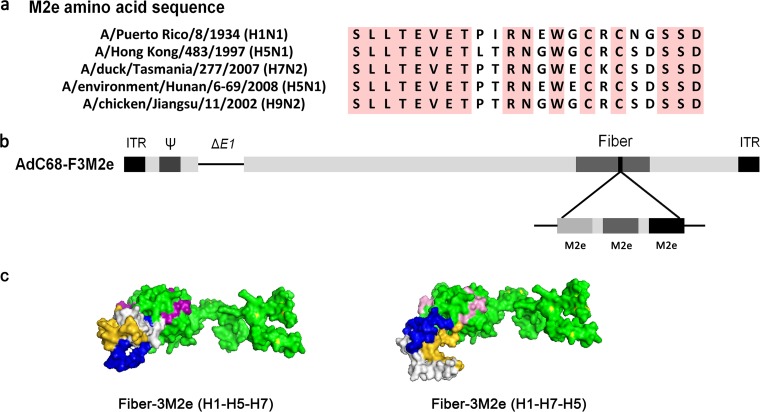
It has been reported that epitopes inserted into AdV fiber elicited the most potent immune responses among capsid proteins, in spite of their rarity on the virion surface (17). Therefore, we decided to incorporate three divergent M2e epitopes (derived from H1N1, H5N1, and H7N2) previously demonstrated to confer cross-protection (18, 19) into the HI loop of AdV fiber. Using Gly-Ala-Ala as a linker, the epitopes were chained together in the orders H1-H5-H7 and H1-H7-H5. Multiple alignment of amino acid sequences, a schematic presentation of the AdV clones, and structural modeling of chimeric fiber protein are shown in Fig. 1 and specified in Materials and Methods. Recombinant AdVs were rescued and their titers determined, as previously reported (20).
FIG 1.
Construction of recombinant AdC68 with M2e displayed on fiber. (a) Sequence alignment of M2e derived from different IVs used in the study. Residues that were conserved in all sequences are shaded in pink. (b) Schematic presentation of AdC68-F3M2e. (c) Structure modeling of modified fiber proteins with H1-H5-H7 (left) or H1-H7-H5 (right) insertion into the HI loop by PyMol. M2e of H1, H5, and H7 is labeled in blue, gold, and white, respectively. The knob is shown in pink.
Trimerization of modified AdV fiber.
AdV fiber proteins form trimers on the virion surface under native conditions (21). Hence, we investigated whether insertion of a 225-bp foreign gene affected fiber trimerization. We performed Western blotting with both nonreducing and reducing buffer-treated AdVs. AdC68-empty was used as the negative control, following the same protocol. As shown in Fig. 2, both M2e-modified AdV fiber proteins maintained their trimer structure under nonreducing conditions, whereas 2-mercaptoethanol treatment turned trimers into ∼51-kDa monomers. AdC68-empty exhibited no band when probed with M2e-specific antibody. These results demonstrated that 75-mer triple M2e epitopes were incorporated into fiber proteins without damping trimerization. Surprisingly, we observed that the M2e expression of AdC68-F3M2e(H1-H5-H7) was slightly higher than that of AdC68-F3M2e(H1-H7-H5) in either trimer or monomer form. We speculate that two factors may contribute to this difference. First, M2e peptides in AdC68-F3M2e(H1-H5-H7) may have been better exposed and more easily recognized by antibodies than their H1-H7-H5 counterparts. Second, the proportion of fiber protein with incorporated M2e might be higher in AdC68-F3M2e(H1-H5-H7) than in its H1-H7-H5 counterpart.
FIG 2.
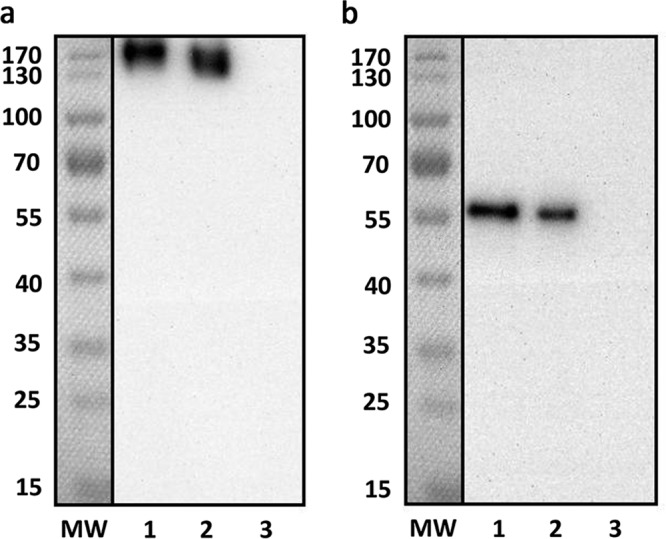
Trimerization of M2e-inserted fiber. Purified AdC68-F3M2e(H1-H5-H7) and AdC68-F3M2e(H1-H7-H5) were electrophoresed using 10% SDS-PAGE under nonreducing (a) or reducing (b) conditions. AdC68-empty (105 PFU) was used as the negative control. MW, molecular weight marker. Lane 1, 105PFU of AdC68-F3M2e(H1-H5-H7); lane 2, 105PFU of AdC68-F3M2e(H1-H7-H5); lane 3, 105 PFU of AdC68-empty. Experiments were repeated three times.
Presentation of M2e on AdC68 fiber.
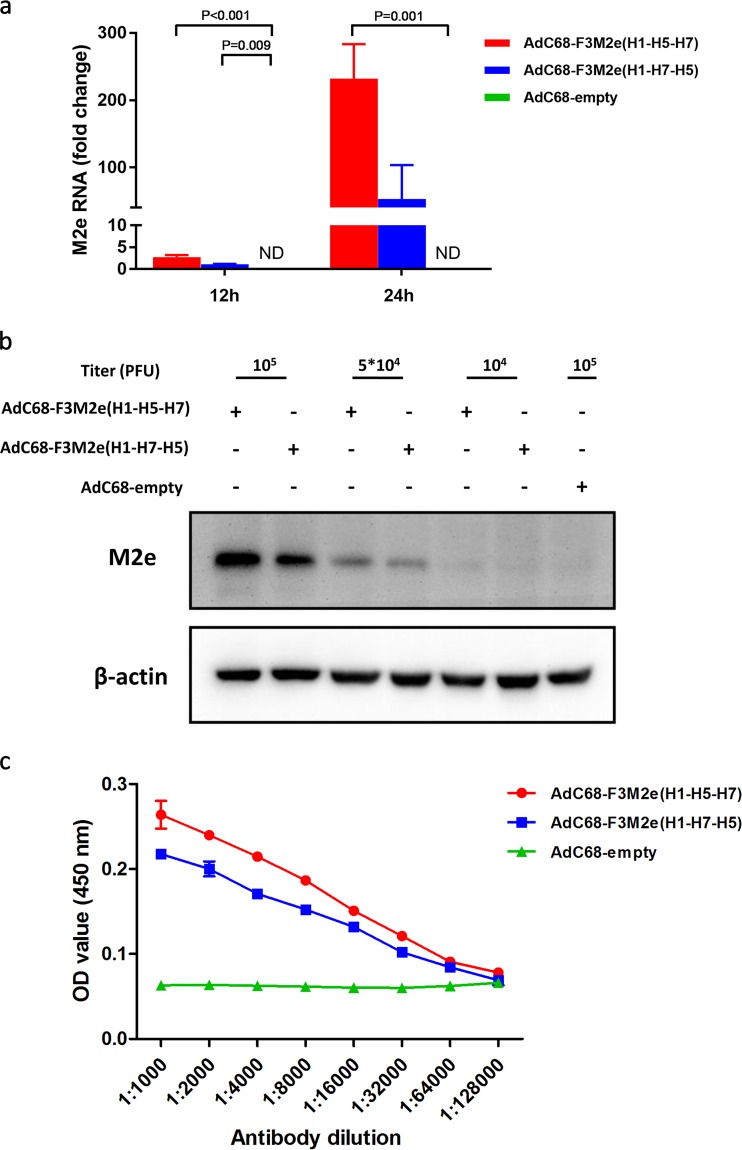
To evaluate the in vitro expression of M2e, we performed additional experiments using real-time PCR (RT-PCR) (Fig. 3a) and Western blotting (Fig. 3b). We first transduced AdVs into human embryonic kidney 293 (HEK293) cells at a multiplicity of infection (MOI) of 0.1 and then determined RNA levels 12 and 24 h later. Figure 3a illustrates the relative M2e expression levels at these two time points. The average fold change values for AdC68-F3M2e(H1-H5-H7) and AdC68-F3M2e(H1-H7-H5) M2e RNA expression were 232.2 and 53.0, respectively, at 24 h postransduction; those levels were enhanced by ∼85- and ∼48-fold compared with expression at 12 h, while AdC68-empty infected cells generated no M2e RNA. We next assessed protein levels via Western blotting after infection using different doses of AdV. AdC68-F3M2e(H1-H5-H7)- or AdC68-F3M2e(H1-H7-H5)-treated cells expressed high levels of M2e protein in a dose-dependent manner (Fig. 3b). However, HEK293 cells transduced with AdC68-F3M2e(H1-H5-H7) exhibited a slightly higher level of expression than their AdC68-F3M2e(H1-H7-H5)-transduced counterparts, indicating that the levels of in vitro expression efficiency might differ.
FIG 3.
Expression of M2e incorporated in AdC68 fiber. (a) HEK293 cells were infected with AdC68-F3M2e(H1-H5-H7), AdC68-F3M2e(H1-H7-H5), or AdC68-empty at an MOI of 0.1. Total RNA was extracted and applied for M2e RNA expression analysis by real-time PCR 12 or 24 h later. Values were normalized to β-actin data and are expressed as average fold changes relative to AdC68-F3M2e(H1-H7-H5) (the reference) ± standard deviations (SD). (b) Different doses of AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) were transduced into HEK293 cells and analyzed for M2e expression (relative to β-actin) by Western blotting 24 h later. AdC68-empty served as a negative control. (c) Plates were coated with 5 × 104 PFU of the indicated adenoviruses, blocked, and incubated with 2-fold serially diluted (from 1:100 to 1:12,800) 14C2. HRP-conjugated anti-mouse IgG served as secondary antibody. Data are shown as mean absorbance ± standard deviation (SD). All experiments were repeated in triplicate.
Results determined in a previous study implied that capsid-incorporated epitopes might present on intact virion for immune recognition (17). Hence, we conducted an adenovirus-based enzyme-linked immunosorbent assay (ELISA) to demonstrate the existence of M2e on the virion surface and to evaluate the binding affinities. ELISA plates were coated with 5 × 104 PFU AdVs and reacted with serially diluted 14C2. As shown in Fig. 3c, AdC68-F3M2e(H1-H5-H7) and AdC68-F3M2e(H1-H7-H5) exhibited significantly higher affinities for 14C2 than AdC68-empty, suggesting that the fiber-presented PR8 M2e epitope was well exposed and accessible to its corresponding antibodies.
Induction of M2e-specific antibody responses.
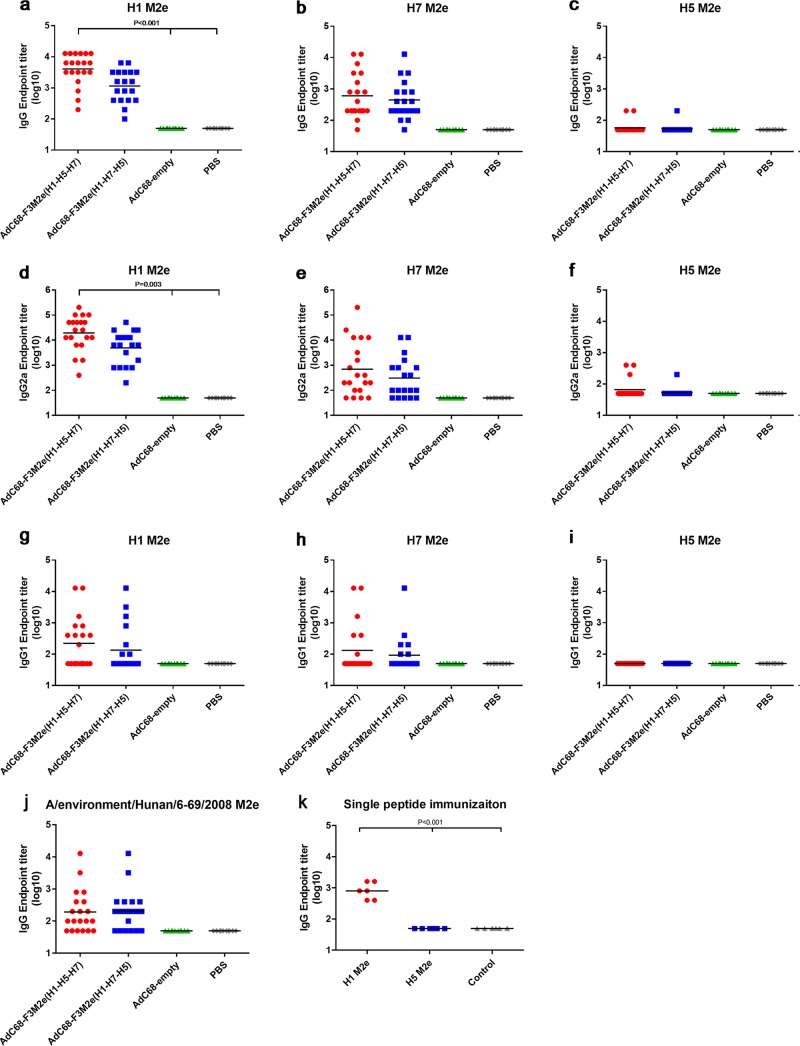
We examined whether the recombinant AdVs with M2e incorporated in fiber could enhance M2e immunogenicity. Therefore, groups of BALB/c mice were immunized intramuscularly twice with 2.5 × 105 PFU of AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) using a 2-week interval, and sera were collected to test M2e-specific immune responses 2 weeks after vaccination. AdC68-empty- or phosphate-buffered saline (PBS)-injected mice acted as controls. Most AdC68-F3M2e(H1-H5-H7)- or AdC68-F3M2e(H1-H7-H5)-vaccinated mice barely generated total IgG against H1, H7, or H5 M2e after the prime. No significant difference existed between fiber-modified AdVs and the corresponding controls (data not shown). After the boost, total levels of IgG against H1 and H7 M2e in both AdC68-F3M2e groups were markedly elevated, while H5 M2e-specific IgG remained at low levels (Fig. 4a to c). The geometric mean antibody titer (GMT) of total IgG for the AdC68-F3M2e(H1-H5-H7) group was 4,079 against H1 M2e (Fig. 4a), which was statistically significantly different from the control GMT (GMT = 50, P < 0.001). The GMT of IgG against H7 M2e was 606.3 in the AdC68-F3M2e(H1-H5-H7) group (P = 0.213). As for AdC68-F3M2e(H1-H7-H5), the GMTs of H1 and H7 M2e were 1,152 and 446.3, respectively (not significant). Although the endpoint titers of the AdC68-F3M2e(H1-H7-H5) group were not significantly higher than those of either control group, the GMTs were elevated by 23- and 8.9-fold relative to the control GMTs. In evaluating M2e-specific immune responses, we found that AdC68-F3M2e(H1-H5-H7) induced stronger antibody responses than AdC68-F3M2e(H1-H7-H5), as evidenced by higher GMTs of H1- and H7-specific IgG and isotype IgG. These results might also have been due to the better-exposed structure or higher proportion of M2e incorporated into AdC68-F3M2e(H1-H5-H7) (Fig. 2 and 3c). Moreover, to our surprise, the total level of IgG targeting H5 M2e hardly rose even after the boost, and the titers of H7 M2e targeting antibody were lower than those of H1 M2e in both AdC68-F3M2e groups. We discuss possible reasons for these phenomena below.
FIG 4.
Antibody responses to M2e in BALB/c mice. Mouse sera were collected 2 weeks after AdV (a to j) or peptide (k) prime-boost and were tested for M2e-specific antibody titers by ELISA. Endpoint titers were determined as described in Materials and Methods. Titers of negative serum samples were assigned a value of 50 (half of the starting dilution). Total IgG levels were measured against H1 (a), H7 (b), H5 (c), or A/environment/Hunan/6-69/2008 (j) M2e peptides. Isotype IgG (IgG2a and IgG1) levels were determined against H1 (d and g), H7 (e and h), and H5 M2e (f and i). As for the single peptide immunization (k), groups of mice (n = 6) were injected i.p. with H1 or H5 M2e peptides (100 μg per dose) in the presence of Freund's complete adjuvant and boosted with Freund's incomplete adjuvant after a 2-week interval. Mice injected with adjuvant alone served as controls. The black lines in all panels indicate the geometric mean titers. One-way ANOVA was applied to compare with the control groups. Experiments were repeated three times.
To better elucidate the type of immune responses elicited by fiber-modified AdVs, we investigated the isotype profiles (IgG2a and IgG1) postvaccination. As depicted in Fig. 4d and g, we observed a substantial induction of H1-specific IgG2a in the AdC68-F3M2e(H1-H5-H7)-treated group (GMT = 19,401, P = 0.003). In contrast, levels of IgG1 isotypes were barely elevated (GMT = 221.9, P = 0.512). Consistent with the results described above, AdC68-F3M2e(H1-H7-H5) also generated a larger amount of H1-specific IgG2a (GMT = 4,958) than IgG1 (GMT = 133.9). The GMTs of H7 M2e IgG2a in AdC68-F3M2e groups were 13.9- and 6.2-fold higher than those in the corresponding controls, but no significant difference existed [AdC68-F3M2e(H1-H5-H7) GMT = 696.4, AdC68-F3M2e(H1-H7-H5) GMT = 309.9, P = 0.384] (Fig. 4e and h). Consistent with total IgG results, most mice barely generated H5-M2e-specific isotype antibodies, as shown in Fig. 4f and i. The isotype profile indicated the activation of a Th1 dominant immune response after vaccination. Total IgG against A/environment/Hunan/6-69/2008, the H5N1 strain that we used to challenge mice, was also analyzed, and the GMT values were 193.2 and 207.4 in the AdC68-F3M2e(H1-H5-H7) and AdC68-F3M2e(H1-H7-H5) groups, respectively (Fig. 4j). To further test the immunogenicity of single M2e, naive mice were directly immunized with H1 or H5 M2e peptides with Freund's adjuvants. Antibody responses to H1 M2e (GMT = 800) were significantly stronger than those to H5 M2e (GMT = 50, P < 0.001) (Fig. 4k), indicating the difference in immunogenicity.
In vivo protection against homologous PR8 challenge in BALB/c mice.
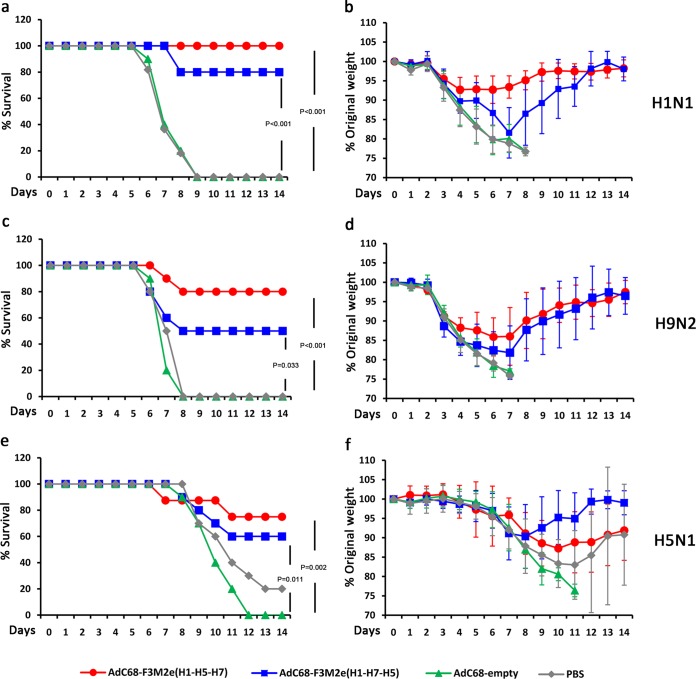
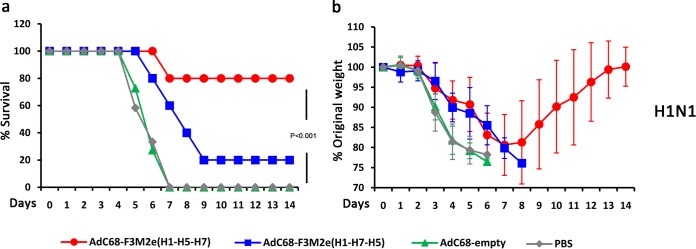
To assess the effect of increased antibody responses on clinical outcomes, we challenged the immunized BALB/c mice [AdC68-F3M2e(H1-H5-H7) (n = 9), AdC68-F3M2e(H1-H7-H5) (n = 10), AdC68-empty (n = 10), and PBS (n = 11)] with 5 50% lethal doses (LD50) of PR8 2 weeks postboost. Clinical symptoms were monitored on a daily basis for 14 days. As shown in Fig. 5a and b, the AdC68-F3M2e(H1-H5-H7), AdC68-F3M2e(H1-H7-H5), and control groups all exhibited similar weight loss patterns at 2 days postchallenge. AdC68-F3M2e(H1-H5-H7)-vaccinated mice lost at most 8% of their initial weight around days 3 to 6 and gradually recovered. All AdC68-F3M2e(H1-H5-H7)-immunized mice survived the lethal challenge (P < 0.001). The weight loss of AdC68-F3M2e(H1-H7-H5)-vaccinated mice peaked at ∼19% by day 7, and 2 of 10 mice succumbed to infection by day 8 (P < 0.001). In contrast, every subject mouse of the two control groups suffered constant weight loss and died or required euthanasia by day 9. Thus, AdC68-F3M2e(H1-H5-H7) protected BALB/c mice completely from lethal challenge with PR8, while AdC68-F3M2e(H1-H7-H5) conferred 80% protection.
FIG 5.
Protection against lethal doses of H1N1, H9N2, and H5N1 challenge in BALB/c mice. BALB/c mice were immunized intramuscularly (i.m.) twice with 2.5 × 105 PFU of AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) and challenged with 5 LD50 of H1N1 (a and b), H9N2 (c and d), and H5N1 (e and f) 2 weeks postboost [H1N1 challenge, AdC68-F3M2e(H1-H5-H7) (n = 9) or PBS (n = 11); H5N1 challenge, AdC68-F3M2e(H1-H5-H7) (n = 8) (others, n = 10)]. The AdC68-empty- and PBS-treated groups served as controls. Mice from each group were monitored for 14 days for survival rates (a, c, and e) and weight loss (b, d, and f). Mean weights ± SD were determined at each time point. The chi-square test was applied to compare survival rates. The survival experiments were repeated twice.
Cross-protection against H5N1 and H9N2 in BALB/c mice.
We further investigated whether AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) could enhance the breadth of protection against heterologous influenza viruses. Antigenically different H5N1 and H9N2 strains were selected for challenge experiments due to their potential to infect humans and unleash pandemics (22, 23). Groups of BALB/c mice were immunized and infected with 5 LD50 of H9N2 (n = 10 for all groups) or H5N1 [AdC68-F3M2e(H1-H5-H7) n = 8, other groups n = 10] as described above. Eight of 10 mice from the AdC68-F3M2e(H1-H5-H7)-vaccinated group survived the lethal H9N2 challenge, although they lost ∼14% of their original weight by day 6 (P < 0.001) (Fig. 5c and d). In the AdC68-F3M2e(H1-H7-H5) group, 50% of mice succumbed to infection (P = 0.033), and this group suffered an average ∼19% decrease from their original weight by day 7. All control mice were sacrificed 8 days postinfection (d.p.i.). In terms of H5N1, AdC68-F3M2e(H1-H5-H7) and AdC68-F3M2e(H1-H7-H5) significantly alleviated symptoms and conferred 75% protection (P = 0.002 compared with AdC68-empty) and 60% protection (P = 0.011 compared with AdC68-empty), respectively (Fig. 5e and f). Survival rates of the AdC68-empty and PBS groups were 0% and 20%, respectively. These results demonstrated that fiber-modified AdVs could confer efficient cross-protection against antigenically distinct avian influenza viruses of high or low pathogenicity.
Protection conferred by passive transfer of AdC68-F3M2e-vaccinated mouse sera.
To determine whether fiber-modified AdV-induced protection depended mainly on humoral immunity, naive BALB/c mice were intraperitoneally (i.p.) injected with 500 μl sera from AdC68-F3M2e(H1-H5-H7)- or AdC68-F3M2e(H1-H7-H5)-immunized mice and were challenged with 5 LD50 of PR8 24 h later. AdC68-empty- or PBS serum-treated mouse served as controls. As depicted in Fig. 6, passive transfer of AdC68-F3M2e(H1-H5-H7) serum protected 80% of the mice from lethal challenge (P < 0.01), while administration of AdC68-F3M2e(H1-H7-H5) sera provided only 20% protection (not significant). All controls succumbed to viral infection by day 7. Compared with control group results, preinjection of AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) sera reduced the extent of weight loss by ∼5% to 10% from day 3 to day 6. Passive transfer results proved that humoral immunity played a crucial role in AdC68-F3M2e-induced protection.
FIG 6.
Protection conferred by passive serum transfer. Naive BALB/c mice were i.p. injected with 500 μl serum from AdC68-F3M2e(H1-H5-H7)- or AdC68-F3M2e(H1-H7-H5)-immunized mice 1 day before infection with 5 LD50 of PR8 (AdC68-F3M2e(H1-H5-H7) (n = 5), AdC68-F3M2e(H1-H7-H5) (n = 5), or AdC68-empty (n = 11) or injection of PBS (n = 12). AdC68-empty- and PBS-treated groups served as controls. Mice from each group were monitored for 14 days for survival rates (a) and weight loss (b). Mean weights ± SD were determined at each time point. The chi-square test was applied to compare survival rates. One mouse in the AdC68-F3M2e(H1-H7-H5) group survived the challenge, but its weight loss was not included in the data in panel b corresponding to the time period after day 10. The experiments described above were repeated twice.
DISCUSSION
Traditional IV vaccines require regular update and large quantities of chicken embryos for production. Consequently, novel universal influenza vaccines produced independently of the use of eggs are in urgent demand. M2e has become a putative candidate because of its conservation, and M2e-specific monoclonal antibodies demonstrably restrict IV replication both in vitro (24) and in vivo (25, 26). However, improving M2e immunogenicity remains a significant challenge. Liu et al. fused 16 tandem copies of M2e peptide with glutathione S-transferase and vaccinated BALB/c mice with three doses in the presence of complete Freund's adjuvant (50 μg antigen per dose) (10). De Filette et al. immunized mice three times with 10 μg M2e-HBc in combination with CTA1-DD, an adjuvant that fused the cholera toxin A1 subunit to a dimer of the Ig-binding D-region of Staphylococcus aureus protein A (27). Although these complexes protected mice from lethal IV challenge, the high doses of antigen and adjuvant were indispensable.
The present study generated replication-defective chimpanzee adenoviruses as platforms to present M2e epitopes. AdVs have been widely modified to function as vaccine vectors for the following advantages: broad tropism, high transduction efficiency, potent immune response induction, and excellent safety profile (28). Moreover, the production of the AdV-based influenza vaccine is independent of chicken embryos (29). We inserted M2e peptides into the HI loop of fiber, one of the AdV surface proteins. In previous studies, the peptide inserted into the HI loop was usually smaller than a 20-mer (17, 21, 30). Here we showed that a 75-mer foreign peptide insertion into the HI loop of fiber protein could be well tolerated. M2e epitopes were well exposed on the virion surface and were able to induce potent M2e-specific antibody responses in mice. Vaccinated mice survived not only the homologous PR8 infection but also infection using two heterologous IV strains that once circulated in poultry.
A major difficulty in displaying epitopes on AdV capsid proteins is that of constructing the adenovirus molecular clone in light of its large genome size and limited insertion size in structural proteins. Previous studies usually adopted multiple steps of PCR amplification, enzyme digestion, and ligation (21, 31). Many shuttle vectors were needed. Hence, the incorporated epitopes were unlikely to be directly modified. Here, we generated the modified AdC68 molecular clone by inserting a PmeI site into the fiber HI loop. Foreign epitopes with certain homologous sequences at both ends could be linked with PmeI-linearized AdC68 vector via one-step isothermal assembly. The process of AdV genetic construction was greatly simplified. In addition, we maintained the I-CeuI and PI-SceI sites in the AdC68 E1 region. It may be feasible to coexpress foreign genes in the E1 region and in fiber at the same time.
One unexpected result of our research was that BALB/c mice immunized with AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) hardly generated antibodies against A/Hong Kong/483/1997(H5N1) (HK483) M2e. Kim et al. reported a similar phenomenon: the avian type II M2e (SLLTEVETLTRNGWGCRCS), whose amino acid sequence was the same as the first 19 amino acids of the H5N1 M2e that we chose, induced less-potent antibody responses, which those authors considered a positional effect (32). In the present study, when we altered the chained order of the three M2e sequences, AdC68-F3M2e(H1-H5-H7) and AdC68-F3M2e(H1-H7-H5) exhibited similar immune response patterns, implying that the location of the H5N1 M2e peptide was not the determinant of these results. Therefore, we speculated that the lack of anti-HK483 immunity was due to the poor immunogenicity of HK483 M2e in BALB/c mice. To test this hypothesis, we directly immunized BALB/c mice with H1 or H5 M2e peptides in the presence of Freund's adjuvants. We observed much stronger antibody responses to H1 M2e than to H5 M2e (Fig. 4k), indicating that immunogenicity differences did exist among these peptides. On the other hand, the total level of IgG against A/environment/Hunan/6-69/2008, the H5N1 strain that we used to challenge mice, was comparable to that seen with H7 M2e (Fig. 4j). The A/environment/Hunan/6-69/2008 amino acid sequence shared 83%, 87%, and 91% similarity with A/Puerto Rico/8/1934, A/Hong Kong/483/1997, and A/duck/Tasmania/277/2007, respectively (Fig. 1a). Therefore, we assumed that the antibody responses to and protection against A/environment/Hunan/6-69/2008 might be conferred by cross-reactivity with H7N2 or H1N1 M2e. As for why A/Hong Kong/483/1997(H5N1) M2e could hardly induce antibody responses, we considered the following two possibilities. The first is the unstable binding between major histocompatibility complex (MHC) class II and peptides, which could alter the display of MHC class II-peptide complexes on antigen-presenting cells (33). It resulted in insufficient differential priming and expansion of the population of H5-M2e-specific CD4 T cells, a key in establishing protective antibody responses. The second possible explanation is the comparatively lower binding affinity between H5 M2e and the B cell receptor (BCR). Therefore, these B cells might undergo apoptosis during selection in germinal centers (34).
The present results demonstrate that AdC68-F3M2e is a feasible universal influenza vaccine and provide guidance in choosing the appropriate M2e as a universal IV vaccine antigen. Additional studies, including studies of the display of highly immunogenic M2e peptides on fiber or coexpression of other conserved antigens in the E1 region of AdC68-F3M2e, e.g., the nucleoprotein (NP) and the hemagglutinin (HA) stalk, are required to further optimize prophylactic efficiency.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney 293 (HEK293) cells were acquired from the Shanghai Cell Bank of the Chinese Academy of Sciences and were maintained in Dulbecco's modified Eagle's medium (HyClone, Beijing, China) supplemented with 10% fetal bovine serum (Gemini Biological Products, Calabasas, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin (HyClone).
Influenza virus.
Influenza virus strains A/Puerto Rico/8/1934 (H1N1), A/environment/Hunan/6-69/2008 (H5N1), and A/chicken/Jiangsu/11/2002 (H9N2) were inoculated into the allantoic cavity of 9-day-old specific-pathogen-free (SPF) chicken embryos, as previously described (18). The titer corresponding to the median lethal dose (LD50) of each virus was determined in adult mice by intranasal inoculation. All experiments with H5N1 were performed in biosafety level 3 (BSL-3) facilities at Fudan University (Shanghai, China); other experiments were conducted under BSL-2 conditions at the Institute Pasteur of Shanghai (Shanghai, China).
Mice.
Female BALB/c mice (6 to 8 weeks of age) were obtained from a Shanghai laboratory animal center and housed in BSL-2 (Institute Pasteur of Shanghai) or BSL-3 (Fudan University) facilities. All animal experiment protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Institute Pasteur of Shanghai.
Adenovirus vector.
The construction of fiber-modified AdV vector was adopted from an optimized isothermal assembly protocol (35, 36). Specifically, the AdC68 molecular clone was modified by inserting two PmeI restriction sites into the HI loop (fiber positions 1161 to 1162 and 1170 to 1171) for linearization. The three tandem M2e sequences (Fig. 1a) (H1-H5-H7 or H1-H7-H5) were synthesized by Genscript (Genscript Corp., Nanjing, China) and amplified by PCR with the addition of homogenous sequences (5′ end, CCCTCAATGGTACTGGGCCC; 3′ end, ACATATTCAATGTCATTTTC) at both ends. The PmeI-linearized AdC68 backbone and amplified M2e sequences were ligated via isothermal assembly to generate HI loop-modified AdC68 clones, namely, pAdC68-F3M2e(H1-H5-H7) and pAdC68-F3M2e(H1-H7-H5) (Fig. 1b). The assembly reaction volume was 20 μl and comprised 10 μl Gibson Assembly master mix (New England BioLabs, Beverly, MA), 50 ng of both fragments, and sterilized distilled water. Mixtures were incubated at 50°C for 60 min. The molecular clones were subsequently verified by sequencing. To recover recombinant adenoviruses, pAdC68-F3M2e(H1-H5-H7) or pAdC68-F3M2e(H1-H7-H5) clones were transfected into HEK293 cells with X-treme Gene HP DNA transfection reagent (Roche, Indianapolis, IN). After formation of plaques, supernatant was collected and further expanded in HEK293 cells. Adenoviruses were purified by cesium chloride density gradient centrifugation, and titers were determined by plaque-forming assay. The control AdV, AdC68-empty, was generated as described above.
Real-time PCR.
The M2e RNA expression was determined by RT-PCR. Total RNA was extracted from HEK293 cells by the use of TRIzol reagent (Invitrogen, Carlsbad, CA) at 12 or 24 h post-AdV infection and was reverse transcribed with a Moloney murine leukemia virus (M-MLV) reverse transcriptase kit (Promega, San Luis Obispo, CA), as previously described (6). The M2e expression level in cDNA was further quantified in triplicate using a 7900HT real-time PCR system (Applied Biosystems, Foster City, CA) and SYBR green I master mix (Roche Diagnostics, Mannheim, Germany) with the following primers: M2e forward, 5′-TCGGTGCAATGGCTCTTCAG-3′; M2e reverse, 5′-TAGCCCCAAATGTTGCTCCAA-3′. Measurements were normalized to β-actin control measurements and compared with the AdC68-F3M2e(H1-H7-H5) reference data. All data were analyzed using 7900HT System SDS software, version 2.4 (Applied Biosystems, Foster City, CA). Experiments were repeated independently three times.
Western blotting.
To evaluate the effect of M2e insertion on fiber protein structure, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with purified AdVs under both nonreducing and reducing conditions. Specifically, purified AdC68-F3M2e(H1-H5-H7), AdC68-F3M2e(H1-H7-H5), and AdC68-empty were lysed without 2-mercaptoethanoland mixed with native gel sample loading buffer (Biyuntian company, Shanghai, China) for nonreducing SDS-PAGE, as described previously (37). As for reduction of samples, AdVs were first treated with radioimmunoprecipitation assay (RIPA) buffer and SDS-PAGE sample loading buffer (Biyuntian) and were subsequently subjected to heat denaturation. Treated samples were analyzed using 10% SDS-PAGE. After transfer, the membrane was blocked using 5% nonfat milk. Protein expression was detected by incubating the membrane with anti-M2e monoclonal antibody (14C2; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight followed by horseradish peroxidase (HRP)-conjugated IgG secondary antibody and ECL (enhanced chemiluminescence) reagent (Sigma-Aldrich, St. Louis, MO).
In vitro expression of M2e was determined by measurement of titers by Western blotting in HEK293 cells. Cells infected with AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) (105 PFU, 5 × 105 PFU, or 104 PFU per well) or with AdC68-empty (105 PFU per well) were harvested and lysed by RIPA 24 h later as described above. Expression levels were determined by 14C2 analysis. β-Actin (Sigma) served as a normalization control for sample loading. Experiments were repeated independently three times.
ELISA.
The existence of M2e on the surface of recombinant AdVs was assessed using enzyme-linked immunosorbent assay (ELISA). AdC68-F3M2e(H1-H5-H7), AdC68-F3M2e(H1-H7-H5), and AdC68-empty (5 × 104 PFU each) were each applied to a microplate. 14C2 that had been serially diluted 2-fold (1:100 to 1:12,800) served as the primary antibody. Plates were incubated with HRP-conjugated IgG (Sigma) and TMB (3,3′,5,5′-tetramethylbenzidine) substrate (Thermo Fisher Scientific Inc., Waltham, MA). The absorbance at 450 nm was determined using a Varioskan Flash microplate reader (Thermo).
Endpoint titers of systemic antibody responses of immunized mice were determined by M2e peptide-based ELISA. In brief, a 96-well plate was coated with 100 ng/well of H1, H5, or H7 M2e peptide synthesized by Genscript. The peptide sequences are displayed in Fig. 1a. Mouse sera were 2-fold serially diluted from 1:100, added to each well, and incubated at 37°C for 2 h followed by addition of HRP-conjugated IgG (Sigma), IgG1, or IgG2a (Southern Biotechnology Associates, Birmingham, AL). Assays of each sample were repeated in triplicate. The antibody endpoint titer was determined as the reciprocal of the highest serum dilution whose absorbance was 0.1 optical density (OD) units above those of both controls (absorbance of AdC68-empty- or phosphate-buffered saline-treated samples). The experiments described above were repeated independently three times.
Vaccination and challenge.
Groups of mice were immunized intramuscularly twice with AdC68-F3M2e(H1-H5-H7) or AdC68-F3M2e(H1-H7-H5) using a 2-week interval. Mice injected with AdC68-empty or phosphate-buffered saline (PBS) served as controls. To assess systemic antibody responses, serum samples were collected at week 2 and week 4. Two weeks after the boost, mice were anesthetized and challenged intranasally with 5 LD50 of the indicated influenza virus diluted in 30 μl PBS. Body weight and survival rates were determined daily for 14 days postinfection (d.p.i.). Mice that lost over 25% of their initial weight were euthanized. The survival experiments were repeated twice.
To compare the immunogenicity levels of different M2e peptides, groups of mice (n = 6) were immunized i.p. with H1 or H5 M2e peptides (100 μg per dose) in the presence of Freund's complete adjuvant and boosted with Freund's incomplete adjuvant using a 2-week interval. Mice injected with adjuvant alone served as controls. Mouse sera were collected 2 weeks after prime-boost and tested for M2e-specific antibody titers by ELISA.
Passive immunization.
Sera were collected 14 days postboost from BALB/c mice immunized following the aforementioned protocols. Groups of naive BALB/c mice [AdC68-F3M2e(H1-H5-H7) (n = 5), AdC68-F3M2e(H1-H7-H5) (n = 5), AdC68-empty (n = 11), and PBS (n = 12)] were intraperitoneally (i.p.) injected with 500 μl pooled serum 1 day prior to PR8 challenge (5 LD50) and observed as described above.
Statistical analyses.
Statistical analyses were conducted with SPSS 16.0 (Chicago, IL). Differences in fold RNA expression levels and antibody responses were analyzed by one-way analysis of variance (ANOVA) between groups. The chi-square test was applied to compare survival rates. Differences were considered statistically significant when P values were <0.05.
ACKNOWLEDGMENTS
This project was supported by grants from the National Natural Science Foundation of China (31370929), the “China 863 program” (2014AA021003), the “Knowledge Innovation Program,” and the “100 Talent Program” from the Chinese Academy of Sciences.
REFERENCES
- 1.Thompson WW, Comanor L, Shay DK. 2006. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis 194:S82–S91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- 2.Pica N, Palese P. 2013. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med 64:189–202. doi: 10.1146/annurev-med-120611-145115. [DOI] [PubMed] [Google Scholar]
- 3.Tompkins SM, Lo CY, Tumpey TM, Epstein SL. 2004. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci U S A 101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 5.CDC. 2 March 2015. CDC presents updated estimates of flu vaccine effectiveness for the 2014–2015 season. CDC, Atlanta, GA: http://www.cdc.gov/flu/news/updated-vaccine-effectiveness-2014-15.htm. [Google Scholar]
- 6.Zhang H, Tang X, Zhu C, Song Y, Yin J, Xu J, Ertl HC, Zhou D. 23 April 2015. Adenovirus-mediated artificial MicroRNAs targeting matrix or nucleoprotein genes protect mice against lethal influenza virus challenge. Gene Ther doi: 10.1038/gt.2015.31. [DOI] [PubMed] [Google Scholar]
- 7.Liu WL, Zou P, Ding J, Lu Y, Chen YH. 2005. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect 7:171–177. doi: 10.1016/j.micinf.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahimi SM, Tebianian M. 2011. Influenza A viruses: why focusing on M2e-based universal vaccines. Virus Genes 42:1–8. doi: 10.1007/s11262-010-0547-7. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Zhang M, Mozdzanowska K, Zharikova D, Hoff H, Wunner W, Couch RB, Gerhard W. 2006. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol J 3:102. doi: 10.1186/1743-422X-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Peng Z, Liu Z, Lu Y, Ding J, Chen YH. 2004. High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine 23:366–371. doi: 10.1016/j.vaccine.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Fan JA, Liang XP, Horton MS, Perry HC, Citron MP, Heidecker GJ, Fu TM, Joyce J, Przysiecki CT, Keller PM, Garsky VM, Ionescu R, Rippeon Y, Shi L, Chastain MA, Condra JH, Davies ME, Liao J, Emini EA, Shiver JW. 2004. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine 22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. 2008. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur J Immunol 38:114–126. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- 13.Eliasson DG, El Bakkouri K, Schon K, Ramne A, Festjens E, Lowenadler B, Fiers W, Saelens X, Lycke N. 2008. CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine. Vaccine 26:1243–1252. doi: 10.1016/j.vaccine.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, Hogan RJ, Wu Z, Benton KA, Tumpey TM, Epstein SL. 2007. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis 13:426–435. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, Adler-Moore JP, Fujii G. 2006. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine 24:5158–5168. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. 2011. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A 108:757–761. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause A, Joh JH, Hackett NR, Roelvink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG, Worgall S. 2006. Epitopes expressed in different adenovirus capsid proteins induce different levels of epitope-specific immunity. J Virol 80:5523–5530. doi: 10.1128/JVI.02667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou D, Wu TL, Lasaro MO, Latimer BP, Parzych EM, Bian A, Li Y, Li H, Erikson J, Xiang Z, Ertl HC. 2010. A universal influenza A vaccine based on adenovirus expressing matrix-2 ectodomain and nucleoprotein protects mice from lethal challenge. Mol Ther 18:2182–2189. doi: 10.1038/mt.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, Wu TL, Emmer KL, Kurupati R, Tuyishime S, Li Y, Giles-Davis W, Zhou X, Xiang Z, Liu Q, Ratcliffe SJ, Ertl HCJ. 2013. Hexon-modified recombinant E1-deleted adenovirus vectors as dual specificity vaccine carriers for influenza virus. Mol Ther 21:696–706. doi: 10.1038/mt.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou DM, Zhou XY, Bian A, Li H, Chen H, Small JC, Li Y, Giles-Davis W, Xiang ZQ, Ertl HCJ. 2010. An efficient method of directly cloning chimpanzee adenovirus as a vaccine vector. Nat Protoc 5:1775–1785. doi: 10.1038/nprot.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kritz AB, Nicol CG, Dishart KL, Nelson R, Holbeck S, Von Seggern DJ, Work LM, Mcvey JH, Nicklin SA, Baker AH. 2007. Adenovirus 5 fibers mutated at the putative HSPG-binding site show restricted retargeting with targeting peptides in the HI loop. Mol Ther 15:741–749. doi: 10.1038/sj.mt.6300094. [DOI] [PubMed] [Google Scholar]
- 22.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 23.Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. 1999. Human infection with influenza H9N2. Lancet 354:916–917. doi: 10.1016/S0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 24.Zebedee SL, Lamb RA. 1988. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol 62:2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treanor JJ, Tierney EL, Zebedee SL, Lamb RA, Murphy BR. 1990. Passively transferred monoclonal antibody to the M2 protein inhibits influenza A virus replication in mice. J Virol 64:1375–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos EL, Mitcham JL, Koller TD, Bonavia A, Usner DW, Balaratnam G, Fredlund P, Swiderek KM. 2015. Efficacy and safety of treatment with an anti-m2e monoclonal antibody in experimental human influenza. J Infect Dis 211:1038–1044. doi: 10.1093/infdis/jiu539. [DOI] [PubMed] [Google Scholar]
- 27.De Filette M, Ramne A, Birkett A, Lycke N, Lowenadler B, Min Jou W, Saelens X, Fiers W. 2006. The universal influenza vaccine M2e-HBc administered intranasally in combination with the adjuvant CTA1-DD provides complete protection. Vaccine 24:544–551. doi: 10.1016/j.vaccine.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 28.Coughlan L, Mullarkey C, Gilbert S. 2015. Adenoviral vectors as novel vaccines for influenza. J Pharm Pharmacol 67:382–399. doi: 10.1111/jphp.12350. [DOI] [PubMed] [Google Scholar]
- 29.Vemula SV, Mittal SK. 2010. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther 10:1469–1487. doi: 10.1517/14712598.2010.519332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma A, Krause A, Xu YQ, Sung BI, Wu W, Worgall S. 2013. Adenovirus-based vaccine with epitopes incorporated in novel fiber sites to induce protective immunity against Pseudomonas aeruginosa. PLoS One 8:e56996. doi: 10.1371/journal.pone.0056996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koizumi N, Mizuguchi H, Utoguchi N, Watanabe Y, Hayakawa T. 2003. Generation of fiber-modified adenovirus vectors containing heterologous peptides in both the HI loop and C terminus of the fiber knob. J Gene Med 5:267–276. doi: 10.1002/jgm.348. [DOI] [PubMed] [Google Scholar]
- 32.Kim M-C, Song J-M, Eunju O, Kwon Y-M, Lee Y-J, Compans RW, Kang S-M. 2013. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther 21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazarski CA, Chaves FA, Jenks SA, Wu SH, Richards KA, Weaver JM, Sant AJ. 2005. The kinetic stability of MHC class II: peptide complexes is a key parameter that dictates immunodominance. Immunity 23:29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Batista FD, Neuberger MS. 1998. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8:751–759. doi: 10.1016/S1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 35.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:U343–U341. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Chi Y, Tang X, Ertl HC, Zhou D. 2016. Rapid, efficient, and modular generation of adenoviral vectors via isothermal assembly. Curr Protoc Mol Biol 16.26.11–16.26.18. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Wang W, Yang L, Ren H, Kimata JT, Zhou P. 2013. Trimeric glycosylphosphatidylinositol-anchored HCDR3 of broadly neutralizing antibody PG16 is a potent HIV-1 entry inhibitor. J Virol 87:1899–1905. doi: 10.1128/JVI.01038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]