ABSTRACT
Plasmacytoid dendritic cells (pDC) play a central role in the antiviral immune response, both in the innate response and in shaping the adaptive response, mainly because of their ability to produce massive amounts of type I interferon (TI-IFN). Here, we report that cells infected with the live attenuated Bartha vaccine strain of porcine alphaherpesvirus pseudorabies virus (PRV) trigger a dramatically increased TI-IFN response by porcine primary pDC compared to cells infected with wild-type PRV strains (Becker and Kaplan). Since Bartha is one of the relatively few examples of a highly successful alphaherpesvirus vaccine, identification of factors that may contribute to its efficacy may provide insights for the rational design of other alphaherpesvirus vaccines. The Bartha vaccine genome displays several mutations compared to the genome of wild-type PRV strains, including a large deletion in the unique short (US) region, encompassing the glycoprotein E (gE), gI, US9, and US2 genes. Using recombinant PRV Becker strains harboring the entire Bartha US deletion or single mutations in the four affected US genes, we demonstrate that the absence of the viral gE/gI complex contributes to the observed increased IFN-α response. Furthermore, we show that the absence of gE leads to an enhanced extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation in pDC, which correlates with a higher TI-IFN production by pDC. In conclusion, the PRV Bartha vaccine strain triggers strongly increased TI-IFN production by porcine pDC. Our data further indicate that the gE/gI glycoprotein complex suppresses TI-IFN production by pDC, which represents the first alphaherpesvirus factor that suppresses pDC activity.
IMPORTANCE Several alphaherpesviruses, including herpes simpex virus, still lack effective vaccines. However, the highly successful Bartha vaccine has contributed substantially to eradication of the porcine alphaherpesvirus pseudorabies virus (PRV) in several countries. The impact of Bartha on the immune response is still poorly understood. Type I interferon (TI-IFN)-producing plasmacytoid dendritic cells (pDC) may play an important role in vaccine development. Here, we show that Bartha elicits a dramatically increased type I interferon (TI-IFN) response in primary porcine pDC compared to wild-type strains. In addition, we found that the gE/gI complex, which is absent in Bartha, inhibits the pDC TI-IFN response. This is the first description of an immune cell type that is differentially affected by Bartha versus wild-type PRV and is the first report describing an alphaherpesvirus protein that inhibits the TI-IFN response by pDC. These data may therefore contribute to the rational design of other alphaherpesvirus vaccines.
KEYWORDS: Bartha, ERK1/2, gE, herpes, immune evasion, interferon, pDC, plasmacytoid dendritic cell, pseudorabies
INTRODUCTION
Pseudorabies virus (PRV) is a member of the Alphaherpesvirinae, which can cause severe respiratory, neurological, and reproductive disorders in pigs. PRV is also an excellent model to study general aspects of alphaherpesvirus biology (1).
Bartha is an attenuated PRV vaccine strain, derived from a field strain that was isolated in Hungary and that was attenuated via multiple passages on cultured chicken cells and embryo's (2), resulting in several mutations (3). The most striking feature in the Bartha genome is a 3-kb deletion in the unique short (US) region, resulting in the partial loss of US2, most of US7 (gI), and complete deletion of US8 (gE) and US9. Although the Bartha vaccine has been highly successful and is well characterized with regard to its reduced neuropathogenesis (1, 3), relatively little is known on the effect of Bartha on the immune system. A better understanding of the unique aspects of the Bartha vaccine that may contribute to its efficacy can provide valuable insights for important alphaherpesviruses that still lack effective vaccines, including herpes simplex virus (HSV) (4–6).
Plasmacytoid dendritic cells (pDC) are a small subpopulation of dendritic cells constituting approximately 0.1% of the total porcine peripheral blood mononuclear cells (PBMC) and are specialized in producing large amounts of type I interferon (TI-IFN). Indeed, pDC produce up to 1,000-fold more TI-IFN than any other cell type (7) and therefore play a crucial role in controlling viral infections. TI-IFN interferes at several stages with the viral replication cycle through the JAK/STAT signaling pathway and thereby limits further spread of the virus through the body. In addition, TI-IFN activates several components of the immune system, including conventional dendritic cells (cDC), NK cells, T cells, and B cells (8). Due to their direct and indirect antiviral effects and their ability to steer the immune response toward an antiviral modus, which all mainly rely on their TI-IFN-producing capacities, interest is growing in the potential role of targeting pDC for vaccination against various (viral) infectious diseases (9–11).
Therefore, in the present report, we investigated the potential of PRV Bartha-infected cells to stimulate TI-IFN production by porcine pDC compared to cells infected with virulent PRV strains. Interestingly, we found that Bartha elicited a dramatically increased TI-IFN response by pDC. Importantly, the absence of the glycoprotein gE/gI complex in the genome of Bartha partly explains this increased TI-IFN response by pDC. In addition, we show that gE modulates extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation in pDC and that this correlates with its suppressive effect on TI-IFN production by pDC.
RESULTS
Efficiency of pDC enrichment and purification.
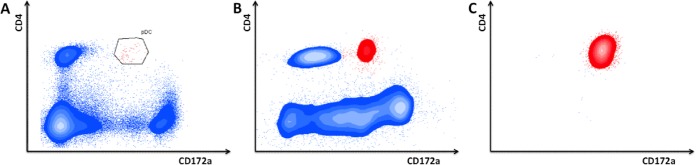
Porcine plasmacytoid dendritic cells (pDC) represent 0.1 to 0.2% of the total PBMC population (Fig. 1A) (12) and are characterized by their low CD172a and high CD4 surface expression. Studies addressing porcine pDC activity typically make use of CD14-depleted and/or CD172a-selected pDC-enriched PBMC populations (7, 12–14). In line with this, we found that depletion of monocytes (CD14+ fraction) followed by enrichment of the myeloid cell population (CD172a+ fraction) resulted in a 20- to 30-fold increase in pDC, leading to a population with 2 to 6% pDC (Fig. 1B). The use of pDC-enriched populations to analyze the TI-IFN-producing capacity of pDC is widely established. However, to make sure that the observed results were specifically due to differences in activity of pDC, we have validated some of our results with fluorescence-activated cell sorter (FACS) purity-sorted pDC. These were derived from the pDC-enriched population based on their CD4high CD172adim expression, resulting in >96% pure pDC populations (Fig. 1C).
FIG 1.
Enrichment and purification of porcine plasmacytoid dendritic cells (pDC). Porcine PBMC were stained for CD4 and CD172a and analyzed by flow cytometry. Porcine pDC (red) are characterized by their high CD4 and low CD172a expression. Freshly isolated PBMC contain 0.1 to 0.2% pDC (A). Depletion of monocytes (CD14+) followed by enrichment of myeloid cells (CD172a+) by MACS leads to a 20- to 30-fold enrichment in pDC (2 to 5% pDC) (B). Subsequent sorting by FACS leads to highly pure pDC populations (>96%) (C).
PRV Bartha triggers strongly increased IFN production by pDC compared to wild-type (WT) PRV strains.
The PRV vaccine strain and two WT PRV strains were tested for their capacity to activate pDC.
Figure 2A shows that whereas mock-infected swine testicle (ST) cells did not trigger detectable IFN-α responses by pDC (below the limit of detection of 10 U/ml IFN-α), ST cells infected with WT PRV strains Becker (588 ± 154 U/ml IFN-α) and Kaplan (326 ± 9 U/ml IFN-α) induced substantial IFN-α production by pDC. Interestingly, however, Bartha-infected cells elicited a much stronger IFN-α response by pDC (3,480 ± 602 U/ml IFN-α) compared to the WT PRV strains (P < 10−4). Repeating the same experiments without pDC always resulted in IFN-α levels that were close to or below the detection limit (data not shown), indicating that the infected ST cells produce very little IFN-α and that the observed IFN-α responses were derived from the pDC. In addition, the observed differences between Bartha and wild-type strains were not due to differences in viral replication between the virus strains, since Western blot analysis revealed no obvious differences in viral protein expression in infected ST cells (Fig. 2B).
FIG 2.
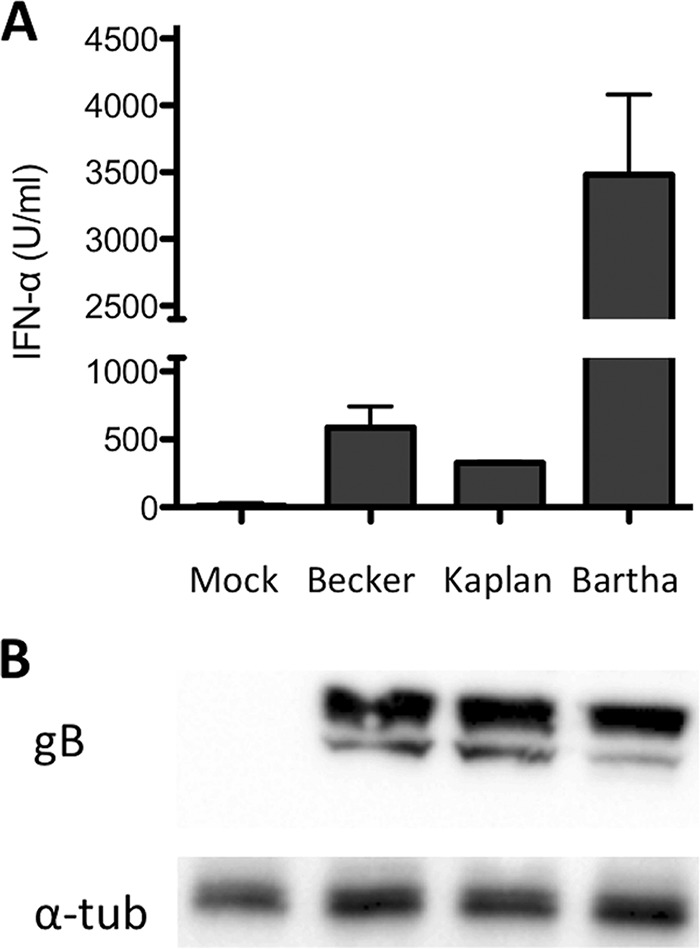
The PRV vaccine strain Bartha triggers increased IFN-α production by pDC. ST cells were infected with virulent (PRV Becker or Kaplan) or attenuated (Bartha) strains of PRV and subsequently (at 2 hpi) coincubated with freshly isolated enriched pDC populations. Supernatants were collected at 24 hpi, and concentrations of IFN-α were determined by ELISA. The data shown represent the average IFN-α production ± standard error of the mean (SEM) obtained from 4 different pigs. PRV Bartha elicits a 5- to 10-fold-higher IFN production by pDC compared to the wild-type PRV strains (A). Lysates of infected ST cells were harvested at 24 hpi. Western blot analysis is shown using antibodies against viral gB (infection control [100 kDa]) and α-tubulin (loading control [57 kDa]) (B).
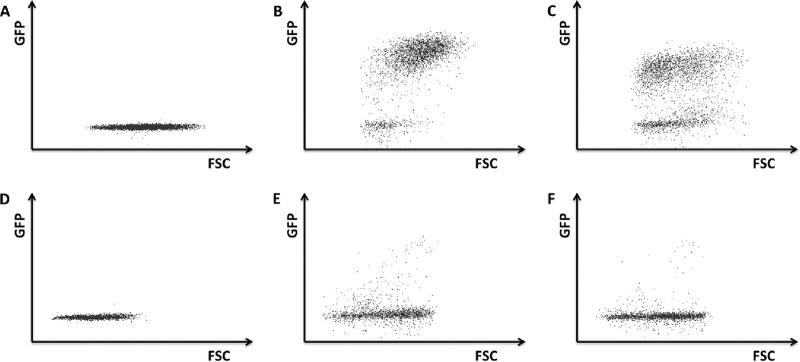
To determine whether pDC are susceptible to PRV infection, Becker and Bartha PRV strains (PRV 151 and PRV 152) that express green fluorescent protein (GFP) under the control of a constitutive cytomegalovirus (CMV) promoter were used (15, 16). The experimental setup for these assays was similar to the experimental setup to determine pDC-mediated IFN-α responses. In brief, ST cells were infected with PRV 151 or 152, the inoculum was removed at 2 h postinfection (hpi), and pDC were added. At 24 hpi, pDC were collected and analyzed by flow cytometry for GFP expression. As a positive control, monocytes, which have been shown before to be susceptible to PRV infection (17), were used in the same experimental setup. Flow cytometry results are shown in Fig. 3. As a negative control, pDC and monocytes were coincubated with noninfected ST cells, which as expected, did not trigger a GFP signal (Fig 3A and D). Furthermore as expected, monocytes incubated with PRV 151- or PRV 152-infected ST cells did show GFP expression (3B and C). However, pDC incubated with Becker- or Bartha-infected ST cells did not show obvious GFP expression (Fig. 3E and F).
FIG 3.
pDC are not obviously permissive to PRV infection. ST cells were either mock infected (A and D) or infected with PRV Becker (B and E) or PRV Bartha (C and F) strains that express GFP. At 2 hpi, the inoculum was removed, and freshly isolated monocytes (A to C) or pDC (D to F) were added. At 24 hpi, GFP expression in myeloid cells was analyzed by flow cytometry.
Moreover, we found that PRV strains lacking viral proteins that are essential for host cell infection (ΔgB, ΔgD, ΔgH, and ΔgL PRV) also induce adequate IFN-α responses, further supporting the statement that infection is not required for pDC-mediated IFN-α production in response to PRV (data not shown).
The absence of gE/gI contributes to the hyperactivating effect of PRV Bartha on pDC.
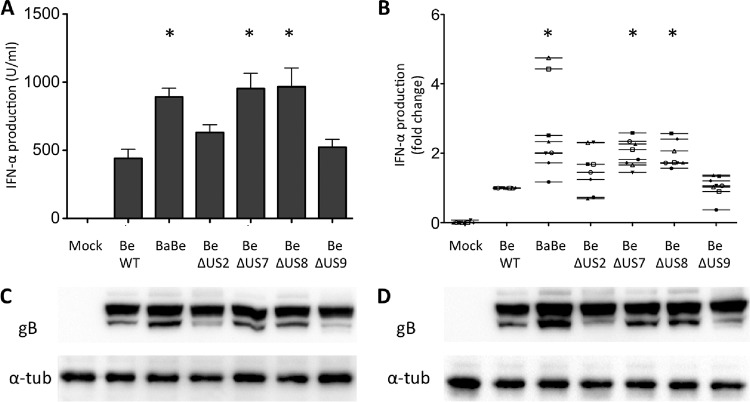
Next, we tested whether the large genomic deletion in the US region of the Bartha vaccine strain is involved in the massively increased IFN-α response by pDC. To this end, ST cells were infected with Becker or Becker mutant strains with mutations that correspond to parts of the deletion that affects 4 genes in the US region. Becker-derived strain BaBe contains the same deletion in the US region as Bartha (affecting US2, US7 [gI], US8 [gE], and US9), while the other viruses each lack expression of one of the four respective proteins. Figure 4A displays the IFN-α production observed for these different strains and shows that the Bartha deletion region indeed significantly influences pDC activity (strain BaBe triggers a statistically significant 2- to 4-fold increase in IFN-α production compared to WT Becker), although this strain did not induce IFN-levels comparable to the Bartha vaccine strain (Fig. 2A). Of the 4 genes affected by the US deletion, individual deletions in US7 (gI) and US8 (gE) resulted in a significantly increased IFN-α production (analysis of variance [ANOVA] with Tukey's post hoc comparison; P < 0.05) that was comparable to the increase observed with PRV BaBe, while deletions in US2 and US9 did not significantly affect IFN-α levels. To increase the biological significance of these data, the experiments with the different isogenic strains were repeated with pDC-enriched populations derived from 8 different pigs. Performing experiments in 8 biologically independent pigs invariably leads to natural pig-to-pig variability in terms of TI-IFN production by pDC. This has been shown before by other groups working with porcine pDC (18, 19). To reduce this variability, IFN-α levels were set relative to the IFN-α response observed with wild-type PRV Becker (set to 1.00). The corresponding data are shown in Fig. 4B and confirm the data from Fig. 4A. Western blot analysis revealed no obvious differences in viral protein expression in infected ST cells between the isogenic mutants (Fig. 4C and D).
FIG 4.
Effect of the Bartha US deletion on IFN-α production by pDC. At 2 hpi, ST cells infected with different isogenic PRV mutants were coincubated for 22 h with MACS-enriched pDC, and IFN-α levels in the supernatant were determined by ELISA. BaBe is a Becker strain that lacks the same 3-kb US region that is absent in PRV Bartha. The other strains lack expression of any of the 4 individual genes affected by the US deletion in Bartha (US2, US7, US8, and US9). Bars represent the means ± SEM from triplicate independent assays from 1 pig. Significant differences in IFN-α responses by pDC compared to the WT Becker strain are indicated (*) (A). Using the same experimental setup as in panel A, data were generated from 8 different pigs. The data shown represent the mean ± SEM fold change in IFN-α production by pDC for each strain relatively to the IFN-α production observed using wild-type Be (set to 1.0). Significant differences in IFN-α responses by pDC compared to the WT Becker strain are indicated (*) (B). Lysates of infected ST cells were harvested at 12 hpi (C) or 24 hpi (D) and subjected to Western blot analysis for viral protein gB (infection control [100 kDa]) and α-tubulin (loading control [57 kDa]).
In conclusion, these data show that the US deletion contributes partially to the hyperactivating effect of PRV Bartha on pDC and that this can be attributed to the deletion of the gE/gI complex.
The TI-IFN-inducing effect of Bartha and ΔgE PRV is also observed in purified pDC populations.
The enriched pDC populations still contain a large amount of non-pDC (among others, T and B cells). Although control experiments showed that none of these other populations triggered substantial TI-IFN responses upon contact with PRV/Bartha-infected cells (data not shown), to ensure that the observed effects were specifically due to differences in pDC activity, we repeated some experiments using FACS-sorted pDC. For these experiments, equal numbers of pDC (1,840 cells/condition) were added to the infected ST cells as the numbers of pDC present in the enriched magnetically activated cell sorting (MACS)-derived population (80,000 cells containing 2.3% pDC). TI-IFN data obtained using purified pDC populations were strikingly similar to the data obtained with pDC-enriched populations (Fig. 5), which shows that the observed TI-IFN responses are specifically derived from the pDC population and are a consequence of direct activation of pDC without the need for other leukocytes and/or the cytokines that they produce.
FIG 5.
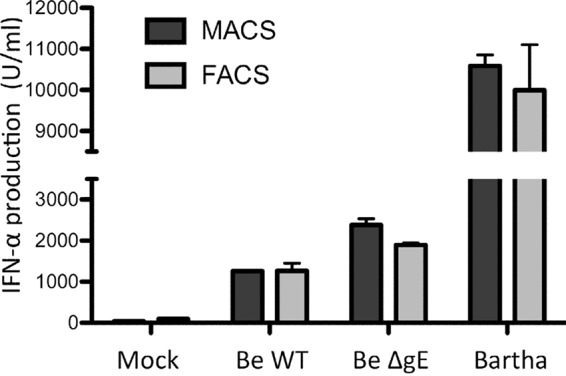
The interferon production profiles of enriched and purified pDC populations are highly similar. ST cells were infected with Becker WT, Becker ΔgE, or the vaccine strain Bartha and subsequently coincubated (at 2 hpi) with freshly isolated enriched (MACS) or purity-sorted (FACS) pDC populations. Supernatant was collected at 24 hpi, and levels of IFN-α were determined by ELISA. Bars represent the mean ± standard deviation (SD) of duplicates from 1 pig.
gE modulates ERK1/2 activity in pDC, which correlates with its ability to suppress TI-IFN production by pDC.
The above data indicate that expression of gE in PRV-infected cells suppresses the TI-IFN response by pDC. We found earlier that PRV gE modulates phosphorylation of the ERK1/2 signaling molecule in different cell types, including primary isolated porcine T cells, Jurkat cells, and epithelial PK-15 cells (20, 21). Since in human pDC, increased ERK1/2 activation has been linked with increased TI-IFN production (22), we investigated whether gE modulates ERK1/2 phosphorylation in pDC and whether this correlates with its ability to suppress TI-IFN production by these cells.
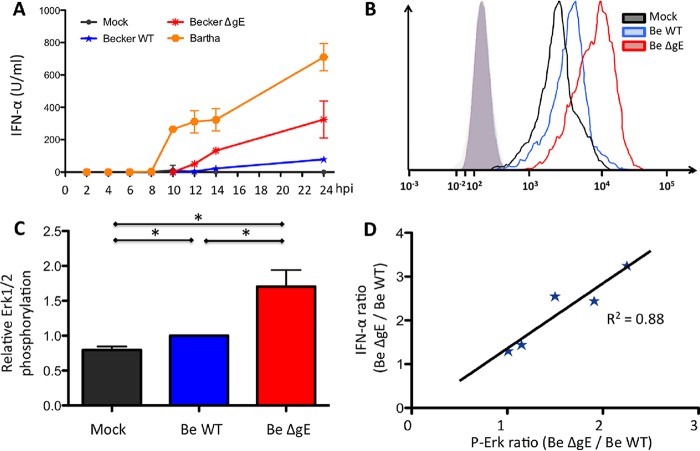
To determine an accurate time point for analysis of ERK1/2 phosphorylation, the kinetics of TI-IFN production by pDC upon contact with PRV-infected ST cells were determined. To this end, enriched pDC populations were cocultured with infected ST cells (mock infected or infected with Bartha, Becker, or ΔgE Becker) as described before, and supernatant was collected at different time points. IFN-α levels were determined by enzyme-linked immunosorbent assay (ELISA) and are shown in Fig. 6A. In line with our previous results, ST cells infected with PRV Becker triggered substantial TI-IFN by pDC, but ΔgE Becker and particularly Bartha induced much stronger TI-IFN responses. A clear difference in TI-IFN production between wild-type and ΔgE PRV could be observed at 14 hpi. Therefore, this time point was chosen to assess putative differences in ERK1/2 phosphorylation in pDC via flow cytometry. Figures 6B and C show that pDC showed a statistically significant increase in ERK1/2 phosphorylation upon contact with ST cells that were infected with ΔgE Becker compared to ST cells that were infected with wild-type Becker. In addition, results obtained using purity-sorted pDC populations of different pigs showed that the increase in ERK1/2 phosphorylation in pDC using ΔgE Becker compared to wild-type PRV correlates very well with the increase in TI-IFN production by pDC (Fig. 6D [R2 = 0.88]). This was statistically confirmed by Spearman's rank order test (P < 0.05). These data show that gE expression in infected cells modulates ERK1/2 phosphorylation in pDC and that this modulation correlates with the ability of gE to suppress TI-IFN production in these cells.
FIG 6.
PRV gE suppresses ERK1/2 phosphorylation in pDC and increased ERK1/2 phosphorylation in pDC by ΔgE PRV correlates with increased IFN-α production. Enriched (MACS) pDC populations were coincubated with ST cells that were mock infected or infected with PRV (wild-type PRV, ΔgE PRV, or Bartha). Supernatants were collected at regular time points, and IFN-α levels were determined by ELISA (A). ST cells were either mock infected, infected with the Becker WT (Be WT) or Becker ΔgE (Be ΔgE) strain and subsequently coincubated (2 hpi) with freshly purity-sorted pDC populations. At 14 hpi, pDC were harvested, fixed, permeabilized, and stained for ERK1/2 phosphorylation (p-Erk). An overlay of the fluorescence intensity (mean fluorescence intensity [MFI]) of the different samples (open histograms) and isotype controls (shaded histogram) of one representative pig shows enhanced ERK1/2 phosphorylation in pDC stimulated by Becker ΔgE-infected cells, compared to Becker WT-infected cells (B). Graphs show MFI values of ERK1/2 phosphorylation relative to the Becker WT (set to 1.0). Data represent the mean ± SEM from 4 biologically independent repeats (C). The ratio of IFN-α production observed using ΔgE PRV versus Becker WT was plotted against the ratio of ERK1/2 phosphorylation observed using ΔgE PRV versus the Becker WT for 5 different pigs, showing correlation between ERK1/2 phosphorylation in and IFN-α production by pDC (D).
DISCUSSION
In the present study, we found that cells infected with the attenuated PRV vaccine strain Bartha trigger a much higher TI-IFN production by primary porcine pDC compared to cells infected with the wild-type PRV strains Becker and Kaplan. Using this finding as a lead, we discovered that the PRV glycoprotein gE/gI complex displays a previously uncharacterized pDC-suppressing activity.
Although only low numbers of pDC surveil mucosal epithelia in noninfected hosts, upon infection, many pDC may migrate from the bloodstream toward the infection site (23–26). There, pDC may get into contact with infected epithelial cells and fibroblasts (27, 28) and stimulate other components of the immune system (8). The potential of the attenuated PRV Bartha vaccine strain to induce a strong TI-IFN production by pDC may contribute to the efficacy of this highly successful vaccine. Indeed, several reports point to the importance of pDC in vaccine development. For example, the efficacy of the live attenuated yellow fever vaccine 17D (YF-17D), one of the most effective vaccines ever generated, has been shown to rely heavily on its ability to activate dendritic cell subsets, including pDC (9). Furthermore, imiquimod (a Toll-like receptor 7 [TLR7] agonist) and CpG-rich DNA (TLR9 agonist), which both stimulate TI-IFN production by pDC, represent successful adjuvants in numerous vaccines toward different types of cancer (e.g., melanoma) and infectious disease (e.g., malaria, hepatitis B, influenza, and anthrax) (29). In line with this, depletion of pDC in mice resulted in a lowered activity of the adjuvant polyUs21, a TLR7 agonist (30). Of particular interest, CpG is being used in the development of promising new vaccines against HSV-2 (5). Our current findings may contribute to the effective priming of pDC during vaccination. Indeed, in addition to the development of adjuvants that trigger a strong pDC response, vaccine strains may be rationally designed to display impaired viral pDC-suppressing mechanisms.
The observation that the attenuated Bartha PRV strain triggers a much stronger TI-IFN response by pDC compared to WT PRV strains Kaplan and Becker indicates that wild-type PRV strains encode pDC evasion strategies that are mutated or absent in Bartha. Although the exact mechanism underlying these pDC evasion strategies remains to be investigated, we have discovered that the glycoprotein complex gE/gI, which is deleted in the Bartha genome, contributes to pDC evasion.
Our report is the first to identify an alphaherpesvirus protein that suppresses pDC activity. All tested alphaherpesviruses, including PRV (31), trigger TI-IFN production by pDC (24). Research has mainly focused on TLR9-dependent (and -independent) herpesvirus-mediated activation of pDC, but much less on potential pDC evasion (32). Nevertheless, one report indicates that human pDC may be inactivated by varicella-zoster virus (VZV), possibly by infection of the pDC (33). However, in line with reports on HSV and human pDC (34, 35), in our assays porcine pDC appear not to be susceptible to PRV infection (Fig. 3). Other viruses have been reported to inhibit pDC activation via different, but often poorly understood mechanisms, including human viruses such as HIV (36, 37), hepatitis C virus (HCV) (38), respiratory syncytial virus, and measles virus (39) and porcine viruses such as classical swine fever virus (14) and porcine reproductive and respiratory syndrome virus (PRRSV) (31). pDC contain several surface receptors to inhibit the production of TI-IFN, including PTPRS, NKp44, BDCA-2, and ILT7 (24, 40–42). The gp120 protein of HIV (BDCA-2), hemagglutinin of human influenza virus (NKp44), and glycoprotein E2 of HCV (BDCA-2) have been shown to interact directly with a pDC inhibitory receptor (36, 38, 43).
Our data suggest a mechanism for how the gE/gI protein complex affects the TI-IFN response by pDC. The gE/gI complex has been reported before to modulate signaling of the mitogen-activated protein kinase (MAPK) ERK1/2 in different cell types (20, 21). MAPK signaling, particularly ERK1/2 signaling, plays an important role in IFN-α production by human pDC (22, 38, 44, 45). We found that pDC exposed to cells infected with wild-type PRV showed reduced ERK1/2 phosphorylation compared to pDC exposed to cells infected with ΔgE PRV. These differences in ERK1/2 phosphorylation correlated with the observed differences in TI-IFN production and therefore indicate that ERK1/2 signaling also plays a role in TI-IFN responses in porcine pDC. Besides gE, the US2 tegument protein of PRV, which is also absent in Bartha, is also known to interact with the ERK signaling pathway (20, 21, 46, 47). Although ΔUS2 PRV did not trigger significantly higher pDC-mediated IFN-α production, results with this strain showed substantial pig variability (Fig. 4B). Hence, it may be worthwhile to investigate whether, under certain conditions, US2 may affect pDC activity toward PRV-infected cells: e.g., via modulation of cell surface presentation of viral proteins/viral particles (46).
Interestingly, in primary porcine T cells and in epithelial cells, PRV gE has been reported to increase ERK1/2 phosphorylation (20, 21), while our current data indicate that gE is associated with reduced ERK1/2 phosphorylation in pDC. This suggests that gE may lead to activation or suppression of the ERK1/2 signaling axis, depending on the cell type and perhaps other circumstances. Of possible interest in this context is that different inhibitory receptors on pDC fulfill activating functions on other cell types and vice versa. For example, NKp44, a potent activating receptor on NK cells, serves as an inhibitory receptor on pDC (48). Although speculative at this point, it will be interesting to investigate whether gE may interact with a particular (inhibitory) receptor on pDC to suppress TI-IFN production. It has been hypothesized before that the ectodomain of the gE/gI complex may bind a cellular receptor that influences viral virulence (49). Although the identity of such a putative receptor remains elusive, PRV gE/gI, like HSV gE/gI, has been reported to bind the Fc domain of IgG antibodies, indicating that it may interact with immunoglobulin-like protein domains (50, 51). In any way, direct contact between pDC and epithelial cells appears to be required to trigger the observed (differences in) pDC-mediated IFN-α production, as experiments that did not allow cell-cell contact (Transwell assays and the use of supernatant of infected ST cells) did not trigger substantial pDC-mediated IFN-α production (data not shown).
In conclusion, the present report describes that the highly successful attenuated PRV vaccine strain Bartha elicits a much more robust TI-IFN response in pDC than WT PRV strains in vitro. This reveals that WT PRV strains have evolved pDC-suppressing mechanisms that are absent from or mutated in Bartha. Our findings point to a role for the gE/gI glycoprotein complex in suppression of TI-IFN production by pDC. These data may therefore contribute to the rational design of improved vaccines against other alphaherpesviruses that lack effective vaccines, including HSV-1 and HSV-2.
MATERIALS AND METHODS
Antibodies and reagents.
In house monoclonal mouse antibodies against PRV gB (1C11) and PRV gD (13D12) were described earlier (52). Mouse anti-CD172a (clone 74-22-15 [53]) and anti-CD4 (clone 74-12-4 [53]) antibodies were a kind gift from A. Saalmüller (University of Vienna, Austria), and mouse anti-CD14 (MIL-2 [54]) antibodies were kindly donated by K. Haverson (Bristol University, United Kingdom). B. Charley (INRA, France) kindly provided mouse antibodies against porcine IFN-α (clones K9 and F17 [55]). Rabbit polyclonal antibodies against phospho-ERK (phospho-p44/42 MAPK antibody 9101) and horseradish peroxidase (HRP)-conjugated anti-α-tubulin antibodies (DM1A-HRP; AB40742) were obtained from Cell Signaling and Abcam, respectively. Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG, phycoerythrin (PE)-conjugated goat anti-mouse IgG, and goat anti-mouse IgG1, Alexa Fluor 647-conjugated, goat anti-mouse IgG2b, Alexa Fluor 488-conjugated goat anti-rabbit IgG, the mouse IgG1 and IgG2b isotype controls PE-conjugated streptavidin, and the LIVE/DEAD stain SytoxBlue were purchased from Life Technologies. HRP-conjugated goat anti-mouse IgG was obtained from Dako.
Recombinant porcine IFN-α was purchased from R&D Systems and streptavidin-HRP from Thermo Scientific. Type A CpG oligonucleotide D32 (13) was synthesized by Integrated DNA Technologies. The mouse anti-CD4 and anti-IFN-α K9 antibodies were biotinylated using EZ-Link sulfo-NHS-biotin (Life Technologies) according to the manufacturer's instructions. 3,3′,5,5′-Tetramethylbenzidine (TMB) one-component substrate was obtained from Bethyl Laboratories.
Cells and viruses.
Epithelial ST cells (56–58) were cultured in Earle's minimal essential medium with 10% fetal calf serum, 0.3 mg/ml glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.05 mg/ml gentamicin, and 1 mM sodium pyruvate (Life Technologies). pDC and monocytes were cultured in RPMI medium (Life Technologies), containing the same supplements plus 1 mM nonessential amino acids and 20 μM β-mercaptoethanol.
All viruses used in the present study have been described before. PRV Bartha is an attenuated vaccine strain, obtained by extensive passaging of an Aujeszky strain isolated in Hungary (2). PRV Becker is a 1967 field isolate with subsequent laboratory passage in cell culture (59). PRV BaBe is a Becker strain lacking the same genomic 3-kb deletion in the US region as in Bartha, resulting in the partial deletion of US2 and US7 (gI) and a complete absence of US8 (gE) and US9 (60). Becker ΔUS2, ΔgI, ΔgE, and ΔUS9 have also all been described before (60–62). PRV 151 and PRV 152 are Becker and Bartha strains, respectively, that contain the CMV-enhanced green fluorescent protein (EGFP) reporter gene cassette inserted into the gG locus of the viral genome (15, 16). PRV strain Becker, its isogenic mutant strains, and PRV 152 were kind gifts from L. Enquist (Princeton University, USA). PRV Kaplan (63) was a kind gift from T. Mettenleiter (Friedrich-Loeffler Institute, Germany). All viral stocks were grown and titers determined on monolayers of ST cells.
Enrichment and purification of pDC.
Blood was collected from 2- to 6-month-old pigs, kept at the Faculty of Veterinary Medicine (Ghent University; EC2013/62) as described before (64). PBMC were isolated from whole blood using Lymphoprep (1.077 g/liter; Axis-Shield) density gradient. Subsequently, red blood cells were lysed in Tris-buffered ammonium chloride. Next, PBMC were separated using a magnetic cell sorting (MACS) system (Miltenyi Biotech). pDC were further enriched as described with some modifications (12). First, PBMC were depleted of CD14+ cells, using anti-mouse IgG microbeads and an LS column (Miltenyi Biotec), followed by a positive selection for CD172a+ cells, using anti-mouse IgG1 microbeads and an LD column (Myltenyi Biotec). The obtained enriched population was used for type I IFN induction assays.
When specified, pDC were subsequently purity sorted. The enriched population was first incubated (20 min, 4°C) with anti-CD172a and biotinylated anti-CD4 antibodies (stock, 1 mg/ml; used at 1/300), washed three times, and incubated (20 min, 4°C) with AF647-conjugated goat anti-mouse IgG1 (used at 1/200) and PE-conjugated streptavidin (used at 1/500). After subsequent incubation with the LIVE/DEAD stain SytoxBlue (1/1,000), pDC were purity sorted based on CD4++ and CD172adim expression (18) using a BD FACS Aria III cell sorter (BD Biosciences).
Type I IFN Induction assay.
ST cells were inoculated with the respective viruses at a multiplicity of infection (MOI) of 10 in culture medium. After incubation for 2 h at 37°C, the inoculum was aspirated, and ST cells were washed three times to remove any cell-free virus. Next, freshly isolated pDC were added (enriched populations of 400,000 cells/ml and purity-sorted cell populations of 20,000 cells/ml, unless indicated otherwise). After 22 h of coincubation of pDC with the infected ST cells (at 24 h postinoculation [hpi]), cell-free supernatants were collected and stored at −80°C. As a positive control for interferon induction, pDC were incubated for 22 h in the presence of 10 μg/ml CpG D32 (13).
ELISA.
The amount of IFN-α secreted in the supernatant was measured by ELISA as described before with some modifications (65). In brief, ELISA microplates (Maxisorp plates; Thermo Scientific Nunc) were coated with mouse anti-porcine IFN-α F17 (5 5g/ml in 0.1 M NaHCO3) overnight at room temperature. The plates were then incubated for 1 h at 37 °C with blocking buffer (phosphate-buffered saline [PBS] containing 0.05% Tween 20 and 0.5% bovine serum albumin [Merck Millipore]). Samples and standard (recombinant porcine IFN-α) were diluted in blocking buffer, added to the plates, and incubated for 2.5 h at room temperature. Following incubation with biotinylated mouse anti-porcine IFN-α K9 (1.5 h, room temperature) and HRP-conjugated streptavidin (1 h, 37°C), TMB was added to the wells. When peroxidase activity was revealed, 1 M H2SO4 was added to stop the reaction, and absorption (450 nm) was measured by a SpectraFluor spectrophotometer (Tecan) and analyzed by DeltaSoft JV (DeltaSoft Inc.).
Western blotting.
ST cells were collected on ice, washed in TNE buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA [pH 6.8]), and lysed in TNE lysis buffer (TNE with 10% NP-40 [Roche] and protease inhibitor cocktail [Sigma-Aldrich]) for 1 h at 4°C as described before (66). Cell lysates were fractionated on a 10% polyacrylamide gel by SDS-PAGE and then transferred to a Hybond-P polyvinylidene difluoride (PVDF) membrane (GE Healthcare). After blotting, the membranes were blocked in blocking buffer (5% milk [Nestli]), 0.1% Tween 20 [Sigma-Aldrich], PBS) overnight at 4°C. Next, they were incubated with antibodies against PRV-specific proteins (mouse anti-gB; used at 1/100) and actin (DM1A-HRP; used at 1/5,000) for 1 h at room temperature. Following incubation with HRP-conjugated secondary antibodies (HRP-conjugated goat anti-mouse; used at 1/2,000) for 1 h at room temperature, blots were developed using chemiluminescence. All antibodies were diluted in blocking buffer.
Flow cytometry.
Purity-sorted pDC and ST cells were harvested at 14 hpi (12 h of cocultivation) and subsequently fixed and permeabilized using BD Cytofix/Cytoperm (BD Bioscience) according to the manufacturer's instructions. Next, cells were incubated with phospho-ERK antibodies (used at 1/1,000) at 4°C for 30 min, washed three times with BD Perm/Wash buffer (BD Bioscience), and incubated for 30 min at 4°C with Alexa Fluor 488-conjugated, goat anti-rabbit IgG antibodies (used at 1/200). Cells were measured using the BD FACS Aria III. For analysis, pDC were distinguished from ST cells based on their size (forward scatter [FSC]) and granularity (side scatter [SSC]), using the BD FACS Diva software.
Statistics.
Statistical analysis was performed using S-PLUS (TIBCO Software, Inc.). Data were analyzed for statistical differences by analysis of variance (ANOVA) at the 5% significance level. Post hoc comparisons between different conditions were performed by Tukey's range test, unless indicated otherwise in the results, in which case, a Student's t test at the 5% significance level was performed. To investigate correlation between data, Spearman's rank correlation test was used.
ACKNOWLEDGMENTS
We thank Lynn Enquist (Princeton University, USA) and Thomas Mettenleiter (Friedrich-Loeffler-Institut, Germany) for donating the different PRV strains. Furthermore, we thank Rudy Cooman for animal caretaking and Charlotte Helsmoortel and Stéphanie Vervaeke for excellent technical assistance.
This research was supported by grants from the Special Research Fund of Ghent University (grant no. 01G01311 and 01G01317), F.W.O.-Vlaanderen (grant no. G.0176.15N), and the Belgian Science Policy (BELSPO) via the BELVIR consortium (IAP, phase VII). Bert Devriendt is supported by a postdoctoral fellowship from F.W.O.-Vlaanderen.
REFERENCES
- 1.Pomeranz LE, Reynolds AE, Hengartner CJ. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev 69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartha A. 1961. Experimental reduction of virulence of Aujeszky's disease virus. Magy Allatorv Lapja 16:42–45. [Google Scholar]
- 3.Szpara ML, Tafuri YR, Parsons L, Shamim SR, Verstrepen KJ, Legendre M, Enquist LW. 2011. A wide extent of inter-strain diversity in virulent and vaccine strains of alphaherpesviruses. PLoS Pathog 7:e1002282. doi: 10.1371/journal.ppat.1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolland S, Pierce SK. 2015. Ups and downs in the search for a herpes simplex virus vaccine. eLife 4:e06883. doi: 10.7554/eLife.06883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu XP, Muhammad ZS, Wang JG, Lin W, Guo SK, Zhang W. 2014. HSV-2 vaccine: current status and insight into factors for developing an efficient vaccine. Viruses 6:371–390. doi: 10.3390/v6020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vissani MA, Thiry E, Dal Pozzo F, Barrandeguy M. 2016. Antiviral agents against equid alphaherpesviruses: current status and perspectives. Vet J 207:38–44. doi: 10.1016/j.tvjl.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Summerfield A, Guzylack-Piriou L, Schaub A, Carrasco CP, Tache V, Charley B, McCullough KC. 2003. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology 110:440–449. doi: 10.1111/j.1365-2567.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathan TS, Figdor CG, Buschow SI. 2013. Human plasmacytoid dendritic cells: from molecules to intercellular communication network. Front Immunol 4:372. doi: 10.3389/fimmu.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyama S, Aoshi T, Tanimoto T, Kumagai Y, Kobiyama K, Tougan T, Sakurai K, Coban C, Horii T, Akira S, Ishii KJ. 2010. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med 2:25ra24. doi: 10.1126/scitranslmed.3000759. [DOI] [PubMed] [Google Scholar]
- 11.Gungor B, Yagci FC, Tincer G, Bayyurt B, Alpdundar E, Yildiz S, Ozcan M, Gursel I, Gursel M. 2014. CpG ODN nanorings induce IFNalpha from plasmacytoid dendritic cells and demonstrate potent vaccine adjuvant activity. Sci Transl Med 6:235ra61. doi: 10.1126/scitranslmed.3007909. [DOI] [PubMed] [Google Scholar]
- 12.Lannes N, Python S, Summerfield A. 2012. Interplay of foot-and-mouth disease virus, antibodies and plasmacytoid dendritic cells: virus opsonization under non-neutralizing conditions results in enhanced interferon-alpha responses. Vet Res 43:64. doi: 10.1186/1297-9716-43-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzylack-Piriou L, Balmelli C, McCullough KC, Summerfield A. 2004. Type-A CpG oligonucleotides activate exclusively porcine natural interferon-producing cells to secrete interferon-alpha, tumour necrosis factor-alpha and interleukin-12. Immunology 112:28–37. doi: 10.1111/j.1365-2567.2004.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiebach AR, Guzylack-Piriou L, Python S, Summerfield A, Ruggli N. 2011. Classical swine fever virus N(pro) limits type I interferon induction in plasmacytoid dendritic cells by interacting with interferon regulatory factor 7. J Virol 85:8002–8011. doi: 10.1128/JVI.00330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demmin GL, Clase AC, Randall JA, Enquist LW, Banfield BW. 2001. Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. J Virol 75:10856–10869. doi: 10.1128/JVI.75.22.10856-10869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. 2000. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A 97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favoreel HW, Nauwynck HJ, Halewyck HM, Van Oostveldt P, Mettenleiter TC, Pensaert MB. 1999. Antibody-induced endocytosis of viral glycoproteins and major histocompatibility complex class I on pseudorabies virus-infected monocytes. J Gen Virol 80:1283–1291. doi: 10.1099/0022-1317-80-5-1283. [DOI] [PubMed] [Google Scholar]
- 18.Calzada-Nova G, Schnitzlein W, Husmann R, Zuckermann FA. 2010. Characterization of the cytokine and maturation responses of pure populations of porcine plasmacytoid dendritic cells to porcine viruses and toll-like receptor agonists. Vet Immunol Immunopathol 135:20–33. doi: 10.1016/j.vetimm.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auray G, Keller I, Python S, Gerber M, Bruggmann R, Ruggli N, Summerfield A. 2016. Characterization and transcriptomic analysis of porcine blood conventional and plasmacytoid dendritic cells reveals striking species-specific differences. J Immunol 197:4791–4806. doi: 10.4049/jimmunol.1600672. [DOI] [PubMed] [Google Scholar]
- 20.Setas Pontes M, Devriendt B, Favoreel HW. 2015. Pseudorabies virus triggers glycoprotein gE-mediated ERK1/2 activation and ERK1/2-dependent migratory behavior in T cells. J Virol 89:2149–2156. doi: 10.1128/JVI.02549-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontes MS, Van Waesberghe C, Nauwynck H, Verhasselt B, Favoreel HW. 2016. Pseudorabies virus glycoprotein gE triggers ERK1/2 phosphorylation and degradation of the pro-apoptotic protein Bim in epithelial cells. Virus Res 213:214–218. doi: 10.1016/j.virusres.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. 2008. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci U S A 105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summerfield A, McCullough KC. 2009. The porcine dendritic cell family. Dev Comp Immunol 33:299–309. doi: 10.1016/j.dci.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baranek T, Zucchini N, Dalod M. 2009. Plasmacytoid dendritic cells and the control of herpesvirus infections. Viruses 1:383–419. doi: 10.3390/v1030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swiecki M, Colonna M. 2015. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuster P, Boscheinen JB, Tennert K, Schmidt B. 2011. The role of plasmacytoid dendritic cells in innate and adaptive immune responses against alpha herpes virus infections. Adv Virol 2011:679271. doi: 10.1155/2011/679271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamote JA, Glorieux S, Nauwynck HJ, Favoreel HW. 2016. The US3 protein of pseudorabies virus drives viral passage across the basement membrane in porcine respiratory mucosa explants. J Virol 90:10945–10950. doi: 10.1128/JVI.01577-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glorieux S, Favoreel HW, Meesen G, de Vos W, Van den Broeck W, Nauwynck HJ. 2009. Different replication characteristics of historical pseudorabies virus strains in porcine respiratory nasal mucosa explants. Vet Microbiol 136:341–346. doi: 10.1016/j.vetmic.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Steinhagen F, Kinjo T, Bode C, Klinman DM. 2011. TLR-based immune adjuvants. Vaccine 29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajagopal D, Paturel C, Morel Y, Uematsu S, Akira S, Diebold SS. 2010. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood 115:1949–1957. doi: 10.1182/blood-2009-08-238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calzada-Nova G, Schnitzlein WM, Husmann RJ, Zuckermann FA. 2011. North American porcine reproductive and respiratory syndrome viruses inhibit type I interferon production by plasmacytoid dendritic cells. J Virol 85:2703–2713. doi: 10.1128/JVI.01616-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A 101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huch JH, Cunningham AL, Arvin AM, Nasr N, Santegoets SJ, Slobedman E, Slobedman B, Abendroth A. 2010. Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection. J Virol 84:4060–4072. doi: 10.1128/JVI.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaghy H, Bosnjak L, Harman AN, Marsden V, Tyring SK, Meng TC, Cunningham AL. 2009. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol 83:1952–1961. doi: 10.1128/JVI.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster P, Donhauser N, Pritschet K, Ries M, Haupt S, Kittan NA, Korn K, Schmidt B. 2010. Co-ordinated regulation of plasmacytoid dendritic cell surface receptors upon stimulation with herpes simplex virus type 1. Immunology 129:234–247. doi: 10.1111/j.1365-2567.2009.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, Fauci AS. 2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo CC, Schwartz JA, Johnson DJ, Yu M, Aidarus N, Mujib S, Benko E, Hyrcza M, Kovacs C, Ostrowski MA. 2012. HIV delays IFN-alpha production from human plasmacytoid dendritic cells and is associated with SYK phosphorylation. PLoS One 7:e37052. doi: 10.1371/journal.pone.0037052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florentin J, Aouar B, Dental C, Thumann C, Firaguay G, Gondois-Rey F, Soumelis V, Baumert TF, Nunes JA, Olive D, Hirsch I, Stranska R. 2012. HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells. Blood 120:4544–4551. doi: 10.1182/blood-2012-02-413286. [DOI] [PubMed] [Google Scholar]
- 39.Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol 79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. 2009. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med 206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunin A, Sisirak V, Ghosh HS, Grajkowska LT, Hou ZE, Miron M, Yang C, Ceribelli M, Uetani N, Chaperot L, Plumas J, Hendriks W, Tremblay ML, Hacker H, Staudt LM, Green PH, Bhagat G, Reizis B. 2015. Protein tyrosine phosphatase PTPRS is an inhibitory receptor on human and murine plasmacytoid dendritic cells. Immunity 43:277–288. doi: 10.1016/j.immuni.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao M, Liu YJ. 2013. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell 4:40–52. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol 31:2680–2689. doi:. [DOI] [PubMed] [Google Scholar]
- 44.Iparraguirre A, Tobias JW, Hensley SE, Masek KS, Cavanagh LL, Rendl M, Hunter CA, Ertl HC, von Andrian UH, Weninger W. 2008. Two distinct activation states of plasmacytoid dendritic cells induced by influenza virus and CpG 1826 oligonucleotide. J Leukoc Biol 83:610–620. [DOI] [PubMed] [Google Scholar]
- 45.Kuo CH, Hsieh CC, Kuo HF, Huang MY, Yang SN, Chen LC, Huang SK, Hung CH. 2013. Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation. Allergy 68:870–879. doi: 10.1111/all.12162. [DOI] [PubMed] [Google Scholar]
- 46.Lyman MG, Randall JA, Calton CM, Banfield BW. 2006. Localization of ERK/MAP kinase is regulated by the alphaherpesvirus tegument protein Us2. J Virol 80:7159–7168. doi: 10.1128/JVI.00592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang MH, Banfield BW. 2010. Pseudorabies virus tegument protein Us2 recruits the mitogen-activated protein kinase extracellular-regulated kinase (ERK) to membranes through interaction with the ERK common docking domain. J Virol 84:8398–8408. doi: 10.1128/JVI.00794-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonaccorsi I, Cantoni C, Carrega P, Oliveri D, Lui G, Conte R, Navarra M, Cavaliere R, Traggiai E, Gattorno M, Martini A, Mingari MC, Moretta A, Ferlazzo G. 2010. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNalpha production. PLoS One 5:e15080. doi: 10.1371/journal.pone.0015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tirabassi RS, Enquist LW. 2000. Role of the pseudorabies virus gI cytoplasmic domain in neuroinvasion, virulence, and posttranslational N-linked glycosylation. J Virol 74:3505–3516. doi: 10.1128/JVI.74.8.3505-3516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Favoreel HW, Nauwynck HJ, Van Oostveldt P, Mettenleiter TC, Pensaert MB. 1997. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J Virol 71:8254–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson DC, Frame MC, Ligas MW, Cross AM, Stow ND. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol 62:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nauwynck HJ, Pensaert MB. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch Virol 140:1137–1146. doi: 10.1007/BF01315422. [DOI] [PubMed] [Google Scholar]
- 53.Pescovitz MD, Lunney JK, Sachs DH. 1984. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol 133:368–375. [PubMed] [Google Scholar]
- 54.Haverson K, Bailey M, Higgins VR, Bland PW, Stokes CR. 1994. Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues. J Immunol Methods 170:233–245. doi: 10.1016/0022-1759(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 55.L'Haridon RM, Bourget P, Lefevre F, La Bonnardiere C. 1991. Production of an hybridoma library to recombinant porcine alpha I interferon: a very sensitive assay (ISBBA) allows the detection of a large number of clones. Hybridoma 10:35–47. doi: 10.1089/hyb.1991.10.35. [DOI] [PubMed] [Google Scholar]
- 56.McClurkin AW, Norman JO. 1966. Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can J Comp Med Vet Sci 30:190–198. [PMC free article] [PubMed] [Google Scholar]
- 57.Favoreel HW, Van Minnebruggen G, Nauwynck HJ, Enquist LW, Pensaert MB. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J Virol 76:6845–6851. doi: 10.1128/JVI.76.13.6845-6851.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres JM, Alonso C, Ortega A, Mittal S, Graham F, Enjuanes L. 1996. Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. J Virol 70:3770–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Platt KB, Mare CJ, Hinz PN. 1979. Differentiation of vaccine strains and field isolates of pseudorabies (Aujeszky's disease) virus: thermal sensitivity and rabbit virulence markers. Arch Virol 60:13–23. doi: 10.1007/BF01318093. [DOI] [PubMed] [Google Scholar]
- 60.Card JP, Whealy ME, Robbins AK, Enquist LW. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol 66:3032–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whealy ME, Card JP, Robbins AK, Dubin JR, Rziha HJ, Enquist LW. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol 67:3786–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brideau AD, Card JP, Enquist LW. 2000. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J Virol 74:834–845. doi: 10.1128/JVI.74.2.834-845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaplan AS, Vatter AE. 1959. A comparison of herpes simplex and pseudorabies viruses. Virology 7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 64.Baert K, De Geest BG, De Greve H, Cox E, Devriendt B. 2016. Duality of beta-glucan microparticles: antigen carrier and immunostimulants. Int J Nanomedicine 11:2463–2469. doi: 10.2147/IJN.S101881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diaz de Arce H, Artursson K, L'Haridon R, Perers A, La Bonnardiere C, Alm GV. 1992. A sensitive immunoassay for porcine interferon-alpha. Vet Immunol Immunopathol 30:319–327. doi: 10.1016/0165-2427(92)90102-V. [DOI] [PubMed] [Google Scholar]
- 66.Deruelle M, Geenen K, Nauwynck HJ, Favoreel HW. 2007. A point mutation in the putative ATP binding site of the pseudorabies virus US3 protein kinase prevents Bad phosphorylation and cell survival following apoptosis induction. Virus Res 128:65–70. doi: 10.1016/j.virusres.2007.04.006. [DOI] [PubMed] [Google Scholar]