ABSTRACT
Rabies continues to present a public health threat in most countries of the world. The most efficient way to prevent and control rabies is to implement vaccination programs for domestic animals. However, traditional inactivated vaccines used in animals are costly and have relatively low efficiency, which impedes their extensive use in developing countries. There is, therefore, an urgent need to develop single-dose and long-lasting rabies vaccines. However, little information is available regarding the mechanisms underlying immunological memory, which can broaden humoral responses following rabies vaccination. In this study, a recombinant rabies virus (RABV) that expressed murine interleukin-7 (IL-7), referred to here as rLBNSE-IL-7, was constructed, and its effectiveness was evaluated in a mouse model. rLBNSE-IL-7 induced higher rates of T follicular helper (Tfh) cells and germinal center (GC) B cells from draining lymph nodes (LNs) than the parent virus rLBNSE. Interestingly, rLBNSE-IL-7 improved the percentages of long-lived memory B cells (Bmem) in the draining LNs and plasma cells (PCs) in the bone marrow (BM) for up to 360 days postimmunization (dpi). As a result of the presence of the long-lived PCs, it also generated prolonged virus-neutralizing antibodies (VNAs), resulting in better protection against a lethal challenge than that seen with rLBNSE. Moreover, consistent with the increased numbers of Bmem and PCs after a boost with rLBNSE, rLBNSE-IL-7-immunized mice promptly produced a more potent secondary anti-RABV neutralizing antibody response than rLBNSE-immunized mice. Overall, our data suggest that overexpressing IL-7 improved the induction of long-lasting primary and secondary antibody responses post-RABV immunization.
IMPORTANCE Extending humoral immune responses using adjuvants is an important method to develop long-lasting and efficient vaccines against rabies. However, little information is currently available regarding prolonged immunological memory post-RABV vaccination. In this study, a novel rabies vaccine that expressed murine IL-7 was developed. This vaccine enhanced the numbers of Tfh cells and the GC responses, resulting in upregulated quantities of Bmem and PCs. Moreover, we found that the long-lived PCs that were elicited by the IL-7-expressing recombinant virus (rLBNSE-IL-7) were able to sustain VNA levels much longer than those elicited by the parent rLBNSE virus. Upon reexposure to the pathogen, the longevous Bmem, which maintained higher numbers for up to 360 dpi with rLBNSE-IL-7 compared to rLBNSE, could differentiate into antibody-secreting cells, resulting in rapid and potent secondary production of VNAs. These results suggest that the expression of IL-7 is beneficial for induction of potent and long-lasting humoral immune responses.
KEYWORDS: IL-7, humoral immunity, rabies virus
INTRODUCTION
Rabies virus (RABV) is a nonsegmented, negative-sense RNA virus that is a member of the Lyssavirus genus in the Rhabdoviridae family. Rabies is a public health threat that causes more than 59,000 human deaths around the world each year, and most of these deaths occur in the developing countries of Asia and Africa (1, 2). Globally, over 3 billion humans are threatened with exposure to rabies because they live in areas where rabies is endemic in domestic or wild animals (3). More than 95% of human rabies cases are related to dog bites, indicating that it is critical to control rabies in domestic animals, especially dogs, in order to control and eliminate rabies in humans. The mass (>70%) vaccination of domestic dogs has nearly eliminated cases of human rabies in developed and some developing countries. However, the traditional inactivated animal vaccines induce protective antibody (Ab) responses only after multiple shots and generally require repeated booster doses to provide long-lasting protection in preexposure settings (4, 5), which increases the operational costs and restricts their wide use in developing countries. Therefore, there is still an urgent need to develop a single-dose and long-lasting RABV vaccine that can induce robust antibodies, especially virus-neutralizing antibodies (VNAs), to protect animals from rabies.
Vaccines usually induce antibody responses via pathways that involve T cell-independent and T cell-dependent B cell mechanisms. An early study showed that antibodies produced in T cell-independent responses peaked at around 5 to 7 days postimmunization (dpi) and did not generate memory B cells (Bmem) with a replication-deficient RABV-based vaccine, suggesting that humoral immunity induced by cooperation between specialized populations of B cells and CD4+ T cells may hold the key to the development of a relatively prolonged antibody response (6). During the development of vaccine-induced humoral immunity, CD4+ T cells are primed by dendritic cells (DCs), are loaded with antigens in the T cell zone, and move toward the B cell follicles (7). When follicular B cells acquire antigen, they migrate toward the border of the T cell zone and further differentiate into short-lived plasma cells (PCs) and early Bmem or return to the follicle and undergo rapid proliferation to form a germinal center (GC) (8). Within the GCs, B cells acquire an antigen by synapsing with antigen-presenting cells such as DCs and macrophages and with specialized stromal cells known as follicular dendritic cells (FDCs) (9) and via contact with additional signals produced by T follicular helper (Tfh) cells (10, 11). GC B cells emigrate from the follicle and differentiate into long-lived PCs or Bmem (8). Humoral immune responses require long-lived PCs that produce copious amounts of antibodies capable of neutralizing pathogenic antigens over time (12). Long-lived Bmem renew antibody responses by rapidly differentiating into antibody-secreting cells upon reexposure to antigen (13).
Interleukin-7 (IL-7) is a nonhematopoietic, cell-derived cytokine that plays a central role in the proliferation and maintenance of both T and B lymphocytes in the adaptive immune system. A previous report demonstrated that IL-7 is produced by human stromal cells in adult bone marrow (BM) and that the IL-7-induced expansion of the pro-B compartment becomes gradually more essential for B cell differentiation throughout development (14). IL-7 has been administered as a vaccine adjuvant to extend immunity by augmenting responses to subdominant antigens and thereby improving the survival of the CD8+ T cell memory (Tmem) pool (15). IL-7 is more efficient than IL-2 and IL-15 at increasing B cell differentiation from CD34+ cord blood mononuclear cells in the presence of the same combination of stem cell factor and Flt3 ligand (16). The role of IL-7 in regulating the generation of Tfh cells and in the formation of GC B cells was defined in a recent study in which a mouse Fc-fused IL-7 was administered with a vaccine to combat challenge with a lethal influenza virus (17). To our knowledge, no previous studies have associated the expression of IL-7 with RABV immunization. In this study, a recombinant RABV (rRABV) that expressed mouse IL-7 was constructed, and its role in the induction of humoral immune responses was evaluated. It was found that overexpressing IL-7 improved the production of long-lasting primary and secondary antibody responses to RABV infection.
RESULTS
Construction and characterization of recombinant RABV-expressing murine IL-7 or IL-7(-).
To investigate whether expression of murine IL-7 could affect RABV-induced immune responses, the murine IL-7 gene was cloned into the RABV genome. To control for any changes in pathogenicity due to the increased size of the RABV genome, we inserted an inactive IL-7 gene with substitution of STOP codons for the START codons of the IL-7 gene into the rLBNSE genome [named IL-7(-)] (Fig. 1A), and the recombinant virus was rescued as described previously (18). The insertion of the mouse IL-7 or IL-7(-) gene was confirmed by real-time PCR (RT-PCR) and sequencing. The BSR cells (Fig. 1B) and neuroblastoma (NA) cells (Fig. 1C) were infected at a multiplicity of infection (MOI) of 0.01, and it was found that both rLBNSE-IL-7 and rLBNSE-IL-7(-) replicated as efficiently as the parent virus rLBNSE in both cell types. These results indicated that viral replication and spreading were not affected by the insertion of an extra IL-7 or IL-7(-) gene. The recombinant RABV (rRABV) encoding the IL-7 or IL-7(-) gene was demonstrated to be stable for at least 10 generations in BSR cells, and the results were confirmed by RT-PCR and sequencing (data not shown). IL-7 was highly expressed in a dose-dependent manner in the BSR cells that were infected with rLBNSE-IL-7 but not in those that were infected with rLBNSE or rLBNSE-IL-7(-), as measured by enzyme-linked immunosorbent assay (ELISA) (Fig. 1D). Cell viability assays in BSR cells showed that rLBNSE-IL-7-infected cells had levels of cell growth and viability that were similar to those seen with the rLBNSE- or rLBNSE-IL-7(-)-infected cells, suggesting that overexpression of IL-7 has no side effect on BSR cells (Fig. 1E).
FIG 1.
Construction and characterization of the recombinant RABV expressing IL-7 or IL-7(-) in vitro. (A) Schematic diagram describing the construction of rLBNSE, rLBNSE-IL-7(-), and rLBNSE-IL-7. The pLBNSE plasmid was derived from the SAD-B19 vaccine strain by deleting the long, noncoding region of the G gene and adding BsiWI and NheI sites between the G and L genes. Murine IL-7 was inserted into the RABV genome between the G and L genes instead of into the deleted long noncoding region. rLBNSE-IL-7(-) was constructed by the substitution of STOP codons for the START codons within IL-7 gene. (B and C) Multiple-step growth curves for rRABVs on BSR cells (B) and NA cells (C) at a multiplicity of infection (MOI) of 0.01 are shown. (D) The expression level of murine IL-7 was determined by ELISA. Briefly, BSR cells were infected with rLBNSE or rLBNSE-IL-7(-) or rLBNSE-IL-7 (MOI = 1, 0.1, 0.01, or 0.001) for 24 h, and the cell culture supernatants were then harvested to determine the quantity of murine IL-7 by using a commercial ELISA kit. (E) BSR cell viability of rLBNSE-IL-7 was similar to that of rLBNSE-IL-7(-) and rLBNSE. Error bars represent the standard deviations (SD; n = 3), and data are representative of results of two independent experiments. OD, optical density.
Pathogenicity of recombinant viruses.
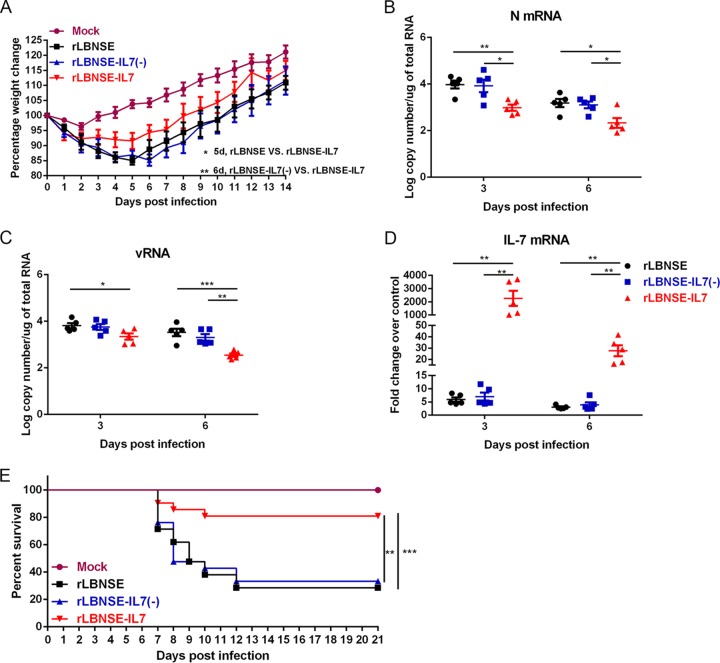
To evaluate any adverse effects on animals that might have been caused by the expression of IL-7, four groups of 6-week-old female ICR mice were inoculated intracerebrally (i.c.) with 107 focus-forming units (FFU) of rLBNSE, rLBNSE-IL-7(-), or rLBNSE-IL-7 or were mock infected with Dulbecco's modified Eagle's medium (DMEM). The infected ICR mice (n = 10) were observed daily for 2 weeks to monitor body weight loss and clinical symptoms. The rLBNSE-IL-7-infected mice exhibited significantly less weight loss than the rLBNSE-infected mice at 5 dpi (P = 0.0442) or than the rLBNSE-IL-7(-)-infected mice at 6 dpi (P = 0.0084) (Fig. 2A). No clinical symptoms were observed in the mice after inoculation with any of the rRABVs mentioned above. Brain tissues from infected ICR mice (n = 5) were collected, and viral N mRNA levels (Fig. 2B) and genomic viral RNA (vRNA) levels (Fig. 2C) and IL-7 mRNA levels (Fig. 2D) were quantified using quantitative RT-PCR (qRT-PCR) at 3 and 6 days postinfection (dpi). Significantly lower levels of N mRNA and vRNA were detected in the rLBNSE-IL-7-infected mouse brains than in the rLBNSE- or rLBNSE-IL-7(-)-infected mouse brains at 3 and 6 dpi. Increased amounts of IL-7 transcripts were detected in the rLBNSE-IL-7-infected mouse brains at 3 and 6 dpi.
FIG 2.
Virulence of rRABVs in adult and suckling mice. (A) Groups of 6-week-old female ICR mice (n = 10) were i.c. inoculated with 107 FFU of rLBNSE or rLBNSE-IL-7(-) or rLBNSE-IL-7 or were mock infected with DMEM, and their body weight loss was then monitored daily for 2 weeks. d, days. (B to D) Groups of 6-week-old female ICR mice (n = 5) were i.c. infected with 107 FFU of rLBNSE, rLBNSE-IL-7(-), or rLBNSE-IL-7 or with DMEM. The levels of N mRNA (B), viral genomic RNA (vRNA) (C), and IL-7 mRNA (D) in the brains were quantified using qRT-PCR. (E) Litters of 5-day-old ICR mice (n = 21) were infected i.c. with 100 FFU of rRABV for clinical signs of rabies, and mortality rates were recorded daily for 21 days. Error bars represent the standard errors (SE), and data are representative of results of two independent experiments.
To examine whether the presence of IL-7 genes further decreases pathogenicity in immunocompromised mice, groups of 5-day-old ICR mice (n = 21) were inoculated i.c. with 100 FFU of different viruses and observed for the occurrence of clinical signs of rabies. Around 70% of the sucking mice injected with rLBNSE and rLBNSE-IL-7(-) succumbed to infection at between 7 and 12 days, while around 80% of the mice infected with rLBNSE-IL-7 did not develop any clinical signs of rabies and survived (Fig. 2E). To confirm that the sucking mice succumbed by reason of RABV, their brains were harvested and homogenized in DMEM and were then analyzed using a direct fluorescence antibody test as described previously (19) (data not shown). Together, the results demonstrate that IL-7 expression attenuates RABV pathogenicity in vivo.
The presence of IL-7 leads to enhanced numbers of Tfh cells.
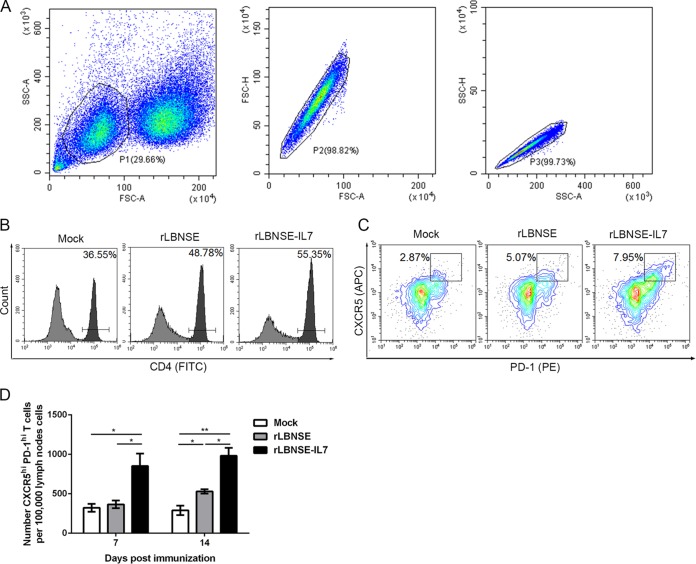
Because a previous report suggested that IL-7 facilitates the generation of Tfh cells (17), the induction of Tfh cells post-rRABV immunization was investigated. BALB/c mice (n = 3) were intramuscularly (i.m.) inoculated with 106 FFU rRABVs or DMEM in the hind legs. At 7 and 14 dpi, single-cell suspensions were prepared from the inguinal lymph nodes (LNs) and analyzed using flow cytometry, which focused on the populations and numbers of cells of interest (Fig. 3A). The gating strategies used to detect CD4+ T cells (Fig. 3B) and Tfh cells (CD4+ CXCR5hi PD-1hi) (Fig. 3C) have been previously described (20, 21). Significantly more Tfh cells were observed in the mice that were vaccinated with rLBNSE-IL-7 than in those vaccinated with rLBNSE at 7 (P = 0.0407) and 14 (P = 0.012) dpi (Fig. 3D). Taken together, these data suggest that the expression of IL-7 enhances the numbers of Tfh cells in the draining LNs post-rRABV immunization.
FIG 3.
IL-7 enhances the numbers of Tfh cells in the draining LNs. Groups of 6-week-old female BALB/c mice were i.m. immunized with 106 FFU rRABVs or with DMEM. The quantity of Tfh cells was analyzed at the indicated time points postimmunization. Draining LNs were collected at 7 and 14 dpi. Single-cell suspensions were prepared and stained using antibodies for Tfh cells (CD4+ CXCR5hi PD-1hi). (A) The population of interest was gated, and the LN cells were then visualized on dot plots showing their forward-scatter (FSC) and side-scatter (SSC) signals in relation to the size and granularity of the cells, respectively. (B and C) Representative gating strategies for the methods used to differentiate CD4+ T cells from the lymphocyte population (B) and Tfh cells (CD4+ CXCR5hi PD-1hi) from the CD4+ T cell population (C) are shown. (D) The number of Tfh cells in the total CD4+ T cell population was measured at 7 and 14 dpi. Error bars represent the SE (n = 3), and data are representative of results of two independent experiments.
IL-7 augments the GC B cell populations.
Because Tfh cells directly associate with GC B cells (17), the effect of IL-7 expressed by rRABV on GC B cells was evaluated. The inguinal LNs were collected at 7 and 14 dpi from BALB/c mice (n = 3) that were individually immunized with 106 FFU of one of the rRABVs or with DMEM alone. The gating strategies used to measure the levels of B cells (B220+) (Fig. 4A) and GC B cells (B220+ GL7hi CD95/Fashi) (Fig. 4B) are shown as previously reported (22, 23). The rLBNSE-IL-7-immunized mice had a significantly higher number of GC B cells in the LNs than the rLBNSE-immunized mice at 7 (P = 0.0084) and 14 dpi (P = 0.0059) (Fig. 4C).
FIG 4.
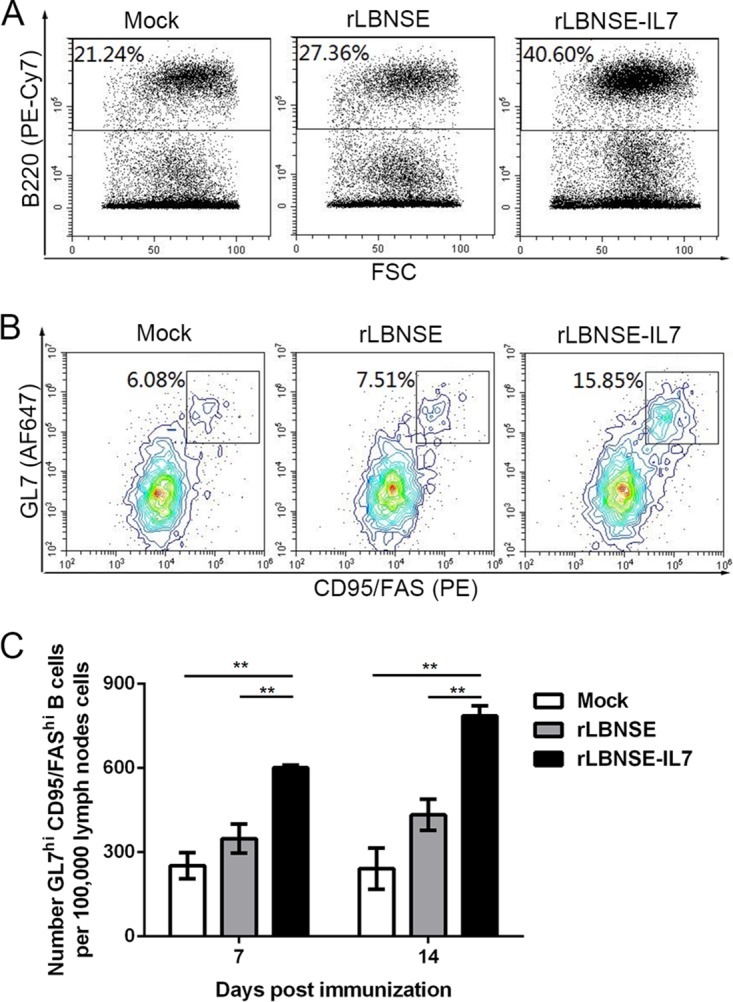
IL-7 augments the population of GC B cells in the draining LNs. Total mouse draining LN cells, as described for Fig. 3, were stained for surface B220, GL7, and CD95/Fas expression for the GC B cell by FACS. (A and B) The representative flow cytometry gating strategy that was used to identify B220+ B cells in the lymphocyte population (A) and GL7hi CD95/Fashi cells (GC B cells) in the B220+ B cell population (B). (C) The number of GC B cells per 105 live LN cells was determined at 7 and 14 dpi. Error bars represent the SE (n = 3), and data are representative of results of two independent experiments.
IL-7 improves the quantity of Bmem and PCs.
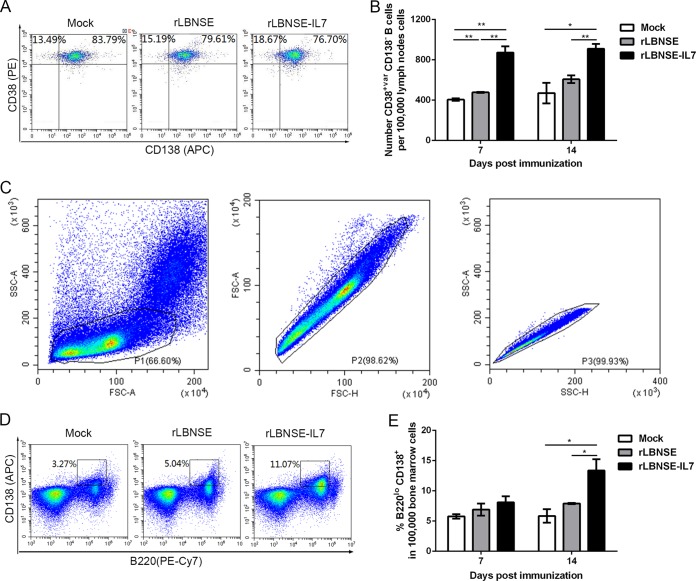
Since GC B cells differentiate into Bmem and PCs, we next determined whether IL-7 increased the quantity of Bmem. Bmem (B220+ CD38+ CD138−) in the inguinal LNs were collected from rRABV- or mock-immunized mice (n = 3). The representative flow cytometry data for Bmem are shown in Fig. 5A (24). Interestingly, significantly more Bmem were induced by rLBNSE-IL-7 than by rLBNSE at 7 (P = 0.0034) and 14 dpi (P = 0.0083) in the LNs (Fig. 5B).
FIG 5.
IL-7 increases Bmem levels in the draining LNs and PCs in the BM. Total mouse draining LN cells, as described for Fig. 3, were stained for Bmem (B220+ CD38+ CD138−). Total mouse BM cells from the mice described in the Fig. 3 legend were stained to analyze the proportion of PCs (B220lo CD138+) using flow cytometry. (A) Representative flow cytometry plots of Bmem (B220+ CD38+ CD138−) in draining LNs are shown. (B) The number of Bmem per 105 live LN cells was determined at 7 and 14 dpi. (C) Cell populations of interest were gated and are presented as BM visualized on dot plots that show their FSC and SSC signals in relation to the size and complexity of the cells, respectively. (D) The results of flow cytometric analysis of B220lo CD138+ cells in the lymphocyte population in the BM are shown. (E) The percentage of PCs per 105 live BM cells was determined at 7 and 14 dpi. Error bars represent the SE (n = 3), and data are representative of results of two independent experiments.
Antibodies produced by PCs play important roles in combating various viruses and bacteria (25), so the potential effects of IL-7 on PC populations were investigated. BM cells were collected from rRABV- or mock-immunized mice (n = 3) at the indicated time points, and approximately 105 live cells were stained to identify PCs (Fig. 5C). The CD138-positive cells obtained from the B220lo B cell population were gated as shown in Fig. 5D. By 14 dpi, over 10% of the BM cells were PCs in the mice immunized with rLBNSE-IL-7, and this proportion was significantly higher than that observed in the cells obtained from rLBNSE-immunized mice (P = 0.0212) (Fig. 5E). All together, these data illustrate that expression of IL-7 increased the Bmem and PC populations.
IL-7 induces long-lasting antibody responses and provides better protection against rabies in mice after primary vaccination.
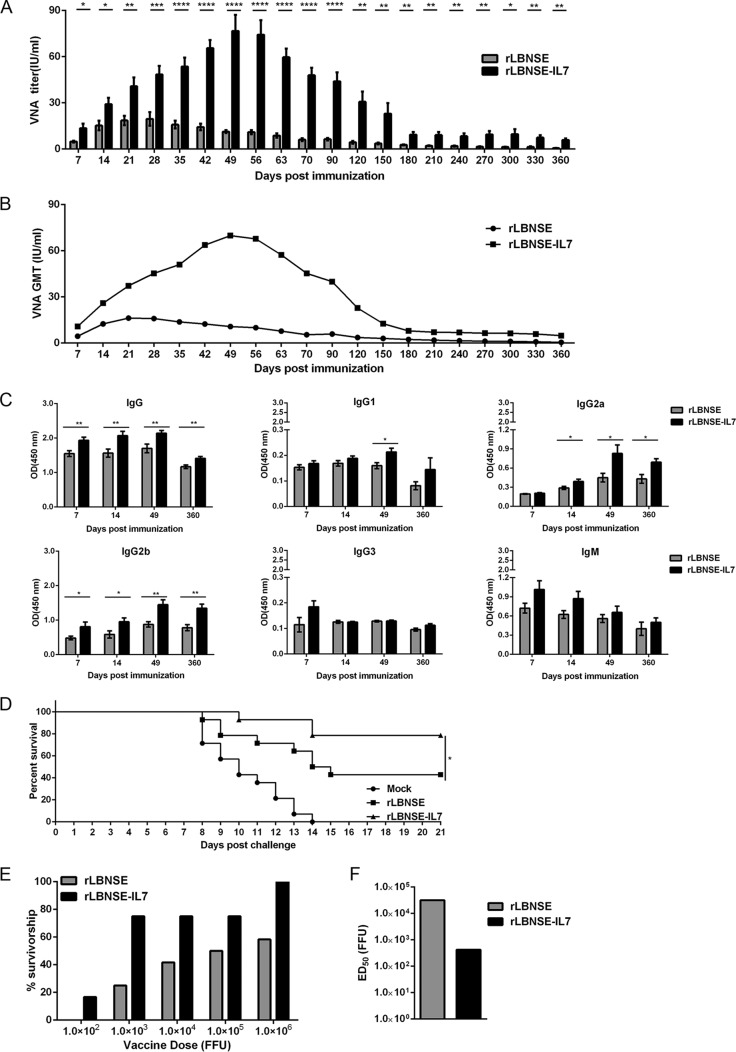
Because IL-7 expression significantly increases the quantity of PCs, the effect of IL-7 on antibody production was investigated. Three groups of ICR mice (n = 10) were immunized i.m. with 106 FFU of rLBNSE-IL-7 or rLBNSE or with DMEM, and blood samples were then collected to measure VNA levels at the indicated time points postimmunization. The mice immunized with rLBNSE-IL-7 maintained significantly higher levels of VNA titers than were observed in the rLBNSE-immunized mice at all time points up to 360 dpi (Fig. 6A). The geometric mean titer (GMT) of VNA that was induced by rLBNSE-IL-7 reached a maximum of 69.892 IU/ml at 49 dpi, and this was much higher than the highest titer of VNA (16.213 IU/ml) that was induced by rLBNSE at 21 dpi (Fig. 6B). In addition to VNA levels, different isotypes of antibodies against RABV G were also analyzed using ELISA (Fig. 6C). Notably, immunization with rLBNSE-IL-7 induced high levels of IgG, IgG2a, and IgG2b for up to 360 days and of IgG1 for up to 49 days. RABV G-specific IgG3 and IgM levels were not significantly increased at any of the indicated time points.
FIG 6.
IL-7 extends the humoral immunity and improves the protection against rabies. (A and B) Groups of 6-week-old female ICR mice (n = 10) were i.m. immunized with 106 FFU of rRABV or with DMEM. At the indicated time points postimmunization, serum were collected from peripheral blood samples to quantify VNA titers (A) and GMT (B), which were determined using FAVN as described in Materials and Methods. (C) Optical density (OD) values of total IgG, IgG1, IgG2a, IgG2b, IgG3, or IgM against RABV G were determined by ELISA. (D) At 360 days after the primary vaccination, the immunized mice (n = 14) were i.c. challenged with 50 LD50 CVS-24 and observed twice daily for 3 weeks. The number of survivors in each group was recorded. (E) Seven weeks after the primary vaccination with serial 10-fold dilutions of the rRABVs, ICR mice (n = 12) were infected i.c. with 50 LD50 of CVS-24 and the percentages of survivors in the different immunization groups were recorded. (F) The ED50 values were calculated from the survivor ratio in the two vaccination groups as described previously (51). Error bars represent the SE, and data are representative of results of two independent experiments.
We further evaluated the effect of IL-7 on long-term protection against pathogenic RABV challenge. At 360 dpi, all of the mice (n = 14) were i.c. challenged with 50 50% lethal doses (LD50) of CVS-24, and clinical symptoms were then monitored for another 3 weeks (Fig. 6D). All the mice in the mock-immunized group succumbed to rabies within 2 weeks, while 78% of the mice immunized with rLBNSE-IL-7 were protected from the lethal challenge compared with only 42% of the mice in the rLBNSE group. Even vaccination with 1 × 103 FFU rLBNSE-IL-7 protected 75% of the mice against a lethal RABV challenge (Fig. 6E). The 50% effective dose (ED50) of rLBNSE was 3.16 × 104 FFU, approximate 75 times higher than that of rLBNSE-IL-7 (4.2 × 102 FFU) (Fig. 6F). Taken together, the results showed that IL-7 expression can sustain antibody production and provide long-term protection.
IL-7 improves secondary antibody responses.
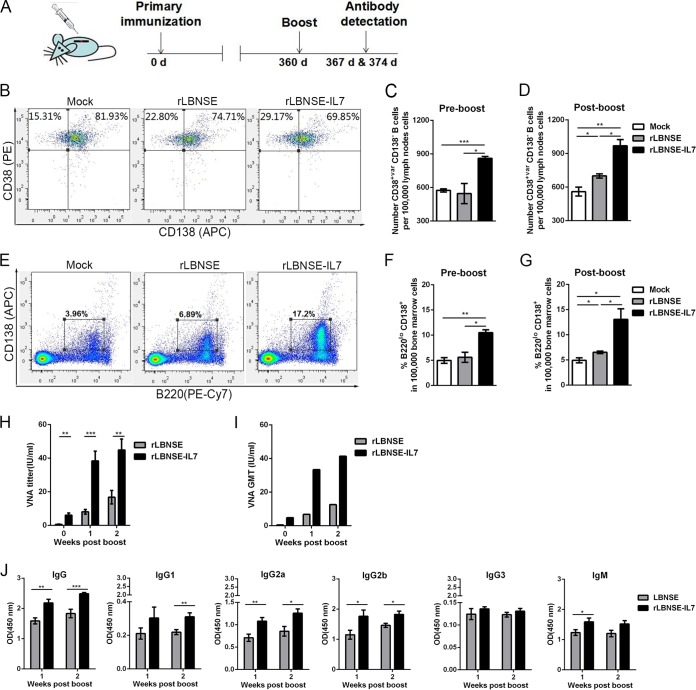
In order to evaluate the effect of IL-7 on the secondary antibody responses, mice (n = 3) were boosted with 106 FFU of rLBNSE or mock boosted with DMEM at 360 dpi. The timeline for the key immunizations and immune analysis is shown in Fig. 7A. The numbers of Bmem in LN cultures (Fig. 7B) and the percentages of PCs in BM cultures (Fig. 7E) collected prior to the boost and 14 days postboost were subjected to fluorescence-activated cell sorter (FACS) analysis. Significantly more Bmem were observed in rLBNSE-IL-7-immunized mice than in rLBNSE-immunized mice at 360 days after the primary vaccination (P = 0.0262) (Fig. 7C) and at 14 days postboost (P = 0.0105) (Fig. 7D). The percentage of PCs in the mice immunized with rLBNSE-IL-7 was maintained at a significantly higher level than in the rLBNSE group for up to 360 days after the primary vaccination (P = 0.0129) (Fig. 7F) and for 14 days postboost (P = 0.0368) (Fig. 7G).
FIG 7.
IL-7 improved secondary antibody responses. At 360 days after the primary immunization, mice were boosted with 106 FFU of rLBNSE or mock boosted with DMEM. (A) The timeline shows the key immunization and immune analysis steps. (B and E) The total draining LN cells and BM cells of BALB/c mice (n = 3) were collected for measurement of Bmem (B) and PCs (E), respectively, prior to the boost and at 14 days after the boost immunization. (C and D) The number of Bmem in 105 total LN cells was determined at 360 days after the primary vaccination (C) and at 14 days postboost (D). (F and G) The percentage of PCs in 105 live BM cells was determined by FACS prior to the boost (F) and at 14 days postboost (G). (H and I) At 1 and 2 weeks postboost, sera were collected and VNA titers (H) and GMTs (I) (n = 8) were analyzed by FAVN testing. (J) OD values of total IgG, IgG1, IgG2a, IgG2b, IgG3, or IgM against RABV G were determined by ELISA. Error bars represent the SE, and data are representative of results of two independent experiments.
Three groups of female ICR mice (n = 8) were boosted with 106 FFU of rLBNSE or mock boosted with DMEM at 360 days after the primary immunization. Blood samples were collected to determine VNA titers and the levels of IgG isotypes at 1 and 2 weeks postboost, respectively. As expected, rLBNSE-IL-7 quickly induced significantly higher levels of VNA titers than rLBNSE at 7 (P = 0.0002) and 14 (P = 0.0023) dpi (Fig. 7H and I). RABV G-specific antibody subclasses, especially the IgG2a and IgG2b isotypes, were significantly upregulated in the rLBNSE-IL-7 group at 1 and 2 weeks postboost (Fig. 7J). Collectively, these results indicate that IL-7 helps to maintain Bmem and PCs to confer the long-term primary and enhanced secondary antibody responses.
DISCUSSION
Our previous studies with rRABVs expressing cytokines/chemokines such as MIP-1a (26) or granulocyte-macrophage colony-stimulating factor (GM-CSF) or flagellin (27) showed that these immune factors help to induce rapid and enhanced humoral immune responses by recruiting and activating DCs, which activate B cells and result in the enhanced production of antibodies, especially VNAs. However, the duration of the VNA production and maintenance is relatively short. Hence, there is still a need for research efforts aimed at exploring novel vaccines that can act over relatively long periods of time. Interestingly, a novel rRABV expressing IL-7 developed in this study can confer long-lasting humoral immunity and provide better protection against virulent rabies challenge. IL-7 has multifunctional roles as an immune modulator in adaptive immunity, especially in T and B cell survival and development, so it has been employed as an adjuvant to enhance the immunogenicity of many viral vectors (28–30). IL-7 signaling could augment the pool of antigen-specific T cells by promoting survival of naive CD4+ T cells and CD8+ T cells or by preventing apoptosis of activated T cells in the proliferative pool (31, 32). IL-7 is also known to costimulate T cell receptor (TCR) signaling (15, 33) and to sensitize TCR thresholds (34), which may facilitate the proliferation of CD4+ T cells into Tfh cells. In our present study, expressing IL-7 significantly increased the populations of Tfh cells, which are specialized supporters of B cells and important for the formation of GCs.
The size and quality of the GC response are directly affected by Tfh cells, which provide growth and differentiation signals to GC B cells and mediate positive selection of high-affinity B cell clones in the GCs (35, 36). In our study, significantly more GC B cells were detected in the mice immunized with rLBNSE-IL-7 than in the mice immunized with rLBNSE. This might be attributable to the increased Tfh cell populations and might lead to increased interactions between Tfh cells and GCs. FDCs serve as long-term reservoirs of intact antigens (37) and support GCs by secreting chemokines and cytokines that attract and sustain B cells (13). A previous report provided evidence that FDCs in the B cell follicles were a major source of IL-7 in the tonsillar B cells (38), and IL-7 acted as a prominent stimulus for the development in B cells (39–41). GL-7+ GC B cells were positive for immunoreactive IL-7R, which may be mainly supplied from FDCs, and blockade of IL-7R by monoclonal antibodies (MAb) may specifically suppress the GC reaction under conditions of immunization with the trinitrophenyl-keyhole limpet hemocyanin (42), which illustrated that IL-7 signal might play a crucial role in the organization of the GC response. Hence, within a pool of increased B cells, exogenous IL-7 may further facilitate GC formation with the assistance of increased levels of Tfh cells.
Despite extensive evidence demonstrating that IL-7 is a critical player in the expansion of CD4+ or CD8+ Tmem immune responses (43–45), identification of the specific role of IL-7 during the development of Bmem responses remains elusive. Interestingly, in our study, in the context of boosting with rLBNSE, the rLBNSE-IL-7 group was able to rapidly generate a robust VNA response, possibly due to the increased numbers of long-lived Bmem. The function of IL-7 has been found to occur in an IL-7R-dependent manner (46). Hence, the IL-7R-expressing cells that are involved in the increased levels of Bmem seen following enhancement with GC B cells might be prime candidates. The high levels of IL-7R expressed by CD4+ T cells may indicate the relevance of these cells to the ability of IL-7 to expand levels of Tfh cells and then to initiate the process of differentiation of augmented B cells into GC-derived B cells. These cells subsequently differentiate into Bmem and PCs after an extensive selection step (47). The role of IL-7 in the development of Bmem and PCs in the context of RABV-mediated immune responses appears to be important, but the details of this process remain to be fully explored.
Interestingly, we found that expression of IL-7 could attenuate RABV pathogenicity by restricting RABV transcription and replication in mouse brains. In a previous study, IL-7 helped to clear persistent infection by lymphocytic choriomeningitis virus 13 (LCMV-13) in mouse livers by downregulating a critical repressor of cytokine signaling, suppressor of cytokine signaling 3 (Socs3) (48). The suppression of Socs3 resulted in amplified cytokine production and increased T cell effector function. Additionally, IL-7 promoted the production of cytoprotective IL-22, which helped to relieve LCMV-induced liver pathology. Previous studies have demonstrated that antigen-specific Tmem could secret cytokines to recruit NK cells as effectors of adaptive immunity to the immediate vaccine-specific cytokine and cytotoxic recall response following rabies virus vaccination (49). The previous results suggested that IL-7 could augment the accumulation of functional virus-specific Tmem during recall responses (30), which might be followed by activation of NK cells to make a contribution to the clearance of pathogenic viruses. IL-7 expression may also upregulate some chemokines, resulting in the influx of leukocytes and clearance of the infection (50). The details of the mechanism underlying the IL-7-induced attenuation of RABV infection remain to be fully investigated.
In summary, our data indicate that the recombinant RABV expressing IL-7 designed for this study demonstrated an ability to attenuate the pathogenicity and high potency of the virus by inducing robust and long-lasting humoral responses. This recombinant RABV therefore has the potential to be used as a single-dose, long-lasting avirulent vaccine for animals.
MATERIALS AND METHODS
Viruses, cell lines, antibodies, and animals.
Recombinant RABV strain LBNSE was generated from the SAD-B19 strain as previously described (18, 27). Rabies challenge virus CVS-24 was propagated in suckling ICR mouse brains. BSR cells, which are a cloned cell line derived from BHK-21 cells, were cultured in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY). Mouse neuroblastoma (NA) cells were maintained in RPMI 1640 medium (Mediatech, Herndon, VA) enriched with 10% FBS. Fluorescein isothiocyanate (FITC)-conjugated antibodies against the RABV N protein were purchased from Fujirebio Diagnostics, Inc. (Malvern, PA). The following antibodies were purchased from BD Biosciences and used to label cells for flow cytometry: phycoerythrin (PE)-Cy7-B220 (clone RA3-6B2), allophycocyanin (APC)-CXCR5 (clone 2G8), APC-CD138 (clone 281-2), PE-CD95/Fas (clone Jo2), Alexa Fluor 647-GL7 (clone GL7), FITC-CD4 (clone RM4-5), PE-PD1 (clone J43), and PE-CD38 (clone 90; eBioscience). Five-day-old ICR mice and 6-week-old female ICR and BALB/c mice were purchased from the Hubei Center for Disease Control, Wuhan, China, and handled according to protocols approved by the Scientific Ethics Committee of Huazhong Agricultural University (permit number HZAUMO-2015-008).
Construction and rescue of recombinant RABVs that expressed murine IL-7.
rRABV vector pLBNSE was constructed as previously described (18). A transcription unit containing the BsiWI and NheI restriction sites was created and inserted between the G- and L-coding sequences by deleting the pseudogene. The rLBNSE-IL-7 or rLBNSE-IL-7(-) cDNA clone was generated from pLBNSE as previously described (18). Briefly, the pLBNSE vector was digested with BsiWI and NheI between the G and L genes. The murine IL-7 cDNA was subjected to RT-PCR amplification from RNA that was extracted from RABV-infected mouse brain tissues. The IL-7(-) gene was synthesized by the substitution of STOP codons for START codons within IL-7 gene. The IL-7 or IL-7(-) gene was then inserted into pLBNSE, resulting in pLBNSE-IL-7 or pLBNSE-IL-7(-), respectively. The primers used for PCR are listed in Table 1. The full-length clone of pLBNSE-IL-7 or pLBNSE-IL-7(-) and four helper plasmids that expressed the N, P, G, and L genes of the LBNSE parent virus were separately transfected into BSR cells using SuperFect transfection reagent (Qiagen, Valencia, CA) according to procedures described in previous studies (18). The rescued rRABVs were detected with FITC-conjugated antibodies against RABV N under an Olympus IX51 fluorescence microscope.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| IL-7-BsiWI-F | TTGCGTACGATGTTCCATGTTTCTTTTAG |
| IL-7-NheI-R | CTAGCTAGCTTATATACTGCCCTTC |
| IL-7-F | GAAAACATGTATGTGATGAT |
| IL-7-R | GGAAACATGCATCATTCT |
| vRNA-F | CCTCGTCGTCAGAGTTGACA |
| vRNA-R | GAGGAATTCTTCGGGAAAGG |
| mRNA-F | GAGGAATTCTTCGGGAAAGG |
| mRNA-R | TCATCTGCCAGTGCTACGTC |
BsiWI and NheI sites were underlined.
Virus titration.
Virus titers were determined using a direct fluorescent antibody assay as previously described (18). Briefly, a serial 10-fold dilution of the virus was inoculated into BSR cells in 96-well microplates in procedures performed in quadruplicate, and the cells were then incubated at 37°C for 48 h. After incubation, the cells were fixed with 80% ice-cold acetone and stained with FITC-conjugated RABV N protein-specific antibodies for 1 h. Antigen-positive foci were observed under an Olympus IX51 fluorescence microscope, and virus titers were calculated and are presented as numbers of focus-forming units per milliliter.
ELISA.
ELISA was performed to quantify the amount of IL-7 in the NA cell culture supernatants. Commercially available mouse IL-7 ELISA kits (RayBiotech, Atlanta, GA) were used following the manufacturer's instructions.
Cell viability assay.
Cell viability assays were performed using Cell Titer 96 AQueous One Solution cell proliferation assay kits (Promega, Madison, WI) according to the manufacturer's instructions.
Pathogenicity studies.
Six-week-old female ICR mice were inoculated i.c. with rLBNSE, rLBNSE-IL-7(-), or rLBNSE-IL-7 at a dose of 107 FFU or with DMEM in a volume of 30 μl under conditions of isoflurane anesthesia. Five-day-old ICR mice were inoculated i.c. with 100 FFU of different viruses in 10 μl DMEM. Mouse body weight loss or survival data were recorded daily for 3 weeks. Moribund mice were euthanized using CO2.
Mouse immunization and challenge test.
Six-week-old female ICR mice were randomly divided into two groups and immunized with a 100-μl solution containing 106 FFU of rLBNSE or rLBNSE-IL-7 or containing DMEM via an intramuscular (i.m.) route. Each of the groups was then further subdivided into two subgroups. The mice in the first of the two subgroups received a challenge infection with 30 μl of 50 LD50 CVS-24 via an i.c. route at 360 days after the primary immunization. The mice in the second subgroup, except the DMEM-injected mice, all received 106 FFU of rLBNSE via the i.m. route as a booster dose at an interval of 360 days postimmunization. Mice that were seen to be moribund or that had lost more than 30% of their starting body weight were humanely euthanized.
Vaccine potency test.
For analysis of vaccine potency against rabies, groups of 6-week-old female ICR mice were inoculated i.m. with 100 μl of serial 10-fold dilutions (102 to 106 FFU per dose) of rRABVs. Seven weeks after immunization, mice were challenged with an i.c. injection of 50 LD50 CVS-24 under conditions of isoflurane anesthesia and were observed for 3 weeks for clinical signs of rabies. The 50% effective dose (ED50) was calculated from the mortality rates in the different vaccine dilution groups as described previously (51).
Virus-neutralizing antibody (VNA) test.
VNA titers were evaluated in mouse serum using the fluorescent antibody virus neutralization (FAVN) test as previously described (52). Briefly, 50 μl of serial 3-fold dilutions of test serum and standard serum was prepared in 96-well plates in a total of 100 μl of cell culture medium. These experiments were performed in quadruplicate. Around 100 FFU of the rabies challenge virus (CVS-11) suspended in a 50-μl solution was added to each well. After the solution was incubated at 37°C for 1 h, 2 × 104 BSR cells were added to each well, and the solutions were incubated at 34°C for 72 h. The cells were then fixed with 80% ice-cold acetone for 30 min and stained with FITC-conjugated antibodies against the RABV N protein. Florescence was observed under an Olympus IX51 fluorescence microscope. The values for fluorescence were compared to the values of a reference serum (obtained from the National Institute for Biological Standards and Control, Hertfordshire, UK), and the results were normalized and quantified in international units per milliliter.
Evaluation of RABV-specific antibody isotypes using ELISA.
ELISA plates were coated with 500 ng/well RABV glycoprotein (RABV G) coating buffer (5 mM Na2CO3, pH 9.6) overnight at 4°C. The plates were then washed three times in phosphate-buffered saline (PBS)-Tween and blocked in 5% low-fat milk–PBS for 2 h at 37°C. The serum samples were diluted 1:30 in 100 μl PBS, and then one sample was added per well to the RABV G-containing wells. The samples were incubated for 2 h at 37°C. After incubation, the plates were washed three times in PBS-Tween, and then 100 μl of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:1,000), IgG1 (1:1,500), IgG2a (1:1,500), IgG2b (1:2,000), IgG3 (1:1,500), or IgM (1:2,000) (Boster, Wuhan, China) was added to each well. The plates were incubated at 37°C for 45 min. Postincubation, the plates were washed three times with PBS-Tween, and then tetra-methyl-benzidine (TMB) substrate was prepared and added to the wells according to the instructions of the manufacturer (Boster, Wuhan, China). The plates were incubated for 30 min at 37°C in the dark, and the reaction was then stopped by adding 2 M H2SO4. Optical density was then read at 450 nm using a SpectraMax 190 spectrophotometer (Molecular Devices, Sunnyvale, CA).
Flow cytometric analysis.
Groups of 6-week-old female BALB/c mice were inoculated i.m. with a 100-μl volume of 106 FFU of rLBNSE or rLBNSE-IL-7 or were subjected to mock infection with DMEM. The draining LNs and BM cells were collected 7 or 14 days postimmunization. Single-cell suspensions (105 cells/sample) were incubated in Stain Buffer (BD Biosciences, San Jose, CA) with fluorescence-conjugated antibodies for 30 min at 4°C in the dark. After incubation, the cells were washed 2 times with FACS buffer and then fixed in 1% paraformaldehyde–PBS for 30 min. Flow cytometry was completed using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA), and the results were analyzed using CytExpert software (Beckman Coulter, Brea, CA).
Quantitative real-time PCR (qRT-PCR).
To determine the levels of virus replication and RNA transcription in each mouse sample, virus-specific N mRNA and genomic RNA levels were analyzed in BALB/c mice that were i.c. infected with rLBNSE, rLBNSE-IL-7(-), or rLBNSE-IL-7 or injected with DMEM. qRT-PCR was performed using an ABI Prism 7500 fast sequence detector system with Power SYBR green PCR master mix (Applied Biosystems). The primers that were used are listed in Table 1. The brains were removed from the infected mice at predetermined time points and flash frozen on dry ice before they were stored at −80°C. Reverse transcriptase and DNA polymerase were used to perform a reaction with a one-step Brilliant II SYBR green qRT-PCR master mix kit (Stratagene). Each reaction was carried out in duplicate using approximately 100 ng of RNA and a 5 nM concentration of each primer (1 pair per sample), as shown in Table 1. The following program was used: one cycle at 50°C for 5 min and 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 65°C for 1 min. cDNA standards were made using the RABV N gene. Standard curves were constructed using threshold cycle (CT) values that were obtained using dilutions of the synthetic standards. The mRNA and genomic RNA copy numbers in each sample were normalized to the respective copy numbers that were derived from the standard curve.
Statistical analysis.
All data were analyzed using GraphPad Prism software (GraphPad Software, Inc., CA). For the percent survival tests, Kaplan-Meier survival curves were analyzed using the log rank test. For the other data, an unpaired two-tailed t test was used to determine statistical significance. For all tests, the following notations are used to indicate significant differences between groups: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
ACKNOWLEDGMENTS
This work was partially supported by the National Program on key Research Project of China (2016YFD0500400), the National Natural Science Foundation of China (31372419 and 31522057), and the European Union's Seventh Framework Programme LinkTADs (613804, to L.Z.) and by the Ministry of Science and Technology of China (863 program; 2011AA10A212) and the Ministry of Agriculture of China (special fund for Agro-scientific research in the Public Interest; 201303042, to Z.F.F.).
REFERENCES
- 1.Fooks AR, Banyard AC, Horton DL, Johnson N, McElhinney LM, Jackson AC. 2014. Current status of rabies and prospects for elimination. Lancet 384:1389–1399. doi: 10.1016/S0140-6736(13)62707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkhouse DA, Faber M, Hooper DC. 2015. Pre- and post-exposure safety and efficacy of attenuated rabies virus vaccines are enhanced by their expression of IFNgamma. Virology 474:174–180. doi: 10.1016/j.virol.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wunner WH, Briggs DJ. 2010. Rabies in the 21 century. PLoS Negl Trop Dis 4:e591. doi: 10.1371/journal.pntd.0000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schutsky K, Curtis D, Bongiorno EK, Barkhouse DA, Kean RB, Dietzschold B, Hooper DC, Faber M. 2013. Intramuscular inoculation of mice with the live-attenuated recombinant rabies virus TriGAS results in a transient infection of the draining lymph nodes and a robust, long-lasting protective immune response against rabies. J Virol 87:1834–1841. doi: 10.1128/JVI.02589-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiraishi R, Nishimura M, Nakashima R, Enta C, Hirayama N. 2014. Neutralizing antibody response in dogs and cats inoculated with commercial inactivated rabies vaccines. J Vet Med Sci 76:605–609. doi: 10.1292/jvms.13-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, McGettigan JP. 2012. Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection. J Virol 86:11533–11540. doi: 10.1128/JVI.00615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O'Garra A, Cahalan MD, Cyster JG. 2005. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol 3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nutt SL, Tarlinton DM. 2011. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol 12:472–477. [DOI] [PubMed] [Google Scholar]
- 9.Cyster JG. 2010. B cell follicles and antigen encounters of the third kind. Nat Immunol 11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 10.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. 2010. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett BE, Ciocca ML, Goenka R, Barnett LG, Wu JM, Laufer TM, Burkhardt JK, Cancro MP, Reiner SL. 2012. Asymmetric B cell division in the germinal center reaction. Science 335:342–344. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connor BP, Gleeson MW, Noelle RJ, Erickson LD. 2003. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev 194:61–76. doi: 10.1034/j.1600-065X.2003.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Victora GD, Nussenzweig MC. 2012. Germinal centers. Annu Rev Immunol 30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 14.Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW, Sahakian E, Kagoda M, Huang G, Hao QL, Sevilla Y, Barsky LW, Zielinska E, Price MA, Wall NR, Dovat S, Payne KJ. 2009. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol 182:4255–4266. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. 2005. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest 115:1177–1187. doi: 10.1172/JCI200523134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliyari Z, Alami F, Mostafavi T, Taiefi Nasrabadi H, Soleimanirad J, Nozad Charoudeh H. 2015. The roles of IL-2, IL-7, and IL15 ligands in B cells development from cord blood mononuclear cells. Iran J Ped Hematol Oncol 5:155–160. [PMC free article] [PubMed] [Google Scholar]
- 17.Seo YB, Im SJ, Namkoong H, Kim SW, Choi YW, Kang MC, Lim HS, Jin HT, Yang SH, Cho ML, Kim YM, Lee SW, Choi YK, Surh CD, Sung YC. 2014. Crucial roles of interleukin-7 in the development of T follicular helper cells and in the induction of humoral immunity. J Virol 88:8998–9009. doi: 10.1128/JVI.00534-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen YJ, Wang HL, Wu H, Yang FH, Tripp RA, Hogan RJ, Fu ZF. 2011. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J Virol 85:1634–1644. doi: 10.1128/JVI.01552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean MKA DJ, Atanasiu P. 1996. The fluorescent antibody test, p 88–95. In Meslin FX, Kaplan MM, Koprowski H (ed), Laboratory techniques in rabies, 4th ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 20.Goenka R, Barnett LG, Silver JS, O'Neill PJ, Hunter CA, Cancro MP, Laufer TM. 2011. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol 187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, Yu D, Fagarasan S, Tarlinton DM, Cunningham AF, Vinuesa CG. 2011. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med 208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. 2010. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med 207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. 2011. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One 6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP. 2013. Investigating the role for IL-21 in rabies virus vaccine-induced immunity. PLoS Negl Trop Dis 7:e2129. doi: 10.1371/journal.pntd.0002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramakrishna C, Stohlman SA, Atkinson RD, Shlomchik MJ, Bergmann CC. 2002. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J Immunol 168:1204–1211. doi: 10.4049/jimmunol.168.3.1204. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Toriumi H, Wang H, Kuang Y, Guo X, Morimoto K, Fu ZF. 2010. Expression of MIP-1alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J Virol 84:9642–9648. doi: 10.1128/JVI.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou M, Zhang GQ, Ren GP, Gnanadurai CW, Li ZG, Chai QQ, Yang Y, Leyson CM, Wu WX, Cui M, Fu ZF. 2013. Recombinant rabies viruses expressing GM-CSF or flagellin are effective vaccines for both intramuscular and oral immunizations. PLoS One 8:e63384. doi: 10.1371/journal.pone.0063384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombetti S, Levy F, Chapatte L. 2009. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood 113:6629–6637. doi: 10.1182/blood-2008-05-155309. [DOI] [PubMed] [Google Scholar]
- 29.Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, Assouline B, Lahl K, Sparwasser T, Ohashi PS, Mak TW. 2009. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med 15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 30.Nanjappa SG, Walent JH, Morre M, Suresh M. 2008. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J Clin Invest 118:1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 32.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A 98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fry TJ, Mackall CL. 2005. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol 174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 34.Deshpande P, Cavanagh MM, Le Saux S, Singh K, Weyand CM, Goronzy JJ. 2013. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. J Immunol 190:1416–1423. doi: 10.4049/jimmunol.1201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linterman MA, Hill DL. 2016. Can follicular helper T cells be targeted to improve vaccine efficacy? F1000Res 5(F1000 Faculty Rev):88. doi: 10.12688/f1000research.7388.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. 2013. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Mandel TE, Phipps RP, Abbot AP, Tew JG. 1981. Long-term antigen retention by dendritic cells in the popliteal lymph node of immunized mice. Immunology 43:353–362. [PMC free article] [PubMed] [Google Scholar]
- 38.Kröncke R, Loppnow H, Flad HD, Gerdes J. 1996. Human follicular dendritic cells and vascular cells produce interleukin-7: a potential role for interleukin-7 in the germinal center reaction. Eur J Immunol 26:2541–2544. doi: 10.1002/eji.1830261040. [DOI] [PubMed] [Google Scholar]
- 39.Lee G, Namen AE, Gillis S, Ellingsworth LR, Kincade PW. 1989. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol 142:3875–3883. [PubMed] [Google Scholar]
- 40.Corfe SA, Paige CJ. 2012. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin Immunol 24:198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Monroe JG, Allman D. 2004. Keeping track of pro-B cells: a new model for the effects of IL-7 during B cell development. Eur J Immunol 34:2642–2646. doi: 10.1002/eji.200425532. [DOI] [PubMed] [Google Scholar]
- 42.Hikida M, Nakayama Y, Yamashita Y, Kumazawa Y, Nishikawa SI, Ohmori H. 1998. Expression of recombination activating genes in germinal center B cells: involvement of interleukin 7 (IL-7) and the IL-7 receptor. J Exp Med 188:365–372. doi: 10.1084/jem.188.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. 2004. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc Natl Acad Sci U S A 101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colpitts SL, Dalton NM, Scott P. 2009. IL-7 receptor expression provides the potential for long-term survival of both CD62Lhigh central memory T cells and Th1 effector cells during Leishmania major infection. J Immunol 182:5702–5711. doi: 10.4049/jimmunol.0803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui GL, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu JX, Kaech SM. 2015. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8(+) T cell longevity. Cell 161:750–761. doi: 10.1016/j.cell.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobs SR, Michalek RD, Rathmell JC. 2010. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol 184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesin L, Ersching J, Victora GD. 2016. Germinal center B cell dynamics. Immunity 45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW. 2011. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. 2010. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol 185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 50.Michlmayr D, McKimmie CS, Pingen M, Haxton B, Mansfield K, Johnson N, Fooks AR, Graham GJ. 2014. Defining the chemokine basis for leukocyte recruitment during viral encephalitis. J Virol 88:9553–9567. doi: 10.1128/JVI.03421-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilbur LA, Aubert MFA. 1996. The NIH test for potency, p 360–368. In Meslin FX, Kaplan MM, Koprowski H (ed), Laboratory techniques in rabies, 4th ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 52.Cliquet F, Aubert M, Sagne L. 1998. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods 212:79–87. doi: 10.1016/S0022-1759(97)00212-3. [DOI] [PubMed] [Google Scholar]