SUMMARY
Respiratory syncytial virus (RSV) is an important etiological agent of respiratory infections, particularly in children. Much information regarding the immune response to RSV comes from animal models and in vitro studies. Here, we provide a comprehensive description of the human immune response to RSV infection, based on a systematic literature review of research on infected humans. There is an initial strong neutrophil response to RSV infection in humans, which is positively correlated with disease severity and mediated by interleukin-8 (IL-8). Dendritic cells migrate to the lungs as the primary antigen-presenting cell. An initial systemic T-cell lymphopenia is followed by a pulmonary CD8+ T-cell response, mediating viral clearance. Humoral immunity to reinfection is incomplete, but RSV IgG and IgA are protective. B-cell-stimulating factors derived from airway epithelium play a major role in protective antibody generation. Gamma interferon (IFN-γ) has a strongly protective role, and a Th2-biased response may be deleterious. Other cytokines (particularly IL-17A), chemokines (particularly CCL-5 and CCL-3), and local innate immune factors (including cathelicidins and IFN-λ) contribute to pathogenesis. In summary, neutrophilic inflammation is incriminated as a harmful response, whereas CD8+ T cells and IFN-γ have protective roles. These may represent important therapeutic targets to modulate the immunopathogenesis of RSV infection.
KEYWORDS: immunology, respiratory syncytial virus
INTRODUCTION
Respiratory syncytial virus (RSV) is an enveloped single-stranded RNA virus belonging to the Pneumoviridae family of the Mononegavirales order. Infections occur worldwide, with outbreaks in temperate climates occurring primarily during the winter months. RSV is an important etiological agent of respiratory infections, particularly in children, causing a spectrum of illness encompassing upper respiratory tract infections (URTI) and lower respiratory tract infections (LRTI), including pneumonia and bronchiolitis, which are associated with greater morbidity and mortality. Natural infection results in incomplete immunity, permitting recurrent infection in childhood as well as infections in adults, including the elderly. Much information regarding the immune response to RSV comes from murine and other animal models and in vitro human cell culture studies. While important for hypothesis generation, these methodologies may not provide a completely accurate reflection of the immune response during infection in humans. Here, we provide a comprehensive description of the human immune response to RSV infection, based on a systematic literature review exclusively of clinical, ex vivo, and postmortem data from naturally and experimentally infected humans.
In this review, we consider the existing data describing the major cellular and humoral components of the immune response to RSV, distinguishing events occurring systemically from those occurring locally within the respiratory tract (Fig. 1). First we describe the behavior of all major immune cell types, encompassing neutrophils, dendritic cells (DCs), monocytes, macrophages, eosinophils, and T lymphocytes. Second, the anti-RSV antibody response and its regulation is discussed. Next, the distinct Th1 and Th2 responses to RSV and the effect of their balance on disease progression are considered. Several chemokines, cytokines, and other immune molecules have been demonstrated to be involved in the immune response and are reviewed. The global host transcriptional response is also discussed in the context of immune-related pathways. Certain key pathogen-host interactions described herein may represent targets for the development of novel therapeutics. For completeness, we summarize the association between RSV infection and subsequent asthma and also key differences between immune responses in humans and those in animals used in model systems of infection.
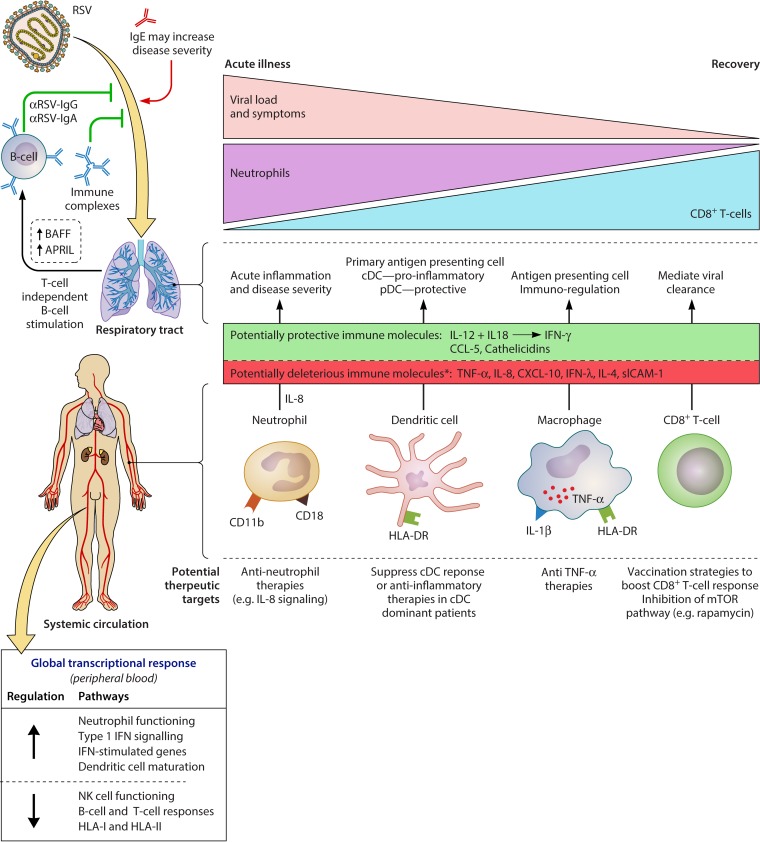
FIG 1.
Summary of the human immune response to RSV and potential novel therapeutic targets. The roles of major cell types (neutrophils, dendritic cells, macrophages, CD8+ T cells, and B cells) are summarized, in addition to key antibody, cytokine, chemokine, and other immune molecule responses. Major transcriptional changes (in peripheral blood) of immune-related pathways are shown. The deleterious role of neutrophilic inflammation and the protective role of CD8+ T-cell-mediated viral clearance are emphasized. Finally, we highlight areas where novel therapeutic interventions could potentially modulate the immune response in favor of the host. ↑, immune cell recruitment to the respiratory tract; *, association with increased disease severity.
METHODS OF SYSTEMATIC LITERATURE REVIEW
We conducted a systematic literature review following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (PROSPERO registration number CRD42016047320). An electronic literature search of Medline, Embase, and Web of Science was performed using the following search terms: (RSV[Title] OR respiratory syncytial virus[Title]) AND (immune response OR T cell OR B cell OR lymphocyte OR macrophage OR neutrophil OR monocyte OR natural killer cell OR dendritic cell OR immunoglobulin OR IgG OR IgA OR IgE OR cytokine OR chemokine OR interleukin OR interferon) AND (human OR clinical OR experimental OR neonate OR infant OR children OR adult OR elderly).
The last search was conducted on 16 May 2016. The results from the databases were merged and duplicates removed. The combined results of the electronic database search were assessed independently by two authors, and discrepancies were discussed and agreed upon according to the inclusion and exclusion criteria. Publications in all languages describing primary research in humans (clinical, ex vivo, or postmortem) were included. Editorials, reviews, commentaries, and opinion pieces were excluded. Articles were limited to those published after 1990. Additional articles of interest were identified from reviewing the bibliographies of relevant articles. The literature search resulted in 2,541 publications after removal of duplicates and pre-1990 publications. Two authors reviewed titles and abstracts and identified 268 records that then underwent full text review. Of these, 166 met the inclusion criteria. A further 9 articles were identified through other sources, including bibliographies of identified articles.
SYSTEMIC AND PULMONARY IMMUNE CELL RESPONSES TO RSV INFECTION
Neutrophils
RSV infection elicits a strong systemic and especially respiratory tract neutrophil response (1–4). Neutrophils are the predominant cell type in bronchoalveolar lavage (BAL) fluid from the lungs of ventilated infants with severe RSV bronchiolitis and those with milder infection (5). These cells are activated during the initial pathogenesis of RSV LRTI, producing neutrophil elastase (6, 7) and expressing activation markers (CD11b, CD18, and CD54 [ICAM-1]) (8, 9). The peak neutrophil response coincides with maximum clinical severity and viral load, and by the time infants with severe infection are discharged from the intensive care unit (ICU) after ventilation, neutrophil counts in peripheral blood have normalized (10). Widespread neutrophil infiltration is seen in lung tissue from fatal cases of RSV LRTI (3, 11).
During severe infection, the virus interacts directly with neutrophils. Cells from peripheral blood and BAL fluid express RSV proteins F, G, and N proportionately, implying stoichiometric expression and thus intact intracellular virions (12). RSV genomic RNA and mRNA are also present intracellularly (12, 13). This could be explained by phagocytosis of virions or replication of RSV within neutrophils. These RSV-containing neutrophils detected in the peripheral blood may have transmigrated from the lungs into the circulation.
Neutrophil apoptosis and neutrophil extracellular trap (NET) formation (“NETosis,” a unique form of neutrophil cell death) are active during infection. Proteins involved in apoptosis (annexin V and the Fas death receptor CD95) are upregulated in nasopharyngeal fluid, and NETs are present in BAL fluid from ventilated children (8, 14). NETs may prevent spread of infectious virions and comprise a web-like DNA backbone studded with histones and cytotoxic/antimicrobial proteins.
Natural Killer Cells
RSV infection results in reduced total systemic natural killer (NK) cell counts, albeit with an increase in an activated subset that lacks expression of CD94 (15, 16). Circulating NK cells have higher expression of the inhibitory leukocyte immunoglobulin-like receptor subfamily B member (LILRB1), suggesting that they may contribute to regulation of inflammation during infection (17). Lower systemic total counts correlate with greater severity of infection, and NK cells are sparse in lung tissue from fatal cases (3, 15, 18, 19). In contrast, there is accumulation of granzyme B-expressing NK cells in the respiratory tracts of infants ventilated due to severe RSV bronchiolitis (BAL fluid and tracheal aspirate), possibly suggesting migration to the lungs (20, 21).
Dendritic Cells
Conventional dendritic cells (cDCs) and plasmacytoid DCs (pDCs) are mobilized from the circulation to the nasal mucosa early during infection, with a further increase in DC counts during subsequent convalescence (22, 23). The RSV fusion protein is present within HLA-DR+ DCs in the nasal mucosa, and the selective emigration of DCs, but not monocytes, highlights their likely role as the primary antigen-presenting cell during RSV infection (23). Low numbers of blood pDCs have been associated with the development of RSV bronchiolitis, suggesting either increased emigration to the respiratory tract or an insufficient pDC response in severe RSV infection (24).
cDCs and pDCs have also been found in the lower airways of infants ventilated due to severe RSV bronchiolitis, where cDCs exhibit an activated proinflammatory phenotype (20). Circulating cDCs express the activation marker CD83 and the costimulatory molecule CD40. Concentrations of innate immune proinflammatory cytokines (interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], and IL-8) and T-cell-derived cytokines (gamma interferon [IFN-γ], IL-13, IL-10, and IL-2) in BAL fluid correlate with cDC counts. In subsets of infants with severe RSV bronchiolitis (preterm infants and infants aged 4 months or more), pulmonary pDC counts are low compared to those in term-born and younger infants, suggesting an inadequate antiviral response as a factor in severe RSV disease (20).
Macrophages and Monocytes
Alveolar macrophages obtained from BAL fluid from RSV-infected infants and adult transplant recipients coexpress RSV surface glycoproteins, HLA-DR molecules, IL-1β, and cytoplasmic TNF-α, suggesting a local immune-regulatory and antigen-presenting role (25, 26). The cells appear to be infected productively, as viral replication from the cells can be confirmed ex vivo (25).
CD69+ monocytes are present in lung tissue from fatal cases of RSV infection (11). In the peripheral blood, monocytes display reduced Toll-like receptor 8 (TLR8) expression and TNF-α production during acute RSV infection, which subsequently normalizes in convalescence (27). In contrast, circulating monocytes increase their expression of TLR4 in RSV infection (28).
Eosinophils
Eosinophils are activated during the acute phase of RSV LRTI and may contribute to recovery. Expression of the myeloid activation marker CD11b on circulating eosinophils from infants with RSV LRTI is increased and inversely correlates with the required duration of supplemental oxygen (29). In comparison to children hospitalized due to influenza virus or adenovirus infection, those with RSV infection have higher systemic eosinophil counts during recovery but not at presentation (30). Despite a lack of data demonstrating significant eosinophil recruitment to the respiratory tract, there is evidence of eosinophil activity during bronchiolitis. Leukotriene C4, eosinophil-derived neurotoxin (EDN), and eosinophil cationic protein (ECP) are elevated in the respiratory tract in RSV bronchiolitis, detectable in nasal fluid (leukotriene C4 and ECP) and lower airway secretions (EDN and ECP) (31–33), while one study did not find increased ECP levels (34). Nasopharyngeal ECP concentrations are also elevated in children with RSV LRTI (not specifically bronchiolitis) and URTI (35–39). Nasal ECP concentrations correlate with nasal concentrations of the neutrophil chemoattractant CCL-3 (MIP-1α) and systemic neutrophil and eosinophil counts (37, 39). Concentrations of CCL-5 (RANTES) (an eosinophil chemoattractant), ECP, and eotaxin all increase during the progression from acute illness to recovery in RSV LRTI and correlate with respiratory tract eosinophil counts, suggesting that this response may have a role in resolution (30, 38, 40, 41). In contrast to the apparent proresolution role of eosinophils themselves during RSV infection, it seems that a Th2-biased response, of which eosinophilia is a component, may be associated with more severe disease, and this is discussed in detail in “Th2 Responses” below.
T Lymphocytes
An initial transient systemic T-cell lymphopenia occurs during RSV LRTI. Counts of CD8+, CD4+, CD3+, and γδ-T cells are all reduced compared to those during convalescence and in noninfected infants (2, 15, 16, 18, 19, 30, 42–44). There is no increased expression of CD11a (LFA-1α) in circulating T cells, suggesting that these cells are not activated, nor is there increased expression of CTLA-4, a marker of downregulated T-cell activation (45, 46). Absolute T-cell counts during RSV infection are inversely associated with age; thus, T-cell lymphopenia is more pronounced in younger patients (42). Children with more severe illness and those requiring ventilation have reduced circulating T-cell counts (all subsets) compared to those with less severe infection, and in lung tissue from fatal cases, CD4+ and CD8+ T cells are sparse (3, 16, 43, 47, 48). During the course of disease, circulating CD8+ T-cell counts increase (16, 49). In mechanically ventilated infants with severe RSV LRTI, systemic effector CD8+ T-cell counts are low during maximum symptoms and viral load and then peak during convalescence (after the systemic neutrophil response) (10, 49). At the time of ICU discharge, circulating CD8+ T-cell counts are temporarily elevated, whereas neutrophils are normal.
Circulating FOXP3 mRNA and counts of FOXP3+ CD4+ regulatory T cells (comprising suppressive resting Treg cells [CD45RA+ FOXP3lo] and suppressive activated Treg cells [CD45RA+ FOXP3hi]) are reduced in infants hospitalized with RSV bronchiolitis and for at least 3 weeks following acute infection (50, 51). Whether this represents apoptosis or recruitment to the lungs is unknown. Absolute counts of circulating regulatory T cells do not correlate with disease severity (52).
CD4+ and CD8+ T cells are present in BAL fluid obtained from infants with RSV LRTI, with a predominance of CD4+ T cells (4, 5). During the course of infection, the expansion of CD8+ T cells is greater than that of CD4+ T cells, and the CD8+ T cells exhibit an effector phenotype (HLA-DR+ granzyme B+ CD38+). Lower respiratory tract (tracheal aspirate and BAL fluid) granzyme A and B levels are elevated in ventilated patients, and granzyme B is expressed by CD8+ T cells (21). In bronchiolitis specifically, peripheral blood RSV-specific cell-mediated cytotoxic immune responses are more frequent in infants with mild infection than in those with severe infection (53). In experimental RSV infection of adults, the arrival of CD8+ T cells to the lungs (in BAL fluid) is associated with a reduction in pulmonary viral load (54). The frequency of preexisting RSV-specific pulmonary CD8+ T cells in BAL fluid is inversely associated with pulmonary viral load and symptom severity.
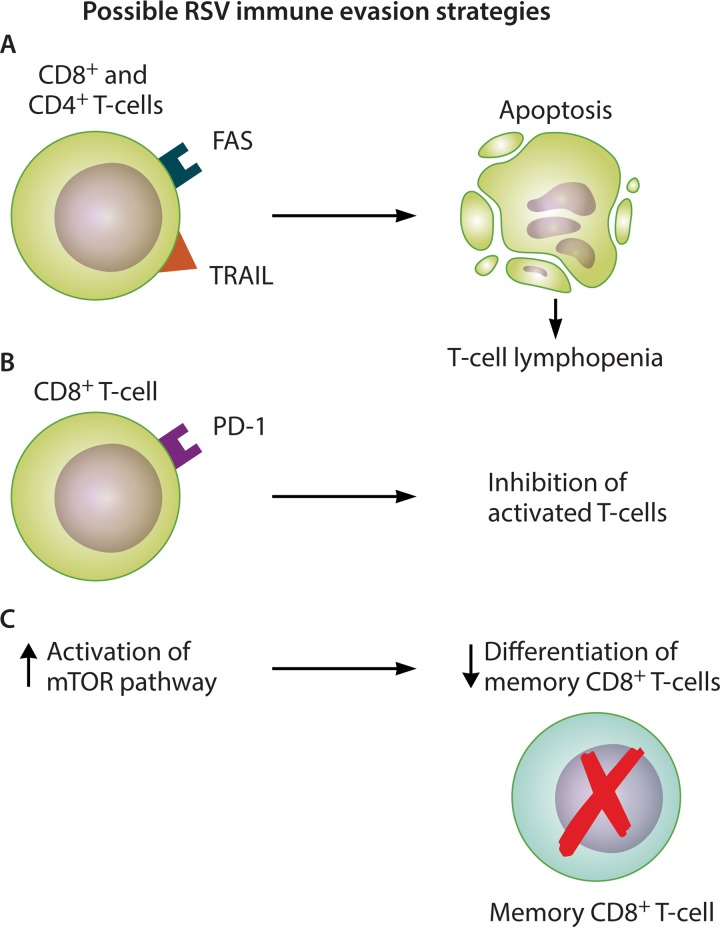
During acute infection, there is upregulation of Fas and TRAIL receptor expression on circulating CD4+ and CD8+ T cells compared to that in in convalescence (42). Systemic concentrations of soluble Fas ligand and caspase-1 are elevated. An inverse correlation exists between CD4+ T-cell Fas expression and cell counts. Therefore, one mechanism underlying systemic lymphopenia may be the induction of T-cell apoptosis as a viral immune evasion strategy (Fig. 2A). Furthermore, programmed cell death 1 (PD-1) protein expression is specifically upregulated on pulmonary CD8+ T cells during RSV LRTI (55). PD-1 is a T-cell-coinhibitory receptor that is inhibitory to activated T cells; therefore, PD-1 upregulation could be another immune evasion strategy to blunt the cytotoxic T-cell response (Fig. 2B). RSV infection may also impair differentiation of CD8+ T cells into memory cells by inducing mammalian target of rapamycin (mTOR) activation (Fig. 2C) (56). mTOR mRNA expression is increased in the lungs of infants with RSV bronchiolitis compared to those with human metapneumovirus and rhinovirus infection (and healthy controls), and the RSV cases have a higher proportion of CD8+ mTORser2448+ T cells, indicating activation of the mTOR pathway by phosphorylation on serine 2448 (56). Higher prolactin and lower leptin levels have been associated with lymphopenia in severe RSV infection, suggesting a neuroendocrine component, although these hormonal differences could also be explained by the systemic effects of critical illness (57).
FIG 2.
Mechanisms of RSV T-cell interference as a potential immune evasion strategy. RSV infection is associated with an initial systemic T-cell lymphopenia that is quantitatively associated with disease severity. RSV may interfere with T-cell responses by inducing apoptosis (CD4+ and CD8+ T cells) (A), inducing increased expression of the programmed cell death 1 (PD-1) protein, which is inhibitory to activated T cells (CD8+ T cells) (B), and promoting activation of the mammalian target of rapamycin (mTOR) pathway, thus preventing memory CD8+ T-cell formation (C).
Defective T-cell responses.
Deficits in systemic CD4+ and CD8+ T-cell responses may contribute to RSV susceptibility in the elderly, as these subjects have lower levels of RSV-specific CD4+ and CD8+ T cells than younger adults (58, 59). Interestingly, there is no decrease in the level of influenza virus-specific CD8+ T cells with increasing age (59). Furthermore, immunosuppressant drugs prescribed for solid organ transplant recipients (glucocorticoids, calcineurin inhibitors, azathioprine, mycophenolate mofetil, and sirolimus) all have inhibitory activity against T cells, thus impairing the ability of these patients to clear opportunistic RSV infection, resulting in more severe RSV disease (60). Similarly, hematopoietic stem cell transplant recipients are also at increased risk of severe RSV disease, and peripheral blood lymphopenia has been identified as a specific risk factor for RSV LRTI (61).
Cellular Response in Term and Preterm Infants
Total cellularity, neutrophil counts, macrophage counts, and lymphocyte counts in BAL fluid from infants ventilated due to RSV bronchiolitis are all higher in term than in preterm infants, which possibly is related to immune system maturation (62).
B-LYMPHOCYTE RESPONSES AND ANTIBODY PRODUCTION DURING RSV INFECTION
Antibody Production and B-Lymphocyte Stimulation
There is an increase in circulating B cells, including mature (CD19+ CD5+) and precursor (CD19+ CD10+) cells, in infants with RSV LRTI, and CD20+ B cells and IgM+, IgG+, and IgA+ plasma cells are prominent in postmortem lung tissue from infants with fatal RSV bronchiolitis (43, 63, 64). Antibody responses target the F and G glycoproteins and increase between the acute and convalescent phases of natural primary infection of infants (65). Bronchiolitis may lead to a greater IgG response (66). Type I interferon (IFN) is implicated in early antiviral B-cell responses, and type I IFN-induced proteins (myxovirus resistance protein A and 2′,5′-oligoadenylate synthetase 1) are present in high concentrations in bronchiolar and alveolar epithelial cells from RSV-infected infants (63). The B-cell-stimulating factors, a proliferation-inducing ligand (APRIL) and B-cell-activating factor (BAFF), are also present, colocalized to infected epithelial cells. APRIL and BAFF receptors are expressed on a subset of perialveolar plasma cells. In infants ventilated due to severe RSV bronchiolitis, pulmonary BAFF levels are increased (67, 68). BAFF mRNA levels are elevated in bronchial brushings, further suggesting that airway epithelial cells are the source (67). RSV IgA, IgG, and IgM are present in the lungs of infants with RSV LRTI, together with higher quantities of BAFF and APRIL but lower levels of T-cell-dependent cytokines (IL-2, IL-4, and IL-10) (63, 69). APRIL concentrations correlate positively with RSV IgA and IgM levels and inversely with hypoxia. Thus, the pulmonary antibody response to RSV seems to be driven predominantly by T-cell-independent antibody production via B-cell-stimulating factors (APRIL and BAFF), likely derived from infected pulmonary epithelial cells. In adults with RSV infection, a longer duration of virus shedding is associated with prolonged presence of circulating RSV-specific plasma cells, suggesting that persistent antigenic stimulation in the lung drives B-cell stimulation (70). Similarly, in elderly adults with nosocomial RSV infection, the highest IgG and IgA responses postinfection are seen in patients with more severe illness, perhaps correlating with viral load (71).
In comparison to healthy controls and rotavirus-infected infants, there is a high prevalence of anti HEp-2 (antinuclear) antibodies in infants with RSV LRTI (72). Decay of these autoantibodies was not studied (nor was their presence preinfection), but further investigation of subsequent development of autoimmune disease seems warranted.
Protective Effects of RSV IgG and RSV IgA
In experimental infection of healthy adults, higher preinoculation nasal RSV IgA and serum anti-RSV neutralizing-antibody titers are associated with protection from infection and reduced viral replication (73–77). RSV-specific nasal IgA, serum IgG, and serum neutralizing titers in adults are also all associated with protection against natural RSV reinfection (78, 79). In experimental infections, nasal RSV IgA appears to confer more protection than serum neutralizing antibody, and the response may be more durable (74, 80). Similarly, in infants and children with natural infection, it is the development of the IgA response that appears to correlate with recovery (81). During convalescence, circulating RSV IgG- but not IgA-producing memory B cells are present, in contrast to the case for natural influenza virus infection, where influenza virus IgA-producing memory B cells are detectable (74). Overall, a possible deficit in IgA memory, especially in childhood, when IgA appears to offer important protective immunity, may contribute to recurrent infections (74, 81). In contrast, in elderly patients it is a deficit in circulating serum neutralizing antibodies that appears to predispose to RSV disease (79).
In symptomatic RSV-infected and noninfected children, circulating RSV IgG is present at the highest level in those <1 month old, likely derived from transplacental maternal antibody transfer (82). IgG levels decrease after 3 months until 2 years, when levels increase again. The avidity of IgG is significantly lower among symptomatic RSV-infected infants aged 1 to 3 months than in age-matched controls. Similarly, in children aged ≥24 months, total IgG affinity was lower for children with RSV LRTI than for those with milder URTI. Serum RSV IgG and nasal RSV IgA neutralizing activity is quantitatively higher in children aged 9 to 21 months compared to those aged 4 to 8 months (the age group with a higher incidence of RSV infection) (83). In infants there is a reverse correlation between preexisting serum IgG and the development of nasal IgA following infection, suggesting that maternally derived IgG may suppress the IgA response (84). These observations suggest that good IgG and IgA avidity for RSV contributes to protection against both the development of symptomatic infection and more serious lung involvement. Following natural reinfection in adulthood, there is an 8-fold increase in serum neutralization titer, but this is short-lived, with a 4-fold drop by 1 year in the majority of cases (85).
The serum neutralizing antibody response and nasal IgA and IgG response to the G glycoprotein are RSV group specific (86, 87). In contrast, antibodies to the F glycoprotein are cross-reactive between RSV groups (88).
Other Mechanisms of RSV-Specific Antibody Activity
Maximal cell-bound C3 is present during the convalescent phase and is associated with cell-bound IgG and IgM (89). RSV antigen-containing immune complexes are detectable in the upper airways of infected infants from 3 days after the onset of illness and up to 36 days after (90). The appearance of such immune complexes coincides with the failure to detect RSV antigen in airway epithelial cells, possibly due to antibody-dependent cell-mediated cytotoxicity (ADCC), which occurs in infants with primary RSV infection (91). ADCC activity correlates with the titer of RSV IgG in the upper airways and is greater during reinfection than during primary infection.
Immunoglobulin E
An IgE response is mounted against the RSV F and G glycoproteins and may play a deleterious role (92). In infants with RSV bronchiolitis, there is a higher proportion of circulating CD23+ B cells (CD23 is the low-affinity IgE receptor on mature and activated B cells) than in those with non-RSV bronchiolitis and noninfected infants (93). Nasopharyngeal RSV IgE, histamine, and leukotriene C4 levels are interrelated and associated with bronchiolitis (where peak levels correlate with hypoxia) compared to other manifestations of infection (URTI or pneumonia) (36, 94). In children with RSV bronchiolitis or pneumonia, higher serum IgE at admission has been associated with prolonged fever and worse symptoms and IgE titers and eosinophil counts with the development of wheeze during RSV LRTI (95–97).
Th1 AND Th2 RESPONSES TO RSV INFECTION
Th1 Responses
Th1 responses are characterized by production of IFN-γ, IL-1, IL-2, IL-12, IL-18, and TNF-α. IL-12 induces IFN-γ production and favors Th1 cell differentiation. The Th1 response is proinflammatory and is important in the generation of cell-mediated immunity required for the control of intracellular pathogens. Therefore, it is an inherently appropriate response to viral infection.
Systemic.
Markers of the Th1 response (IFN-γ, soluble tumor necrosis factor receptor II, and soluble interleukin-2 receptor [sCD25]) are elevated in the circulation during RSV LRTI, and systemic IFN-γ exerts a protective effect (97–100). Children ≤6 months with RSV bronchiolitis have a reduced IFN-γ response, possibly contributing to the increased incidence of RSV disease in the younger age group (101). In infants with RSV LRTI, systemic IFN-γ concentrations are lower in those with severe disease (48, 98). Infants with RSV bronchiolitis requiring ventilation have lower IFN-γ concentrations than those with milder disease, and undetectable circulating IFN-γ positively correlates with the need for ventilation (102). Low IFN-γ/IL-10 ratios are associated with hypoxia and wheeze (99). During the acute phase of RSV LRTI, peripheral blood mononuclear cell (PBMC) IFN-γ mRNA expression is lower in hypoxic patients (47). Furthermore, circulating IL-12 levels are lower in severe those with RSV LRTI than in those with mild infections or controls (48, 98).
Respiratory tract.
IFN-γ levels are also elevated in the nasal mucosa (37, 103–106) and in the lung (20, 106). The respiratory tract IFN-γ response exerts a protective effect, with lower IFN-γ production associated with increased severity scores, hypoxia, and need for ventilation (106–110). In RSV LRTI, the nasopharyngeal IFN-γ/IL-10 ratio increases from presentation to discharge, in parallel with clinical recovery, strengthening the association of IFN-γ with protection (41).
Other Th1-associated cytokines are also elevated in the nasal mucosa (IL-1, IL-2, IL-12, IL-18, and TNF-α) (37, 111, 112) and in the lungs (IL-1, IL-2, and TNF-α) (20, 113). TNF-α levels are highest during the acute phase of infection and then decline during recovery (37, 105, 113–115). Raised IL-6 mRNA and protein have been observed in BAL fluid and nasopharyngeal fluid from infants with severe RSV infection, and a high IL-6/TNF-α ratio is associated with reduced disease severity (113, 116). In children with only URTI, there is reduced nasal production of anti-inflammatory IL-10, and this is inversely related to TNF-α production (117). It has been suggested that a reduction in IL-10 production facilitates a robust TNF-α response, limiting the infection to the upper airway.
Increased nasal concentrations of IL-1α are associated with the need for ventilation in children with RSV LRTI (118, 119). There is also an increase in nasal IL-18 concentrations and the number of IL-18-positive cells in children with RSV bronchiolitis compared to those with URTI (117). In bronchiolitis, nasal IL-18 production is associated with nonhypoxic infection, consistent with its role in stimulating IFN-γ production (117, 120).
Th2 Responses
The Th2 response, characterized by IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13 production, is involved in the generation of antibody (in particular IgE) and eosinophil responses. This response is associated with atopy and also protection against parasitic infections, and it may counteract and limit Th1-mediated inflammation.
Systemic.
Systemic IL-4, IL-6, IL-10, and IL-13 levels are elevated in children with RSV LRTI (37, 97, 101, 121–123). Systemic IL-6 and IL-10 levels correlate with disease severity in RSV LRTI, including the requirement for supplemental oxygen (99, 122, 124, 125). In comparison to those during influenza A virus infection, the systemic concentrations of IL-4, IL-5, and CCL-5 are higher during RSV LRTI (126).
Respiratory tract.
Elevated concentrations of IL-4, IL-6, IL-9, IL-10, and IL-13 have been found in nasal washes (37, 109, 127–130) and in the lung (20, 131–133) in children with RSV LRTI. Respiratory tract IL-10 production appears to exert a protective effect in RSV LRTI, with concentrations inversely correlating with the duration of required supplemental oxygen and symptom severity (108, 128, 132). In very young infants (<3 months), this effect appears to be reversed, with IL-10 concentrations correlating with severity (125), and nasal IL-10/CCL-5 ratios are only inversely correlated with duration of mechanical ventilation when infants older than 5 months are considered (134).
IL-6 levels are strongly elevated in BAL fluid from infants ventilated due to severe RSV bronchiolitis (20, 113) and are elevated to a lesser extent in the respiratory tract in infants with milder infection (37, 117, 132). There are inconsistent data associating the nasal IL-6 response with severity. In infants with RSV bronchiolitis, nasal IL-6 concentrations are higher in those requiring ventilation and correlate with the degree of hypoxia (111, 118, 135, 136). Similarly, adults hospitalized due to RSV infection have higher nasal IL-6 concentrations than those not requiring hospitalization (137). In experimentally infected adults, the nasal IL-6 concentration is positively correlated with viral load and symptom severity (138). In contrast, in a cohort of children with RSV bronchiolitis, higher nasal IL-6 concentrations were associated with a shorter requirement for supplemental oxygen (108).
Th1/Th2 Balance
A high nasal and systemic IL-4/IFN-γ ratio, a marker of Th2 bias, is associated with severe (hypoxic) RSV bronchiolitis (103, 123, 139). Independent of the ratio, IFN-γ concentrations are lower and IL-4 concentrations higher in infants with severe bronchiolitis. Also in severe RSV bronchiolitis, circulating CXCR3+ T-cell (Th1) counts are significantly reduced during acute infection compared to convalescence, but CCR4+ T cells (Th2) are not (140). An excessive Th2 or deficient Th1 response may be associated with the development of bronchiolitis compared to milder URTI with RSV: the nasal IL-4/IFN-γ and IL-10/IL-12 ratios are higher in infants with bronchiolitis (141). In a cohort of children with hypoxic RSV LRTI, comparison of systemic and respiratory tract cytokines showed a predominance of Th2 cytokines in nasopharyngeal fluid (higher pulmonary/systemic of IL-4/IL-12, IL-10/IL-2, IL-10/IFN-γ, IL-6/IFN-γ, and IL-6/IL-2 ratios) (37). Overall, these data suggest that a Th2-biased response may be associated with more severe manifestations of RSV infection, consistent with it being either an inappropriate response to acute viral infection or one that is required to limit a potentially detrimental Th-1 response in severe RSV infection.
However, such findings are not entirely consistent throughout the literature, and there are reports of elevated IFN-γ/IL-4 ratios in children with more severe manifestations of RSV infection (bronchiolitis, pneumonia, and any LRTI) compared to controls, albeit not stratified by severity of infection within the groups (98, 142, 143). A heterogeneous polarization of pulmonary Th responses in infants with severe RSV bronchiolitis has also been described, with 25% of infants expressing only IFN-γ and 50% expressing only IL-4, although again overall supporting a Th2 bias in severe disease (144). In comparison to infection with human metapneumovirus (hMPV), infants with RSV infection have similar nasopharyngeal IFN-γ levels but higher IL-4 levels, consistent with a Th2-biased response that is distinct from the response to hMPV (34).
There are lower counts of in vivo RSV-specific T cells in the elderly, and in in vitro experiments, both isolated T cells and peripheral blood mononuclear cells from healthy elderly patients produce less IFN-γ when stimulated with RSV F protein or RSV, respectively (58, 59, 145). Although this finding has not been confirmed by in vivo experiments, it does hint at a defective Th1 response in the elderly which may contribute to the higher incidence of severe RSV disease in this population.
CHEMOKINES, CYTOKINES, AND OTHER IMMUNE MOLECULES EXPRESSED DURING RSV INFECTION
Overview
A comprehensive list of immune and lung structural proteins involved in the response to RSV infection is presented in Table 1. Key molecules are discussed here.
TABLE 1.
Chemokines, cytokines, and other immune molecules involved in the human immune response to RSV infectiona
| Immune molecule(s) | Productionb |
Comments | Reference(s)d | ||
|---|---|---|---|---|---|
| Respiratory tractc |
Systemic | ||||
| Nasal mucosa | Lung | ||||
| Th1 cytokines | |||||
| IFN-γ | + | + | + | Protective | |
| IL-12 | + | + | + | Protective | |
| IL-1α and IL-1β | + | + | Deleterious | ||
| IL-2 | + | + | + | No reported association with severity | |
| TNF-α | + | + | + | Deleterious | |
| IL-18 | + | Protective | |||
| sCD25 | + | Deleterious | |||
| Th2 cytokines | |||||
| IL-4 | + | + | + | Deleterious | |
| IL-6 | + | + | + | Variable association with severity (see text) | |
| IL-9 | + | + | No reported association with severity | ||
| IL-10 | + | + | + | Variable association with severity (see text) | |
| IL-13 | + | + | + | No reported association with severity | |
| Other cytokines | |||||
| IL-8 | + | + | + | Deleterious: neutrophil chemoattractant | |
| IL-17A | + | + | Variable association with severity (see text) | ||
| IL-33 | + | No reported association with severity | 130 | ||
| Chemokines | |||||
| CCL-2 (MCP-1) | + | + | Deleterious | ||
| CCL-3 (MIP-1α) | + | + | Deleterious | ||
| CCL-4 (MIP-1β) | + | Variable association with severity (see text) | |||
| CCL-5 (RANTES) | + | + | Protective | ||
| CXCL-10 (IP-10) | + | + | Deleterious | ||
| Eotaxin | + | + | Deleterious | ||
| Other | |||||
| IFN-λ | + | Deleterious despite stimulating ISG expression | 159, 160 | ||
| IFN-α | + | + | No reported association with severity | 158 | |
| G-CSF | + | + | Circulating levels are higher in infants with RSV LRTI requiring ventilation | 18, 105 | |
| Soluble ICAM-1 | + | + | Levels in nasal fluid positively correlate with severity | 40, 124, 126 | |
| Substance P | + | + | Lower concentrations associated with increased severity | 106 | |
| MBL | − | No reported association with severity | 44 | ||
| Cathelicidin LL-37 | + | Protective: in human exptl infection, higher constitutive nasal levels are associated with reduced development of infection | 177 | ||
| Olfactomedin 4 | + | Greater expression in PBMC was associated with need for ventilation in RSV LRTI | 19 | ||
| Surfactants A, B, D | − | + | The pulmonary level of surfactant A and measurable surfactant activity increase during recovery | 178–181 | |
| MMP-9, MMP-3, PGP | + | Elevated pulmonary levels in ventilated infants are associated with hypoxia and acute lung injury | 182–184 | ||
| KL-6 | + | Circulating levels are greater in infants with RSV LRTI requiring ventilation | 185 | ||
| sTRAIL | + | No reported association with severity | 186 | ||
Abbreviations: CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; G-CSF, granulocyte colony-stimulating factor; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; IP-10, IFN-γ-inducible protein-10; ISG, interferon-stimulated gene; MBL, mannose binding lectin; MCP-1, monocyte chemoattractant protein-1; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinase; PGP, proline-glycine-proline (the product of MMP hydrolysis of collagen); RANTES, regulated on activation, normal T expressed and secreted; sTRAIL, soluble TNF-related apoptosis-inducing ligand; TIMP, tissue inhibitor of metalloproteinase; TNF, tumor necrosis factor.
+, increased production; −, reduced production.
Nasal mucosa, measurements made in nasal fluid or nasopharyngeal aspirate; lung, measurements made in bronchoalveolar lavage fluid or tracheal aspirate.
References are provided for molecules not discussed in detail in the text.
Interleukin-8
Systemic and respiratory tract production of IL-8, a neutrophil chemoattractant, is increased during RSV LRTI, and circulating concentrations normalize during convalescence (37, 102, 105, 111, 121, 122, 133, 146–149). Higher circulating and respiratory tract IL-8 levels are associated with hypoxia and need for ventilation in infants (18, 37, 102, 135, 136, 147). IL-8 production in the nasal mucosa is also higher during LRTI caused by RSV in children than with rhinovirus (150). When comparing term and preterm infants with RSV LRTI of similar severity, nasal IL-8 and leukocyte counts are higher in the term infants, suggesting a more vigorous inflammatory response (151).
Interleukin-17A
Compared to those in infants with non-RSV LRTI, circulating Th17 cell counts and IL-17 levels are higher in infants with RSV bronchiolitis (51). In these infants, nasal concentrations of proinflammatory IL-17A are higher in patients requiring ventilation (118). When patients are ventilated, tracheal IL-17A concentrations positively correlate with neutrophil counts (152). In infants with mild bronchiolitis, although nasal IL-17A levels are lower initially, they increase during the convalescent phase, hinting at a dual role for IL-17A: deleterious in the acute phase, which is possibly related to neutrophil recruitment, but potentially involved in the resolution of milder infections (118).
CC Chemokines
CCL-5 (RANTES), eotaxin, and CCL-3 (MIP-1α) production in the nasal mucosa and lung (in BAL fluid) is increased during RSV LRTI and bronchiolitis (32, 37, 38, 129, 132, 133, 143, 149, 153–155). However, nasal and systemic CCL-5 concentrations are lower in patients requiring ventilation (18, 132), inversely correlating with the duration of ventilation and required supplemental oxygen. In RSV LRTI, the duration of required supplemental oxygen is positively associated with nasal CCL-3 and inversely associated with CCL-4 (MIP-1β) (107, 108). CCL-3 and eotaxin concentrations in the nasal mucosa are higher in hypoxic bronchiolitis than in URTI or nonhypoxic bronchiolitis (103, 155, 156). Nasal CCL-3 concentrations are higher in RSV-infected adults who require hospitalization than in those who do not, and they are associated with symptom severity in experimentally infected adults (137, 138). However, one study of RSV LRTI found that increased nasal CCL-2 (MCP-1), CCL-3, and CCL-4 are all positively associated with severity (119).
Pattern Recognition Receptors
Pattern recognition receptors (PRRs) are involved in innate immune recognition of viral pathogens in order to stimulate interferon and cytokine responses. In comparison to healthy controls or infants with rhinovirus or bocavirus infection, in infants with RSV bronchiolitis there is increased pulmonary expression of TLR7, TLR8, RIG-1, and MDA-5 (157). RIG-1 mRNA in the lungs correlated with RSV viral load (157). Furthermore, an individual's TLR4 genotype influences the severity of RSV bronchiolitis, and this is significantly influenced by environmental lipopolysaccharide exposure (139).
Innate Interferons
IFN-α is produced systemically and in the respiratory tract in response to RSV infection (158). Nasopharyngeal IFN-α titers peak on day 1 of illness, remain elevated for ∼6 days, and then decrease in parallel with nasopharyngeal RSV antigen levels (158). In peripheral blood, IFN-α levels peak by day 2. Infants less than 3 months of age produce the lowest levels of IFN-α in both the nasopharynx and peripheral blood (158). RSV may be a comparatively weak inducer of type I IFN, since nasopharyngeal IFN-α levels are higher in infants with influenza virus, adenovirus, and parainfluenza virus infection (158).
Type III interferons (IFN-λ) are produced in response to viral infection and have type I IFN-like activities. Their receptor complex is expressed primarily on epithelial cells, and IFN-λ responsiveness is greatest in organs with high epithelial content, such as the lungs. There is a IFN-λ response to RSV bronchiolitis, with higher nasal levels of IFN-λ 1 to 3 seen than in rhinovirus infection (159, 160). IFN-λ mRNA levels correlate with IFN-stimulated gene expression (MxA and ISG56) (159). Despite their association with antiviral gene expression, higher nasal IFN-λ 1 levels are associated with increased disease severity (159).
Immunostimulatory defective viral genomes (iDVGs) have been detected in the nasal fluid of around half of RSV-infected children in one study (161). These RSV genomes have large deletions rendering them unable to replicate without the presence of helper virus. The presence of iDVGs correlates with mRNA levels of IFNA4 and the ISGs IFIT1 and RSAD2, suggesting that they are sufficient to stimulate an innate interferon response (161).
microRNA
Viral infection (especially with RNA viruses) can subvert cellular microRNA expression, potentially to the benefit of the virus. A distinct microRNA expression profile is detectable in the nasal mucosa of RSV-infected infants compared to noninfected controls (downregulation of miR-34b, miR-34c, miR-125b, miR-29c, mir125a, miR-429, and miR-27b and upregulation of miR-155, miR-31, miR-203a, miR-16, and let-7d) (162). miR-125a and miR-429 are downregulated in mild but not severe infection; the former has roles in NF-κB signaling and macrophage function (162). miR-26b (which is thought to target TLR4 based on miRNA target prediction software) has been studied in PBMCs from children with RSV bronchiolitis, where it is upregulated, negatively correlating with TLR4 expression (163).
GLOBAL HOST TRANSCRIPTIONAL RESPONSE TO RSV INFECTION
Genes and pathways associated with neutrophil function, interferon signaling (including STAT1, STAT2, IFITM1, OAS2, MX1, IFI27, IFI35, and IFIT3), interferon-inducible proteins (including IFI44, EIF2AK2, IFI44L, IFI6, OAS3, and G1P2), dendritic cell maturation, and inflammation are upregulated in the circulation of children with RSV infection (164–166). Genes and pathways associated with NK cell, B-cell, and T-cell responses, cytotoxic lymphocyte-mediated apoptosis of target cells, HLA class I and II, and antigen presentation are underexpressed (164–166). Underexpression is greater in infants <6 months than in those aged 6 to 24 months (164), which may reflect either low gene expression or migration of peripheral blood immune cells to the infected tissues. In severe disease there is greater upregulation of neutrophil and inflammatory gene expression and greater suppression of T-cell-, NK cell-, and plasma cell-associated genes (164). In comparison, this dysregulation of genes relating to neutrophil, B-cell, and T-cell function is not seen in children with rhinovirus or influenza virus infection (164).
A different transcriptional response is seen in the upper airways of RSV-infected children. In infants requiring supplemental oxygen or mechanical ventilation, the ubiquitin D, tetraspanin 8, mucin 13, and β-microseminoprotein genes are upregulated and the chemokine ligand 7 gene is downregulated compared to those in infants with milder RSV infection (167).
RELATIONSHIP BETWEEN MOLECULAR AND CELLULAR IMMUNE RESPONSES TO RSV AND PATHOPHYSIOLOGY
Molecular and cellular events during RSV infection are reflected in changes in host physiology observed during the course of disease (Fig. 2). The initial development of cough, wheezing, and tachypnoea, usually peaking on days 4 to 5, develops in parallel to the maximal neutrophil response and viral load (10). This is followed by a convalescent period with a CD8+ T-cell predominant response involved in viral clearance, which coincides with the reduction in the above-mentioned respiratory symptoms over a period of 2 to 3 weeks.
Many of the different cytokines, chemokines, and other immune molecules that are involved in the immune response to RSV infection have been associated with protective or deleterious effects, as listed in Table 1, depending on the perceived severity of disease in the studied patients. This is usually based on the need for ICU admission, endotracheal intubation, and mechanical ventilation but also on composite scores of clinical parameters, including respiratory rate, oxygen saturations, the need for supplemental oxygen, or the need for hospitalization.
We know that preexisting differences in immune status may modulate molecular and cellular responses during RSV infection. Younger infants have more pronounced lymphopenia and reduced IFN-γ responses, possibly reflecting the immunological immaturity of early life (42, 101). Term infants seem to have a stronger inflammatory response, with higher leukocyte counts and IL-8 levels, than preterm infants (151). On the other hand, preterm babies may have an inadequate antiviral response, with reduced pulmonary pDC counts (20). These observations may provide an explanation for the increased frequency of severe RSV disease in preterm and younger term-born infants.
Furthermore, early life microbiome changes in the gut and respiratory tract may influence the host immune responses during RSV disease (168), similar to the distinct patterns of nasopharyngeal microbiota development that have been reported in young infants with cystic fibrosis (169). Certainly, associations between the respiratory and gut microbiome, host transcriptional immune responses, RSV load, and clinical status are now evident and require further detailed investigation (170).
RSV INFECTION AND SUBSEQUENT RESPIRATORY HEALTH
RSV bronchiolitis during early life has been associated with an increase in susceptibility to subsequent episodic wheeze, physician-diagnosed asthma, and decreased forced expiratory volume (FEV1) and forced vital capacity (FVC) measurements on pulmonary function testing (171). Evidence for a causal relationship comes from an intervention trial in premature infants (gestational age 33 to 35 weeks) who received either palivizumab, a humanized monoclonal anti-RSV IgG used in the prevention of severe RSV disease, or placebo during RSV season (172). Palivizumab treatment almost halved (−46.4%) the proportion of infants with subsequent recurrent wheeze compared to placebo. Possible molecular and cellular explanations for such a relationship have been described. There are few human data on potential immune mechanisms for the long-term effects of RSV bronchiolitis, but levels of the cytokines IL-3 and IL-12p40 during RSV disease have been found to correlate with subsequent development of recurrent wheeze (133). Furthermore, elevation of vascular endothelial growth factor (VEGF), granulocyte colony-stimulating factor (G-CSF), and IL-10, all mediators that have been related to asthma and postvirus induced wheeze, persists after the RSV episode (173). Higher proportions of nasal pDCs may reflect a heightened antiviral response in the respiratory tract, potentially due to higher viral load, leading to the development of recurrent wheeze and asthma (174). IL-33, although not reported to be associated with severity of disease, has also been implicated in a Th2-biased response to RSV and may relate to RSV-mediated asthma in later life (130).
KEY DIFFERENCES IN THE IMMUNE RESPONSES TO RSV OF ANIMAL MODELS AND HUMANS
Contemporary data from animal models of RSV infection have been comprehensively reviewed by Borchers and colleagues recently (175). Although similarities are evident, the critical fact remains that human RSV has no animal reservoir and has evolved to infect humans as its natural host, not the commonly used rodent models, which require infection doses far in excess of those needed for human RSV infection. Neutrophilic inflammation contributes significantly to pathogenesis in humans (where neutrophils can constitute up to 85% of BAL fluid cell counts) but appears to have a less dominant role in mice (15 to 20% of cells) (67). In humans the contribution of Th1 and Th2 immune responses is variable and related to pathogenesis, whereas in mice there is generally a robust and reliable Th1 (IFN-γ) response (175). The evidence for an important contribution of eosinophils in humans has been reviewed above, but there is no evidence that these cells have a major role in the pathogenesis of disease in mice (175, 176).
CONCLUSION
By synthesizing the results of a systematic literature review of data exclusively from infected humans, we propose the following model to describe our current understanding of the immune response to RSV infection in humans (Fig. 1).
Large quantities of proinflammatory cytokines are produced in the respiratory tract, with an initial strong activated pulmonary and systemic neutrophil response which correlates with disease severity and is mediated by the neutrophil chemoattractant IL-8. Eosinophil degranulation occurs in the lungs during RSV bronchiolitis, and there may also be a role for CCL-5-mediated eosinophil recruitment to the lungs during recovery from RSV LRTI. Dendritic cells migrate into the lungs, where they are the primary antigen-presenting cell. Circulating cDCs exhibit an activated phenotype, and pulmonary cDC counts correlate with proinflammatory and T-cell-derived cytokine concentrations, suggesting that they contribute to the inflammatory response in a potentially deleterious manner. Alveolar macrophages have an immune-regulatory and antigen-presenting role.
Initially, there is a systemic CD4+ and CD8+ T-cell lymphopenia, without evidence for pulmonary sequestration of T cells. There is active T-cell apoptosis, upregulation of the T-cell-coinhibitory molecule PD-1, and mTOR-mediated suppression of memory CD8+ T-cell differentiation, suggesting that T-cell interference is a key viral immune evasion strategy (Fig. 2). Following the initial neutrophilic response, there is a pulmonary CD8+ T-cell response coinciding with clearance of RSV from the lungs. CD8+ T cells are protective, likely mediating viral clearance and therefore enabling resolution of infection. Humoral immunity to RSV reinfection is incomplete, but RSV-specific circulating IgG and secretory IgA are protective against infection and possibly modify the severity of infection. T-cell-independent B-cell antibody production via B-cell stimulating factors (BAFF and APRIL) derived from the airway epithelium seems to play a major role in protective antibody generation. On the other hand, RSV IgE production is associated with bronchiolitis, where it may have a deleterious effect. There is strong evidence that IFN-γ (and, related to this, IL-12 and IL-18, which promote IFN-γ production/Th1 differentiation) has a protective role in RSV infection. In contrast, a Th2-biased response may be associated with more severe disease manifestations. Global host transcriptional profiling reveals upregulation of innate inflammatory (e.g., neutrophil-related) genes and suppression of genes associated with the adaptive immune response. This is exaggerated in severe disease and is specific to RSV infection. Other cytokines (particularly IL-17A), chemokines (particularly CCL-5 and CCL-3), and local innate immune factors (cathelicidins, IFN-λ, G-CSF, and soluble ICAM-1) have also been associated with the course of disease. Elderly patients are at increased risk of severe RSV disease, and this susceptibility may relate to defects in circulating neutralizing antibody titers and RSV-specific CD4+ and CD8+ T cells.
Overall, neutrophilic pulmonary inflammation is incriminated as a damaging process, and protective effects of CD8+ T cells and IFN-γ production are consistently reported. While these processes may be important therapeutic targets to modulate the immunopathogenesis of RSV infection, less well characterized immune processes, especially those occurring in the lower airways and lung, require further investigation.
ACKNOWLEDGMENTS
We thank Ronnie Grant and Patrick Lane for their assistance with the illustrations used in Fig. 1 and 2.
Biographies

Clark D. Russell is a medical trainee in the Regional Infectious Diseases Unit in Edinburgh, Scotland, and an honorary clinical fellow of the University of Edinburgh. His undergraduate medical training was in Edinburgh, with an elective in Vancouver in infectious diseases and microbiology. He graduated from the University of Edinburgh with a B.Med.Sci. in infectious diseases in 2010 and then an M.B.Ch.B. with honors in 2013 and completed the MRCP (UK) diploma in 2016. He is interested in academic infection medicine, and his research experience includes molecular diagnostics, bacterial pathogenesis, host genetics, and descriptive clinical studies.

Stefan A. Unger is a clinical lecturer at the Department of Child Life and Health at the University of Edinburgh and a pediatrician with specializations in respiratory and sleep medicine. Originally from Germany, Dr. Unger qualified in medicine at the University of Edinburgh and trained in pediatrics in Scotland. Dr. Unger conducted a randomized clinical trial of nutritional supplements in acutely unwell children in rural West Africa during his Medical Research Council (MRC) Career Development Fellowship studying the effect on infectious disease presentations with a focus on respiratory disease. After completion of his Ph.D. with the London School of Hygiene and Tropical Medicine (LSHTM), he specialized in pediatric respiratory medicine with an interest in improving clinical management of bronchiolitis. As a clinical lecturer at the University of Edinburgh, his research focuses on the relationship between undernutrition and immune modulation in lower respiratory tract infections in infancy and subsequent respiratory health in high- and low-income settings.

Marc Walton is an undergraduate student at the University of Edinburgh who recently completed the second year of his medical degree (M.B.Ch.B.). He is currently undertaking a one-year “intercalated” degree in neuroscience (honors), after which he will return to clinical training to complete his medical degree. Mr. Walton started working on this systematic review while undertaking a period of laboratory work in Professor Jürgen Schwarze's group. His main research interests lie in neuroscience and pediatrics, and he is currently involved in projects related to the use of outcome measures in intellectual disability, the design of neural implants, and the neuropathology of Alzheimer's disease.

Jürgen Schwarze is the Edward Clark Chair of Child Life and Health at the University of Edinburgh. He is an internationally recognized expert in immune mechanisms of RSV bronchiolitis and associated airway allergy and a pediatrician specializing in allergy and respiratory medicine. After qualifying in medicine from Freiburg University, Germany, and training in pediatrics, Dr. Schwarze started to work on immune responses in RSV bronchiolitis and allergic airway disease as a postdoctoral fellow at National Jewish Medical and Research Center in Denver, CO. He then continued his research in this field at Ruhr-University Bochum (Germany) and as a Wellcome Trust Senior Clinical Fellow at Imperial College London. In 2007 he moved to the MRC Centre for Inflammation Research at the University of Edinburgh. Dr. Schwarze's research focuses on the interface between innate (lung epithelial cells and dendritic cells) and adaptive immunity in RSV infection and subsequent reactive airway disease.
REFERENCES
- 1.Smith PK, Wang SZ, Dowling KD, Forsyth KD. 2001. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health 37:146–151. doi: 10.1046/j.1440-1754.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell DR, Carrington D. 2002. Peripheral blood lymphopenia and neutrophilia in children with severe respiratory syncytial virus disease. Pediatr Pulmonol 34:128–130. doi: 10.1002/ppul.10140. [DOI] [PubMed] [Google Scholar]
- 3.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidema J, Lukens MV, van Maren WWC, van Dijk MEA, Otten HG, van Vught AJ, van der Werff DBM, van Gestel SJP, Semple MG, Smyth RL, Kimpen JLL, van Bleek GM. 2007. CD8(+) T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol 179:8410–8417. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
- 5.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. 1994. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emboriadou M, Hatzistilianou M, Magnisali C, Sakelaropoulou A, Exintari M, Conti P, Aivazis V. 2007. Human neutrophil elastase in RSV bronchiolitis. Ann Clin Lab Sci 37:79–84. [PubMed] [Google Scholar]
- 7.Abu-Harb M, Bell F, Finn A, Rao WH, Nixon L, Shale D, Everard ML. 1999. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J 14:139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang SZ, Smith PK, Lovejoy M, Bowden JJ, Alpers JH, Forsyth KD. 1998. The apoptosis of neutrophils is accelerated in respiratory syncytial virus (RSV)-induced bronchiolitis. Clin Exp Immunol 114:49–54. doi: 10.1046/j.1365-2249.1998.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SZ, Smith PK, Lovejoy M, Bowden JJ, Alpers JH, Forsyth KD. 1998. Shedding of L-selectin and PECAM-1 and upregulation of Mac-1 and ICAM-1 on neutrophils in RSV bronchiolitis. Am J Physiol 275:L983–L989. [DOI] [PubMed] [Google Scholar]
- 10.Lukens MV, van de Pol AC, Coenjaerts FEJ, Jansen NJG, Kamp VM, Kimpen JLL, Rossen JWA, Ulfman LH, Tacke CEA, Viveen MC, Koenderman L, Wolfs TFW, van Bleek GM. 2010. A systemic neutrophil response precedes robust CD8+ T-cell activation during natural respiratory syncytial virus infection in infants. J Virol 84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Modern Pathol 20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 12.Halfhide CP, Flanagan BF, Brearey SP, Hunt JA, Fonceca AM, McNamara PS, Howarth D, Edwards S, Smyth RL. 2011. Respiratory syncytial virus binds and undergoes transcription in neutrophils from the blood and airways of infants with severe bronchiolitis. J Infect Dis 204:451–458. doi: 10.1093/infdis/jir280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yui I, Hoshi A, Shigeta Y, Takami T, Nakayama T. 2003. Detection of human respiratory syncytial virus sequences in peripheral blood mononuclear cells. J Med Virol 70:481–489. doi: 10.1002/jmv.10421. [DOI] [PubMed] [Google Scholar]
- 14.Cortjens B, de Boer OJ, de Jong R, Antonis AFG, Pineros YSS, Lutter R, van Woensel JBM, Bem RA. 2016. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol 238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 15.Larranaga CL, Ampuero SL, Luchsinger VF, Carrion FA, Aguilar NV, Morales PR, Palomino MAM, Tapia LF, Avendano LF. 2009. Impaired immune response in severe human lower tract respiratory infection by respiratory syncytial virus. Pediatr Infect Dis J 28:867–873. doi: 10.1097/INF.0b013e3181a3ea71. [DOI] [PubMed] [Google Scholar]
- 16.De Weerd W, Twilhaar WN, Kimpen JL. 1998. T cell subset analysis in peripheral blood of children with RSV bronchiolitis. Scand J Infect Dis 30:77–80. doi: 10.1080/003655498750002349. [DOI] [PubMed] [Google Scholar]
- 17.Noyola DE, Juarez-Vega G, Monjaras-Avila C, Escalante-Padron F, Rangel-Ramirez V, Cadena-Mota S, Monsivais-Urenda A, Garcia-Sepulveda CA, Gonzalez-Amaro R. 2015. NK cell immunophenotypic and genotypic analysis of infants with severe respiratory syncytial virus infection. Microbiol Immunol 59:389–397. doi: 10.1111/1348-0421.12265. [DOI] [PubMed] [Google Scholar]
- 18.Brand HK, Ferwerda G, Preijers F, de Groot R, Neeleman C, Staal FJT, Warris A, Hermans PWM. 2013. CD4(+)T-cell counts and interleukin-8 and CCL-5 plasma concentrations discriminate disease severity in children with RSV infection. Pediatric Res 73:187–193. doi: 10.1038/pr.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand HK, Ahout IML, de Ridder D, van Diepen A, Li Y, Zaalberg M, Andeweg A, Roeleveld N, de Groot R, Warris A, Hermans PWM, Ferwerda G, Staal FJT. 2015. Olfactomedin 4 serves as a marker for disease severity in pediatric respiratory syncytial virus (RSV) infection. PLoS One 10:e0131927. doi: 10.1371/journal.pone.0131927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerrin A, Fitch P, Errington C, Kerr D, Waxman L, Riding K, McCormack J, Mehendele F, McSorley H, MacKenzie K, Wronski S, Braun A, Levin R, Theilen U, Schwarze J. 16 August 2016. Differential lower airway dendritic cell patterns may reveal distinct endotypes of RSV bronchiolitis. Thorax doi: 10.1136/thoraxjnl-2015-207358. [DOI] [PubMed] [Google Scholar]
- 21.Bem RA, Bos AP, Bots M, Wolbink AM, Van Ham SM, Medema JP, Lutter R, Van Woensel JBM. 2008. Activation of the granzyme pathway in children with severe respiratory syncytial virus infection. Pediatric Res 63:650–655. doi: 10.1203/PDR.0b013e31816fdc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill MA, Long K, Kwon T, Muniz L, Mejias A, Connolly J, Roy L, Banchereau J, Ramilo O. 2008. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. J Infect Dis 198:1667–1676. doi: 10.1086/593018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O. 2005. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis 191:1105–1115. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- 24.Weng KZ, Zhang JX, Mei XQ, Wu A, Zhang BZ, Cai MY, Zheng YH, Ke ZY. 2014. Lower number of plasmacytoid dendritic cells in peripheral blood of children with bronchiolitis following respiratory syncytial virus infection. Influenza Other Respir Viruses 8:469–473. doi: 10.1111/irv.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Midulla F, Villani A, Panuska JR, Dab I, Kolls JK, Merolla R, Ronchetti R. 1993. Respiratory syncytial virus lung infection in infants: immunoregulatory role of infected alveolar macrophages. J Infect Dis 168:1515–1519. doi: 10.1093/infdis/168.6.1515. [DOI] [PubMed] [Google Scholar]
- 26.Panuska JR, Hertz MI, Taraf H, Villani A, Cirino NM. 1992. Respiratory syncytial virus infection of alveolar macrophages in adult transplant patients. Am Rev Respir Dis 145:934–939. doi: 10.1164/ajrccm/145.4_Pt_1.934. [DOI] [PubMed] [Google Scholar]
- 27.Bendelja K, Vojvoda V, Aberle N, Cepin-Bogovic J, Gagro A, Mlinaric-Galinovic G, Rabatic S. 2010. Decreased Toll-like receptor 8 expression and lower TNF-alpha synthesis in infants with acute RSV infection. Respir Res 11:143. doi: 10.1186/1465-9921-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagro A, Tominac M, Krsulovic-Hresic V, Bace A, Matic M, Drazenovic V, Mlinaric-Galinovic G, Kosor E, Gotovac K, Bolanca I, Batinica S, Rabatic S. 2004. Increased Toll-like receptor 4 expression in infants with respiratory syncytial virus bronchiolitis. Clin Exp Immunol 135:267–272. doi: 10.1111/j.1365-2249.2004.02364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindemans CA, Kimpen JLL, Luijk B, Heidema J, Kanters D, van der Ent CK, Koenderman L. 2006. Systemic eosinophil response induced by respiratory syncytial virus. Clin Exp Immunol 144:409–417. doi: 10.1111/j.1365-2249.2006.03084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawasaki Y, Hosoya M, Kanno H, Suzuki H. 2006. Serum regulated upon activation, normal T cell expressed and presumably secreted concentrations and eosinophils in respiratory syncytial virus infection. Pediatrics Int 48:257–260. doi: 10.1111/j.1442-200X.2006.02199.x. [DOI] [PubMed] [Google Scholar]
- 31.Dimova-Yaneva D, Russell D, Main M, Brooker RJ, Helms PJ. 2004. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clin Exp Allergy 34:555–558. doi: 10.1111/j.1365-2222.2004.1918.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim HH, Lee MH, Lee JS. 2007. Eosinophil cationic protein and chemokines in nasopharyngeal secretions of infants with respiratory syncytial virus (RSV) bronchiolitis and non-RSV bronchiolitis. J Korean Med Sci 22:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. 1999. Respiratory syncytical virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med 159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Kim YH, Kwon E, Callaway Z, Fujisawa T, Kim CK. 2015. Different inflammatory mechanisms of human metapneumovirus and respiratory syncytial virus. J Allergy Clin Immunol 135:AB150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garofalo R, Kimpen JL, Welliver RC, Ogra PL. 1992. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr 120:28–32. doi: 10.1016/S0022-3476(05)80592-X. [DOI] [PubMed] [Google Scholar]
- 36.Volovitz B, Welliver RC, De Castro G, Krystofik DA, Ogra PL. 1988. The release of leukotrienes in the respiratory tract during infection with respiratory syncytial virus: role in obstructive airway disease. Pediatr Res 24:504–507. doi: 10.1203/00006450-198810000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Bermejo-Martin JF, Garcia-Arevalol MC, De Lejarazu RO, Ardura J, Eiros JM, Alonso A, Matias V, Pino M, Bernardo D, Arranz E, Blanco-Quiros A. 2007. Predominance of Th2 cytokines, CXC chemokines and innate immunity mediators at the mucosal level during severe respiratory syncytial virus infection in children. Eur Cytokine Netw 18:162–167. [DOI] [PubMed] [Google Scholar]
- 38.Chung HL, Kim SG. 2002. RANTES may be predictive of later recurrent wheezing after respiratory syncytial virus bronchiolitis in infants. Ann Allergy Asthma Immunol 88:463–467. doi: 10.1016/S1081-1206(10)62383-6. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto N, Ikeda M, Okuda M, Sakamoto T, Takasugi M, Takahashi N, Araki T, Morishima T, Yasui K. 2011. Increased eosinophilic cationic protein in nasal fluid in hospitalized wheezy infants with RSV infection. Allergol Int 60:467–472. doi: 10.2332/allergolint.10-OA-0263. [DOI] [PubMed] [Google Scholar]
- 40.Smyth RL, Fletcher JN, Thomas HM, Hart CA. 1997. Immunological responses to respiratory syncytial virus infection in infancy. Arch Dis Child 76:210–214. doi: 10.1136/adc.76.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bermejo-Martin JF, Garcia-Arevalo MC, Alonso A, De Lejarazu RO, Pino M, Resino S, Tenorio A, Bernardo D, Leon AJ, Garrote JA, Ardura J, Dominguez-Gil M, Eiros JM, Blanco-Quiros A, Munoz-Fernandez MA, Kelvin DJ, Arranz E. 2007. Persistence of proinflammatory response after severe respiratory syncytial virus disease in children. J Allergy Clin Immunol 119:1547–1550. doi: 10.1016/j.jaci.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Roe MFE, Bloxham DM, White DK, Ross-Russell RI, Tasker RTC, O'Donnell DR. 2004. Lymphocyte apoptosis in acute respiratory syncytial virus bronchiolitis. Clin Exp Immunol 137:139–145. doi: 10.1111/j.1365-2249.2004.02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roman M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, Gaggero A, Diaz PV. 1997. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respi Crit Care Med 156:190–195. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro LZG, Tripp RA, Rossi LMG, Palma PVB, Yokosawa J, Mantese OC, Oliveira TFM, Nepomuceno LL, Queiroz DAO. 2008. Serum mannose-binding lectin levels are linked with respiratory syncytial virus (RSV) disease. J Clin Immunol 28:166–173. doi: 10.1007/s10875-007-9141-8. [DOI] [PubMed] [Google Scholar]
- 45.Ayukawa H, Matsubara T, Kaneko M, Hasegawa M, Ichiyama T, Furukawa S. 2004. Expression of CTLA-4 (CD152) in peripheral blood T cells of children with influenza virus infection including encephalopathy in comparison with respiratory syncytial virus infection. Clin Exp Immunol 137:151–155. doi: 10.1111/j.1365-2249.2004.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koga M, Matsuoka T, Matsubara T, Katayama K, Furukawa S. 2000. Different expression of ICAM-1 and LFA-1 alpha by peripheral leukocytes during respiratory syncytial virus and influenza virus infection in young children. Scand J Infect Dis 32:7–11. doi: 10.1080/00365540050164146. [DOI] [PubMed] [Google Scholar]
- 47.Aberle JH, Aberle SW, Dworzak MN, Mandl CW, Rebhandl W, Vollnhofer G, Kundi M, Popow-Kraupp T. 1999. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med 160:1263–1268. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 48.Pinto RA, Arredondo SM, Bono MR, Gaggero AA, Diaz PV. 2006. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics 117:e878–e886. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 49.de Waal L, Koopman LP, van Benten IJ, Brandenburg AH, Mulder PGH, de Swart RL, Fokkens WJ, Neijens HJ, Osterhaus A. 2003. Moderate local and systemic respiratory syncytial virus-specific T-cell responses upon mild or subclinical RSV infection. J Med Virol 70:309–318. doi: 10.1002/jmv.10396. [DOI] [PubMed] [Google Scholar]
- 50.Raiden S, Pandolfi J, Payaslian F, Anderson M, Rivarola N, Ferrero F, Urtasun M, Fainboim L, Geffner J, Arruvito L. 2014. Depletion of circulating regulatory T cells during severe respiratory syncytial virus infection in young children. Am J Respi Crit Care Med 189:865–868. doi: 10.1164/rccm.201311-1977LE. [DOI] [PubMed] [Google Scholar]
- 51.Li B, Wu FL, Feng XB, Sun DK, Cui QQ, Zhao ZX. 2012. Changes and the clinical significance of CD4(+) CD25(+) regulatory T cells and Th17 cells in peripheral blood of infants with respiratory syncytial virus bronchiolitis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 28:426–428. [PubMed] [Google Scholar]
- 52.Bacharier LB, Coverstone A, Schweiger T, Gregory G, Yin-DeClue H, Sajol G, Giri T, Sierra O, Atkinson J, Wilson B, Zheng J, Schechtman K, Castro M. 2013. Regulatory T cells in acute severe RSV bronchiolitis, abstr 187. Abstr Am J Respir Crit Care Med Conf. [Google Scholar]
- 53.Isaacs D, Bangham CR, McMichael AJ. 1987. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet ii:769–771. [DOI] [PubMed] [Google Scholar]
- 54.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EHC, Sykes A, Maybeno M, Del Rosario J, Trujillo-Torralbo MB, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, Chiu C. 2015. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun 6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao S, Jiang L, Moser EK, Jewett LB, Wright J, Du J, Zhou B, Davis SD, Krupp NL, Braciale TJ, Sun J. 2015. Control of pathogenic effector T-cell activities in situ by PD-L1 expression on respiratory inflammatory dendritic cells during respiratory syncytial virus infection. Mucosal Immunol 8:746–759. doi: 10.1038/mi.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Souza APD, de Freitas D, Fernandes KEA, da Cunha MD, Fernandes JLA, Gassen RB, Fazolo T, Pinto LA, Scotta M, Mattiello R, Pitrez PM, Bonorino C, Stein RT. 2016. Respiratory syncytial virus induces phosphorylation of mTOR at ser2448 in CD8 T cells from nasal washes of infected infants. Clin Exp Immunol 183:248–257. doi: 10.1111/cei.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tasker RC, Roe MFE, Bloxham DM, White DK, Ross-Russell RI, O'Donnell DR. 2004. The neuroendocrine stress response and severity of acute respiratory syncytial virus bronchiolitis in infancy. Intensive Care Med 30:2257–2262. doi: 10.1007/s00134-004-2470-7. [DOI] [PubMed] [Google Scholar]
- 58.Cherukuri A, Patton K, Gasser RA, Zuo FR, Woo J, Esser MT, Tang RS. 2013. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol 20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Bree GJ, Heidema J, van Leeuwen EMM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RAW, Out TA. 2005. Respiratory syncytial virus-specific CD8(+) memory T cell responses in elderly persons. J Infect Dis 191:1710–1718. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- 60.Duncan MD, Wilkes DS. 2005. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc 2:449–455. doi: 10.1513/pats.200507-073JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ljungman P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ, Brinch L, Brune M, De La Camara R, Dekker A, Pauksen K, Russell N, Schwarer AP, Cordonnier C. 2001. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 62.McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. 2003. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child 88:922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reed JL, Welliver TP, Sims GP, McKinney L, Velozo L, Avendano L, Hintz K, Luma J, Coyle AJ, Welliver RC. 2009. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J Infect Dis 199:1128–1138. doi: 10.1086/597386. [DOI] [PubMed] [Google Scholar]
- 64.Raes M, Peeters V, Alliet P, Gillis P, Kortleven J, Magerman K, Rummens JL. 1997. Peripheral blood T and B lymphocyte subpopulations in infants with acute respiratory syncytial virus brochiolitis. Pediatr Allergy Immunol 8:97–102. doi: 10.1111/j.1399-3038.1997.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 65.Shinoff JJ, O'Brien KL, Thumar B, Shaw JB, Reid R, Hua W, Santosham M, Karron RA. 2008. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J Infect Dis 198:1007–1015. doi: 10.1086/591460. [DOI] [PubMed] [Google Scholar]
- 66.Strannegard O, Cello J, Bjarnason R, Sigurbergsson F, Sigurs N. 1997. Association between pronounced IgA response in RSV bronchiolitis and development of allergic sensitization. Pediatr Allergy Immunol 8:1–6. doi: 10.1111/j.1399-3038.1997.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 67.McNamara PS, Fonceca AM, Howarth D, Correia JB, Slupsky JR, Trinick RE, Al Turaiki W, Smyth RL, Flanagan BF. 2013. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax 68:76–81. doi: 10.1136/thoraxjnl-2012-202288. [DOI] [PubMed] [Google Scholar]
- 68.Fonceca A, McNamara P, Howarth D, Trinick R, Alturaiki W, Smyth R, Flanagan B. 2011. Human respiratory syncytial virus infection in vivo and in vitro induces airway epithelial cell expression of the B cell differentiation factor BAFF. Immunology 135:94. [Google Scholar]
- 69.Jensen IP, Thisted E, Glikmann G, Obel N, Kofoed PE, Sambo M, Valerius NH, Mordhorst CH. 1997. Secretory IgM and IgA antibodies to respiratory syncytial virus in nasopharyngeal aspirates: a diagnostic supplement to antigen detection. Clin Diagn Virol 8:219–226. doi: 10.1016/S0928-0197(97)10002-2. [DOI] [PubMed] [Google Scholar]
- 70.Lee FEH, Falsey AR, Halliley JL, Sanz I, Walsh EE. 2010. Circulating antibody-secreting cells during acute respiratory syncytial virus infection in adults. J Infect Dis 202:1659–1666. doi: 10.1086/657158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agius G, Dindinaud G, Biggar RJ, Peyre R, Vaillant V, Ranger S, Poupet JY, Cisse MF, Castets M. 1990. An epidemic of respiratory syncytial virus in elderly people: clinical and serological findings. J Med Virol 30:117–127. doi: 10.1002/jmv.1890300208. [DOI] [PubMed] [Google Scholar]
- 72.Forster J, Maier O, Lobbert J, Kaufmehl K, Streckert HJ, Werchau H. 1996. Prevalence of antibodies against HEp-2 cell antigen in infants and children hospitalized with respiratory syncytial virus infection. Infection 24:407–411. doi: 10.1007/BF01713039. [DOI] [PubMed] [Google Scholar]
- 73.Bagga B, Cehelsky JE, Vaishnaw A, Wilkinson T, Meyers R, Harrison LM, Roddam PL, Walsh EE, DeVincenzo JP. 2015. Effect of preexisting serum and mucosal antibody on experimental respiratory syncytial virus (RSV) challenge and infection of adults. J Infect Dis 212:1719–1725. doi: 10.1093/infdis/jiv281. [DOI] [PubMed] [Google Scholar]
- 74.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, de Haan CAM, Wrammert J, Openshaw PJM, Chiu C, Mechanisms of Severe Acute Influenza Consortium Investigators. 2015. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med 191:1040–1049. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bagga B, Cehelsky J, Vaishnaw A, Wilkinson T, Meyers R, Harrison L, Roddam P, Walsh EE, DeVincenzo JP. 2011. Effect of serum and mucosal antibody on experimental RSV infections of adults. J Invest Med 59:487–488. [DOI] [PubMed] [Google Scholar]
- 76.Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 77.Lee FEH, Walsh EE, Falsey AR, Betts RE, Treanor JJ. 2004. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res 63:191–196. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Walsh EE, Falsey AR. 2004. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 79.Falsey AR, Walsh EE. 1998. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 177:463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- 80.Medrano Gonzalez L, Jozwik AA, Habibi MS, Openshaw PJM, Chiu C. 2014. Quality of antigen-specific B-cell responses as a correlate of protection against RSV. Immunology 143:75. [Google Scholar]
- 81.Tsutsumi H, Matsuda K, Yamazaki H, Ogra PL, Chiba S. 1995. Different kinetics of antibody responses between IgA and IgG classes in nasopharyngeal secretion in infants and children during primary respiratory syncytial virus infection. Acta Paediatr Japonica 37:464–468. doi: 10.1111/j.1442-200X.1995.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 82.Freitas GRO, Silva DAO, Yokosawa J, Paula NT, Costa LF, Carneiro BM, Ribeiro LZG, Oliveira TFM, Mineo JR, Queiroz DAO. 2011. Antibody response and avidity of respiratory syncytial virus-specific total IgG, IgG1, and IgG3 in young children. J Med Virol 83:1826–1833. doi: 10.1002/jmv.22134. [DOI] [PubMed] [Google Scholar]
- 83.Murphy BR, Graham BS, Prince GA, Walsh EE, Chanock RM, Karzon DT, Wright PF. 1986. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J Clin Microbiol 23:1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamazaki H, Tsutsumi H, Matsuda K, Nagai K, Ogra PL, Chiba S. 1994. Effect of maternal antibody on IgA antibody response in nasopharyngeal secretion in infants and children during primary respiratory syncytial virus infection. J Gen Virol 75:2115–2119. doi: 10.1099/0022-1317-75-8-2115. [DOI] [PubMed] [Google Scholar]
- 85.Falsey AR, Singh HK, Walsh EE. 2006. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol 78:1493–1497. doi: 10.1002/jmv.20724. [DOI] [PubMed] [Google Scholar]
- 86.Sande CJ, Mutunga MN, Medley GF, Cane PA, Nokes DJ. 2013. Group- and genotype-specific neutralizing antibody responses against respiratory syncytial virus in infants and young children with severe pneumonia. J Infect Dis 207:489–492. doi: 10.1093/infdis/jis700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamazaki H, Tsutsumi H, Matsuda K, Nagai K, Ogra PL, Chiba S. 1994. Respiratory syncytial virus group-specific antibody response in nasopharyngeal secretions from infants and children after primary infection. Clin Diagn Lab Immunol 1:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGill A, Greensill J, Marsh R, Craft AW, Toms GL. 2004. Detection of human respiratory syncytial virus genotype specific antibody responses in infants. J Med Virol 74:492–498. doi: 10.1002/jmv.20203. [DOI] [PubMed] [Google Scholar]
- 89.Kaul TN, Welliver RC, Ogra PL. 1982. Appearance of complement components and immunoglobulins on nasopharyngeal epithelial cells following naturally acquired infection with respiratory syncytial virus. J Med Virol 9:149–158. doi: 10.1002/jmv.1890090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaul TN, Welliver RC, Faden HS, Ogra PL. 1984. The development of respiratory syncytial virus-specific immune complexes in nasopharyngeal secretions following natural infection. J Clin Lab Immunol 15:187–190. [PubMed] [Google Scholar]
- 91.Kaul TN, Welliver RC, Ogra PL. 1982. Development of antibody-dependent cell-mediated cytotoxicity in the respiratory tract after natural infection with respiratory syncytial virus. Infect Immun 37:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Russi JC, Delfraro A, Borthagaray MD, Velazquez B, Garcia-Barreno B, Hortal M. 1993. Evaluation of immunoglobulin E-specific antibodies and viral antigens in nasopharyngeal secretions of children with respiratory syncytial virus infections. J Clin Microbiol 31:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rabatic S, Gagro A, Lokar-Kolbas R, Krsulovic-Hresic V, Vrtar Z, Popow-Kraupp T, Drazenovic V, Mlinaric-Galinovic G. 1997. Increase in CD23+ B cells in infants with bronchiolitis is accompanied by appearance of IgE and IgG4 antibodies specific for respiratory syncytial virus. J Infect Dis 175:32–37. doi: 10.1093/infdis/175.1.32. [DOI] [PubMed] [Google Scholar]
- 94.Welliver RC, Wong DT, Sun M, Middleton E Jr, Vaughan RS, Ogra PL. 1981. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med 305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 95.Chung HL, Jang YY. 2016. High serum IgE level in the children with acute respiratory syncytial virus infection is associated with severe disease. J Allergy Clin Immunol 137:AB110. [Google Scholar]
- 96.Welliver RC, Duffy L. 1993. The relationship of RSV-specific immunoglobulin E antibody responses in infancy, recurrent wheezing, and pulmonary function at age 7-8 years. Pediatr Pulmonol 15:19–27. doi: 10.1002/ppul.1950150104. [DOI] [PubMed] [Google Scholar]
- 97.Ye Q, Shao WX, Shang SQ, Pan YX, Shen HQ, Chen XJ. 2015. Epidemiological characteristics and immune status of children with respiratory syncytial virus. J Med Virol 87:323–329. doi: 10.1002/jmv.24047. [DOI] [PubMed] [Google Scholar]
- 98.Hattori S, Shimojo N, Mashimo T, Inoue Y, Ono Y, Kohno Y, Okamoto Y, Hata A, Suzuki Y. 2011. Relationship between RANTES polymorphisms and respiratory syncytial virus bronchiolitis in a Japanese infant population. Jpn J Infect Dis 64:242–245. [PubMed] [Google Scholar]
- 99.Fernandez JA, Roine I, Vazquez A, Caneo M. 2005. Soluble interleukin-2 receptor (sCD25) and interleukin-10 plasma concentrations are associated with severity of primary respiratory syncytial virus (RSV) infection. Eur Cytokine Netw 16:81–90. [PubMed] [Google Scholar]
- 100.Fernandez JA, Tapia L, Palomino MA, Larranaga C, Pena M, Jaramillo H. 2005. Plasma interferon-gamma, interleukin-10 and soluble markers of immune activation in infants with primary adenovirus (ADV) and respiratory syncytial virus (RSV) infection. Eur Cytokine Netw 16:35–40. [PubMed] [Google Scholar]
- 101.Chung HL, Park HJ, Kim SY, Kim SG. 2007. Age-related difference in immune responses to respiratory syncytial virus infection in young children. Pediatr Allergy Immunol 18:94–99. doi: 10.1111/j.1399-3038.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- 102.Bont L, Heijnen CJ, Kavelaars A, van Aalderen WMC, Brus F, Draaisma JTM, Geelen SM, van Vught HJ, Kimpen JLL. 1999. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J 14:144–149. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 103.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. 2001. Macrophage inflammatory protein-1 alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis 184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 104.Kim CK, Callaway Z, Koh YY, Kim SH, Fujisawa T. 2012. Airway IFN-gamma production during RSV bronchiolitis is associated with eosinophilic inflammation. Lung 190:183–188. doi: 10.1007/s00408-011-9349-5. [DOI] [PubMed] [Google Scholar]
- 105.Choi J, Callaway Z, Kim HB, Fujisawa T, Kim CK. 2010. The role of TNF-alpha in eosinophilic inflammation associated with RSV bronchiolitis. Pediatr Allergy Immunol 21:474–479. doi: 10.1111/j.1399-3038.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 106.Semple MG, Dankert HM, Ebrahimi B, Correia JB, Booth JA, Stewart JP, Smyth RL, Hart CA. 2007. Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS One 2:e1038. doi: 10.1371/journal.pone.0001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia C, Soriano-Fallas A, Lozano J, Leos N, Gomez AM, Ramilo O, Mejias A. 2012. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhinovirus bronchiolitis. Pediatr Infect Dis J 31:86–89. doi: 10.1097/INF.0b013e31822dc8c1. [DOI] [PubMed] [Google Scholar]
- 108.Bennett BL, Garofalo RP, Cron SG, Hosakote YM, Atmar RL, Macias CG, Piedra PA. 2007. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis 195:1532–1540. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 109.Kristjansson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, Jonsdottir I. 2005. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol 116:805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 110.Bont L, Heijnen CJ, Kavelaars A, van Aalderen WMC, Brus F, Draaisma JMT, Pekelharing-Berghuis M, van Diemen-Steenvoorde R, Kimpen JLL. 2001. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis 184:355–358. doi: 10.1086/322035. [DOI] [PubMed] [Google Scholar]
- 111.Diaz PV, Gaggero AA, Pinto RA, Mamani R, Uasapud PA, Bono MR. 2013. Levels of inflammatory cytokines and plasma cortisol in respiratory syncytial virus bronchiolitis. Rev Med Chile 141:574–581. doi: 10.4067/S0034-98872013000500004. [DOI] [PubMed] [Google Scholar]
- 112.Giugno KM, Machado DC, Amantea SL, Menna Barreto SS. 2004. Concentrations of interleukin-2 in the nasopharyngeal secretion of children with acute respiratory syncytial virus bronchiolitis. J Pediatria 80:315–320. (In Portuguese.) doi: 10.2223/1206,10.2223/JPED.1206. [DOI] [PubMed] [Google Scholar]
- 113.McNamara PS, Flanagan BF, Selby AM, Hart CA, Smyth RL. 2004. Pro- and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur Respir J 23:106–112. doi: 10.1183/09031936.03.00048103. [DOI] [PubMed] [Google Scholar]
- 114.van Benten IJ, van Drunen CM, Koevoet JLM, Koopman LP, Hop WCJ, Osterhaus A, Neijens HJ, Fokkens WJ. 2005. Reduced nasal IL-10 and enhanced TNF alpha responses during rhinovirus and RSV-induced upper respiratory tract infection in atopic and non-atopic infants. J Med Virol 75:348–357. doi: 10.1002/jmv.20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsuda K, Tsutsumi H, Okamoto Y, Chiba C. 1995. Development of interleukin 6 and tumor necrosis factor alpha activity in nasopharyngeal secretions of infants and children during infection with respiratory syncytial virus. Clin Diagn Lab Immunol 2:322–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hornsleth A, Klug B, Nir M, Johansen J, Hansen KS, Christensen LS, Larsen LB. 1998. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatr Infect Dis J 17:1114–1121. doi: 10.1097/00006454-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 117.van Benten IJ, van Drunen CM, Koopman LP, KleinJan A, van Middelkoop BC, de Waal L, Osterhaus A, Neijens HJ, Fokkens WJ. 2003. RSV-induced bronchiolitis but not upper respiratory tract infection is accompanied by an increased nasal IL-18 response. J Med Virol 71:290–297. doi: 10.1002/jmv.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faber TE, Groen H, Welfing M, Jansen KJG, Bont LJ. 2012. Specific increase in local IL-17 production during recovery from primary RSV bronchiolitis. J Med Virol 84:1084–1088. doi: 10.1002/jmv.23291. [DOI] [PubMed] [Google Scholar]
- 119.Tabarani CM, Bonville CA, Suryadevara M, Branigan P, Wang D, Huang D, Rosenberg HF, Domachowske JB. 2013. Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr Infect Dis J 32:e437–442. doi: 10.1097/INF.0b013e3182a14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garofalo RP, Hintz KH, Hill V, Ogra PL, Welliver RC. 2004. Production of interferon gamma in respiratory syncytial virus infection of humans is not associated with interleukins 12 and 18. J Med Virol 73:289–294. doi: 10.1002/jmv.20089. [DOI] [PubMed] [Google Scholar]
- 121.Mella C, Suarez-Arrabal MC, Lopez S, Stephens J, Fernandez S, Hall MW, Ramilo O, Mejias A. 2013. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis 207:564–573. doi: 10.1093/infdis/jis721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Diaz PV, Pinto RA, Mamani R, Uasapud PA, Bono MR, Gaggero AA, Guerrero J, Goecke A. 2012. Increased expression of the glucocorticoid receptor beta in infants with RSV bronchiolitis. Pediatrics 130:E804–E811. doi: 10.1542/peds.2012-0160. [DOI] [PubMed] [Google Scholar]
- 123.Hassan MA, Eldin AM, Ahmed MM. 2008. T-helper2/T-helper1 imbalance in respiratory syncytial virus bronchiolitis in relation to disease severity and outcome. Egyptian J Immunol 15:153–160. [PubMed] [Google Scholar]
- 124.Vieira RA, Diniz EMA, Mejr C. 2010. Concentrations of inflammatory mediators in Brazilian newborn with respiratory syncytial virus lower respiratory tract infection. J Maternal-Fetal Neonatal Med 23:635–636. [Google Scholar]
- 125.Vieira RA, Diniz EM, Ceccon ME. 2010. Correlation between inflammatory mediators in the nasopharyngeal secretion and in the serum of children with lower respiratory tract infection caused by respiratory syncytial virus and disease severity. J Bras Pneumol 36:59–66. doi: 10.1590/S1806-37132010000100011. [DOI] [PubMed] [Google Scholar]
- 126.Sung RYT, Hui SHL, Wong CK, Lam CWK, Yin J. 2001. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur J Pediatr 160:117–122. doi: 10.1007/s004310000676. [DOI] [PubMed] [Google Scholar]
- 127.Midulla F, Tromba V, Lo Russo L, Mileto F, Sabatino G, Sgarrella M, Panuska JR, Manganozzi L, Korn D, Moret C. 2006. Cytokines in the nasal washes of children with respiratory syncytial virus bronchiolitis. Int J Immunopathol Pharmacol 19:231–235. [PubMed] [Google Scholar]
- 128.Chung HL, Kim WT, Kim JK, Choi EJ, Lee JH, Lee GH, Kim SG. 2005. Relationship between atopic status and nasal interleukin 10 and 11 levels in infants with respiratory syncytial virus bronchiolitis. Ann Allergy Asthma Immunol 94:267–272. doi: 10.1016/S1081-1206(10)61307-5. [DOI] [PubMed] [Google Scholar]
- 129.Murai H, Terada A, Mizuno M, Asai M, Hirabayashi Y, Shimizu S, Morishita T, Kakita H, Hussein MH, Ito T, Kato I, Asai K, Togari H. 2007. IL-10 and RANTES are elevated in nasopharyngeal secretions of children with respiratory syncytial virus infection. Allergol Int 56:157–163. doi: 10.2332/allergolint.O-06-454. [DOI] [PubMed] [Google Scholar]
- 130.Saravia J, You D, Shrestha B, Jaligama S, Siefker D, Lee GI, Harding JN, Jones TL, Rovnaghi C, Bagga B, DeVincenzo JP, Cormier SA. 2015. Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog 11:e1005217. doi: 10.1371/journal.ppat.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McNamara PS, Flanagan BF, Baldwin LM, Newland P, Hart CA, Smyth RL. 2004. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchlolitis. Lancet 363:1031–1037. doi: 10.1016/S0140-6736(04)15838-8. [DOI] [PubMed] [Google Scholar]
- 132.Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sanchez PJ, Ramilo O. 1999. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J 18:115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 133.Bertrand P, Lay MK, Piedimonte G, Brockmann PE, Palavecino CE, Hernandez J, Leon MA, Kalergis AM, Bueno SM. 2015. Elevated IL-3 and IL-12p40 levels in the lower airway of infants with RSV-induced bronchiolitis correlate with recurrent wheezing. Cytokine 76:417–423. doi: 10.1016/j.cyto.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 134.Hornsleth A, Loland L, Larsen LB. 2001. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J Clin Virol 21:163–170. doi: 10.1016/S1386-6532(01)00159-7. [DOI] [PubMed] [Google Scholar]
- 135.Brandenburg AH, Kleinjan A, van't Land B, Moll HA, Timmerman HH, de Swart RL, Neijens HJ, Fokkens W, Osterhaus A. 2000. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J Med Virol 62:267–277. [PubMed] [Google Scholar]
- 136.Diaz PV, Valdivia G, Gaggero AA, Bono MR, Zepeda G, Rivas M, Uasapud P, Pinto RA, Boza ML, Guerrero J. 2015. Pro-inflammatory cytokines in nasopharyngeal aspirate from hospitalized children with respiratory syncytial virus infection with or without rhinovirus bronchiolitis, and use of the cytokines as predictors of illness severity. Medicine 94:e1512. doi: 10.1097/MD.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. 2013. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis 207:1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, Oxford J, Pareek R, Moore R, Walsh E, Studholme R, Dorsett P, Alvarez R, Lambkin-Williams R. 2010. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Caballero MT, Serra ME, Acosta PL, Marzec J, Gibbons L, Salim M, Rodriguez A, Reynaldi A, Garcia A, Bado D, Buchholz UJ, Hijano DR, Coviello S, Newcomb D, Bellabarba M, Ferolla FM, Libster R, Berenstein A, Siniawaski S, Blumetti V, Echavarria M, Pinto L, Lawrence A, Ossorio MF, Grosman A, Mateu CG, Bayle C, Dericco A, Pellegrini M, Igarza I, Repetto HA, Grimaldi LA, Gudapati P, Polack NR, Althabe F, Shi M, Ferrero F, Berge E, Stein RT, Peebles RS, Boothby M, Kleeberger SR, Polack FP. 2015. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest 125:571–582. doi: 10.1172/JCI75183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roe MFE, Bloxham DM, Cowburn AS, O'Donnell DR. 2011. Changes in helper lymphocyte chemokine receptor expression and elevation of IP-10 during acute respiratory syncytial virus infection in infants. Pediatr Allergy Immunol 22:229–234. doi: 10.1111/j.1399-3038.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- 141.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. 2003. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 168:633–639. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 142.Chen ZM, Mao JH, Du LZ, Tang YM. 2002. Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr 91:914–922. doi: 10.1111/j.1651-2227.2002.tb02877.x. [DOI] [PubMed] [Google Scholar]
- 143.Pancham K, Perez GF, Husen S, Jain A, Kurdi B, Rodriguez-Martinez CE, Preciado D, Rose MC, Nino G. 2015. Premature infants have impaired airway antiviral IFN gamma responses to human metapneumovirus compared to respiratory syncytial virus. Pediatr Res 78:389–394. doi: 10.1038/pr.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mobbs KJ, Smyth RL, O'Hea U, Ashby D, Ritson P, Hart CA. 2002. Cytokines in severe respiratory syncytial virus bronchiolitis. Pediatr Pulmonol 33:449–452. doi: 10.1002/ppul.10101. [DOI] [PubMed] [Google Scholar]
- 145.Looney RJ, Falsey AR, Walsh E, Campbell D. 2002. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J Infect Dis 185:682–685. doi: 10.1086/339008. [DOI] [PubMed] [Google Scholar]
- 146.Noah TL, Ivins SS, Murphy P, Kazachkova I, Moats-Staats B, Henderson FW. 2002. Chemokines and inflammation in the nasal passages of infants with respiratory syncytial virus bronchiolitis. Clin Immunol 104:86–95. doi: 10.1006/clim.2002.5248. [DOI] [PubMed] [Google Scholar]
- 147.Smyth RL, Mobbs KJ, O'Hea U, Ashby D, Hart CA. 2002. Respiratory syncytial virus bronchiolitis: disease severity, interleukin-8, and virus genotype. Pediatr Pulmonol 33:339–346. doi: 10.1002/ppul.10080. [DOI] [PubMed] [Google Scholar]
- 148.Biswas S, Friedland JS, Remick DG, Davies EG, Sharland M. 1995. Elevated plasma interleukin 8 in respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J 14:919. doi: 10.1097/00006454-199510000-00027. [DOI] [PubMed] [Google Scholar]
- 149.Noah TL, Becker S. 2000. Chemokines in nasal secretions of normal adults experimentally infected with respiratory syncytial virus. Clin Immunol 97:43–49. doi: 10.1006/clim.2000.4914. [DOI] [PubMed] [Google Scholar]
- 150.Adams O, Weis J, Jasinska K, Vogel M, Tenenbaum T. 2015. Comparison of human metapneumovirus, respiratory syncytial virus and rhinovirus respiratory tract infections in young children admitted to hospital. J Med Virol 87:275–280. doi: 10.1002/jmv.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Assefa D, Amin N, Dozor AJ, Parton LA. 2011. Attenuated interleukin-8/leukocyte immunoresponse in preterm infants compared with term infants hospitalized with respiratory syncytial virus bronchiolitis: a pilot study. Hum Immunol 72:708–711. doi: 10.1016/j.humimm.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 152.Stoppelenburg AJ, Salimi V, Hennus M, Plantinga M, in 't Veld RH, Walk J, Meerding J, Coenjaerts F, Bont L, Boes M. 2013. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS One 8:e78461. doi: 10.1371/journal.pone.0078461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McNamara PS, Flanagan BF, Hart CA, Smyth RL. 2005. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis 191:1225–1232. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- 154.Becker S, Reed W, Henderson FW, Noah TL. 1997. RSV infection of human airway epithelial cells causes production of the beta-chemokine RANTES. Am J Physiol 272:L512–L520. [DOI] [PubMed] [Google Scholar]
- 155.Garofalo RP, Olszewska-Pazdrak B, Ogra PL, Welliver RC. 2001. Beta-chemokines in nasal secretions of infants with respiratory syncytial virus-induced respiratory infections. Pediatr Asthma Allergy Immunol 15:89–96. doi: 10.1089/088318701753436880. [DOI] [Google Scholar]
- 156.Garofalo RP, Hintz KH, Hill V, Patti J, Ogra PL, Welliver RC. 2005. A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J Med Virol 75:282–289. doi: 10.1002/jmv.20268. [DOI] [PubMed] [Google Scholar]
- 157.Scagnolari C, Midulla F, Pierangeli A, Moretti C, Bonci E, Berardi R, De Angelis D, Selvaggi C, Di Marco P, Girardi E, Antonelli G. 2009. Gene expression of nucleic acid-sensing pattern recognition receptors in children hospitalized for respiratory syncytial virus-associated acute bronchiolitis. Clin Vaccine Immunol 16:816–823. doi: 10.1128/CVI.00445-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakayama T, Sonoda S, Urano T, Sasaki K, Maehara N, Makino S. 1993. Detection of alpha-interferon in nasopharyngeal secretions and sera in children infected with respiratory syncytial virus. Pediatr Infect Dis J 12:925–929. doi: 10.1097/00006454-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 159.Selvaggi C, Pierangeli A, Fabiani M, Spano L, Nicolai A, Papoff P, Moretti C, Midulla F, Antonelli G, Scagnolari C. 2014. Interferon lambda 1-3 expression in infants hospitalized for RSV or HRV associated bronchiolitis. J Infect 68:467–477. doi: 10.1016/j.jinf.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nenna R, Ferrara M, Nicolai A, Pierangeli A, Scagnolari C, Papoff P, Antonelli G, Moretti C, Midulla F. 2015. Viral load in infants hospitalized for respiratory syncytial virus bronchiolitis correlates with recurrent wheezing at thirty-six-month follow-up. Pediatr Infect Dis J 34:1131–1132. doi: 10.1097/INF.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 161.Sun Y, Jain D, Koziol-White CJ, Genoyer E, Gilbert M, Tapia K, Panettieri RA, Hodinka RL, Lopez CB. 2015. Immunostimulatory defective viral genomes from respiratory syncytial virus promote a strong innate antiviral response during infection in mice and humans. PLoS Pathog 11:e1005122. doi: 10.1371/journal.ppat.1005122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Inchley CS, Sonerud T, Fjaerli HO, Nakstad B. 2015. Nasal mucosal microRNA expression in children with respiratory syncytial virus infection. BMC Infect Dis 15:150. doi: 10.1186/s12879-015-0878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu S, Gao L, Wang X, Xing Y. 2015. Respiratory syncytial virus infection inhibits TLR4 signaling via up-regulation of miR-26b. Cell Biol Int 39:1376–1383. doi: 10.1002/cbin.10518. [DOI] [PubMed] [Google Scholar]
- 164.Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, Blankenship D, Jordan-Villegas A, Ardura MI, Xu ZH, Banchereau J, Chaussabel D, Ramilo O. 2013. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med 10:e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bucasas KL, Mian AI, Demmler-Harrison GJ, Caviness AC, Piedra PA, Franco LM, Shaw CA, Zhai YJ, Wang XQ, Bray MS, Couch RB, Belmont JW. 2013. Global gene exprssion profiling in infants with acute respiratory syncytial virus broncholitis demonstrates systemic activation of interferon signaling networks. Pediatr Infect Dis J 32:E68–E76. doi: 10.1097/INF.0b013e318278b4b3. [DOI] [PubMed] [Google Scholar]
- 166.Fjaerli HO, Bukholm G, Krog A, Skjaeret C, Holden M, Nakstad B. 2006. Whole blood gene expression in infants with respiratory syncytial virus bronchiolitis. BMC Infect Dis 6:175. doi: 10.1186/1471-2334-6-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van den Kieboom CH, Ahout IML, Zomer A, Brand KH, de Groot R, Ferwerda G, de Jonge MI. 2015. Nasopharyngeal gene expression, a novel approach to study the course of respiratory syncytial virus infection. Eur Respir J 45:718–725. doi: 10.1183/09031936.00085614. [DOI] [PubMed] [Google Scholar]
- 168.Seed PC. 2016. Do bacteria in the gut set the stage for who gets viral bronchiolitis and its severity? Pediatrics 138:e20161377. doi: 10.1542/peds.2016-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Prevaes SM, de Winter-de Groot KM, Janssens HM, de Steenhuijsen Piters WA, Tramper-Stranders GA, Wyllie AL, Hasrat R, Tiddens HA, van Westreenen M, van der Ent CK, Sanders EA, Bogaert D. 2016. Development of the nasopharyngeal microbiota in infants with cystic fibrosis. Am J Respir Crit Care Med 193:504–515. doi: 10.1164/rccm.201509-1759OC. [DOI] [PubMed] [Google Scholar]
- 170.de Steenhuijsen Piters WA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez-Arrabal MC, Chaussabel D, Cohen DM, Sanders EA, Ramilo O, Bogaert D, Mejias A. 2016. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med 194:1104–1115. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zomer-Kooijker K, van der Ent CK, Ermers MJ, Uiterwaal CS, Rovers MM, Bont LJ. 2014. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One 9:e87162. doi: 10.1371/journal.pone.0087162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, Bont L. 2013. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 173.Pino M, Kelvin DJ, Bermejo-Martin JF, Alonso A, Matias V, Tenorio A, Rico L, Eiros JM, Castrodeza J, Blanco-Quiros A, Ardura J, de Lejarazu RO. 2009. Nasopharyngeal aspirate cytokine levels 1 yr after severe respiratory syncytial virus infection. Pediatr Allergy Immunol 20:791–795. doi: 10.1111/j.1399-3038.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 174.Kitcharoensakkul M, Bacharier LB, Yin-Declue H, Schweiger T, Goss CW, Boomer JS, Sajol G, Schectman K, Castro M. 2016. Increased nasal plasmacytoid dendritic cells are associated with recurrent wheezing following severe RSV bronchiolitis. J Allergy Clin Immunol 137:AB411. doi: 10.1016/j.jaci.2015.12.1321. [DOI] [PubMed] [Google Scholar]
- 175.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. 2013. Respiratory syncytial virus—a comprehensive review. Clin Rev Allergy Immunol 45:331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Knudson CJ, Hartwig SM, Meyerholz DK, Varga SM. 2015. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog 11:e1004757. doi: 10.1371/journal.ppat.1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Currie SM, Findlay EG, McFarlane AJ, Fitch PM, Bottcher B, Colegrave N, Paras A, Jozwik A, Chiu C, Schwarze J, Davidson DJ. 2016. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J Immunol 196:2699–2710. doi: 10.4049/jimmunol.1502478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kerr MH, Paton JY. 1999. Surfactant protein levels in severe respiratory syncytial virus infection. Am J Respir Crit Care Med 159:1115–1118. doi: 10.1164/ajrccm.159.4.9709065. [DOI] [PubMed] [Google Scholar]
- 179.Dargaville PA, South M, McDougall PN. 1996. Surfactant abnormalities in infants with severe viral bronchiolitis. Arch Dis Child 75:133–136. doi: 10.1136/adc.75.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Skelton R, Holland P, Darowski M, Chetcuti PA, Morgan LW, Harwood JL. 1999. Abnormal surfactant composition and activity in severe bronchiolitis. Acta Paediatr 88:942–946. doi: 10.1111/j.1651-2227.1999.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 181.Wang SZ, Doyle IR, Nicholas TE, Forsyth KD. 1999. Plasma surfactant protein-B is elevated in infants with respiratory syncytial virus-induced bronchiolitis. Pediatr Res 46:731–734. doi: 10.1203/00006450-199912000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kong MY, Clancy JP, Peng N, Li Y, Szul TJ, Xu X, Oster R, Sullender W, Ambalavanan N, Blalock JE, Gaggar A. 2014. Pulmonary matrix metalloproteinase-9 activity in mechanically ventilated children with respiratory syncytial virus. Eur Respir J 43:1086–1096. doi: 10.1183/09031936.00105613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kong M, Peng N, Jackson P, Clancy J, Blalock E, Gaggar A. 2012. 174: dysregulated matrix metalloproteinase-9 activity and elevated proline-glycine-proline (pgp) levels are observed in pediatric RSV-induced lung injury. Crit Care Med 40:1–328.23213646 [Google Scholar]
- 184.Schuurhof A, Bont L, Hodemaekers HM, de Klerk A, de Groot H, Hofland RW, van de Pol AC, Kimpen JL, Janssen R. 2012. Proteins involved in extracellular matrix dynamics are associated with respiratory syncytial virus disease severity. Eur Respir J 39:1475–1481. doi: 10.1183/09031936.00012311. [DOI] [PubMed] [Google Scholar]
- 185.Kawasaki Y, Aoyagi Y, Abe Y, Go H, Imamura T, Kaneko M, Ito M, Katayose M, Hashimoto K, Hosoya M. 2009. Serum KL-6 levels as a biomarker of lung injury in respiratory syncytial virus bronchiolitis. J Med Virol 81:2104–2108. doi: 10.1002/jmv.21634. [DOI] [PubMed] [Google Scholar]
- 186.Bem RA, Bos AP, Wosten-van Asperen RM, Bruijn M, Lutter R, Sprick MR, van Woensel JB. 2010. Potential role of soluble TRAIL in epithelial injury in children with severe RSV infection. Am J Respir Cell Mol Biol 42:697–705. doi: 10.1165/rcmb.2009-0100OC. [DOI] [PubMed] [Google Scholar]