SUMMARY
Polymyxins are well-established antibiotics that have recently regained significant interest as a consequence of the increasing incidence of infections due to multidrug-resistant Gram-negative bacteria. Colistin and polymyxin B are being seriously reconsidered as last-resort antibiotics in many areas where multidrug resistance is observed in clinical medicine. In parallel, the heavy use of polymyxins in veterinary medicine is currently being reconsidered due to increased reports of polymyxin-resistant bacteria. Susceptibility testing is challenging with polymyxins, and currently available techniques are presented here. Genotypic and phenotypic methods that provide relevant information for diagnostic laboratories are presented. This review also presents recent works in relation to recently identified mechanisms of polymyxin resistance, including chromosomally encoded resistance traits as well as the recently identified plasmid-encoded polymyxin resistance determinant MCR-1. Epidemiological features summarizing the current knowledge in that field are presented.
KEYWORDS: Gram-negative bacteria, MCR-1, lipopolysaccharide, polymyxins, toxicity
INTRODUCTION
Colistin (also known as polymyxin E) is a polypeptide antibiotic that was originally isolated in 1947 from the soil bacterium Paenibacillus polymyxa subsp. colistinus (1). Colistin and polymyxin B belong to the class of polymyxins, which is one of the primary classes of antibiotics with activity against most Gram-negative bacteria.
Structure
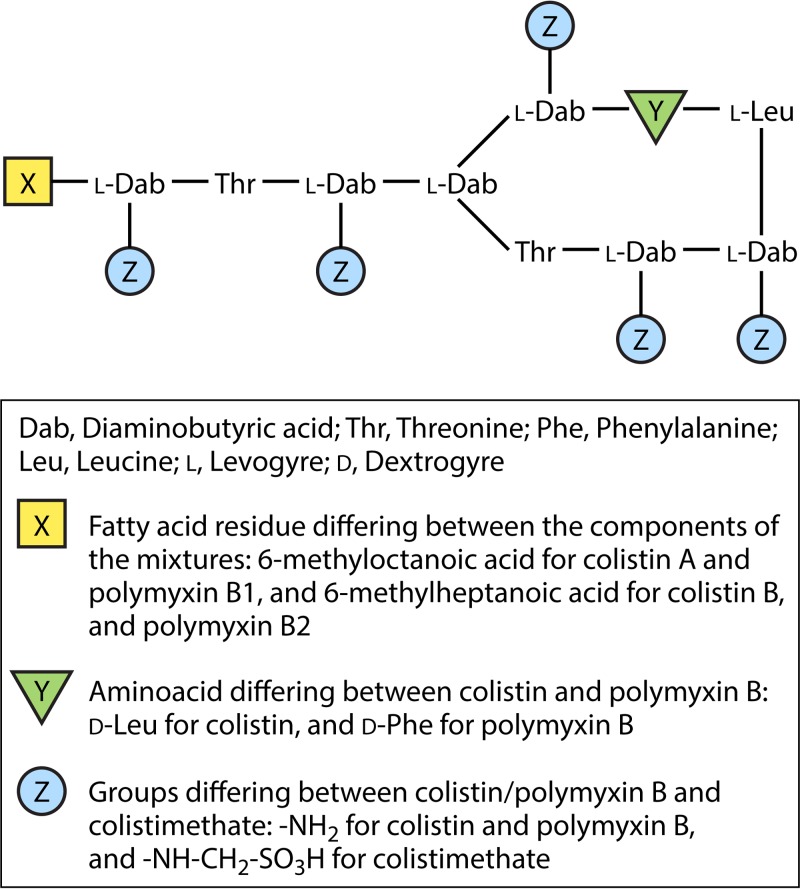
The chemical structure of polymyxins is similar to that of cationic antimicrobial peptides (CAMPs) (defensins and gramicidins), which represent the first line of defense against bacterial colonization in eukaryotic cells (2). Polymyxins are cationic polypeptides that consist of a cyclic heptapeptide possessing a tripeptide side chain acylated at the N terminus by a fatty acid tail (3, 4) (Fig. 1). The inherent toxicity of colistin may be explained by the hydrophobic properties of the N-terminal fatty acyl segment, which also accounts significantly for its antimicrobial activity, and also by positions 6 and 7, which are very important (5, 6).
FIG 1.
Structures of colistin A and B, colistimethate A and B, and polymyxin B1 and B2.
Colistin and polymyxin B differ by only a single amino acid in the peptide ring, with a phenylalanine in polymyxin B and a leucine in colistin (Fig. 1) (7). Polymyxin B is administered directly as an active antibiotic, whereas colistin is administered as an inactive prodrug, colistin methanesulfonate (also known as colistimethate [CMS]) (Fig. 1) (7).
The terms “colistin” and “colistimethate” are not interchangeable, since they correspond to different forms of colistin available for clinical use (4). Indeed, colistimethate sodium is a polyanionic inactive prodrug that is less toxic than colistin sulfate (Fig. 1) (4, 8). Colistimethate is formed by the reaction of colistin with formaldehyde and sodium bisulfite (9). This prodrug is transformed in aqueous media, and also in vivo in biological fluids, and is converted into colistin and several inactive methanesulfonated compounds (10, 11).
Mechanism of Action
The target of polymyxins is the outer membrane of Gram-negative bacteria. Because of an electrostatic interaction occurring between the α,γ-diaminobutyric acid (Dab) residue of the positively charged polymyxin on one side and the phosphate groups of the negatively charged lipid A membrane on the other side, divalent cations (Ca2+ and Mg2+) are displaced from the negatively charged phosphate groups of membrane lipids (12). The lipopolysaccharide (LPS) is therefore destabilized, consequently increasing the permeability of the bacterial membrane, leading to leakage of the cytoplasmic content and ultimately causing cell death (4, 13). Note that even though the LPS is the initial target, the exact mode of action of polymyxins still remains unclear.
Another antibacterial mechanism is the endotoxin effect. The endotoxin of Gram-negative pathogens corresponds to the lipid A portion of the LPS; polymyxins have the ability to bind to and neutralize this LPS molecule released during cell lysis (14).
Finally, another mode of action of the polymyxins is the inhibition of vital respiratory enzymes (inhibition of type II NADH-quinone oxidoreductases [NDH-2]) in the bacterial inner membrane (15).
Spectrum of Activity
Polymyxins have a narrow antibacterial spectrum, mainly against common Gram-negative bacteria. They are active against most members of the Enterobacteriaceae family, including Escherichia coli, Enterobacter spp., Klebsiella spp., Citrobacter spp., Salmonella spp., and Shigella spp. Polymyxins also have significant activity against common nonfermentative Gram-negative bacteria, including Acinetobacter baumannii, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia (13).
Conversely, some species are naturally resistant to polymyxins, including Proteus spp., Morganella morganii, Providencia spp., Serratia marcescens, Pseudomonas mallei, Burkholderia cepacia, Chromobacterium spp., Edwardsiella spp., Brucella, Legionella, Campylobacter, and Vibrio cholerae. Polymyxins are not active against Gram-negative cocci (Neisseria spp.), Gram-positive bacteria, and anaerobic bacteria (13).
Pharmacodynamics
The antibacterial effect of colistin is concentration dependent (4, 16–18). The pharmacokinetic-pharmacodynamic (PK-PD) index that best predicts the antibacterial activity against A. baumannii and P. aeruginosa is the ratio of the area under the concentration-time curve for free drug from 0 to 24 h to the MIC (fAUC0–24/MIC), with this index being superior to the maximum concentration of drug in serum (Cmax)/MIC relationship, suggesting that time-averaged exposure to colistin is more important than the achievement of high peak concentrations (19–21). An average steady-state plasma colistin concentration of 2 μg/ml has been suggested as a reasonable target value for isolates with MICs of ≤1 μg/ml, maximizing the antimicrobial activity while minimizing the risk of nephrotoxicity (22). An inadequate AUC/MIC ratio likely leads to treatment failure. The colistin antibacterial effect is extremely rapid, occurring as early as 5 min after exposure (17, 18, 23, 24).
A postantibiotic effect was observed against Klebsiella pneumoniae, P. aeruginosa, and A. baumannii (25). However, it is important to highlight that polymyxins have minimal postantibiotic effects at clinically relevant concentrations. Despite the major initial killing rate observed against colistin-susceptible strains exposed to colistin alone, regrowth has been reported for A. baumannii (17) and K. pneumoniae (18) in static time-kill studies. Colistin heteroresistance, a phenomenon corresponding to the emergence of a colistin-resistant subpopulation (that can grow in the presence of ≥4 μg/ml of colistin) within a susceptible population (i.e., with a MIC of ≤2 μg/ml), has been observed for A. baumannii (26, 27), K. pneumoniae (18, 28), and P. aeruginosa (23).
USE OF COLISTIN IN HUMAN AND VETERINARY MEDICINE
Use in Human Medicine
After its discovery in 1947, colistin was used in Japan and Europe during the 1950s (29). Then, after its approval by the U.S. FDA in 1959, colistimethate (CMS), the inactive prodrug of colistin, replaced colistin for parenteral administration (29). Colistin and CMS have been used widely for decades for treatment of infections caused by Gram-negative bacteria. However, in the 1970s, because of their toxicity, especially nephrotoxicity (30), their use was reconsidered. They were then replaced by novel, more active and less toxic antibiotics, such as aminoglycosides, quinolones, and β-lactams. For 20 years, the use of colistin was restricted to ophthalmic and topical uses. Systemic or nebulized colistin was used only for cystic fibrosis patients.
However, the increasing prevalence of multidrug-resistant (MDR) Gram-negative bacteria (31), particularly K. pneumoniae, A. baumannii, and P. aeruginosa, has forced physicians to reintroduce systemic polymyxin as a valuable therapeutic option (4, 13, 32).
Considering the paucity of novel antibiotics, colistin is currently often the only effective antibiotic agent against MDR organisms, particularly carbapenemase-producing bacteria.
Commercial formulations.
There are more than 30 polymyxin molecules, among which there are five main chemical compounds (polymyxins A to E), each containing multiple components. Although colistin (polymyxin E) and polymyxin B are both used in clinical practice (33), colistin is the most widely used polymyxin (23). The two most common commercially available parenteral formulations of the colistin prodrug, CMS, are Colomycin (Forest Laboratories UK Limited, Dartford, United Kingdom), primarily employed in Europe, and Coly-Mycin M Parenteral (Monarch Pharmaceuticals, Inc., Bristol, TN), primilarily employed in the United States (34). Unfortunately, the vials of both formulations contain different dry powder quantities, and the two products are differently labeled, with Colomycin being labeled in international units (IU) of CMS and Coly-Mycin M Parenteral being labeled in milligrams of CMS or colistin base activity (CBA) (34). The conversion is as follows: 1 million units (MU) CMS = 80 mg CMS = 30 mg CBA (35). To add to the confusion, some other brands corresponding to generic products are now available (36). The multiplicity of terms used to express contents of vials and dose regimens unfortunately creates confusion and does not allow any meaningful comparison of data collected from studies performed in different parts of the world.
Routes of administration.
Colistin sulfate can be administered orally as tablets and syrup for selective digestive tract decontamination (no absorption) and topically for the treatment of bacterial skin infections (13). CMS, the less toxic prodrug, has different administration routes, i.e., parenteral (including intravenous) and intramuscular, but intrathecal or intraventricular administration is also possible (13). The intramuscular injection is rarely used in clinical practice because it may be very painful locally, and also because its absorption is variable (33). Both colistin sulfate and CMS can be delivered through inhalation by aerosol therapy, but there is a higher frequency of bronchoconstriction with colistin sulfate (33). Delivery of CMS by inhalation and by the intrathecal and intraventricular routes allows much higher concentrations in lung fluid and cerebrospinal fluid, respectively, than those seen with systemic administration. Moreover, those routes of administration lead to negligible plasma exposure and are less toxic (in particularly less nephrotoxic) (22).
In aqueous solutions, colistimethate sodium is transformed into colistin; therefore, it should be administered shortly after reconstitution to avoid the toxicity associated with colistin (37).
Pharmacokinetics.
Because of their discovery and their introduction into clinical use more than 50 years ago, polymyxins were never subjected to the drug development approval process currently required by international drug regulatory authorities. Consequently, the PK and PD data on the rational use of polymyxins (maximizing antibacterial activity and minimizing toxicity and development of resistance) were not available until recently. The fact that, until recently, plasma concentrations of CMS and formed colistin could not be differentiated because of a lack of suitable techniques was another obstacle limiting progress in this area. The recent development of chromatographic methods allowing quantitative assessment of each compound separately significantly contributed to the renewed interest in prescribing colistin and colistimethate (38, 39). It was clearly demonstrated that the observed antimicrobial activity results from the action of colistin itself, which is generated in vivo when CMS is given. For accurate PK information, a prerequisite is to quantify separately the inactive prodrug (CMS) and the active entity (colistin) (34).
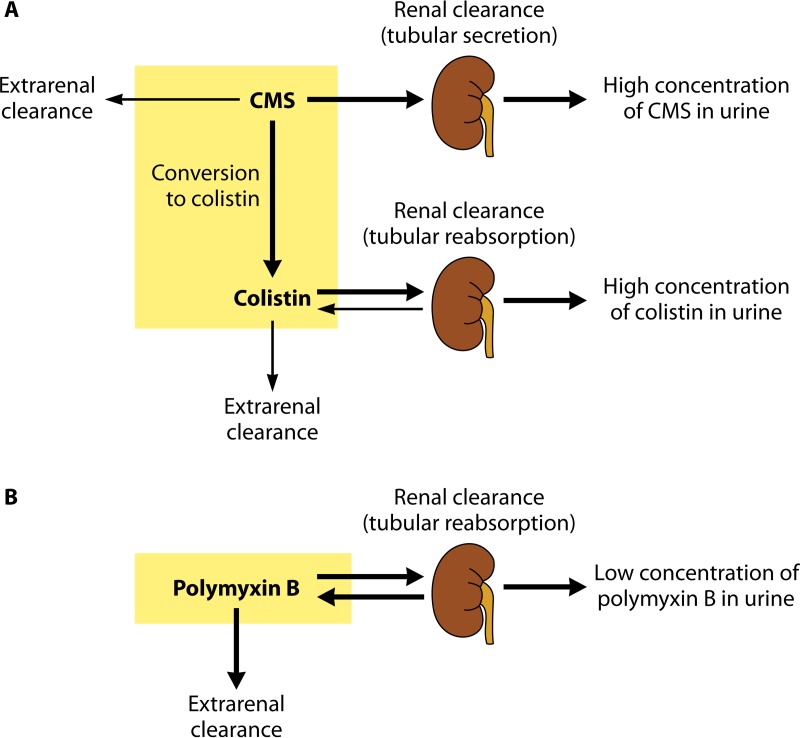
After its parenteral administration, a large proportion of CMS is eliminated mainly through the kidneys by glomerular filtration and tubular secretion (Fig. 2A) (11). Because in a healthy individual the clearance of CMS by the kidneys is much higher than its conversion clearance to colistin, no more than 20 to 25% of a CMS dose is hydrolyzed in vivo into an active colistin entity (7). Consequently, the colistin concentrations resulting from the original CMS administration are low. In contrast to CMS, colistin is eliminated predominantly by a nonrenal way because of its extensive renal tubular reabsorption (Fig. 2A) (11, 40). Although colistin is poorly excreted in urine, the urinary concentration of colistin may be relatively high after administration of CMS due to the conversion of CMS (highly excreted by the kidneys) into colistin within the urinary tract (7).
FIG 2.
Overview of the pharmacokinetic pathways for colistimethate (CMS) and colistin (A) and for polymyxin B (B). The thicknesses of the arrows indicate the relative magnitudes of the respective clearance pathways when kidney function is normal. CMS includes fully and all partially methanesulfonated derivatives of colistin. After administration of CMS, extensive renal excretion of the prodrug occurs, with some of the excreted CMS being converted to colistin within the urinary tract. (The figure is based in part on data from reference 7.)
In contrast to colistin, polymyxin B is administered directly in its active antibacterial form. As for colistin formed from CMS, polymyxin B is subject to very extensive renal tubular reabsorption and is thus eliminated mainly by a nonrenal clearance mechanism(s) (Fig. 2B) (7).
Dosing regimen.
Due to renewed interest in its use in the context of infections caused by multidrug-resistant bacteria, and considering the increasing rates of resistance to colistin currently observed, CMS has to be administered carefully. In particular, the regimens allowing maximal antibacterial activity and minimal development of resistance have to be defined accurately, since the regimens need to minimize adverse effects (23). A study analyzing product data characteristics of intravenous CMS revealed a lack of uniformity between manufacturers, with quite broad variations in term of indications, dose regimens (3 to 12 MU/day), and PK (36). Moreover, dosing regimens given by manufacturers are often discordant with the dosing regimens recommended by the recent literature (21, 34, 41).
(i) Patients with normal renal function.
The currently used dosage regimens of CMS generate suboptimal exposure to colistin in many critically ill patients, in particular in renally competent patients. Two studies reported low plasma colistin Cmax values following administration of 174 mg to 250 mg (2 to 3 MU) of CMS every 8 or 12 h, with steady-state levels of 1.15 to 5.14 μg/ml or 0.68 to 4.65 μg/ml, respectively (42). Moreover, a significant delay in obtaining steady-state plasma concentrations of formed colistin was reported for CMS treatment started without administration of a loading dose (43). In the latter study, concentrations of colistin in the plasma were reported to be below the MIC breakpoint (2 μg/ml), which is a main drawback considering that a delayed initiation of appropriate antibiotic therapy has been shown to be associated with increased mortality rates, in particular for critically ill patients (44). In addition, low colistin concentrations may induce the amplification of colistin-resistant subpopulations (18, 45). Interestingly, on consideration of the current dose range product recommendations for CMS, it was confirmed that its administration at the upper limit to patients with normal renal function resulted in low and potentially suboptimal plasma colistin concentrations, especially when the MIC for the infecting bacterial strain was in the upper range (2 μg/ml) or if the infection was associated with a high bacterial inoculum (21). That study also revealed that steady-state plasma colistin concentrations are highly variable, with up to a 10-fold range achieved across patients at a given creatinine clearance (21).
In contrast, there is a relatively low interpatient variability (3.3-fold) across a wide range of creatinine clearance values following administration of polymyxin B (46). Considering that polymyxin B is not given as a prodrug, it is easier to rapidly achieve a desired plasma concentration of polymyxin B (46).
There is no consensus about dosing regimens, even though recently published dosing suggestions seem to be widely accepted (19). Compared to those suggested by the manufacturers, the regimens in recent studies support the administration of a loading dose and of higher doses of CMS in order to achieve adequate colistin concentrations leading to a better therapeutic effect (21, 41, 47). The dosing regimen currently recommended by the recent literature (for patients with good renal function) is a loading dose of 4.5 MU of CMS followed by maintenance doses of 4.5 MU twice daily (48–50). A colistin-containing combination therapy has to be considered if the infecting pathogen shows an MIC of colistin above 1 μg/ml, if there is a high inoculum, or in dealing with deep-seated infections (e.g., in lungs). One therefore has to consider adding antibiotics to colistin regimens, especially for patients with relatively normal renal function (21, 22).
Data about the pharmacokinetics, effectiveness, and safety of polymyxins were recently reviewed by the European Medicines Agency (EMA). There have been recommended changes in terms of product information in order to ensure the safer use of polymyxins (51). According to the EMA, polymyxins should be reserved for the treatment of serious infections due to aerobic Gram-negative pathogens with limited treatment options (51). Also, they should be given with another suitable antibiotic when possible. The recommended dose for CMS in adults is 9 MU daily in 2 or 3 divided doses as a slow intravenous infusion. For dealing with critically ill patients, a loading dose of 9 MU should be given. For patients with renal impairment, doses should obviously be reduced, with consideration of the creatinine clearance.
Because the efficacy and toxicity of colistin are dose dependent, it is crucial that optimal dose regimens be used to maximize the antimicrobial activity and to minimize adverse effects and the development of resistance. This is especially important for critically ill patients, as they are most at risk for high morbidity and mortality (52).
(ii) Patients with renal insufficiency.
A study showed that colistin levels were elevated in patients with renal insufficiency, presumably due to decreased elimination of the antibiotic generating a higher rate of conversion of CMS to colistin (43). Development of nephrotoxicity is consequently higher in patients with renal insufficiency than in patients with normal renal function (53).
Dalfino et al. (54) suggested a new dose adjustment for high-dose colistin therapy for patients with renal insufficiency. For patients with creatinine clearance of 20 to 50 ml/min, they recommend a loading dose of 9 MU and maintenance doses of 4.5 MU every 24 h. For patients with creatinine clearance of <20 ml/min, they recommend a loading dose of 9 MU and maintenance doses of 4.5 MU every 48 h (21, 55).
Toxicity.
Rates of toxicity following intravenous administration of CMS are considered lower today than those observed in previous studies, and it has to be mentioned that the criteria for defining toxicity have also been updated (56). The lower toxicity may be related to the fact that there are fewer chemical impurities in CMS but also to the fact that monitoring in intensive care units (ICUs) is better nowadays and the coadministration of other nephrotoxic drugs is significantly avoided (33).
Colistin has a narrow therapeutic window, and major adverse effects related to its parenteral use are neurotoxicity and nephrotoxicity. Neurotoxicity is dose dependent and reversible (55) and may cause peripheral and facial paresthesia, weakness, dizziness/vertigo, visual disturbances, confusion, ataxia, and neuromuscular blockade, even leading to respiratory failure or apnea (56). The most common neurotoxicological effect is paresthesia (occurring in 27% of patients), and there is no report of neuromuscular blockade or apnea in the recent literature (56). Nephrotoxicity is the most common and concerning adverse effect, especially with the newly recommended high-dose regimen. Similarly to neurotoxicity, nephrotoxicity is dose dependent. The risk of colistin-associated nephrotoxicity increases with plasma colistin concentrations above 2.5 to 3 μg/ml, as revealed by recent PK-PD analyses (57). Other risk factors for nephrotoxicity include coadministration of other drugs that are also nephrotoxic (anti-inflamatory drugs, vancomycin, or aminoglycoside antibiotics) and patient-related factors (advanced age, male sex, hypoalbuminemia, hyperbilirubinemia, preexisting chronic kidney disease, and severity of illness) (33, 56). Nephrotoxicity is reported to be a rapid-onset effect, with most cases occurring within the first week of treatment, and is mostly reversible (33, 55). Rates of nephrotoxicity in recent studies ranged from 6% to 55% (33). The large range of nephrotoxicity rates may be explained partially by different definitions of renal failure, the dosing regimens used, the concomitant administration of nephrotoxic drugs, and the use of colistin monitoring to adapt dosing regimens. The RIFLE (risk–injury–failure–loss–end-stage renal disease) classification is used to determine colistin-associated nephrotoxicity (58).
Two recent comparative studies involving large numbers of patients showed that the nephrotoxicity rates were lower for polymyxin B than for CMS/colistin (59, 60).
Use in Veterinary Medicine
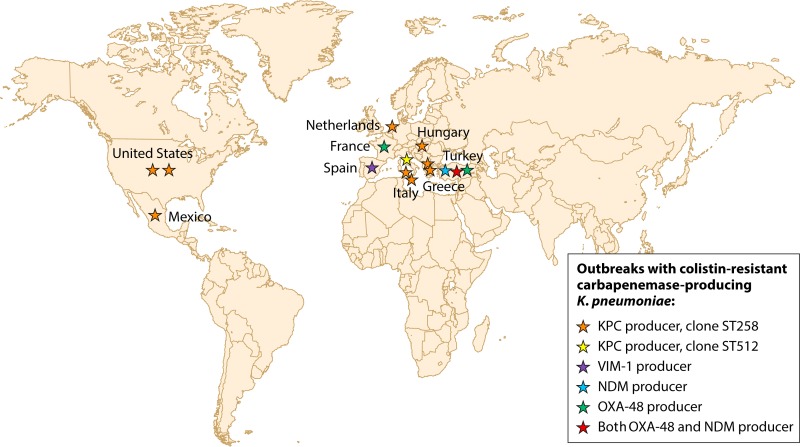
As opposed to the case in human medicine, in veterinary medicine colistin has been used extensively for decades for the treatment and prevention of infectious diseases. The majority of polymyxin consumption corresponds to orally administered forms, with different formulations (premix, powder, or oral solutions). The main usage is related to enterobacterial infections, and in particular to gastrointestinal infections caused by E. coli in poultry and pigs within intensive husbandry systems (61). Apart from this common usage for treating infections caused by Enterobacteriaceae, another usage corresponds to growth promotion, which is a common practice worldwide. Furthermore, the fact that only a thin line exists between oral metaphylactic therapy, preventive starter rations, and growth promotion adds to the problem. In 2011, polymyxins were the fifth most sold class of antimicrobials (7%) for treating food-producing animals in Europe (61).
Despite this extensive use in veterinary medicine, the resistance rate to colistin in E. coli strains recovered from healthy animals remains <1% in many European countries (62). However, resistance to colistin has increasingly been reported (10%) among porcine-pathogenic E. coli strains in Belgium (63), and the emergence of resistance has been described for cattle (64). Moreover, some recent data revealed the possibility of horizontal transmission from farm animals to humans in Asia (65). Given the increasing need to retain the efficacy of colistin to treat MDR infections in humans, the potential for spreading colistin-resistant isolates from animals to humans, and the recent identification of colistin-resistant Enterobacteriaceae organisms harboring a plasmid-borne colistin resistance determinant in animals and food products (see below), the use of colistin in veterinary medicine is being reevaluated. As a very recent example, the formal Ministry of Agriculture of China decided to ban colistin as a feed additive for animals (66). Also, the European Medicines Agency provided a position paper in June 2016, in which updated advice on the use of colistin products in animals within the European Union is provided (67).
METHODS FOR SUSCEPTIBILITY TESTING
Despite such a long term of clinical use (decades), the optimal method for polymyxin susceptibility testing still remains undefined. However, the recent emergence of MDR Gram-negative bacteria and the subsequent increased use of colistin prompted the scientific community to develop rapid and reliable methods to determine the susceptibility of isolates to polymyxins, as this is now an urgent need in clinical laboratories. Polymyxin susceptibility testing is now a major challenge, as human infections with colistin-resistant Gram-negative bacteria are associated with higher patient mortality (68). The difficulties in testing susceptibility to polymyxins are diverse, including poor diffusion of polymyxins into agar, the inherent cationic properties of polymyxins, the occurrence of heteroresistance to polymyxins in many species, and the lack of a reliable reference method that may allow reliable comparisons of commercial tests (69, 70).
Dilution Methods
The aim of dilution methods is to determine the MIC, corresponding to the lowest concentration of polymyxin that inhibits visible bacterial growth after an incubation of 16 to 24 h at 35 ± 2°C.
Broth dilution methods.
Broth dilution is a technique in which a bacterial suspension at a predetermined concentration is tested against various concentrations of antimicrobial agent in a liquid medium with a predetermined formulation. Two types of broth dilution methods are available: (i) the broth macrodilution method, performed with a minimum volume of 2 ml in standard test tubes; and (ii) the broth microdilution (BMD) method, performed with a volume of 0.05 to 0.1 ml in microtitration trays.
(i) Broth microdilution method.
BMD is the reference susceptibility test method. It is currently the only method recommended by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (71, 72) for polymyxin antimicrobial susceptibility testing.
According to CLSI recommendations, BMD is performed with cation-adjusted Mueller-Hinton broth (CA-MHB), a range of 2-fold dilutions of polymyxins (ranging from 0.12 to 512 μg/ml), and a final bacterial inoculum of 5 × 105 CFU/ml in each well (73). BMD is considered to be the optimal method and is currently recommended for susceptibility testing in the recent document proposed by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf).
However, BMD is quite laborious, and manual preparation (if the technique used is not an automated one) of antibiotic solutions may lead to significant errors. It is therefore not adaptable for most clinical microbiology laboratories. Furthermore, nonreproducible and noninterpretable MIC results have been reported due to the presence of skip wells (i.e., wells that exhibit no growth, whereas growth is observed in wells with higher antibiotic concentrations) for Enterobacter species (69), P. aeruginosa (72), and A. baumannii (73). This phenomenon might be caused by heteroresistant subpopulations for Enterobacter spp. (69). In parallel, “skip well” isolates of P. aeruginosa have been found to have increased expression of the pmrAB, phoQ, and arn genes related to changes in the LPS structure, reducing the potential binding sites of polymyxins (74).
Nevertheless, BMD currently remains the reference method for determination of MICs because of its reproducibility, reliability, and possibility of automation.
(ii) Broth macrodilution method (or tube dilution method).
The growth medium (CA-MHB), the inoculum bacterial suspension, the preparation of 2-fold dilutions of polymyxins, the incubation conditions, and the reading of the plate are identical to those for the broth microdilution method. The only difference is the volume of growth medium and the use of test tubes instead of trays. When evaluated against BMD results, the results of the broth macrodilution method showed the highest agreement (83%) compared to other available methods, and no false susceptibility was observed (70).
Agar dilution method.
Agar dilution is another reference method that relies on various concentrations of polymyxin molecules in Mueller-Hinton agar (usually 2-fold serial dilutions), followed by the seeding of a defined bacterial inoculum onto the agar plate. In accordance with the CLSI recommendations, the polymyxin powder is dissolved in sterile water and added to molten MH agar to provide 2-fold dilutions (usually ranging from 0.12 μg/ml to 512 μg/ml) (70, 71). A bacterial inoculum corresponding to a 0.5 McFarland standard (approximately 108 CFU/ml) is prepared, and then 10-fold dilutions are performed. One microliter of this dilution is spotted manually or with an automated system, and each spot consequently inoculates 104 CFU of bacteria.
Agar dilution may theoretically avoid the adsorption of colistin to the plates, but no study has measured the colistin concentration in agar dilution plates to confirm this hypothesis. Numerous studies have demonstrated a strong correlation between agar dilution and BMD (70, 75, 76), with the exception of results obtained with P. aeruginosa and S. maltophilia isolates from cystic fibrosis patients (77, 78). One advantage of the agar dilution method is the ability to test multiple strains on the same plate and the possibility to semiautomate the method. However, the agar dilution method also presents some disadvantages, as it is very laborious if not automated and the plates (not available from commercial sources) must be used within a week of preparation.
Many studies have employed the agar dilution method as a standard; however, BMD remains the primary reference method for polymyxin MIC testing. In a recent document proposed by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group, it is stated that agar dilution is not recommended for susceptibility testing (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf).
Routine Susceptibility Testing Methods
Nonautomatic systems.
(i) DD test (Kirby-Bauer procedure).
The disk diffusion (DD) test refers to the diffusion of a given concentration of polymyxin from disks into MH agar that has been seeded with a defined bacterial inoculum. According to the CLSI and EUCAST guidelines, the disk diffusion test is performed by applying a bacterial inoculum corresponding to a 0.5 McFarland standard (approximately 108 CFU/ml) suspended in 0.85% NaCl onto the entire surface of an MH agar plate by use of a sterile cotton swab. Paper disks impregnated with polymyxin are placed on the inoculated agar surface. Following the CLSI guidelines, the contents of colistin and polymyxin B on the paper disks are 10 μg and 300 U, respectively (72), while following the EUCAST recommendations, the colistin content is 50 μg (71). The growth inhibition zone diameter around the disk is measured after incubation for 16 to 24 h at 35 ± 2°C. The diameter of the inhibition zone is proportional to the bacterial susceptibility to polymyxins and inversely correlates with the MIC of the bacterial strain.
The DD test is easy and cheap and does not require specific equipment. These advantages explain why this method is commonly used as a primary test method to screen large numbers of isolates. However, the poor and slow diffusion of polymyxins through agar gives small zones of inhibition and limits the predictive accuracy of the DD test. In fact, many studies showed that the DD test is a nonreliable method for measuring susceptibility to colistin for Gram-negative rods, giving an unacceptable and very high rate of false susceptibility (up to 35%) compared to that with dilution methods (76, 78–80). A higher concentration of colistin in the disk (50 μg as recommended by EUCAST versus 10 μg as recommended by CLSI) does not improve the reliability of the test (80). Susceptible results should therefore be confirmed by dilution tests. On the other hand, no false resistance results are found with this method (80).
This method is not reliable and should be abandoned. For human medicine, EUCAST recommends that precise MIC determination be mandatory before clinical use and no longer provides disk breakpoints (71). For veterinary medicine, EUCAST recommends precise determination of the MIC each time that the diameter of the inhibition zone is between 15 and 18 mm for a given strain.
(ii) Etest strips.
Etests are thin plastic test strips impregnated with increasing antibiotic concentrations. MICs are read with the concentration scale marked on the upper surface. According to the manufacturer's recommendations, this method is performed by applying a bacterial inoculum of approximately 108 CFU/ml (turbidimetry of a 0.5 McFarland standard) suspended in 0.85% NaCl onto the entire surface of an MH agar plate by use of a sterile cotton swab. Etest strips containing a colistin concentration gradient (ranging from 0.016 to 256 μg/ml) are placed on the inoculated agar surface, and the MIC is determined after incubation for 16 to 24 h at 35 ± 2°C. The MIC value is defined by the intersection of the lower part of the ellipse-shaped growth inhibition area with the test strip. When the intersection occurs around the MIC endpoint, the highest MIC intersection is recorded (75). When small colonies grow within the zone of inhibition, the strain must be considered heteroresistant to colistin, and the highest MIC intersection is recorded (75, 81).
Several studies, notably including few resistant isolates, found an excellent correlation between the Etest and reference techniques (75, 76, 80, 82). However, studies including larger numbers of resistant isolates reported high rates of false susceptibility (up to 32%) for Gram-negative rods compared to those with dilution methods (69, 70, 78, 83). The Etest method may fail to detect resistance to colistin even when isolates exhibit high MICs by dilution methods (70, 83). In addition, there are discrepancies between MICs measured by Etest and MICs measured by dilution methods (70, 82). It has been reported that the Etest strip method underestimates the level of resistance of polymyxin-resistant strains (MIC ≥ 4 μg/ml) and overestimates the MIC values for susceptible strains (MIC < 4 μg/ml) (70).
This method is easy to perform but is relatively expensive and does not reliably detect colistin-resistant isolates. As for the disk diffusion test, susceptibility results obtained by Etest require a 24-h delay.
(iii) UMIC system.
The UMIC system (Biocentric) consists of broth microdilution unitary panels in which the wells contain prediluted lyophilized colistin at concentrations ranging from 0.06 to 64 μg/ml. The inoculation is performed manually, and the required incubation time ranges from 18 to 24 h. The performance of this system has not yet been evaluated.
Automatic systems.
Use of instruments may allow susceptibility testing to be performed in a shorter period than that required for manual methods, as the sensitive optical detection systems of current instruments measure subtle changes in bacterial growth. To date, four automated instruments capable of measuring susceptibility to polymyxins are available. Two of them generate overall rapid (4 to 16 h) susceptibility test results (Vitek 2 and Phoenix), while the others (MicroScan and Sensititre) are overnight systems. These systems are associated with computer software to interpret susceptibility results.
(i) Vitek 2 system.
The Vitek 2 system (bioMérieux) uses plastic reagent cards that contain microliter quantities of antibiotics and test media in wells (84). It tests colistin concentrations ranging from 0.5 to 16 μg/ml and monitors turbidimetry to determine bacterial growth during a period of 4 to 10 h. Compared to dilution methods, the Vitek 2 system displays a low sensitivity for detecting colistin-resistant Gram-negative isolates (83) and is not reliable for detecting heteroresistant subpopulations (76).
(ii) Phoenix automated microbiology system.
The BD Phoenix automated microbiology system (BD Diagnostics) has a large incubator reader. Panels test colistin concentrations ranging from 0.5 to 4 μg/ml, and the inoculation is manual or automatic (84). MIC results are generated in 6 to 16 h. No study has evaluated the performance of Phoenix for detection of colistin resistance among Gram-negative bacteria. The only published study evaluating polymyxin susceptibility by using the Phoenix system unfortunately did not include colistin-resistant strains (85). Nevertheless, we recently evaluated the accuracy of this system by testing 100 enterobacterial isolates (60 colistin-resistant and 40 colistin-susceptible isolates) and found a high rate (15%) of false-susceptible results. We observed a low sensitivity for detecting colistin heteroresistance in K. pneumoniae and Enterobacter cloacae isolates (our unpublished data) but a good sensitivity for detecting plasmid-mediated colistin resistance.
(iii) MicroScan system.
The MicroScan system (Beckman Coulter Diagnostics) uses microdilution trays with colistin concentrations of 2 and 4 μg/ml. The trays are inoculated manually and incubated in the instrument for 16 to 20 h (84). Compared to dilution methods, the categorical agreement of the MicroScan system is 87% for Acinetobacter isolates (86), and the sensitivity is 88% for detection of polymyxin B resistance in K. pneumoniae isolates (87).
(iv) Sensititre system.
The Sensititre system (Thermo Fisher Scientific) is an automated incubation and reading system (84). The tests are standard broth microdilution panels containing prediluted ranges of lyophilized colistin within the wells (0.12 to 128 μg/ml). Inoculation may be performed by using a Sensititre autoinoculator. Growth is measured after an incubation of 18 to 24 h. A single study has evaluated the Sensititre method, and a 96% categorical agreement with BMD was found, with no false susceptibility results reported (70).
Impact of Materials on MIC Determination
Impact of medium.
Polymyxin resistance is regulated by the two-component systems PhoP/PhoQ and PmrA/PmrB (88), which respond to cation (calcium, iron, and magnesium) concentrations and pH variations. These systems are involved in the LPS modifications leading to polymyxin resistance.
There is a high variability of cation concentrations in MH medium depending on the commercial brand, and calcium and magnesium concentrations measured for each brand tested are far below the recommendations of the CLSI (89). This is why the CLSI recommends cation-adjusted MH or supplementation of the culture medium with cations (72, 73).
Iso-Sensitest agar is a well-defined medium with stabilized mineral content that was developed to overcome problems associated with traditional media used for antimicrobial susceptibility tests. Comparison of the agar dilution and Etest methods on MH and Iso-Sentitest agar (76) revealed a lack of detection of the resistant subpopulation of heteroresistant E. cloacae isolates for the Etest performed on MH agar, while Iso-Sensitest agar was more sensitive for detecting the resistant subpopulation with both methods (76).
However, a cation-dependent inhibition of antimicrobial activity has been reported for polymyxin antibiotics (90, 91). In fact, it is suspected that the colistin antimicrobial activity might be overestimated if tested using conventional cation-adjusted MH as recommended by the CLSI. Note that the calcium concentration recommended by the CLSI for determining colistin susceptibility in vitro is 2-fold higher than the concentration found in human interstitial space fluid in vivo (92). A recent study revealed that the MIC of colistin might be misestimated if tested with conventional cation-adjusted growth media (overestimation for P. aeruginosa and A. baumannii and underestimation for E. coli) (92). The use of cation-adjusted or non-cation-adjusted medium therefore remains questionable, and a consensus is still needed.
Impact of powder composition.
MIC testing is performed using commercially available polymyxin B and colistin sulfate powders. The variability in the relative proportions of the mixture components between powder batches and manufacturers is a potential source of variability of the results (93, 94). In parallel, MICs obtained using BMD with purified forms of the major compounds of polymyxin B were within a log2 dilution of the MICs obtained using the U.S. Pharmacopoeia polymyxin B sulfate powder mixture (95). These data suggest that the powder composition may not have an impact on polymyxin susceptibility testing. Note that CMS, as a prodrug, cannot be used for susceptibility testing, as it yields erroneously high MIC values (96).
Impact of the composition and treatment of plates.
Due to their cationic properties, polymyxins adhere to the negative charges of the microtiter trays commonly used for BMD. Karvanen et al. (97) measured the colistin concentrations following incubation in polypropylene, polystyrene, and glass tubes. The adsorption was proportionally higher at lower concentrations of the drug. Consequently, the results of colistin BMD measurements significantly differ if tests are conducted in microtiter plates with different coated wells (98). The amount of colistin adsorbed to the plate surface therefore depends on various factors, such as the coating applied to the plate, and is not consistent from well to well (K. Sei, presented at the January 2012 Meeting of the CLSI Subcommittee on Antimicrobial Susceptibility Testing, Tempe, AZ, 22 to 24 January 2012). Since the nature and treatment of plastics are not addressed in the CLSI recommendations, significant variability is observed between laboratories performing the reference BMD method.
Presence or absence of P-80.
Polysorbate 80 (P-80 or Tween 80) is a surfactant used for the preparation of BMD panels used for susceptibility testing (99). This surfactant has been recommended by the CLSI to prevent or at least mitigate binding of lipoglycopeptides to plastics (72, 73). The presence of 0.002% P-80 mitigates colistin adsorption to polystyrene microplates (Sei, presented at the January 2012 Meeting of the CLSI Subcommittee on Antimicrobial Susceptibility Testing). When P-80 is added to a final concentration of 0.002% in the well, the polymyxin MICs are 4- to 8-fold lower than those obtained without P-80 among isolates with low MICs by BMD testing (70, 99).
It is noteworthy that the effect of P-80 on bacterial viability has not been well evaluated. Also, another concern is that P-80 may act synergistically with polymyxins, consequently giving artificially lower MICs (100). Also, in P. aeruginosa, P-80 increases cell permeability and lyses spheroblasts (101). On the other hand, polymyxins destabilize the outer membrane, allowing P-80 to access the inner membrane and induce cell lysis. Therefore, isolates resistant to polymyxins would not be affected significantly by P-80. Therefore, only isolates with polymyxin MICs of ≤1 μg/ml might be affected (94).
In January 2014, the CLSI subcommittee decided to pursue a recommendation of polymyxin BMD testing without P-80. However, if a susceptibility breakpoint of ≤1 μg/ml is chosen, the ability to detect susceptible isolates without using P-80 may be compromised (94). Since the use of P-80 is still questionable, a solution might be to determine MICs in glass plates, as colistin fixation on glass is less extensive (94). However, glass plates are fragile and expensive.
Impacts of Subcultures and Storage on MICs
Impact of subcultures.
A study by Li et al. (26) revealed a loss of colistin resistance when resistant isolates were subcultured without selective pressure. For instance, about 98% of a colistin-resistant A. baumannii population lost the resistance phenotype after a single passage in a colistin-free medium.
Impact of storage.
A loss of colistin resistance was also observed after 6 to 8 months of storage at −70°C (70). Among 25 isolates that initially tested resistant by a dilution method, five (20%) tested susceptible by the same method after freezing. The availability of easy, rapid, and inexpensive techniques allowing screening of colistin resistance on fresh cultures in routine laboratories is consequently a real clinical need.
Interpretive Criteria
There is a lack of consensus between the two organizations setting up breakpoints for polymyxins, namely, the CLSI in the United States (72) and EUCAST in Europe (71). The zone diameter and MIC interpretive criteria given by those two organizations for colistin and polymyxin B are shown in Table 1. However, recent data related to PK suggest that the current breakpoints might be too high (21).
TABLE 1.
Colistin and polymyxin B breakpoints according to CLSI and EUCAST in 2014
| Criteria and bacterial group | Colistin |
Polymyxin B |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disk content (μg) | Zone diam interpretative criteria (mm)a |
MIC interpretative criteria (μg/ml)a |
Disk content (IU) | Zone diam interpretative criteria (mm)a |
MIC interpretative criteria (μg/ml)a |
|||||||
| Sensitive (S) | Resistant (R) | S | Intermediate (I) | R | S | R | S | I | R | |||
| CLSI criteria | ||||||||||||
| Enterobacteriaceae | — | — | — | — | — | 300 | —b | —b | — | — | — | |
| Acinetobacter spp. | — | — | ≤2 | — | ≥4 | — | —b | —b | ≤2 | — | ≥4 | |
| Pseudomonas spp. | 10 | ≥11 | ≤10 | ≤2 | 4 | ≥8 | 300 | ≥12 | ≤11 | ≤2 | 4 | ≥8 |
| EUCAST criteria | ||||||||||||
| Enterobacteriaceae | 50 | ≥18b,c | <15b,c | ≤2 | — | >2 | — | —b | —b | ≤2 | — | >2 |
| Acinetobacter spp. | —c | —c | ≤2 | — | >2 | — | —b | —b | ≤2 | — | >2 | |
| Pseudomonas spp. | —c | —c | ≤4 | — | >4 | — | —b | —b | — | — | — | |
—, not determined or absent.
Zone diameter interpretative criteria for Enterobacteriaceae given by EUCAST are only for veterinary medicine; MIC determination is required for diameters ranging from 15 to 18 mm.
No zone diameter interpretative criteria for human medicine; the MIC must be determined before use.
Quality Controls
Quality control organisms are required during susceptibility testing in order to ensure accuracy and standardization of the procedures. Quality control can be assessed using the E. coli ATCC 25922 (NCTC 12241; CIP 76.24) and P. aeruginosa ATCC 27853 (NCTC 12903; CIP 76110) reference strains. The disk diffusion and MIC quality control ranges for these strains determined by the CLSI are shown in Table 2 (72).
TABLE 2.
Zone diameter and MIC quality control ranges for polymyxins according to CLSI guidelines
| Strain | Zone diam (mm) range |
MIC (μg/ml) range |
||
|---|---|---|---|---|
| Colistin | Polymyxin B | Colistin | Polymyxin B | |
| E. coli ATCC 25922 | 11–17 | 13–19 | 0.25–2 | 0.25–2 |
| P. aeruginosa ATCC 27853 | 11–17 | 14–18 | 0.5–4 | 0.5–2 |
Correlation between MICs of Colistin and Polymyxin B
Despite the high similarity of the molecular structures of colistin and polymyxin B, a recent study including 15,377 Gram-negative bacteria revealed differences between the MICs of colistin and polymyxin B (102). MIC values determined by the Sensititre system were 2-fold higher for polymyxin B than for colistin for 55% and 53% of Klebsiella species isolates (n = 4,177) and E. coli isolates (n = 6,311), respectively. However, a categorical agreement of >99% was obtained for enterobacterial strains when breakpoints of ≤2/≥4 for both colistin and polymyxin B were applied. That study showed a high level of agreement between MICs of colistin and polymyxin B for P. aeruginosa and Acinetobacter spp.
Qualitative Detection Techniques
Rapid detection of heterogeneous populations among colistin-resistant Gram-negative bacteria by use of capillary electrophoresis.
Sautrey et al. (103) proposed a capillary electrophoresis method for rapid detection of heterogeneous populations of colistin-resistant strains. However, further development is required for such applications to be used in clinical laboratories on a daily basis.
Rapid detection of colistin-resistant A. baumannii isolates by use of the Micromax assay.
The Micromax assay is based on the detection of released nucleotides, indicating cell wall damage, in the presence of colistin (104). After incubation with 0.5 μg/ml of colistin, strains are considered resistant to colistin if ≤11% of bacteria present cell wall damage. Bacteria are incubated for 90 min in Mueller-Hinton broth to achieve exponential growth and then incubated for 60 min with colistin at concentrations of 0 and 0.5 μg/ml, respectively. Bacteria embedded in agarose are incubated with a lysis solution removing only weakened cell walls. The released fragmented DNA may be stained with the fluorochrome SYBR gold (Molecular Probes, Eugene, OR) and visualized by fluorescence microscopy (45 min to 60 min of technical processing and scoring under the microscope). This method is faster than the routine automatic microdilution procedure (3 h 30 min versus 6 to 8 h) and is accurate for detecting colistin resistance in A. baumannii (100% sensitivity and 96% specificity). Another advantage is that it can be automated. However, the manual task and the cost of the materials (fluorochrome and epifluorescence microscope) are disadvantages for its routine use.
Rapid detection of colistin-resistant Enterobacteriaceae isolates by use of the Rapid Polymyxin NP test.
We developed the Rapid Polymyxin NP test, which is based on the detection of bacterial growth in the presence of a defined polymyxin concentration (105). Detection is based on detection of glucose metabolism upon bacterial growth. Glucose metabolism induces the formation of acid, leading to a color change of the red phenol used as a pH indicator. The test is performed with a final concentration of bacteria of ca. 108 CFU/ml in each well (or tube), and the final concentration of polymyxin is 3.75 μg/ml. Visual inspection of the tray is made after 10 min and then every hour for 2 h. The test is considered positive (indicating polymyxin resistance) if the isolate grows in the presence of colistin (color change from orange to yellow), while it is considered negative (indicating polymyxin susceptibility) if the isolate does not grow in the presence of polymyxin (no color change). This test is rapid (less than 2 h) and easy to perform.
By testing a total of 200 enterobacterial isolates exhibiting either resistance (intrinsic or acquired, or various) or susceptibility to polymyxins, the specificity and sensitivity of this test were evaluated at 99.3% and 95.4%, respectively, compared to BMD as the reference method (105). Note that the Rapid Polymyxin NP test identified the isolates exhibiting a heteroresistance phenotype as well as those producing the plasmid-mediated MCR-1 determinant (see below).
For the Rapid Polymyxin NP test, the adequate culture media for culturing the bacteria prior to the test were Mueller-Hinton agar, Luria-Bertani agar, Columbia agar plus 5% sheep blood, chocolate agar, UriSelect 4 agar, and eosin methylene blue agar.
The Rapid Polymyxin NP test may also detect colistin-resistant Enterobacteriaceae directly from blood cultures (106). Results are obtained within 4 h.
Selective medium.
So far, no selective medium allowing screening for any type of polymyxin-resistant Gram-negative isolates (with intrinsic, chromosomally encoded, or plasmid-mediated polymyxin resistance) has been available. Neither commercial nor in-house screening culture media had been designed that might permit screening of patients possibly colonized by polymyxin-resistant isolates. Therefore, we developed SuperPolymyxin, a selective culture medium for detection of any type of polymyxin-resistant Gram-negative organism (107). The SuperPolymyxin medium prevents swarming of Proteus spp. (intrinsically resistant to polymyxins) and also the growth of Gram-positive bacteria and fungi, by addition of daptomycin and amphotericin B, respectively. Its base corresponds to the eosin methylene blue medium (Levine's medium) (108) selective for Gram-negative bacteria, which differentiates lactose fermenters (black colonies) from nonfermenters (colorless or light lavender colonies). In addition, differentiation of lactose fermenters is also possible to some extent. The SuperPolymyxin medium contains a colistin concentration (3.5 μg/ml) that allows clear categorization between polymyxin-resistant and -susceptible isolates. The sensitivity and specificity of this medium have been found to be 100% (107).
Genotypic Methods
Although the mechanisms underlying resistance to polymyxins have not all been elucidated, acquisition of colistin resistance in Gram-negative bacteria has been attributed to lipopolysaccharide (LPS) modifications via diverse routes, including (i) the addition of cationic groups to the LPS reducing the overall negative charge of the LPS and consequently preventing the fixation of polymyxins; (ii) loss of the LPS and, consequently, loss of the polymyxin target; (iii) the overproduction of capsule polysaccharide (CPS) hiding polymyxin binding sites; and (iv) the release of CPS trapping polymyxins. Specific modifications of outer membrane porins and overexpression of efflux pump systems have also been described (88).
Several molecular mechanisms have been associated with colistin resistance in Gram-negative bacteria, such as alterations in the PmrA/PmrB, PhoP/PhoQ, ParR/ParS, ColR/ColS, and CprR/CprS two-component systems and alterations in the mgrB gene, which encodes a negative regulator of PhoPQ. Mutations leading to the addition of cationic groups on lipid A result in a less anionic lipid A and, consequently, to less fixation of polymyxins (88).
Similarly, alterations in the lpxA, lpxC, and lpxD genes of A. baumannii result in inactivation of lipid A biosynthesis, leading to a complete loss of LPS and, consequently, to a loss of the polymyxin target (109).
The mechanisms of polymyxin resistance can be identified by sequencing those specific genes. However, molecular techniques cannot be envisioned in the near future considering that (i) many chromosomally encoded mechanisms of resistance remain to be identified, (ii) it is difficult to extrapolate whether some substitutions identified in proteins known to be involved in LPS biosynthesis lead to resistance, and (iii) the levels of expression of the corresponding genes may vary and consequently influence the level of resistance to polymyxins.
There is an exception that corresponds to the recent identification of the plasmid-borne mcr-1/mcr-2 genes, whose products confer resistance to polymyxins (see below). According to the current knowledge on the topic, identification of these genes may be considered a signature of resistance or reduced susceptibility to polymyxins. This is why identifying the gene makes sense in this case, since qualitative genetic results may be translated directly into a nonsusceptibility phenotype. Screening of both mcr-1 and mcr-2 can be performed by using a standard PCR protocol using the primers MCR-1/2-Fw (5′-TAT CGC TAT GTG CTA AAG CC-3′) and MCR-1/2-Rv (5′-TCT TGG TAT TTG GCG GTA TC-3′), giving rise to a 715-bp amplicon. Also, a SYBR green-based real-time PCR assay that provides a simple, specific, sensitive, and rapid molecular tool for detection of mcr-1-positive isolates was recently published (110). That technique was validated on human and animal isolates and may be applied to extensive surveillance studies.
Porin mutations and overexpression of efflux pump systems may also be involved in colistin resistance (88), and it is very likely that phenotypic resistance to polymyxins in clinical isolates often results from combined resistance mechanisms (e.g., defects in outer membrane proteins combined with structural modification of the LPS). Phenotypic methods, such as the Rapid Polymyxin NP test, are consequently very relevant for determining the subsequent therapeutic decision, since they actually concretely determine the susceptibility or lack thereof of isolates, in contrast to genotypic methods, which detect only potential resistance and require sequencing of multiple genes.
RESISTANCE MECHANISMS IN ENTEROBACTERIACEAE
Intrinsic Resistance Mechanisms in Proteus mirabilis and Serratia marcescens
In P. mirabilis and S. marcescens, naturally occurring resistance to polymyxins is linked to the constitutive expression of the arnBCADTEF operon and/or the eptB gene, causing addition of phosphoethanolamine (pEtN) and/or 4-amino-4-deoxy-l-arabinose (l-Ara4N) cationic groups to the LPS. This modification increases the charge of the LPS, which is the initial target of the polymyxins, and therefore decreases polymyxin binding, giving rise to intrinsic resistance of these species (111–113).
Mechanisms Responsible for Acquired Resistance in Enterobacteriaceae
Acquired resistance to polymyxins has been identified in several genera of the Enterobacteriaceae, such as Klebsiella, Escherichia, Enterobacter, and Salmonella. Colistin resistance mechanisms remain unknown for some bacterial species, but several molecular mechanisms have been identified. The most common is modification of the LPS via cationic substitution, similar to that observed in bacteria with intrinsic resistance to polymyxins. A single transferable mechanism of resistance has been identified so far (see below), with most of the resistance mechanisms being encoded chromosomally.
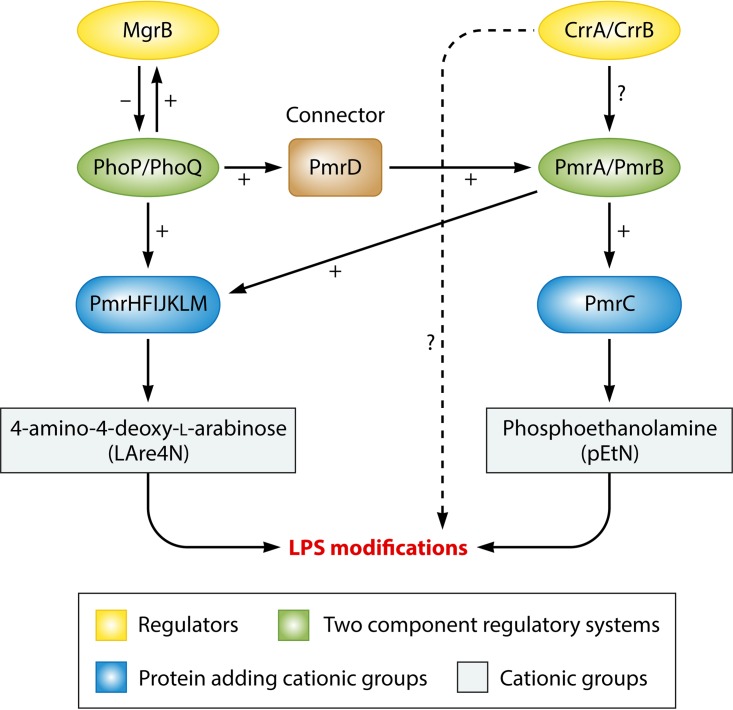
Similar to what is observed in strains that are naturally resistant to colistin, addition of cationic groups (l-Ara4N and pEtN) to the LPS is responsible for acquisition of colistin resistance in Enterobacteriaceae. A large panel of genes and operons are involved in qualitative modification of the LPS (Fig. 3), including genes and operons coding for enzymes that are directly involved in LPS modifications (genes responsible for synthesis of cationic groups and/or their addition to the LPS), i.e., the pmrC gene, the pmrE gene, and the pmrHFIJKLM operon; regulatory genes, such as those encoding proteins involved in the PmrAB and PhoPQ two-component systems; and the regulators of these two-component systems, i.e., the mgrB gene, which negatively regulates the PhoPQ system, and the newly described crrAB two-component regulatory system, which regulates the PmrAB system.
FIG 3.
Regulation pathways of LPS modifications in Klebsiella pneumoniae.
Genes encoding LPS-modifying enzymes.
(i) The pmrC gene.
The pmrCAB operon codes for three proteins, namely, the phosphoethanolamine (pEtN) phosphotransferase PmrC, the response regulator PmrA (also called BasR), and the sensor kinase protein PmrB (also called BasS) (114). The phosphoethanolamine phosphotransferase PmrC adds a pEtN group to the LPS (Fig. 3) (114).
(ii) The pmrHFIJKLM operon and the pmrE gene.
The pmrHFIJKLM operon (also called the arnBCADTEF or pbgPE operon) codes for a total of seven proteins (115). The pmrE gene and the pmrHFIJKLM operon are responsible for the synthesis of the l-aminoarabinose group (l-Ara4N) and its fixation to lipid A (Fig. 3) (115).
(iii) The pmrA and pmrB genes, which encode the PmrAB two-component system.
Environmental stimuli, such as macrophage phagosomes, ferric (Fe3+) iron, aluminum (Al3+), and low pH (e.g., pH 5.5), mediate activation of PmrB through its periplasmic domain (114). The PmrAB and PhoPQ two-component systems are normally activated when bacteria are phagocytized into macrophages, allowing bacterial survival (114).
PmrB is a protein with tyrosine kinase activity that activates PmrA by phosphorylation. PmrA in turn activates the transcription of the pmrCAB operon, the pmrHFIJKLM operon, and the pmrE gene involved in LPS modification (pEtN and l-Ara4N addition) (Fig. 3) (114).
Specific mutations within the pmrA and pmrB genes have been described as being responsible for acquired colistin resistance in K. pneumoniae (105, 116–120), Enterobacter aerogenes (121), and Salmonella enterica (122, 123) (Table 3). These mutations are responsible for constitutive activation of the PmrAB two-component system, leading to upregulation of the pmrCAB operon, the pmrHFIJKLM operon, and the pmrE gene, and thus to the synthesis of pEtN and l-Ara4N and their transfer to lipid A (Fig. 3).
TABLE 3.
Chromosomal mutations and amino acid deletions responsible for acquired colistin resistance in Klebsiella pneumoniae, Enterobacter aerogenes, Escherichia coli, Salmonella enterica, P. aeruginosa, and A. baumannii isolates
| Bacterial group and species | Protein (normal length [aa]) | Domain involved (residues)a,b | Amino acid changed | Reference(s) |
|---|---|---|---|---|
| Enterobacteriaceae | ||||
| K. pneumoniae | PmrA (223) | REC (1–112) | S42N | 120 |
| G53C | 105, 120 | |||
| G53S | 105 | |||
| Trans_reg_C (145–216) | ||||
| PmrB (365) | TM (13–35) | ΔR14 | 118 | |
| L17Q | 105 | |||
| HAMP (90–142) | L82R | 116 | ||
| S85R | 120 | |||
| T140P | 120 | |||
| HisKA (143–203) | T157P | 117–119 | ||
| S208N | 118 | |||
| ΔY209 | 118 | |||
| HATPase_c (250–358) | R256G | 117 | ||
| PhoP (223) | REC (1–112) | V3F | 117 | |
| L26Q | 120 | |||
| S86L | 117 | |||
| Trans_reg_C (145–220) | D191Y | 81 | ||
| PhoQ (488) | PhoQ sensor (10–189) | R16C | 105 | |
| L26P | 117 | |||
| L96P | 120 | |||
| D150G | 117 | |||
| S174N | 118 | |||
| HAMP (195–263) | V258F | 117 | ||
| HisKA (267–330) | ||||
| L348Q | 120 | |||
| HATPase_c (375–482) | G385S | 120 | ||
| D434N | 128 | |||
| MgrB (47) | K3* | 105 | ||
| L9* | 120 | |||
| I13* | 120 | |||
| A14S | 120 | |||
| W20R | 105 | |||
| L24H | 130 | |||
| V26* | 120 | |||
| M27K | 105 | |||
| C28F | 120 | |||
| C28Y | 117, 120, 128, 130 | |||
| C28* | 105, 120 | |||
| Q30* | 105, 120 | |||
| D31N | 120 | |||
| Q33* | 105 | |||
| F35I | 120 | |||
| G37S | 130 | |||
| C39Y | 105 | |||
| N42Y/K43I | 105 | |||
| I45T | 105 | |||
| W47R | 105 | |||
| W47* | 105 | |||
| *48Y | 117 | |||
| CrrB (353) | Q10L | 128, 137 | ||
| TM (12–34) | Y31H | 137 | ||
| HAMP (81–135) | L94 M | 128 | ||
| HisKA (136–200) | W140R | 137 | ||
| N141I | 137 | |||
| P151S | 137 | |||
| S195N | 137 | |||
| E. aerogenes | PmrA | G53S | 121 | |
| E. coli | PmrA (222) | REC (1–112) | R81Sc | 125 |
| Trans_reg_C (145–216) | ||||
| PmrB (363) | Δ7–12c | 124 | ||
| TM1 (15–37) | ||||
| TM2 (69–91) | ||||
| HAMP (92–144) | ||||
| HisKA (145–205) | T156Kc | 124 | ||
| A159Vc | 124 | |||
| V161Gc | 125 | |||
| HATPase_c (252–360) | ||||
| PhoQ_sensor (10–189) | ||||
| HAMP (195–263) | ||||
| HisKA (267–330) | ||||
| PhoP (223) | REC (1–112) | |||
| Trans_reg_C (145–220) | ||||
| PhoQ (486) | PhoQ_sensor (10–189) | |||
| HAMP (195–263) | ||||
| HisKA (267–330) | ||||
| HATPase_c (374–480) | E375Kc | 65 | ||
| S. enterica | PmrA (222) | REC (1–112) | G15Rc | 123 |
| G53Ec | 123 | |||
| G53Rc | 123 | |||
| R81Cc | 123 | |||
| R81Hc | 123 | |||
| Trans_reg_C (145–216) | ||||
| PmrB (356) | TM (13–35) | Δ11–14c | 122 | |
| L14Fc | 123 | |||
| L14Sc | 123 | |||
| M15Lc | 122 | |||
| L22Pc | 123 | |||
| S29Rc | 123 | |||
| HAMP (89–141) | T92Ac | 123 | ||
| P94Qc | 123 | |||
| E121Ac | 123 | |||
| S124Pc | 123 | |||
| N130Yc | 123 | |||
| HisKA (142–202) | T147Pc | 123 | ||
| R155Pc | 123 | |||
| T156Mc | 123 | |||
| T156Pc | 123 | |||
| V161Gc | 123 | |||
| V161Lc | 123 | |||
| V161Mc | 123 | |||
| E166Kc | 123 | |||
| M186Ic | 123 | |||
| HATPase_c (249–356) | G206Rc | 123 | ||
| G206Wc | 123 | |||
| S305Rc | 123 | |||
| Nonfermentative bacilli | ||||
| P. aeruginosa | PmrA (221) | REC (1–112) | ||
| Trans_reg_C (145–216) | L157Q | 166 | ||
| PmrB (477) | L14P | 167 | ||
| TM1 (15–37) | ||||
| PD (38–160) | ΔD45 | 74, 167 | ||
| A54V | 167 | |||
| TM2 (161–183) | L167P | 166 | ||
| HAMP (186–238) | G188D | 167 | ||
| F237L | 118 | |||
| HisKA (239–304) | L243Q | 167 | ||
| A247T | 168 | |||
| A248V | 167 | |||
| S257N | 167 | |||
| M292I | 167 | |||
| M292T | 169 | |||
| HATPase_c (348–459) | ||||
| PhoQ (448) | R6C | 170 | ||
| TM1 (7–29) | ||||
| ΔV57–Q332 | 170 | |||
| PD (30–166) | N104I | 118 | ||
| K123Q | 166 | |||
| K123E | 118 | |||
| Q133E | 118 | |||
| A143V | 166 | |||
| V152* | 168 | |||
| TM2 (167–189) | V184G | 118 | ||
| A207R | 118 | |||
| R214H | 118 | |||
| H223R | 168 | |||
| HisKA (238–300) | V260G | 163, 247 | ||
| HATPase_c (343–448) | ΔL364–G365 | 170 | ||
| I421* | 170 | |||
| Fr at I421 | 170 | |||
| D433* | 170 | |||
| R444C | 170 | |||
| ParR (235) | REC (7–117) | L18I | 118 | |
| N24S | 118 | |||
| S24N | 118 | |||
| M59I | 171 | |||
| Trans_reg_C (152–228) | E156K | 171 | ||
| ParS (428) | TM1 (5–27) | L14Q | 171 | |
| PD (28–131) | V101 M | 171 | ||
| TM2 (132–154) | L137P | 171 | ||
| HAMP (155–207) | ||||
| HisKA (208–273) | Q232E | 118 | ||
| HATPase_c (318–428) | G361R | 118 | ||
| H398R | 247 | |||
| ColS | A106V | 172 | ||
| CprS | R241C | 172 | ||
| A. baumannii | PmrA (224) | REC (2–112) | E8D | 177, 180 |
| M12I | 174 | |||
| P102H | 173 | |||
| S119T | 174 | |||
| Trans_reg_C (150–221) | ||||
| PmrB (444) | TM1 (10–29) | T13N | 173 | |
| S14L | 175 | |||
| S17R | 177 | |||
| Fr at F26 | 176 | |||
| PD (30–141) | ΔA32–E35 | 174 | ||
| D64V | 174 | |||
| A80V | 174 | |||
| L87F | 175 | |||
| Y116H | 177 | |||
| I121F | 178 | |||
| TM2 (142–164) | M145K | 175 | ||
| ΔL160 | 174 | |||
| P170L | 174, 179 | |||
| P170Q | 174 | |||
| A183T | 178 | |||
| A184V | 178 | |||
| P190S | 178 | |||
| T192I | 178 | |||
| L208F | 174 | |||
| HisKA (218–280) | A226V | 174 | ||
| A227V | 173, 175, 176 | |||
| Q228P | 178 | |||
| R231L | 174 | |||
| T232I | 177 | |||
| P233S | 118, 173–176, 179 | |||
| P233T | 173 | |||
| T235I | 174 | |||
| N256I | 174 | |||
| A262P | 173 | |||
| R263C | 174 | |||
| R263L | 177 | |||
| R263P | 174 | |||
| Q277H | 174 | |||
| G315D | 174 | |||
| HATPase_c (326–437) | N353Y | 175 | ||
| P377L | 174 | |||
| F387Y | 175 | |||
| S403F | 175 | |||
| LpxA (262) | Fr at I25 | 109 | ||
| G68D | 109 | |||
| Q72K | 109 | |||
| Fr at H121 | 109 | |||
| Fr at D130 | 109 | |||
| H159D | 109 | |||
| Q234* | 109 | |||
| LpxC (276) | P30L | 109 | ||
| Fr at D45 | 109 | |||
| Fr at T285 | 109 | |||
| LpxD (356) | Fr at K317 | 109 |
Domains predicted in SMART by using protein sequences of Escherichia coli K-12 substrain MG1655, Klebsiella pneumoniae subsp. pneumoniae MGH 78578, Salmonella enterica serovar Typhimurium LT2, P. aeruginosa PAO1, and A. baumannii AB0057. REC, CheY-homologous receiver domain; Trans_reg_C, transcriptional regulatory protein, C-terminal domain; TM, transmembrane domain; TM1, first transmembrane domain; TM2, second transmembrane domain; PD, periplasmic domain; HAMP, histidine kinases, adenylyl cyclases, methyl-binding proteins, and phosphatases; HisKA, histidine kinase A (phosphoacceptor) domain; HATPase_c, histidine kinase-like ATPases; PhoQ sensor, phosphorelay signal transduction system.
A periplasmic domain (PD) was not predicted in SMART but was assumed to be between TM1 and TM2.
The involvement of the mutation in the colistin resistance profile was determined by in silico analysis.
Δ, deletion; Fr, frameshift; *, stop codon.
Some polymorphism in the pmrAB genes of colistin-resistant E. coli has been reported (124, 125), but the involvement of these mutations in the colistin resistance phenotype has not formally been demonstrated, since no complementation or site-directed mutagenesis has been performed.
(iv) The phoP and phoQ genes, which encode the PhoPQ two-component system.
The phoPQ operon codes for two proteins, namely, the regulator protein PhoP and the sensor protein kinase PhoQ. Environmental stimuli, such as macrophage phagosomes, low magnesium (Mg2+), and low pH (e.g., pH 5.5), mediate activation of PhoQ through its periplasmic domain (114). The PhoPQ two-component system allows the expression of genes that code for magnesium transport, enzymes that modify the LPS to allow resistance to cationic antimicrobial peptides, and enzymes that decrease the cell stress caused by acidic pH or some virulence factors (126, 127). The PhoPQ two-component system therefore allows bacterial survival under conditions of low magnesium or acidic pH or in the presence of cationic antimicrobial peptides.
PhoQ is a protein with tyrosine kinase activity that activates PhoP by phosphorylation. PhoP in turn activates the transcription of the pmrHFIJKLM operon, involved in the addition of l-Ara4N to the LPS (Fig. 3) (126, 127). PhoP can also activate the PmrA protein, either directly or indirectly via the PmrD connector protein, causing the addition of pEtN to the LPS.
Several mutations in the phoP and phoQ genes are responsible for acquired resistance to polymyxins in K. pneumoniae (81, 105, 117, 118, 120, 128) (Table 3). One mutation potentially involved in colistin resistance in E. coli has also been described (65). These mutations are responsible for constitutive activation of the PhoPQ two-component system, leading to upregulation of the pmrHFIJKLM operon and thus to the synthesis of l-Ara4N and its transfer to lipid A (Fig. 3).
Regulators of the PmrAB and PhoPQ Two-Component Systems
The mgrB gene.
MgrB (also called YobG) is a small transmembrane protein of 47 amino acids (129). Upon activation of PhoP, the mgrB gene is upregulated. The MgrB protein in turn represses the expression of the PhoQ-encoding gene, leading to negative regulation of the PhoPQ two-component system (Fig. 3) (129). Inactivation of the mgrB gene (the negative regulator of the PhoPQ two-component system) leads to overexpression of the phoPQ operon, thus causing pmrHFIJKLM operon activation, leading to the production of l-Ara4N responsible for the acquisition of colistin resistance.
Several missense mutations resulting in amino acid substitutions and nonsense mutations and therefore leading to a truncated MgrB protein may be responsible for acquired resistance to colistin in K. pneumoniae (Table 3). Other alterations, such as insertions or deletions of small nucleotide sequences in the mgrB gene, or even some complete deletions of the mgrB locus, have been reported (120, 130, 131). Insertional inactivation caused by diverse insertion sequences (IS), belonging to several families and inserted at different locations within the mgrB gene, is often responsible for acquired resistance to colistin in K. pneumoniae (105, 117, 120, 130–132) and Klebsiella oxytoca (133, 134). Recently, the transposition of genes encoding extended-spectrum β-lactamases (ESBLs) or carbapenemases, leading to disruption of the chromosomal mgrB gene, was reported as a source of resistance to colistin (135, 136). Notably, selective pressure with β-lactams leading to the acquisition of β-lactamase genes may therefore be responsible for coselection of colistin resistance. Despite the high homology observed among mgrB gene sequences of Enterobacteriaceae organisms (129), disruption of this gene has so far not been found to be responsible for acquired resistance to colistin in genera other than Klebsiella.
The crrAB operon.
The crrAB (colistin resistance regulation) operon codes for two proteins, namely, the regulatory protein CrrA and the sensor protein kinase CrrB. The physiological role of the crrAB operon is still unknown. However, inactivation of the crrB gene leads to overexpression of the pmrAB operon, thus causing activation of the pmrHFIJKLM operon and of the pmrC and pmrE genes, consequently leading to the production of l-Ara4N and pEtN, both of which are responsible for the acquisition of colistin resistance (Fig. 3) (128). CrrB inactivation may also modify lipid A through activation of a glycosyltransferase-like protein (128).
Six amino acid substitutions in the CrrB protein have been identified as being responsible for acquired resistance to polymyxins in K. pneumoniae (Table 3) (128, 137).
The Intrinsic Regulator RamA
The intrinsic regulator RamA of K. pneumoniae is known to play a significant role in the overall response to antimicrobials. It regulates genes that are linked to permeability barriers and therefore may be involved in reduced susceptibility to antibiotics. It was recently shown that increased levels of this regulator caused LPS alterations and consequently reduced susceptibility to polymyxins (138).
Plasmid-Mediated Resistance to Polymyxins
The plasmid-mediated mcr-1 gene, responsible for horizontal transfer of colistin resistance, was described first for E. coli and K. pneumoniae isolates recovered in China between 2011 and 2014 (139). The encoded MCR-1 protein is a member of the phosphoethanolamine transferase enzyme family, as its acquisition results in the addition of phosphoethanolamine to lipid A, and consequently in a more cationic LPS, similarly to the chromosomal mutations mentioned above.
Overall, production of MCR-1 in E. coli leads to 4- to 8-fold increases of the MICs of polymyxins. Therefore, without additional resistance mechanisms, production of MCR-1 is enough to confer resistance to colistin in E. coli and other enterobacterial species, such as K. pneumoniae (our unpublished data). Note that despite the fact that polymyxins actually share the same mechanism of action as that of the cationic antimicrobial peptides (CAMPs) cathelicidin LL-37, α-defensin 5 (HD5), and β-defensin 3 (HDB3), which are normal components of the immune system, coresistance to CAMPs and polymyxins has not beeen observed (J. Dobias, L. Poirel, and P. Nordmann, submitted for publication).
Apart from resistance to polymyxin antibiotics, production of MCR-1 was shown to confer resistance to lysozyme (140). The structure of MCR-1 was recently solved at a 1.32-Å resolution, revealing that its active site is similar to that of related phosphoethanolamine transferases (141). Threonine 285 was identified as the putative nucleophile for catalysis, as it was phosphorylated in the catalytic domain of MCR-1 (cMCR-1). Four zinc ions were identified in the active site of cMCR-1, which is thus a metalloenzyme. The binding sites for the lipid A and phosphatidylethanolamine substrates were not apparent in the cMCR-1 structure, likely indicating that they were present in the membrane domain.
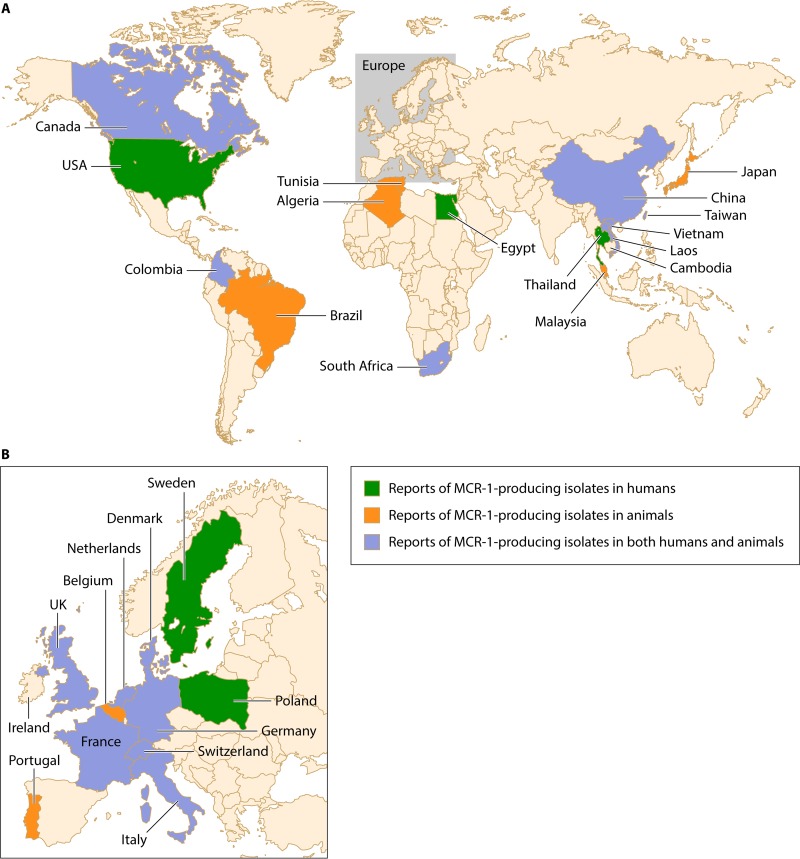
Following these initial findings, the mcr-1 gene was reported worldwide and beyond China, on all continents (Fig. 4; Table 4). The earliest mcr-1-positive strain was collected from chickens in China 3 decades ago (142), when colistin first started to be used in food-producing animals. The mcr-1 gene has been found in various genera of the Enterobacteriaceae (Escherichia, Klebsiella, Enterobacter, Cronobacter, Salmonella, Shigella, and Kluyvera) isolated from the environment, vegetable and meat foods, animals, and human beings (Fig. 4; Table 4). Note that the occurrence of MCR-1-producing E. coli in the environment in Switzerland but also in Asian imported vegetables in the same country highlights the likely wide occurrence of that resistance trait in many different environments (143).
FIG 4.
Reports of MCR-1-producing isolates in humans, animals, and both humans and animals.
TABLE 4.
Worldwide reports of Enterobacteriaceae isolates harboring a plasmid-mediated mcr-1 gene
| Species | Country of isolation | Country of origin or traveled region | Sample origin | Period (yr) | Plasmid featuresa |
Reference(s) | |||
|---|---|---|---|---|---|---|---|---|---|
| Inc type | Size (kb) | Mobile element | Other antimicrobial resistance(s) | ||||||
| E. coli | China | China | Chickens | 1980–2014 | — | — | — | — | 142 |
| China | China | Human (fecal carriage) | Before 2011 | — | — | — | — | 218, 219 | |
| China | China | Chickens, pigs, and humans (infections) | 2011–2014 | IncI2 | 64 | ISApl1 | No | 139 | |
| China | China | 2011–2014 | IncHI2 | 251 | ISApl1 | Resistance to cefotaxime (blaCTX-M-14), aminoglycosides, florfenicol, olaquindox, cotrimoxazole, fosfomycin (fosA3), and ciprofloxacin (oqxAB) | 220 | ||
| China | China | Chicken meat | 2014 | IncI2 | 65 | — | 146 | ||
| China | China | Human (blood) | 2014–2015 | — | — | — | — | 147 | |
| China | China | Human (fecal carriage) | 2015 | — | — | — | — | 221 | |
| China | USA | Human (fecal carriage) | 2016 | IncFI | 33 | — | Resistance to β-lactams (blaCTX-M-15 and blaTEM-1) | 148 | |
| Laos | Laos | Human (fecal carriage) and pigs | 2012 | — | — | — | — | 222 | |
| Thailand | Thailand | Human (fecal carriage) | 2012 | — | — | — | — | 223 | |
| Vietnam | Vietnam | Pigs (fecal carriage) | 2014–2015 | — | — | ISApl1 | Resistance to cefotaxime (blaCTX-M-55), trimethoprim (dfrA12), tetracycline (tetA), aminoglycosides [aadA3, aph(3′)-IA], phenicol (cmlA1), quinolones (qnrS1, oqxA), lincosamides [inu(F)], and sulfonamides (sul2, sul3) | 224 | |
| Cambodia | Cambodia | Human (feces) | 2012 | — | — | ISApl1 | — | 224 | |
| Malaysia | Malaysia | Water, chickens, pigs | 2013 | — | — | ISApl1 or not | — | 225 | |
| Japan | Japan | Cattle (mastitis) | 2008–2013 | Incl2 | 60–61 | — | — | 226 | |
| France | France | Veal calves (feces) | 2005–2014 | IncHI2 | — | — | Resistance to cefotaxime (blaCTX-M-1), sulfonamides, and tetracyclines | 227 | |
| France | France | Broiler, turkeys, pigs | 2007–2014 | — | — | — | — | 228 | |
| Italy | Italy | Human (urine, surgical wound) | 2013–2015 | — | — | — | — | 229 | |
| Italy | Italy | Human (rectal swabs) | 2015 | NT | 35 | — | — | 230 | |
| United Kingdom | United Kingdom or Egypt | Human (blood, stools) | 2012–2015 | IncHI2, IncI2 | — | ISApl1 or not | — | 231 | |
| Switzerland | — | River water | 2012 | — | — | — | — | 143 | |
| Switzerland | Thailand and Vietnam | Vegetables | 2014 | — | — | — | — | 143 | |
| Switzerland | Switzerland | Human (urinary tract infection) | 2015 | — | 60 | — | Resistance to chloramphenicol, florfenicol, and cotrimoxazole | 149 | |
| Switzerland | Switzerland | Human (blood) | 2016 | IncFIB | 30 and 80 | — | — | 156 | |
| Belgium | Belgium | Calves and piglets (feces) | 2011–2012 | IncP | 80 | Truncated ISApl1 | Resistance to trimethoprim (dfrA1), tetracycline (tetA), aminoglycosides [aadA1, aph6-Id/strA, and aph(3′)-Ib/strB], and sulfonamides (sul1) | 223 | |
| Netherlands | Europe | Chicken meat | 2009–2014 | — | — | — | — | 232 | |
| Netherlands | Netherlands | Chickens, veal calves, turkeys | 2010–2015 | — | — | — | — | 233 | |
| Netherlands | Dutch fresh meat and imported frozen meat | Retail meat (mostly chicken and turkey) | 2009–2016 | — | — | — | — | 233 | |
| Netherlands | Netherlands | Human (blood) | 2011 | — | — | — | — | 233 | |
| Netherlands | Travel in Tunisia, South America, China, Southeast Asia | Human (fecal carriage) | 2012–2013 | — | — | — | — | 234 | |
| Netherlands | Germany | Veal valves, broilers, and turkey | 2010–2015 | IncHI2 or IncX4 | — | ISApl1 | — | 235 | |
| Germany | Pigs and human (wound infection) | 2010–2015 | IncHI2 or IncX4 | — | ISApl1 or not | — | 150 | ||
| Denmark | Europe | Chicken meat | 2012–2014 | IncI2 or IncX4 | — | — | — | 236 | |
| Denmark | Denmark | Human (bloodstream infection) | 2015 | IncI2 | — | — | — | 236 | |
| Canada | Unknown | Beef meat | 2010 | IncHI2A | — | — | — | 151 | |
| Canada | Lived in Egypt for 5 years | Human (gastrostomy site and rectum) | 2011 | IncI2 | — | — | — | 151 | |
| USA | USA | Human (urine) | 2016 | IncF | 225 | ISApl1 | Resistance to β-lactams (blaCTX-M-55) | 237 | |
| Algeria | Algeria | Chickens | 2015 | — | — | — | — | 222 | |
| Tunisia | Tunisia/France | Chickens | 2015 | IncHI2 | — | — | Resistance to β-lactams (blaCTX-M-1) | 238 | |
| Egypt | Egypt | Human (sputum) | 2015 | — | >90 | — | — | 239 | |
| South Africa | South Africa | Human | 2014–2015 | IncI2 | 65, 70 | ISApl1 or not | — | 154 | |
| IncHI2 | 150 | 154 | |||||||
| IncX4 | 30 | 154 | |||||||
| South Africa | South Africa | Broiler chicken | 2008–2014 | IncI2 | 62 | ISApl1 | — | 240 | |
| Brazil | Brazil | Chicken, swine | 2003–2015 | — | — | — | — | 241 | |
| K. pneumoniae | China | China | Human (surgical wound, peritoneal fluid) | 2015 | — | — | — | — | 147 |
| China | China | Food animals and human (infections) | 2011–2014 | Incl2 | 64 | ISApl1 | No | 139 | |
| Denmark | Denmark | Human (unknown) | 2014 | — | — | — | — | 224 | |
| Enterobacter cloacae | China | China | Human (urine) | 2014 | IncFI | 70 | — | — | 242 |
| Enterobacter aerogenes | China | China | Human (vaginal secretion) | 2014 | IncFI | 65 | — | Resistance to β-lactams (blaTEM-1 and blaCTX-M-15) | 242 |
| Cronobacter sakazakii | China | China | Chicken (diarrhea) | 2015 | Incl2 | 65 | ISApl1 | — | 152 |
| Salmonella enterica serotype Typhimurium | Portugal | Portugal | Food animals | 2011 | IncHI2 | — | ISApl1 | — | 218, 225, 243 |
| S. enterica | Japan | Japan | Swine (septicemia) | 2013 | IncI2 | 58 | — | — | 226 |
| Salmonella Paratyphi B | France | France | Chicken meat, guinea fowl pie | 2012 | IncX4 | — | — | — | 244 |
| Salmonella Derby | France | France | Pork sausage | 2013 | IncP | — | — | — | 244 |
| Salmonella 1,4[5],12:i:− | France | France | Broilers | 2013 | IncP | — | — | — | 244 |
| Salmonella Typhimurium and Salmonella Virchow | United Kingdom | United Kingdom | Human (feces) | 2012–2015 | IncHI2, IncX4 | — | No | — | 231 |
| Salmonella Typhimurium and Salmonella Paratyphi B var. Java | United Kingdom | Travel in Asia and United Arab Emirates | Human (feces) | 2012–2015 | IncHI2, IncI2 | — | ISApl1 or not | — | 231 |
| Salmonella Java | Netherlands | — | Chicken meat | 2010–2015 | IncX4 | 30 | — | — | 235 |
| Salmonella Anatum | Netherlands | — | Turkey meat | 2013 | IncX4 | 30 | — | — | 235 |
| Salmonella Schwartzengrund | Netherlands | — | Turkey meat | 2015 | IncX4 | 30 | — | — | 235 |
| Salmonella Paratyphi B var. Java | United-Kingdom | Europe | Poultry meat | 2012–2015 | IncHI2 | — | No | — | 231 |
| Shigella sonnei | Vietnam | Vietnam | Human (feces) | 2008 | IncI2 | — | — | — | 145 |
| Kluyvera ascorbata | China | China | Hospital sewage | 2015 | IncI2 | 57 | — | — | 216 |
NT, nontypeable; —, unknown.
The hypothesis that animals, particularly pigs and cattle, might be a main source of MCR-1 producers is very strong. Indeed, several features are in accordance with such a hypothesis, including the high selective pressure in veterinary practice and the wide occurrence of that resistance trait in isolates recovered from animals (144).
The genetics of acquisition of the mcr-1 gene has been investigated extensively. This gene was found in plasmids possessing various backbones (IncI2, IncHI2, IncP, IncX4, IncFI, and IncFIB) and of various sizes (58 to 251 kb). Upstream of the mcr-1 gene, the ISApl1 insertion sequence element is inconstantly identified (Table 4) (139). Thanh et al. (145) described an mcr-1 gene disrupted by a 22-bp duplication in a Shigella sonnei isolate. This isolate was colistin susceptible, but under selective pressure with colistin, one copy of the 22-bp tandem repeat could be deleted, restoring the open reading frame of mcr-1 and leading to colistin resistance. This deactivated version of the colistin resistance gene mcr-1 suggests a fitness cost for the active mcr-1 gene. Some but not all plasmids bearing the mcr-1 gene carry other antimicrobial resistance genes encoding resistance to clinically relevant antibiotics for human medicine, such as β-lactams, aminoglycosides, quinolones, fosfomycin, sulfonamides, and tetracyclines. The location of the mcr-1 gene on multidrug resistance plasmids is worrying because the use of antimicrobials other than polymyxins can participate in the coselection of isolates carrying mcr-1 and in their spread. More worryingly, the plasmid-mediated mcr-1 gene has been identified in highly drug-resistant Enterobacteriaceae isolates harboring plasmids encoding carbapenemase genes (blaNDM-1, blaNDM-5, blaNDM-9, blaOXA-48, blaKPC-2, and blaVIM-1) (146–152). Note that the mcr-1 gene was recently identified on the chromosome of an E. coli strain in Switzerland, suggesting that this resistance gene might be integrated and therefore stabilized in the genome in some isolates (153).
Further investigations are required to better understand the process of acquisition of the mcr-1 gene; however, we recently showed that it was located within a 2,600-bp genetic structure, defined as the “mcr-1 cassette,” that might have been mobilized by transposition (154). The cassette was found to carry its own promoter sequences driving the expression of mcr-1. In addition, it was shown that several isolates may possess the mcr-1 gene located in a composite transposon structure made of two copies of ISApl1 (155). However, that structure has not been identified systematically, and therefore further investigations are still required to better understand the process of acquisition of that gene from an unknown progenitor in plasmids replicating in Enterobacteriaceae.
MCR-1-producing enterobacterial isolates have often been identified as colonizers in either humans or animals. Nevertheless, there are some reported cases of infections, including two patients with bacteremia in Switzerland (156).
A functional variant of mcr-1 (Q3L) encoding MCR-1.2 was detected in KPC-3-producing K. pneumoniae in Italy (157), likely sharing the same activity as MCR-1.
In addition, the plasmid-mediated colistin resistance gene mcr-2 was identified in E. coli strains recovered from piglets in Belgium (158). It shared 77% nucleotide identity with mcr-1 and was carried on an IncX4 plasmid.
Other Mechanisms Contributing to Polymyxin Resistance in Enterobacteriaceae
Hyperproduction of CPS.
A study showed that the capsule polysaccharide (CPS) acts as a protective barrier against polymyxins in K. pneumoniae (159). The upregulation of capsular biosynthesis genes indeed reduces the interactions of polymyxins with the bacterial surface, leading to polymyxin resistance.
K. pneumoniae is able to release anionic capsular polysaccharides from its surface (160). This release leads to the trapping of cationic antimicrobial peptides, such as polymyxins, thus decreasing the amount of antibiotic reaching the bacterial surface. The CPS is connected to the bacterial surface through an ionic interaction with the LPS, and this interaction is stabilized by divalent cations (161). As a consequence, the release of CPS in the presence of polymyxins is likely due to perturbation of the cation-dependent bridges between the molecules of LPS.
Role of porins.
It has been shown that a periplasmic protein (YdeI) regulated by the PhoPQ and PmrAB two-component systems can interact with the OmpD porin to increase bacterial resistance to polymyxins in Salmonella enterica (162).
Role of efflux pumps.
The role of efflux in colistin resistance is not well understood, but several studies suggested the involvement of efflux pumps in colistin resistance. Mutations in kpnEF and acrAB, encoding components of efflux pumps, may actually lead to a 2-fold decrease of the MIC of colistin and increase bacterial survival in the presence of a low concentration of polymyxins (163, 164). Addition to the test medium of low doses of the efflux pump inhibitor carbonyl cyanide m-chlorophenylhydrazone (CCCP) decreased the MICs for resistant strains (128- to 512-fold reductions) and partially or completely inhibited the regrowth of resistant subpopulations (165). However, this observation should be considered with caution owing to the nonspecific effect of CCCP on efflux systems, with a likely wider impact on bacterial metabolism.
Mechanisms of Polymyxin Resistance in Pseudomonas aeruginosa and Acinetobacter baumannii
Pseudomonas aeruginosa.
The colistin resistance in P. aeruginosa is mediated by five two-component systems that regulate LPS modifications. As for the Enterobacteriaceae, alterations in the PmrAB (74, 118, 166–169) and PhoPQ (118, 166, 168, 170) two-component systems have been shown to be responsible for acquired resistance to colistin. Mutations in these two-component systems cause constitutive alterations and consequently activate transcription of the pmrHFIJKLM operon and the subsequent addition of l-Ara4N to the LPS, finally leading to colistin resistance. Notably, unlike what is observed in K. pneumoniae, the colistin resistance mediated by PhoPQ modifications does not depend on the PmrAB system.
Three other two-component systems have been proved to contribute to colistin resistance in P. aeruginosa, namely, ParRS, ColRS, and CprRS. The ParRS (polymyxin adaptive resistance) two-component system is involved in adaptative resistance to polymyxins (118, 166, 171). Mutations in this system cause constitutive expression of the pmrHFIJKLM operon and thus lead to the addition of l-Ara4N to the LPS, leading to colistin resistance. Additionally, mutations in the ColRS and CprRS two-component regulatory systems may also contribute to polymyxin resistance, since the association of mutations in the phoQ gene and mutations in the colS or cprS gene confers a high level of colistin resistance (172). The action of the ColRS and CprRS systems may occur through the activation of the phoQ gene and/or through other genes that have not yet been identified.
Acinetobacter baumannii.
The main mechanism of colistin resistance in A. baumannii corresponds to the addition of cationic groups to the LPS (qualitative modification of the LPS); nevertheless, acquired resistance to colistin may also be the consequence of a complete loss of LPS production (quantitative modification of the LPS).
The addition of cationic groups in A. baumannii is mediated by mutations in PmrAB (118, 173–180). Mutations in the pmrA and pmrB genes have been shown to cause colistin resistance through upregulation of the pmrCAB operon, leading to pEtN synthesis but not to l-Ara4N synthesis (unlike in Enterobacteriaceae).
The second mechanism of colistin resistance in A. baumannii corresponds to the complete loss of LPS caused by alterations of the lipid A biosynthesis genes, namely, the lpxA, lpxC, and lpxD genes. Mutations identified in those genes were either substitutions, truncations, frameshifts (109), or insertional inactivation by the insertion sequence ISAba11 (181).
EPIDEMIOLOGY OF RESISTANCE TO POLYMYXINS
General Epidemiology of Resistance to Polymyxins
Although polymyxins currently retain significant in vitro activity against most Gram-negative organisms, resistance to these antibiotics is increasingly being reported among clinical isolates (29, 182).
The SENTRY antimicrobial surveillance program carried out a worldwide survey in 2009 and reported low rates of resistance to polymyxins among Gram-negative pathogens (Acinetobacter spp., P. aeruginosa, E. coli, and Klebsiella spp.) (<0.1% to 1.5%) (183). However, a rising trend was observed in a 2006-2009 study period focusing on K. pneumoniae isolates (resistance rates of 1.2% in 2006 and 1.8% in 2009), probably because of the extensive and/or inadequate usage of polymyxins worldwide for treating infections with MDR Gram-negative bacteria.
Colistin resistance in K. pneumoniae represents a growing public health concern, since this bacterial species is one of the main pathogens of nosocomial infection and has gathered a wide range of resistance mechanisms to broad-spectrum antibiotics over the years. Table 5 shows the populations studied (mainly carbapenem-resistant K. pneumoniae [CR-KP] clinical isolates), along with the methods that have been used to determine the rate of colistin resistance, since some methods are now known to underestimate the level of colistin resistance and therefore may significantly bias the proposed rates. The occurrence of colistin resistance in K. pneumoniae has been reported in surveillance studies and clinical case reports worldwide (Table 5) (183). Many studies report an increase of the resistance rate among multidrug-resistant K. pneumoniae isolates, particularly among CR-KP isolates, with high colistin resistance rates reported (Table 5). More worryingly, multiple outbreaks with carbapenem- and colistin-resistant isolates have been reported in North America and Europe (Table 6; Fig. 5).
TABLE 5.
Studies reporting prevalences of colistin resistance among K. pneumoniae clinical isolatesd
| Study type and period | Country | Setting | Test method | Total no. of isolates | Carbapenemase producers (no. [%]) | Polymyxin-resistant isolates (no. [%]) | Reference(s) |
|---|---|---|---|---|---|---|---|
| Studies reporting colistin resistance rates among overall clinical isolates | |||||||
| 2006–2009 | Worldwide | Worldwide surveillance | BMD | 9,774 | NA | (1.5) | 183 |
| 2007–2008 | Canada | National | BMD | 515 | NA | 15 (2.9) | 184 |
| 2013–2014 | United States | Multicenter | BMD | 1,205 | NA | (4) | 185 |
| 2006–2007 | South Korea | Multicenter (9 hospitals) | BMD | 221a | 15 (13.3) | 24 (10.9)b | 206 |
| 2004–2005 | Singapore | Single center | Agar dilution | 16 | NA | 1 (6) | 207 |
| Studies reporting colistin resistance rates among CR-KP isolates | |||||||
| 2008–2011 | Canada | Multicenter | Etest | 30 | 30 (100) | 2 (6.7) | 184, 186 |
| 2013–2014 | United States | Multicenter | BMD | 69 | 69 (100) | (18)c | 185 |
| 2003–2004 | United States | Multicenter | BMD or agar dilution | 96 | 96 (100) | (9) | 187 |
| 2010–2013 | Greece | Single center (only ICU) | Vitek2, Etest | 92 | 92 (100) | 20 (21.7) | 202 |
| 2010 | Greece | Single center | Vitek2, Etest | 120 | 120 (100) | 25 (20.8) | 203 |
| 2014 | Italy | Single center (only ICU) | Vitek2, Etest | 214 | 214 (100) | 47 (21.9) | 199 |
| 2013 | Italy | Single center (only ICU) | BMD | 25 | 24 (100) | 6 (24.0) | 200 |
| 2013–2014 | Italy | National | BMD | 178 | 178 (100) | 76 (43) | 201 |
| 2010–2011 | Italy | Multicenter (9 hospitals) | Vitek2, BMD | 97 | 97 (100) | 35 (36.1) | 68 |
| 2010–2012 | Spain | Hospital | Agar dilution | 79 | 79 (100) | 18 (22.8) | 204 |
| 2014 | France | National | BMD | 561 | 561 (100) | 35 (6.2) | 205 |
| 2012–2013 | Turkey | Single center | Vitek2, Etest | 37 | 36 (98) | (2.7) | 245 |
| 2006–2007 | Israel | Single center | ? | 88 | 88 (100) | (4.5) | 246 |
| 2009–2010 | China | Single center | Agar dilution | 68 | 68 (100) | 3 (4.4) | 208 |
| 2012 | Taiwan | National | Sensititre | 247 | 55 (22.3) | (12.1) | 209 |
Isolated from blood samples.
Number of colistin-resistant isolates according to EUCAST breakpoints.
Eighteen percent colistin resistance among 4 E. coli and 69 K. pneumoniae isolates harboring carbapenemases.
NA, not applicable.
TABLE 6.
Studies reporting outbreaks of colistin-resistant and carbapenemase-producing isolatese
| Study period | Country | Setting | Test method | Colistin MIC (μg/ml) | Resistance mechanism | Total no. of cases | Beta-lactamase(s) | Sequence type | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | United States | One single hospital (ICU and medical ward) | Agar dilution | >128 | ND | 5 | KPC-2 | ST258 | 188 |
| 2009 | United States | Two hospitals and a long-term acute care setting in Detroit, MI | Etest | 8–64 | ND | 4 | KPC | ND | 189 |
| 2012–2013 | Mexico | One single hospital in Mexico City | BMD | 4 | ND | 15 | KPC-2 | ST258 | 190 |
| 2013 | Netherlands | One hospital and a nursing home | Vitek2, Etest | ND | ND | 6 | KPC-2, SHV-12 | ST258 | 192 |
| 2008–2009 | Hungary | Three hospitals in Miskolc | Etest | 16–32 | ND | 8 | KPC-2, SHV-12, TEM-1, SHV-11 | ST258 | 193 |
| 2008 | Greece | ICUs of two distinct hospitals | Etest | 12–128 | ND | 6 | KPC-2, SHV-12 | ST258 | 194 |
| 2004–2005 | Greece | One single hospital in Athens (ICU) | Etest | 12–>1,024 | ND | 13 (multiclonal) | ND | ND | 195 |
| 2010 | Italy | Two hospitals in Catania, Sicily | BMD | 8–64 | ND | 8 | KPC-3, SHV-11, TEM-1, OXA-9 | ST258 | 196 |
| 2011 | Italy | One single hospital in Palermo, Sicily | Etest | 28 (multiclonal)a | 197 | ||||
| 3–128 | ND | 24 | KPC-3, SHV-11, TEM-1, OXA-9 | ST258 | |||||
| 3–32 | ND | 3 | KPC-3, SHV-12, TEM-1 | ST273 | |||||
| 4–12 | ND | 2 | KPC-3, SHV-28, TEM-1, CTX-M-15 | ST15 | |||||
| 2010–2013 | Italy | One single hospital (22 different wards) | Vitek2, Sensititre | 4–>16 | mgrB Δnt109/119 | Multiclonalb | 198 | ||
| Unknownc | 50 | KPC-3 | ST512 | ||||||
| Unknownc | 5 | KPC-3 | ST512 | ||||||
| 2 | KPC-2 | ST101 | |||||||
| 2010–2012 | Spain | One hospital | Agar dilution | ND | ND | 14 | VIM-1 | ST22 | 204 |
| 2014 | France | Hospital in Picardie | BMD | 4–64 | Unknownd | 15 | OXA-48, CTX-M-15 | ST11 | 205 |
One patient with two colistin-resistant clones (belonging to ST-258 and ST-273).
There was a total of 93 bloodstream infections, but only isolates recovered in 2013 were investigated further.
No mutation in the mgrB, pmrA, or pmrB gene was responsible for colistin resistance.
No mutation in the mgrB gene was responsible for colistin resistance.
ND, not determined.
FIG 5.
Outbreaks caused by colistin-resistant, carbapenemase-producing K. pneumoniae isolates. Each star indicates a single report.
North America.
Multicenter surveys showed low rates of resistance among K. pneumoniae isolates in Canada (2.9%) (184) and the United States (4%) (185) (Table 5). However, the colistin resistance rate was higher (6.7 to 18%) among carbapenemase-producing isolates (185–187). In addition, outbreaks with colistin-resistant, KPC-producing K. pneumoniae, mostly attributed to the international epidemic clone type ST258, have been reported in the United States (188, 189) and Mexico (190) (Table 6; Fig. 5).
South America.
The results from the SENTRY antimicrobial surveillance program showed a moderate resistance rate in Latin America in 2009 (3%) (183); however, the emergence of colistin-resistant K. pneumoniae isolates has been reported in Argentina (191), Colombia (119), and Brazil (87).
Europe.
Multiple outbreaks of both carbapenem- and colistin-resistant K. pneumoniae isolates have been reported in Europe (Table 6; Fig. 5). Outbreaks with KPC-producing K. pneumoniae isolates attributed to the international epidemic clone type ST258 have been reported in the Netherlands (192), Hungary (193), Greece (194, 195), and Italy (196, 197).
In addition to the two outbreaks attributed to the ST258 clone (196, 197), a large nosocomial outbreak of colistin-resistant and KPC-producing ST512 K. pneumoniae isolates was reported in Italy (198) (Table 6; Fig. 5). More worryingly, colistin resistance was recently reported at a high level (>20%) among carbapenemase-producing isolates in ICUs of two Italian hospitals (199, 200), with an even higher rate (36.1%) in hospitals in Rome (68) (Table 6). Moreover, a national study reported a countrywide level of colistin resistance among KPC-producing K. pneumoniae isolates, with 43% of isolates being resistant to colistin (201) (Table 5).
In Greece, several outbreaks caused by KPC-producing, colistin-resistant K. pneumoniae isolates have been reported (194, 195) (Table 6; Fig. 5). Studies performed in two Greek hospitals reported a huge increase in colistin resistance within a few years (<3.5% incidence before 2010 and >20% incidence after 2010) (202, 203).
During a 2-year period (2010 to 2012) in Spain, a study showed an increase of the prevalence of colistin resistance among carbapenemase-producing K. pneumoniae isolates, from 13.5 to 31.7%, and an outbreak of colistin-resistant, VIM-1-producing K. pneumoniae was reported (204).
In France, a national survey revealed a low rate of colistin resistance (6.2%) among carbapenemase-producing K. pneumoniae isolates; neverthess, an outbreak of OXA-48 carbapenemase-producing and colistin-resistant K. pneumoniae isolates was reported (205).
Middle East.
In Turkey and Israel, low rates of resistance to colistin among CR-KP isolates have been reported (2.7% and 4.5%, respectively). However, multiclonal outbreaks with OXA-48-, NDM-, and both OXA-48- and NDM-producing K. pneumoniae are currently ongoing in Turkey (our unpublished data).
Africa.
A very low colistin resistance rate was reported among K. pneumoniae isolates in Tunisia (1.2%), but this rate was probably underestimated because susceptibility to colistin was primarily screened using a DD method generating high false susceptibility rates (205). The emergence of colistin resistance was reported for K. pneumoniae isolates recovered in South Africa (81, 119) and Nigeria (120).
Asia.
Moderate rates of colistin resistance (about 6 to 11%) have been reported for K. pneumoniae isolates from South Korea (206) and Singapore (207), and similar resistance rates (4.4 to 12.1%) were found among CR-KP isolates in China (208) and Taiwan (209). Colistin-resistant K. pneumoniae isolates have also been reported in Laos and Thailand (120).
Risk Factors
The use of colistin was found to be an independent risk factor for the occurrence of resistance in Gram-negative bacteria (210, 211). Important increases of colistin resistance rates among ESBL-producing K. pneumoniae isolates (from 0 to 71% and from 11.1 to 75%) were reported after the introduction of selective digestive tract decontamination in two intensive care units (212, 213). Moreover, this decontamination failed to prevent colonization by ESBL-producing Enterobacteriaceae, and such a strategy should be abandoned. Note that the inappropriate use of colistin (such as suboptimal dosing or prolonged monotherapy) has been shown to be a source of colistin resistance selection (214, 215). The occurrence of colistin resistance in P. aeruginosa was most effectively prevented by 8-h dosing intervals compared to 12- or 24-h dosing intervals (45).
Specific Epidemiology of the Plasmid-Mediated mcr-1 Resistance Gene
Notably, plasmid-borne resistance to polymyxins has been reported for few different enterobacterial species so far, mainly among E. coli isolates and rarely for Salmonella enterica, Enterobacter spp., and K. pneumoniae. There are also some scattered reports of MCR-1-producing isolates in other species, such as Cronobacter sakazakii (152) and Kluyvera ascorbata (216). According to the current literature on the subject, the distribution of MCR-1 appears to be worldwide, covering all continents (217). It remains to be determined if the identification of the mcr-1 gene worldwide corresponds to subsequent spread from an original source (China?) or to simultaneous gene mobilization events in different parts of the world. Ongoing epidemiological surveys should provide some important clues.
It is actually speculated that the original source of the gene, or at least of its mobilization and emergence, might be the animal world. This speculation is based on the fact that MCR-1-producing E. coli isolates have been identified in several animals and animal food products, including chickens and chicken meat, pigs and piglets, cattle, calves, and turkeys, but also in humans (Fig. 4). The corresponding samples were collected from many Asian countries (Cambodia, China, Japan, Laos, Malaysia, Taiwan, Singapore, and Vietnam) but also from Europe (Belgium, Denmark, France, Germany, Portugal, Italy, the Netherlands, Spain, Sweden, Switzerland, and the UK), the Americas (Argentina, Brazil, and Canada), and Africa (Algeria, Egypt, South Africa, and Tunisia) (Table 4). Note that a study conducted in Switzerland identified MCR-1-producing E. coli isolates in vegetables imported from Asia (143), and positive isolates were also identified in environmental water samples in Switzerland and Malaysia (Table 4).
The speculation of an animal origin of the mcr-1 gene is also based on genetic features, since this gene is often associated with the insertion sequence ISApl1, identified in Pasteurella multocida, which is a common animal pathogen, and also with the blaCMY-2 and florR genes, which are often identified in animal enterobacterial isolates (144). Finally, another feature suggesting an animal source of the problem is the heavy usage of polymyxins in veterinary medicine, with usage on many different animal species.
Dating the emergence of MCR-1-positive strains remains quite difficult; however, a Chinese study retrospectively identified positive isolates recovered from chickens during the 1980s (142), and they were discovered as early as 2005 in veal calves in France (144). It therefore seems that the emergence of MCR-positive isolates, at least in animals, is not a recent event. Very likely, there has been some silent dissemination of that resistance mechanism throughout the last few decades, and the current situation shows an ongoing further dissemination rather than an emerging phenomenon.
CONCLUSIONS
Polymyxins are gaining increasing interest because of the current epidemiological situation, with MDR Gram-negative bacteria spreading worldwide and with a paucity of novel marketed antibiotics. In some areas where infections caused by carbapenem-resistant Enterobacteriaceae are now common (such as Greece or Italy), the use of polymyxins (alone or often in combination with other antibiotics) is becoming crucial and may even be considered first-line therapy. The reevaluation of some critical issues in relation to polymyxins (accurate susceptibility testing, defining correct breakpoints, and better appreciating the toxicity issues) now opens new perspectives for its use. Studies that may permit a better evaluation of the PK-PD data, the toxicity level, and appropriate drug combinations are therefore crucial.
The recent identification of plasmid-mediated mechanisms of resistance to polymyxins also modifies the perspective. Indeed, epidemiological studies have to be initiated in order to better evaluate the extent of dissemination of this resistance in human and veterinary medicine and the impact of its occurrence. The perspective of nosocomial dissemination of MDR Gram-negative organisms possessing resistance determinants to all main antibiotics is frightening, in particular for K. pneumoniae, which is one of the main nosocomial pathogens. Whether veterinary medicine is affecting the epidemiological situation by providing selective pressure with polymyxins has to be precisely determined. Whether discontinuing some specific usages of these drugs (prophylaxis or metaphylaxis in animals and decontamination of MDR bacteria in humans) should be considered is therefore an open debate.
Ultimately, reinforcing the detection of polymyxin-resistant isolates must be encouraged. Prospective epidemiological surveys are needed, since the current knowledge on this issue is very scarce. Actually, the recent development of a rapid diagnostic test for detection of polymyxin resistance, along with the development of a screening agar medium, will contribute to facilitating those surveys.
ACKNOWLEDGMENTS
This work was funded by the University of Fribourg, Switzerland, and by a grant from the ANIWHA ERA-NET project, Switzerland (ANIWHA).
Biographies

Laurent Poirel, Ph.D., is an Associate Professor in the Medical and Molecular Microbiology Unit, Department of Medicine, University of Fribourg, Switzerland. His research interests focus on antibiotic resistance in Gram-negative bacteria, with a special focus on the identification of molecular mechanisms responsible for acquisition of carbapenem and polymyxin resistance and the genetics of acquisition of resistance genes.

Aurélie Jayol, Pharm.D., is a Microbiologist currently preparing a Ph.D. thesis at the University of Fribourg, Switzerland. Her research interests focus on antibiotic resistance, especially the molecular mechanisms responsible for acquisition of colistin resistance in Enterobacteriaceae and the use of diagnostic tests for detection of colistin resistance.

Patrice Nordmann, M.D., Ph.D., is Chair Professor and Head of the Medical and Molecular Microbiology Unit of the French INSERM European Unit (University of Fribourg) of the National Reference Center for Emerging Antibiotic Resistance (Switzerland), Department of Medicine, University of Fribourg, Switzerland. His research interests focus on antibiotic resistance in Gram-negative bacteria, encompassing identification of molecular mechanisms, performance of epidemiological surveys, development of rapid diagnostic tests for antibiotic resistance, and development of novel antibiotic molecules.
REFERENCES
- 1.Benedict RG, Langlykke AF. 1947. Antibiotic activity of Bacillus polymyxa. J Bacteriol 54:24. [PubMed] [Google Scholar]
- 2.Hancock RE. 1997. Peptide antibiotics. Lancet 349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Rafailidis PI, Matthaiou DK. 2010. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updat 13:132–138. doi: 10.1016/j.drup.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 5.Brink AJ, Richards GA, Colombo G, Bortolotti F, Colombo P, Jehl F. 2014. Multicomponent antibiotic substances produced by fermentation: implications for regulatory authorities, critically ill patients and generics. Int J Antimicrob Agents 43:1–6. doi: 10.1016/j.ijantimicag.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Gallardo-Godoy A, Muldoon C, Becker B, Elliott AG, Lash LH, Huang JX, Butler MS, Pelingon R, Kavanagh AM, Ramu S, Phetsang W, Blaskovich MA, Cooper MA. 2016. Activity and predicted nephrotoxicity of synthetic antibiotics based on polymyxin B. J Med Chem 59:1068–1077. doi: 10.1021/acs.jmedchem.5b01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nation RL, Velkov T, Li J. 2014. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett M, Bushby SR, Wilkinson S. 1964. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother 23:552–574. doi: 10.1111/j.1476-5381.1964.tb01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. 2003. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother 47:1364–1370. doi: 10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2004. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother 53:837–840. doi: 10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- 12.Dixon RA, Chopra I. 1986. Leakage of periplasmic proteins from Escherichia coli mediated by polymyxin B nonapeptide. Antimicrob Agents Chemother 29:781–788. doi: 10.1128/AAC.29.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis 40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents 25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Deris ZZ, Akter J, Sivanesan S, Roberts KD, Thompson PE, Nation RL, Li J, Velkov T. 2013. A secondary mode of action of polymyxins against Gram-negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J Antibiot (Tokyo) 67:147–151. doi: 10.1038/ja.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulitta JB, Yang JC, Yohonn L, Ly NS, Brown SV, D'Hondt RE, Jusko WJ, Forrest A, Tsuji BT. 2010. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob Agents Chemother 54:2051–2062. doi: 10.1128/AAC.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen RJ, Li J, Nation RL, Spelman D. 2007. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother 59:473–477. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 18.Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, Nation RL, Li J. 2008. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother 62:1311–1318. doi: 10.1093/jac/dkn425. [DOI] [PubMed] [Google Scholar]
- 19.Bergen PJ, Landersdorfer CB, Zhang J, Zhao M, Lee HJ, Nation RL, Li J. 2012. Pharmacokinetics and pharmacodynamics of ‘old’ polymyxins: what is new? Diagn Microbiol Infect Dis 74:213–223. doi: 10.1016/j.diagmicrobio.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheah SE, Wang J, Nguyen VTT, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 21.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landersdorfer CB, Nation RL. 2015. Colistin: how should it be dosed for the critically ill? Semin Respir Crit Care Med 36:126–135. doi: 10.1055/s-0034-1398390. [DOI] [PubMed] [Google Scholar]
- 23.Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin—back to basic PK/PD. Curr Opin Pharmacol 11:464–469. doi: 10.1016/j.coph.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. 2003. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 47:905–909. doi: 10.1128/AAC.47.3.905-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John EB, Bennett RD, Blaser MJ. 2015. Polymyxins (polymyxin B and colistin), p 549–555. In Bennett JE, Dolin R, Blaser MJ (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 8th ed Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 26.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yau W, Owen RJ, Poudyal A, Bell JM, Turnidge JD, Yu HH, Nation RL, Li J. 2009. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infect 58:138–144. doi: 10.1016/j.jinf.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Meletis G, Tzampaz E, Sianou E, Tzavaras I, Sofianou D. 2011. Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother 66:946–947. doi: 10.1093/jac/dkr007. [DOI] [PubMed] [Google Scholar]
- 29.Bialvaei AZ, Samadi Kafil H. 2015. Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin 31:707–721. doi: 10.1185/03007995.2015.1018989. [DOI] [PubMed] [Google Scholar]
- 30.Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. 1970. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med 72:857–868. [DOI] [PubMed] [Google Scholar]
- 31.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 32.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. 2012. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther 10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 33.Yahav D, Farbman L, Leibovici L, Paul M. 2012. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect 18:18–29. doi: 10.1111/j.1469-0691.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 34.Ortwine JK, Kaye KS, Li J, Pogue JM. 2015. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy 35:11–16. doi: 10.1002/phar.1484. [DOI] [PubMed] [Google Scholar]
- 35.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2014. Consistent global approach on reporting of colistin doses to promote safe and effective use. Clin Infect Dis 58:139–141. doi: 10.1093/cid/cit680. [DOI] [PubMed] [Google Scholar]
- 36.Theuretzbacher U. 2014. Product information for parenteral colistin varies substantially across Europe. J Antimicrob Chemother 69:1987–1992. doi: 10.1093/jac/dku064. [DOI] [PubMed] [Google Scholar]
- 37.Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL. 2008. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother 52:3047–3051. doi: 10.1128/AAC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 761:167–175. doi: 10.1016/S0378-4347(01)00326-7. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother 46:3304–3307. doi: 10.1128/AAC.46.10.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 47:1766–1770. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 56:4241–4249. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markou N, Markantonis SL, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, Rafailidis P, Apostolakos H, Baltopoulos G. 2008. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, Gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Ther 30:143–151. doi: 10.1016/j.clinthera.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by Gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Simon D, Peters C, Ahsan M, Chateau D. 2009. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136:1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 45.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. 2008. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother 61:636–642. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 46.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 47.Vicari G, Bauer SR, Neuner EA, Lam SW. 2013. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant Gram-negative bacteremia. Clin Infect Dis 56:398–404. doi: 10.1093/cid/cis909. [DOI] [PubMed] [Google Scholar]
- 48.Cheng CY, Sheng WH, Wang JT, Chen YC, Chang SC. 2010. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant Gram-negative bacterial infections. Int J Antimicrob Agents 35:297–300. doi: 10.1016/j.ijantimicag.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Michalopoulos AS, Falagas ME. 2011. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care 1:30. doi: 10.1186/2110-5820-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts JA, Lipman J. 2012. Editorial commentary: closing the loop—a colistin clinical study to confirm dosing recommendations from PK/PD modeling. Clin Infect Dis 54:1727–1729. doi: 10.1093/cid/cis311. [DOI] [PubMed] [Google Scholar]
- 51.European Medicines Agency. 2014. European Medicines Agency completes review of polymyxin-based medicines: recommendations issued for safe use in patients with serious infections resistant to standard antibiotics. European Medicines Agency, London, United Kingdom. [Google Scholar]
- 52.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee YJ, Wi YM, Kwon YJ, Kim SR, Chang SH, Cho S. 2015. Association between colistin dose and development of nephrotoxicity. Crit Care Med 43:1187–1193. doi: 10.1097/CCM.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 54.Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N. 2012. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 54:1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ordooei Javan A, Shokouhi S, Sahraei Z. 2015. A review on colistin nephrotoxicity. Eur J Clin Pharmacol 71:801–810. doi: 10.1007/s00228-015-1865-4. [DOI] [PubMed] [Google Scholar]
- 56.Falagas ME, Kasiakou SK. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10:R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorli L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, Alvarez-Lerma F, Knobel H, Benito N, Horcajada JP. 2013. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis 13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellomo R, Kellum JA, Ronco C. 2007. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med 33:409–413. doi: 10.1007/s00134-006-0478-x. [DOI] [PubMed] [Google Scholar]
- 59.Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. 2013. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis 57:1300–1303. doi: 10.1093/cid/cit453. [DOI] [PubMed] [Google Scholar]
- 60.Phe K, Lee Y, McDaneld PM, Prasad N, Yin T, Figueroa DA, Musick WL, Cottreau JM, Hu M, Tam VH. 2014. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob Agents Chemother 58:2740–2746. doi: 10.1128/AAC.02476-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catry B, Cavaleri M, Baptiste K, Grave K, Grein K, Holm A, Jukes H, Liebana E, Navas AL, Mackay D, Magiorakos AP, Romo MA, Moulin G, Madero CM, Pomba MC, Powell M, Pyorala S, Rantala M, Ruzauskas M, Sanders P, Teale C, Threlfall EJ, Torneke K, van Duijkeren E, Edo JT. 2015. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): development of resistance in animals and possible impact on human and animal health. Int J Antimicrob Agents 46:297–306. doi: 10.1016/j.ijantimicag.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Kempf I, Fleury MA, Drider D, Bruneau M, Sanders P, Chauvin C, Madec JY, Jouy E. 2013. What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int J Antimicrob Agents 42:379–383. doi: 10.1016/j.ijantimicag.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Boyen F, Vangroenweghe F, Butaye P, De Graef E, Castryck F, Heylen P, Vanrobaeys M, Haesebrouck F. 2010. Disk prediffusion is a reliable method for testing colistin susceptibility in porcine E. coli strains. Vet Microbiol 144:359–362. doi: 10.1016/j.vetmic.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Kieffer N, Poirel L, Nordmann P, Madec JY, Haenni M. 2015. Emergence of colistin resistance in Klebsiella pneumoniae from veterinary medicine. J Antimicrob Chemother 70:1265–1267. doi: 10.1093/jac/dku485. [DOI] [PubMed] [Google Scholar]
- 65.Olaitan AO, Thongmalayvong B, Akkhavong K, Somphavong S, Paboriboune P, Khounsy S, Morand S, Rolain JM. 2015. Clonal transmission of a colistin-resistant Escherichia coli from a domesticated pig to a human in Laos. J Antimicrob Chemother 70:3402–3404. doi: 10.1093/jac/dkv252. [DOI] [PubMed] [Google Scholar]
- 66.Walsh TR, Wu Y. 2016. China bans colistin as a feed additive for animals. Lancet Infect Dis 16:1102–1103. doi: 10.1016/S1473-3099(16)30329-2. [DOI] [PubMed] [Google Scholar]
- 67.European Medicines Agency. 26 May 2016. Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. EMA/231573/2016. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/05/WC500207233.pdf Accessed 5 September 2016.
- 68.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N. 2013. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 19:E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 69.Landman D, Salamera J, Quale J. 2013. Irreproducible and uninterpretable polymyxin B MICs for Enterobacter cloacae and Enterobacter aerogenes. J Clin Microbiol 51:4106–4111. doi: 10.1128/JCM.02129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hindler JA, Humphries RM. 2013. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol 51:1678–1684. doi: 10.1128/JCM.03385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2014. Breakpoint tables for interpretation of MICs and zone diameters, version 2.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_2.0_120221.pdf.
- 72.Clinical and Laboratory Standards Institute (CLSI). 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 73.Clinical and Laboratory Standards Institute (CLSI). 2012. Methods for dilution of antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed Document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 74.Schurek KN, Sampaio JL, Kiffer CR, Sinto S, Mendes CM, Hancock RE. 2009. Involvement of pmrAB and phoPQ in polymyxin B adaptation and inducible resistance in non-cystic fibrosis clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:4345–4351. doi: 10.1128/AAC.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Behera B, Mathur P, Das A, Kapil A, Gupta B, Bhoi S, Farooque K, Sharma V, Misra MC. 2010. Evaluation of susceptibility testing methods for polymyxin. Int J Infect Dis 14:e596–e601. doi: 10.1016/j.ijid.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Lo-Ten-Foe JR, de Smet AM, Diederen BM, Kluytmans JA, van Keulen PH. 2007. Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother 51:3726–3730. doi: 10.1128/AAC.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hogardt M, Schmoldt S, Gotzfried M, Adler K, Heesemann J. 2004. Pitfalls of polymyxin antimicrobial susceptibility testing of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother 54:1057–1061. doi: 10.1093/jac/dkh470. [DOI] [PubMed] [Google Scholar]
- 78.Moskowitz SM, Garber E, Chen Y, Clock SA, Tabibi S, Miller AK, Doctor M, Saiman L. 2010. Colistin susceptibility testing: evaluation of reliability for cystic fibrosis isolates of Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother 65:1416–1423. doi: 10.1093/jac/dkq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan TY, Ng LS. 2006. Comparison of three standardized disc susceptibility testing methods for colistin. J Antimicrob Chemother 58:864–867. doi: 10.1093/jac/dkl330. [DOI] [PubMed] [Google Scholar]
- 80.Maalej SM, Meziou MR, Rhimi FM, Hammami A. 2011. Comparison of disc diffusion, Etest and agar dilution for susceptibility testing of colistin against Enterobacteriaceae. Lett Appl Microbiol 53:546–551. doi: 10.1111/j.1472-765X.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- 81.Jayol A, Nordmann P, Brink A, Poirel L. 2015. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob Agents Chemother 59:2780–2784. doi: 10.1128/AAC.05055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arroyo LA, Garcia-Curiel A, Pachon-Ibanez ME, Llanos AC, Ruiz M, Pachon J, Aznar J. 2005. Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43:903–905. doi: 10.1128/JCM.43.2.903-905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan TY, Ng SY. 2007. Comparison of Etest, Vitek and agar dilution for susceptibility testing of colistin. Clin Microbiol Infect 13:541–544. doi: 10.1111/j.1469-0691.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 84.Jorgensen JH, Ferraro MJ. 2009. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 85.Sinirtas M, Akalin H, Gedikoglu S. 2009. Investigation of colistin sensitivity via three different methods in Acinetobacter baumannii isolates with multiple antibiotic resistance. Int J Infect Dis 13:e217–e220. doi: 10.1016/j.ijid.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 86.Lee SY, Shin JH, Lee K, Joo MY, Park KH, Shin MG, Suh SP, Ryang DW, Kim SH. 2013. Comparison of the Vitek 2, MicroScan, and Etest methods with the agar dilution method in assessing colistin susceptibility of bloodstream isolates of Acinetobacter species from a Korean university hospital. J Clin Microbiol 51:1924–1926. doi: 10.1128/JCM.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perez LR. 2015. Evaluation of polymyxin susceptibility profile among KPC-producing Klebsiella pneumoniae using Etest and MicroScan WalkAway automated system. APMIS 123:951–954. doi: 10.1111/apm.12438. [DOI] [PubMed] [Google Scholar]
- 88.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Girardello R, Bispo PJ, Yamanaka TM, Gales AC. 2012. Cation concentration variability of four distinct Mueller-Hinton agar brands influences polymyxin B susceptibility results. J Clin Microbiol 50:2414–2418. doi: 10.1128/JCM.06686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen CC, Feingold DS. 1972. Locus of divalent cation inhibition of the bactericidal action of polymyxin B. Antimicrob Agents Chemother 2:331–335. doi: 10.1128/AAC.2.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newton BA. 1953. Reversal of the antibacterial activity of polymyxin by divalent cations. Nature 172:160–161. doi: 10.1038/172160a0. [DOI] [PubMed] [Google Scholar]
- 92.Matzneller P, Strommer S, Osterreicher Z, Mitteregger D, Zeitlinger M. 2015. Target site antimicrobial activity of colistin might be misestimated if tested in conventional growth media. Eur J Clin Microbiol Infect Dis 34:1989–1994. doi: 10.1007/s10096-015-2441-7. [DOI] [PubMed] [Google Scholar]
- 93.He J, Ledesma KR, Lam WY, Figueroa DA, Lim TP, Chow DS, Tam VH. 2010. Variability of polymyxin B major components in commercial formulations. Int J Antimicrob Agents 35:308–310. doi: 10.1016/j.ijantimicag.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Humphries RM. 2015. Susceptibility testing of the polymyxins: where are we now? Pharmacotherapy 35:22–27. doi: 10.1002/phar.1505. [DOI] [PubMed] [Google Scholar]
- 95.Tam VH, Cao H, Ledesma KR, Hu M. 2011. In vitro potency of various polymyxin B components. Antimicrob Agents Chemother 55:4490–4491. doi: 10.1128/AAC.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin Microbiol Rev 21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karvanen MC, Mohamad A, Lagerback P. 2011. Colistin is extensively lost during normal experimental conditions, abstr D-690, p 160 Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 98.Albur M, Noel A, Bowker K, Macgowan A. 2014. Colistin susceptibility testing: time for a review. J Antimicrob Chemother 69:1432–1434. doi: 10.1093/jac/dkt503. [DOI] [PubMed] [Google Scholar]
- 99.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74:412–414. doi: 10.1016/j.diagmicrobio.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 100.Brown MR, Winsley BE. 1968. Synergistic action of polysorbate 80 and polymyxin B sulphate on Pseudomonas aeruginosa. J Gen Microbiol 50(Suppl):ix. [PubMed] [Google Scholar]
- 101.Brown MR, Geaton EM, Gilbert P. 1979. Additivity of action between polysorbate 80 and polymyxin B towards spheroplasts of Pseudomonas aeruginosa NCTC 6750. J Pharm Pharmacol 31:168–170. doi: 10.1111/j.2042-7158.1979.tb13463.x. [DOI] [PubMed] [Google Scholar]
- 102.Sader HS, Rhomberg PR, Farrell DJ, Jones RN. 2015. Differences in potency and categorical agreement between colistin and polymyxin B when testing 15,377 clinical strains collected worldwide. Diagn Microbiol Infect Dis 83:379–381. doi: 10.1016/j.diagmicrobio.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 103.Sautrey G, Duval RE, Chevalley A, Fontanay S, Clarot I. 2015. Capillary electrophoresis for fast detection of heterogeneous population in colistin-resistant Gram-negative bacteria. Electrophoresis 36:2630–2633. doi: 10.1002/elps.201500064. [DOI] [PubMed] [Google Scholar]
- 104.Tamayo M, Santiso R, Otero F, Bou G, Lepe JA, McConnell MJ, Cisneros JM, Gosalvez J, Fernandez JL. 2013. Rapid determination of colistin resistance in clinical strains of Acinetobacter baumannii by use of the Micromax assay. J Clin Microbiol 51:3675–3682. doi: 10.1128/JCM.01787-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nordmann P, Jayol A, Poirel L. 2016. Rapid detection of polymyxin resistance in Enterobacteriaceae. Emerg Infect Dis 22:1038–1043. doi: 10.3201/eid2206.151840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jayol A, Dubois V, Poirel L, Nordmann P. 2016. Rapid detection of polymyxin-resistant Enterobacteriaceae from blood cultures. J Clin Microbiol 54:2273–2277. doi: 10.1128/JCM.00918-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nordmann P, Jayol A, Poirel L. 2016. A universal culture medium for screening polymyxin-resistant Gram-negative isolates. J Clin Microbiol 54:1395–1399. doi: 10.1128/JCM.00446-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levine M. 1943. The effect of concentration of dyes on differentiation of enteric bacteria on eosin-methylene-blue agar. J Bacteriol 45:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bontron S, Poirel L, Nordmann P. 2016. Real-time PCR for detection of plasmid-mediated polymyxin resistance (mcr-1) from cultured bacteria and stools. J Antimicrob Chemother 71:2318–2320. doi: 10.1093/jac/dkw139. [DOI] [PubMed] [Google Scholar]
- 111.Aquilini E, Merino S, Knirel YA, Regue M, Tomas JM. 2014. Functional identification of Proteus mirabilis eptC gene encoding a core lipopolysaccharide phosphoethanolamine transferase. Int J Mol Sci 15:6689–6702. doi: 10.3390/ijms15046689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang SS, Liu MC, Teng LJ, Wang WB, Hsueh PR, Liaw SJ. 2010. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob Agents Chemother 54:1564–1571. doi: 10.1128/AAC.01219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin QY, Tsai YL, Liu MC, Lin WC, Hsueh PR, Liaw SJ. 2014. Serratia marcescens arn, a PhoP-regulated locus necessary for polymyxin B resistance. Antimicrob Agents Chemother 58:5181–5190. doi: 10.1128/AAC.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gunn JS. 2008. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol 16:284–290. doi: 10.1016/j.tim.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 115.Yan A, Guan Z, Raetz CR. 2007. An undecaprenyl phosphate-aminoarabinose flippase required for polymyxin resistance in Escherichia coli. J Biol Chem 282:36077–36089. doi: 10.1074/jbc.M706172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cannatelli A, Di Pilato V, Giani T, Arena F, Ambretti S, Gaibani P, D'Andrea MM, Rossolini GM. 2014. In vivo evolution to colistin resistance by PmrB sensor kinase mutation in KPC-producing Klebsiella pneumoniae is associated with low-dosage colistin treatment. Antimicrob Agents Chemother 58:4399–4403. doi: 10.1128/AAC.02555-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng YH, Lin TL, Pan YJ, Wang YP, Lin YT, Wang JT. 2015. Colistin resistance mechanisms in Klebsiella pneumoniae strains from Taiwan. Antimicrob Agents Chemother 59:2909–2913. doi: 10.1128/AAC.04763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi MJ, Ko KS. 2014. Mutant prevention concentrations of colistin for Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae clinical isolates. J Antimicrob Chemother 69:275–277. doi: 10.1093/jac/dkt315. [DOI] [PubMed] [Google Scholar]
- 119.Jayol A, Poirel L, Brink A, Villegas MV, Yilmaz M, Nordmann P. 2014. Resistance to colistin associated with a single amino acid change in protein PmrB among Klebsiella pneumoniae isolates of worldwide origin. Antimicrob Agents Chemother 58:4762–4766. doi: 10.1128/AAC.00084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olaitan AO, Diene SM, Kempf M, Berrazeg M, Bakour S, Gupta SK, Thongmalayvong B, Akkhavong K, Somphavong S, Paboriboune P, Chaisiri K, Komalamisra C, Adelowo OO, Fagade OE, Banjo OA, Oke AJ, Adler A, Assous MV, Morand S, Raoult D, Rolain JM. 2014. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents 44:500–507. doi: 10.1016/j.ijantimicag.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 121.Diene SM, Merhej V, Henry M, El Filali A, Roux V, Robert C, Azza S, Gavory F, Barbe V, La Scola B, Raoult D, Rolain JM. 2013. The rhizome of the multidrug-resistant Enterobacter aerogenes genome reveals how new “killer bugs” are created because of a sympatric lifestyle. Mol Biol Evol 30:369–383. doi: 10.1093/molbev/mss236. [DOI] [PubMed] [Google Scholar]
- 122.Olaitan AO, Dia NM, Gautret P, Benkouiten S, Belhouchat K, Drali T, Parola P, Brouqui P, Memish Z, Raoult D, Rolain JM. 2015. Acquisition of extended-spectrum cephalosporin- and colistin-resistant Salmonella enterica subsp. enterica serotype Newport by pilgrims during Hajj. Int J Antimicrob Agents 45:600–604. doi: 10.1016/j.ijantimicag.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 123.Sun S, Negrea A, Rhen M, Andersson DI. 2009. Genetic analysis of colistin resistance in Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother 53:2298–2305. doi: 10.1128/AAC.01016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olaitan AO, Morand S, Rolain JM. 2016. Emergence of colistin-resistant bacteria in humans without colistin usage: a new worry and cause for vigilance. Int J Antimicrob Agents 47:1–3. doi: 10.1016/j.ijantimicag.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 125.Quesada A, Porrero MC, Tellez S, Palomo G, Garcia M, Dominguez L. 2015. Polymorphism of genes encoding PmrAB in colistin-resistant strains of Escherichia coli and Salmonella enterica isolated from poultry and swine. J Antimicrob Chemother 70:71–74. doi: 10.1093/jac/dku320. [DOI] [PubMed] [Google Scholar]
- 126.Groisman EA. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park SY, Groisman EA. 2014. Signal-specific temporal response by the Salmonella PhoP/PhoQ regulatory system. Mol Microbiol 91:135–144. doi: 10.1111/mmi.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wright MS, Suzuki Y, Jones MB, Marshall SH, Rudin SD, van Duin D, Kaye K, Jacobs MR, Bonomo RA, Adams MD. 2015. Genomic and transcriptomic analyses of colistin-resistant clinical isolates of Klebsiella pneumoniae reveal multiple pathways of resistance. Antimicrob Agents Chemother 59:536–543. doi: 10.1128/AAC.04037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lippa AM, Goulian M. 2009. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet 5:e1000788. doi: 10.1371/journal.pgen.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cannatelli A, Giani T, D'Andrea MM, Di Pilato V, Arena F, Conte V, Tryfinopoulou K, Vatopoulos A, Rossolini G. 2014. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother 58:5696–5703. doi: 10.1128/AAC.03110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Poirel L, Jayol A, Bontron S, Villegas MV, Ozdamar M, Turkoglu S, Nordmann P. 2015. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother 70:75–80. doi: 10.1093/jac/dku323. [DOI] [PubMed] [Google Scholar]
- 132.Lopez-Camacho E, Gomez-Gil R, Tobes R, Manrique M, Lorenzo M, Galvan B, Salvarelli E, Moatassim Y, Salanueva IJ, Pareja E, Codoner FM, Alvarez-Tejado M, Garcillan-Barcia MP, De la Cruz F, Mingorance J. 2014. Genomic analysis of the emergence and evolution of multidrug resistance during a Klebsiella pneumoniae outbreak including carbapenem and colistin resistance. J Antimicrob Chemother 69:632–636. doi: 10.1093/jac/dkt419. [DOI] [PubMed] [Google Scholar]
- 133.Jayol A, Poirel L, Villegas MV, Nordmann P. 2015. Modulation of mgrB gene expression as a source of colistin resistance in Klebsiella oxytoca. Int J Antimicrob Agents 46:108–110. doi: 10.1016/j.ijantimicag.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 134.Olaitan AO, Rolain JM. 2015. Interruption of mgrB in the mediation of colistin resistance in Klebsiella oxytoca. Int J Antimicrob Agents 46:354–355. doi: 10.1016/j.ijantimicag.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 135.Jayol A, Nordmann P, Desroches M, Descousser JW, Poirel L. 2016. Acquisition of broad-spectrum cephalosporin resistance leading to colistin resistance in Klebsiella pneumoniae. Antimicrob Agents Chemother 60:3199–3201. doi: 10.1128/AAC.00237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zowawi HM, Forde BM, Alfaresi M, Alzarouni A, Farahat Y, Chong TM, Yin WF, Chan KG, Li J, Schembri MA, Beatson SA, Paterson DL. 2015. Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci Rep 5:15082. doi: 10.1038/srep15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng YH, Lin TL, Lin YT, Wang JT. 2016. Amino acid substitutions of CrrB responsible for resistance to colistin through CrrC in Klebsiella pneumoniae. Antimicrob Agents Chemother 60:3709–3716. doi: 10.1128/AAC.00009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.De Majumdar S, Yu J, Fookes M, McAteer SP, Llobet E, Finn S, Spence S, Monahan A, Kissenpfennig A, Ingram RJ, Bengoechea J, Gally DL, Fanning S, Elborn JS, Schneiders T. 2015. Elucidation of the RamA regulon in Klebsiella pneumoniae reveals a role in LPS regulation. PLoS Pathog 11:e1004627. doi: 10.1371/journal.ppat.1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 140.Sherman EX, Hufnagel DA, Weiss DS. 2016. MCR-1 confers cross-resistance to lysozyme. Lancet Infect Dis 16:1226–1227. doi: 10.1016/S1473-3099(16)30395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stojanoski V, Sankaran B, Prasad BV, Poirel L, Nordmann P, Palzkill T. 2016. Structure of the catalytic domain of the colistin resistance enzyme MCR-1. BMC Biol 14:81. doi: 10.1186/s12915-016-0303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shen Z, Wang Y, Shen Y, Shen J, Wu C. 2016. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect Dis 16:293. doi: 10.1016/S1473-3099(16)00061-X. [DOI] [PubMed] [Google Scholar]
- 143.Zurfuh K, Poirel L, Nordmann P, Nuesch-Inderbinen M, Hachler H, Stephan R. 2016. Occurrence of the plasmid-borne mcr-1 colistin resistance gene in ESBL-producing Enterobacteriacae in river water and imported vegetable samples in Switzerland. Antimicrob Agents Chemother 60:2594–2595. doi: 10.1128/AAC.00066-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poirel L, Nordmann P. 2016. Plasmid-encoded colistin resistance: the animal world as the culprit? J Antimicrob Chemother 71:2326–2327. doi: 10.1093/jac/dkw074. [DOI] [PubMed] [Google Scholar]
- 145.Thanh DP, Tuyen HT, Nguy T, Nguyen ET, The HC, Wick RR, Thwaites G, Baker S, Holt KE. 2016. Inducible colistin resistance via a disrupted plasmid-borne mcr-1 gene in a 2008 Vietnamese Shigella sonnei isolate. J Antimicrob Chemother 71:2314–2317. doi: 10.1093/jac/dkw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yao X, Doi Y, Zeng L, Lv L, Liu JH. 2016. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect Dis 16:288–289. doi: 10.1016/S1473-3099(16)00057-8. [DOI] [PubMed] [Google Scholar]
- 147.Du H, Chen L, Tang YW, Kreiswirth BN. 2016. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis 16:287–288. doi: 10.1016/S1473-3099(16)00056-6. [DOI] [PubMed] [Google Scholar]
- 148.Zhong LL, Zhang YF, Doi Y, Huang X, Zhang XF, Zeng KJ, Shen C, Patil S, Xing Y, Zou Y, Tian GB. 2017. Coproduction of MCR-1 and NDM-1 by colistin-resistant Escherichia coli isolated from a healthy individual. Antimicrob Agents Chemother 61:e01962-16. doi: 10.1128/AAC.01962-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Poirel L, Kieffer N, Liassine N, Thanh D, Nordmann P. 2016. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect Dis 16:281. doi: 10.1016/S1473-3099(16)00006-2. [DOI] [PubMed] [Google Scholar]
- 150.Falgenhauer L, Waezsada SE, Yao Y, Imirzalioglu C, Kasbohrer A, Roesler U, Michael GB, Schwarz S, Werner G, Kreienbrock L, Chakraborty T. 2016. Colistin resistance gene mcr-1 in extended-spectrum beta-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis 16:282–283. doi: 10.1016/S1473-3099(16)00009-8. [DOI] [PubMed] [Google Scholar]
- 151.Mulvey MR, Mataseje LF, Robertson J, Nash JH, Boerlin P, Toye B, Irwin R, Melano RG. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:289–290. doi: 10.1016/S1473-3099(16)00067-0. [DOI] [PubMed] [Google Scholar]
- 152.Liu BT, Song FJ, Zou M, Hao ZH, Shan H. 2017. Emergence of colistin resistance gene mcr-1 in Cronobacter sakazakii producing NDM-9 and Escherichia coli from the same animal. Antimicrob Agents Chemother 61:e01444-16. doi: 10.1128/AAC.01444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zurfluh K, Tasara T, Poirel L, Nordmann P, Stephan R. 2016. Draft genome sequence of Escherichia coli S51, a chicken isolate harboring a chromosomally encoded mcr-1 gene. Genome Announc 4:e00796-16. doi: 10.1128/genomeA.00796-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poirel L, Kieffer N, Brink A, Coetze J, Jayol A, Nordmann P. 2016. Genetic features of MCR-1-producing colistin-resistant Escherichia coli isolates, South Africa. Antimicrob Agents Chemother 60:4394–4397. doi: 10.1128/AAC.00444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Snesrud E, He S, Chandler M, Dekker JP, Hickman AB, McGann P, Dyda F. 2016. A model for transposition of the colistin resistance gene mcr-1 by ISApl1. Antimicrob Agents Chemother 60:6973–6976. doi: 10.1128/AAC.01457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nordmann P, Lienhard R, Kieffer N, Clerc O, Poirel L. 2016. Plasmid-mediated colistin-resistant Escherichia coli in bacteremia in Switzerland. Clin Infect Dis 62:1322–1323. doi: 10.1093/cid/ciw124. [DOI] [PubMed] [Google Scholar]
- 157.Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S, Giani T, Menichetti F, Rossolini GM. 2016. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother 60:5612–5615. doi: 10.1128/AAC.01075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, Malhotra-Kumar S. 2016. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill 21:30280. doi: 10.2807/1560-7917.ES..21.27.30280. [DOI] [PubMed] [Google Scholar]
- 159.Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA. 2004. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun 72:7107–7114. doi: 10.1128/IAI.72.12.7107-7114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Llobet E, Tomas JM, Bengoechea JA. 2008. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 154:3877–3886. doi: 10.1099/mic.0.2008/022301-0. [DOI] [PubMed] [Google Scholar]
- 161.Fresno S, Jimenez N, Izquierdo L, Merino S, Corsaro MM, De Castro C, Parrilli M, Naldi T, Regue M, Tomas JM. 2006. The ionic interaction of Klebsiella pneumoniae K2 capsule and core lipopolysaccharide. Microbiology 152:1807–1818. doi: 10.1099/mic.0.28611-0. [DOI] [PubMed] [Google Scholar]
- 162.Pilonieta MC, Erickson KD, Ernst RK, Detweiler CS. 2009. A protein important for antimicrobial peptide resistance, YdeI/OmdA, is in the periplasm and interacts with OmpD/NmpC. J Bacteriol 191:7243–7252. doi: 10.1128/JB.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Padilla E, Llobet E, Domenech-Sanchez A, Martinez-Martinez L, Bengoechea JA, Alberti S. 2010. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother 54:177–183. doi: 10.1128/AAC.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Srinivasan VB, Rajamohan G. 2013. KpnEF, a new member of the Klebsiella pneumoniae cell envelope stress response regulon, is an SMR-type efflux pump involved in broad-spectrum antimicrobial resistance. Antimicrob Agents Chemother 57:4449–4462. doi: 10.1128/AAC.02284-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ni W, Li Y, Guan J, Zhao J, Cui J, Wang R, Liu Y. 2016. Effects of efflux pump inhibitors on colistin resistance in multidrug resistant Gram-negative bacteria. Antimicrob Agents Chemother 60:3215–3218. doi: 10.1128/AAC.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee JY, Ko KS. 2014. Mutations and expression of PmrAB and PhoPQ related with colistin resistance in Pseudomonas aeruginosa clinical isolates. Diagn Microbiol Infect Dis 78:271–276. doi: 10.1016/j.diagmicrobio.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 167.Moskowitz SM, Brannon MK, Dasgupta N, Pier M, Sgambati N, Miller AK, Selgrade SE, Miller SI, Denton M, Conway SP, Johansen HK, Høiby N. 2012. PmrB mutations promote polymyxin resistance of Pseudomonas aeruginosa isolated from colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother 56:1019–1030. doi: 10.1128/AAC.05829-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barrow K, Kwon DH. 2009. Alterations in two-component regulatory systems of phoPQ and pmrAB are associated with polymyxin B resistance in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:5150–5154. doi: 10.1128/AAC.00893-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abraham N, Kwon DH. 2009. A single amino acid substitution in PmrB is associated with polymyxin B resistance in clinical isolate of Pseudomonas aeruginosa. FEMS Microbiol Lett 298:249–254. doi: 10.1111/j.1574-6968.2009.01720.x. [DOI] [PubMed] [Google Scholar]
- 170.Miller AK, Brannon MK, Stevens L, Johansen HK, Selgrade SE, Miller SI, Høiby N, Moskowitz SM. 2011. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother 55:5761–5769. doi: 10.1128/AAC.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Muller C, Plesiat P, Jeannot K. 2011. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and beta-lactams in Pseudomonas aeruginosa. Antimicrob Agents Chemother 55:1211–1221. doi: 10.1128/AAC.01252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gutu AD, Sgambati N, Strasbourger P, Brannon MK, Jacobs MA, Haugen E, Kaul RK, Johansen HK, Høiby N, Moskowitz SM. 2013. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob Agents Chemother 57:2204–2215. doi: 10.1128/AAC.02353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother 53:3628–3634. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim Y, Bae IK, Lee H, Jeong SH, Yong D, Lee K. 2014. In vivo emergence of colistin resistance in Acinetobacter baumannii clinical isolates of sequence type 357 during colistin treatment. Diagn Microbiol Infect Dis 79:362–366. doi: 10.1016/j.diagmicrobio.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 177.Lesho E, Yoon EJ, McGann P, Snesrud E, Kwak Y, Milillo M, Onmus-Leone F, Preston L, St Clair K, Nikolich M, Viscount H, Wortmann G, Zapor M, Grillot-Courvalin C, Courvalin P, Clifford R, Waterman PE. 2013. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis 208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- 178.Park YK, Choi JY, Shin D, Ko KS. 2011. Correlation between overexpression and amino acid substitution of the PmrAB locus and colistin resistance in Acinetobacter baumannii. Int J Antimicrob Agents 37:525–530. doi: 10.1016/j.ijantimicag.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 179.Pournaras S, Poulou A, Dafopoulou K, Chabane YN, Kristo I, Makris D, Hardouin J, Cosette P, Tsakris A, Dé E. 2014. Growth retardation, reduced invasiveness, and impaired colistin-mediated cell death associated with colistin resistance development in Acinetobacter baumannii. Antimicrob Agents Chemother 58:828–832. doi: 10.1128/AAC.01439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rolain JM, Diene SM, Kempf M, Gimenez G, Robert C, Raoult D. 2013. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob Agents Chemother 57:592–596. doi: 10.1128/AAC.01314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. 2011. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother 55:3022–3024. doi: 10.1128/AAC.01732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ah YM, Kim AJ, Lee JY. 2014. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents 44:8–15. doi: 10.1016/j.ijantimicag.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 183.Gales AC, Jones RN, Sader HS. 2011. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J Antimicrob Chemother 66:2070–2074. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- 184.Walkty A, DeCorby M, Nichol K, Karlowsky JA, Hoban DJ, Zhanel GG. 2009. In vitro activity of colistin (polymyxin E) against 3,480 isolates of Gram-negative bacilli obtained from patients in Canadian hospitals in the CANWARD study, 2007–2008. Antimicrob Agents Chemother 53:4924–4926. doi: 10.1128/AAC.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sutherland CA, Nicolau DP. 2015. Susceptibility profile of ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from U.S. hospitals. Clin Ther 37:1564–1571. doi: 10.1016/j.clinthera.2015.05.501. [DOI] [PubMed] [Google Scholar]
- 186.Tijet N, Sheth PM, Lastovetska O, Chung C, Patel SN, Melano RG. 2014. Molecular characterization of Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae in Ontario, Canada, 2008–2011. PLoS One 9:e116421. doi: 10.1371/journal.pone.0116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bratu S, Tolaney P, Karumudi U, Quale J, Mooty M, Nichani S, Landman D. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother 56:128–132. doi: 10.1093/jac/dki175. [DOI] [PubMed] [Google Scholar]
- 188.Bogdanovich T, Adams-Haduch JM, Tian GB, Nguyen MH, Kwak EJ, Muto CA, Doi Y. 2011. Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin Infect Dis 53:373–376. doi: 10.1093/cid/cir401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marchaim D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, Endimiani A, Navon-Venezia S, Hothi J, Slim J, Blunden C, Shango M, Lephart PR, Salimnia H, Reid D, Moshos J, Hafeez W, Bheemreddy S, Chen TY, Dhar S, Bonomo RA, Kaye KS. 2011. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 55:593–599. doi: 10.1128/AAC.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Garza-Ramos U, Barrios H, Reyna-Flores F, Sanchez-Perez A, Tamayo-Legorreta E, Ibarra-Pacheco A, Salazar-Salinas J, Nunez-Ceballos R, Silva-Sanchez J. 2014. Characteristics of KPC-2-producing Klebsiella pneumoniae (ST258) clinical isolates from outbreaks in 2 Mexican medical centers. Diagn Microbiol Infect Dis 79:483–485. doi: 10.1016/j.diagmicrobio.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 191.Arduino SM, Quiroga MP, Ramirez MS, Merkier AK, Errecalde L, Di Martino A, Smayevsky J, Kaufman S, Centron D. 2012. Transposons and integrons in colistin-resistant clones of Klebsiella pneumoniae and Acinetobacter baumannii with epidemic or sporadic behaviour. J Med Microbiol 61:1417–1420. doi: 10.1099/jmm.0.038968-0. [DOI] [PubMed] [Google Scholar]
- 192.Weterings V, Zhou K, Rossen JW, van Stenis D, Thewessen E, Kluytmans J, Veenemans J. 2015. An outbreak of colistin-resistant Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae in the Netherlands (July to December 2013), with inter-institutional spread. Eur J Clin Microbiol Infect Dis 34:1647–1655. doi: 10.1007/s10096-015-2401-2. [DOI] [PubMed] [Google Scholar]
- 193.Toth A, Damjanova I, Puskas E, Janvari L, Farkas M, Dobak A, Borocz K, Paszti J. 2010. Emergence of a colistin-resistant KPC-2-producing Klebsiella pneumoniae ST258 clone in Hungary. Eur J Clin Microbiol Infect Dis 29:765–769. doi: 10.1007/s10096-010-0921-3. [DOI] [PubMed] [Google Scholar]
- 194.Kontopoulou K, Protonotariou E, Vasilakos K, Kriti M, Koteli A, Antoniadou E, Sofianou D. 2010. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 beta-lactamase resistant to colistin. J Hosp Infect 76:70–73. doi: 10.1016/j.jhin.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 195.Antoniadou A, Kontopidou F, Poulakou G, Koratzanis E, Galani I, Papadomichelakis E, Kopterides P, Souli M, Armaganidis A, Giamarellou H. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J Antimicrob Chemother 59:786–790. doi: 10.1093/jac/dkl562. [DOI] [PubMed] [Google Scholar]
- 196.Mezzatesta ML, Gona F, Caio C, Petrolito V, Sciortino D, Sciacca A, Santangelo C, Stefani S. 2011. Outbreak of KPC-3-producing, and colistin-resistant, Klebsiella pneumoniae infections in two Sicilian hospitals. Clin Microbiol Infect 17:1444–1447. doi: 10.1111/j.1469-0691.2011.03572.x. [DOI] [PubMed] [Google Scholar]
- 197.Mammina C, Bonura C, Di Bernardo F, Aleo A, Fasciana T, Sodano C, Saporito MA, Verde MS, Tetamo R, Palma DM. 2012. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Euro Surveill 17:20248. [PubMed] [Google Scholar]
- 198.Giani T, Arena F, Vaggelli G, Conte V, Chiarelli A, Henrici De Angelis L, Fornaini R, Grazzini M, Niccolini F, Pecile P, Rossolini GM. 2015. Large nosocomial outbreak of colistin-resistant, carbapenemase-producing Klebsiella pneumoniae traced to clonal expansion of an mgrB deletion mutant. J Clin Microbiol 53:3341–3344. doi: 10.1128/JCM.01017-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Parisi SG, Bartolini A, Santacatterina E, Castellani E, Ghirardo R, Berto A, Franchin E, Menegotto N, De Canale E, Tommasini T, Rinaldi R, Basso M, Stefani S, Palu G. 2015. Prevalence of Klebsiella pneumoniae strains producing carbapenemases and increase of resistance to colistin in an Italian teaching hospital from January 2012 to December 2014. BMC Infect Dis 15:244. doi: 10.1186/s12879-015-0996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mezzatesta ML, Caio C, Gona F, Cormaci R, Salerno I, Zingali T, Denaro C, Gennaro M, Quattrone C, Stefani S. 2014. Carbapenem and multidrug resistance in Gram-negative bacteria in a single centre in Italy: considerations on in vitro assay of active drugs. Int J Antimicrob Agents 44:112–116. doi: 10.1016/j.ijantimicag.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 201.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S, Grundmann H, Pantosti A, Rossolini GM. 2014. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill 19:20939. doi: 10.2807/1560-7917.ES2014.19.42.20939. [DOI] [PubMed] [Google Scholar]
- 202.Meletis G, Oustas E, Botziori C, Kakasi E, Koteli A. 2015. Containment of carbapenem resistance rates of Klebsiella pneumoniae and Acinetobacter baumannii in a Greek hospital with a concomitant increase in colistin, gentamicin and tigecycline resistance. New Microbiol 38:417–421. [PubMed] [Google Scholar]
- 203.Zagorianou A, Sianou E, Iosifidis E, Dimou V, Protonotariou E, Miyakis S, Roilides E, Sofianou D. 2012. Microbiological and molecular characteristics of carbapenemase-producing Klebsiella pneumoniae endemic in a tertiary Greek hospital during 2004–2010. Euro Surveill 17:20088. [PubMed] [Google Scholar]
- 204.Pena I, Picazo JJ, Rodriguez-Avial C, Rodriguez-Avial I. 2014. Carbapenemase-producing Enterobacteriaceae in a tertiary hospital in Madrid, Spain: high percentage of colistin resistance among VIM-1-producing Klebsiella pneumoniae ST11 isolates. Int J Antimicrob Agents 43:460–464. doi: 10.1016/j.ijantimicag.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 205.Jayol A, Poirel L, Dortet L, Nordmann P. 2016. National survey of colistin resistance among carbapenemase-producing Enterobacteriaceae and outbreak caused by colistin-resistant OXA-48-producing Klebsiella pneumoniae, France, 2014. Euro Surveill 21:30339. doi: 10.2807/1560-7917.ES.2016.21.37.30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Suh JY, Son JS, Chung DR, Peck KR, Ko KS, Song JH. 2010. Nonclonal emergence of colistin-resistant Klebsiella pneumoniae isolates from blood samples in South Korea. Antimicrob Agents Chemother 54:560–562. doi: 10.1128/AAC.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tan TY, Ng SY. 2006. The in-vitro activity of colistin in Gram-negative bacteria. Singapore Med J 47:621–624. [PubMed] [Google Scholar]
- 208.Chen S, Hu F, Zhang X, Xu X, Liu Y, Zhu D, Wang H. 2011. Independent emergence of colistin-resistant Enterobacteriaceae clinical isolates without colistin treatment. J Clin Microbiol 49:4022–4023. doi: 10.1128/JCM.01233-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chiu SK, Wu TL, Chuang YC, Lin JC, Fung CP, Lu PL, Wang JT, Wang LS, Siu LK, Yeh KM. 2013. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One 8:e69428. doi: 10.1371/journal.pone.0069428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Matthaiou DK, Michalopoulos A, Rafailidis PI, Karageorgopoulos DE, Papaioannou V, Ntani G, Samonis G, Falagas ME. 2008. Risk factors associated with the isolation of colistin-resistant Gram-negative bacteria: a matched case-control study. Crit Care Med 36:807–811. doi: 10.1097/CCM.0B013E3181652FAE. [DOI] [PubMed] [Google Scholar]
- 211.Kontopidou F, Plachouras D, Papadomichelakis E, Koukos G, Galani I, Poulakou G, Dimopoulos G, Antoniadou A, Armaganidis A, Giamarellou H. 2011. Colonization and infection by colistin-resistant Gram-negative bacteria in a cohort of critically ill patients. Clin Microbiol Infect 17:E9–E11. doi: 10.1111/j.1469-0691.2011.03649.x. [DOI] [PubMed] [Google Scholar]
- 212.Halaby T, Al Naiemi N, Kluytmans J, van der Palen J, Vandenbroucke-Grauls CM. 2013. Emergence of colistin resistance in Enterobacteriaceae after the introduction of selective digestive tract decontamination in an intensive care unit. Antimicrob Agents Chemother 57:3224–3229. doi: 10.1128/AAC.02634-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Strenger V, Gschliesser T, Grisold A, Zarfel G, Feierl G, Masoud L, Hoenigl M, Resch B, Muller W, Urlesberger B. 2011. Orally administered colistin leads to colistin-resistant intestinal flora and fails to prevent faecal colonisation with extended-spectrum beta-lactamase-producing enterobacteria in hospitalised newborns. Int J Antimicrob Agents 37:67–69. doi: 10.1016/j.ijantimicag.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 214.Durante-Mangoni E, Del Franco M, Andini R, Bernardo M, Giannouli M, Zarrilli R. 2015. Emergence of colistin resistance without loss of fitness and virulence after prolonged colistin administration in a patient with extensively drug-resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis 82:222–226. doi: 10.1016/j.diagmicrobio.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 215.Silvestri L, Taylor N, van Saene HK, Bakker J. 2014. Colistin, SDD and resistance: nihil novi sub sole. Intensive Care Med 40:1065. doi: 10.1007/s00134-014-3321-9. [DOI] [PubMed] [Google Scholar]
- 216.Zhao F, Zong Z. 2016. Kluyvera ascorbata strain from hospital sewage carrying the mcr-1 colistin resistance gene. Antimicrob Agents Chemother 60:7498–7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Skov RL, Monnet DL. 2016. Plasmid-mediated colistin resistance (mcr-1 gene): three months later, the story unfolds. Euro Surveill 21:30155. doi: 10.2807/1560-7917.ES.2016.21.9.30155. [DOI] [PubMed] [Google Scholar]
- 218.Hu Y, Liu F, Lin IY, Gao GF, Zhu B. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:146–147. doi: 10.1016/S1473-3099(15)00533-2. [DOI] [PubMed] [Google Scholar]
- 219.Ruppe E, Chatelier EL, Pons N, Andremont A, Ehrlich SD. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:290–291. doi: 10.1016/S1473-3099(16)00066-9. [DOI] [PubMed] [Google Scholar]
- 220.Zhi C, Lv L, Yu LF, Doi Y, Liu JH. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:292–293. doi: 10.1016/S1473-3099(16)00063-3. [DOI] [PubMed] [Google Scholar]
- 221.Zhang R, Huang Y, Chan EW, Zhou H, Chen S. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:291–292. doi: 10.1016/S1473-3099(16)00062-1. [DOI] [PubMed] [Google Scholar]
- 222.Olaitan AO, Chabou S, Okdah L, Morand S, Rolain JM. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:147. doi: 10.1016/S1473-3099(15)00540-X. [DOI] [PubMed] [Google Scholar]
- 223.Malhotra-Kumar S, Xavier BB, Das AJ, Lammens C, Hoang HT, Pham NT, Goossens H. 2016. Colistin-resistant Escherichia coli harbouring mcr-1 isolated from food animals in Hanoi, Vietnam. Lancet Infect Dis 16:286–287. doi: 10.1016/S1473-3099(16)00014-1. [DOI] [PubMed] [Google Scholar]
- 224.Stoesser N, Mathers AJ, Moore CE, Day NP, Crook DW. 2016. Colistin resistance gene mcr-1 and pHNSHP45 plasmid in human isolates of Escherichia coli and Klebsiella pneumoniae. Lancet Infect Dis 16:285–286. doi: 10.1016/S1473-3099(16)00010-4. [DOI] [PubMed] [Google Scholar]
- 225.Petrillo M, Angers-Loustau A, Kreysa J. 2016. Possible genetic events producing colistin resistance gene mcr-1. Lancet Infect Dis 16:280. doi: 10.1016/S1473-3099(16)00005-0. [DOI] [PubMed] [Google Scholar]
- 226.Suzuki S, Ohnishi M, Kawanishi M, Akiba M, Kuroda M. 2016. Investigation of a plasmid genome database for colistin-resistance gene mcr-1. Lancet Infect Dis 16:284–285. doi: 10.1016/S1473-3099(16)00008-6. [DOI] [PubMed] [Google Scholar]
- 227.Haenni M, Poirel L, Kieffer N, Chatre P, Saras E, Metayer V, Dumoulin R, Nordmann P, Madec JY. 2016. Co-occurrence of extended spectrum β-lactamase and MCR-1 encoding genes on plasmids. Lancet Infect Dis 16:281–282. doi: 10.1016/S1473-3099(16)00007-4. [DOI] [PubMed] [Google Scholar]
- 228.Perrin-Guyomard A, Bruneau M, Houee P, Deleurme K, Legrandois P, Poirier C, Soumet C, Sanders P. 2016. Prevalence of mcr-1 in commensal Escherichia coli from French livestock, 2007 to 2014. Euro Surveill 21:30135. doi: 10.2807/1560-7917.ES.2016.21.6.30135. [DOI] [PubMed] [Google Scholar]
- 229.Cannatelli A, Giani T, Antonelli A, Principe L, Luzzaro F, Rossolini GM. 2016. First detection of the mcr-1 colistin resistance gene in Escherichia coli, Italy. Antimicrob Agents Chemother 60:3257–3258. doi: 10.1128/AAC.00246-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Giufrè M, Monaco M, Accogli M, Pantosti A, Cerquetti M, PAMURSA Study Group. 2016. Emergence of the colistin resistance mcr-1 determinant in commensal Escherichia coli from residents of long-term-care facilities in Italy. J Antimicrob Chemother 71:2329–2331. doi: 10.1093/jac/dkw195. [DOI] [PubMed] [Google Scholar]
- 231.Doumith M, Godbole G, Ashton P, Larkin L, Dallman T, Day M, Day M, Muller-Pebody B, Ellington MJ, de Pinna E, Johnson AP, Hopkins KL, Woodford N. 2016. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J Antimicrob Chemother 71:2300–2305. doi: 10.1093/jac/dkw093. [DOI] [PubMed] [Google Scholar]
- 232.Kluytmans-van den Bergh MF, Huizinga P, Bonten MJ, Bos M, De Bruyne K, Friedrich AW, Rossen JW, Savelkoul PH, Kluytmans JA. 2016. Presence of mcr-1-positive Enterobacteriaceae in retail chicken meat but not in humans in the Netherlands since 2009. Euro Surveill 21:30149. doi: 10.2807/1560-7917.ES.2016.21.9.30149. [DOI] [PubMed] [Google Scholar]
- 233.Bonten MJ. 2016. Antimicrobial resistance: is it really all going wrong now? Ned Tijdschr Geneeskd 160:D81. [PubMed] [Google Scholar]
- 234.Arcilla MS, van Hattem JM, Matamoros S, Melles DC, Penders J, de Jong MD, Schultsz C. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:147–149. doi: 10.1016/S1473-3099(15)00541-1. [DOI] [PubMed] [Google Scholar]
- 235.Veldman K, van Essen-Zandbergen A, Rapallini M, Wit B, Heymans R, van Pelt W, Mevius D. 2016. Location of colistin resistance gene mcr-1 in Enterobacteriaceae from livestock and meat. J Antimicrob Chemother 71:2340–2342. doi: 10.1093/jac/dkw181. [DOI] [PubMed] [Google Scholar]
- 236.Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agerso Y, Zankari E, Leekitcharoenphon P, Stegger M, Kaas RS, Cavaco LM, Hansen DS, Aarestrup FM, Skov RL. 2015. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill 20:30085. doi: 10.2807/1560-7917.ES.2015.20.49.30085. [DOI] [PubMed] [Google Scholar]
- 237.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, Hinkle M, Whitman T, Lesho E, Schaecher KE. 2016. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother 60:4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Grami R, Mansour W, Mehri W, Bouallegue O, Boujaafar N, Madec JY, Haenni M. 2016. Impact of food animal trade on the spread of mcr-1-mediated colistin resistance, Tunisia, July 2015. Euro Surveill 21:30144. doi: 10.2807/1560-7917.ES.2016.21.8.30144. [DOI] [PubMed] [Google Scholar]
- 239.Elnahriry SS, Khalifa HO, Soliman AM, Ahmed AM, Moustafa AH, Shimamoto T. 2016. Emergence of plasmid-mediated colistin resistance gene, mcr-1, in a clinical Escherichia coli isolate from Egypt. Antimicrob Agents Chemother 60:3249–3250. doi: 10.1128/AAC.00269-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Perreten V, Strauss C, Collaud A, Gerber D. 2016. Colistin resistance gene mcr-1 in avian pathogenic Escherichia coli in South Africa. Antimicrob Agents Chemother 60:4414–4415. doi: 10.1128/AAC.00548-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fernandes MR, Moura Q, Sartori L, Silva KC, Cunha MP, Esposito F, Lopes R, Otutumi LK, Gonçalves DD, Dropa M, Matté MH, Monte DF, Landgraf M, Francisco GR, Bueno MF, de Oliveira Garcia D, Knöbl T, Moreno AM, Lincopan N. 2016. Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Euro Surveill 21:30214. doi: 10.2807/1560-7917.ES.2016.21.17.30214. [DOI] [PubMed] [Google Scholar]
- 242.Zeng KJ, Doi Y, Patil S, Huang X, Tian GB. 2016. Emergence of plasmid-mediated mcr-1 gene in colistin-resistant Enterobacter aerogenes and Enterobacter cloacae. Antimicrob Agents Chemother 60:3862–3863. doi: 10.1128/AAC.00345-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tse H, Yuen KY. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:145–146. doi: 10.1016/S1473-3099(15)00532-0. [DOI] [PubMed] [Google Scholar]
- 244.Webb HE, Granier SA, Marault M, Millemann Y, den Bakker HC, Nightingale KK, Bugarel M, Ison SA, Scott HM, Loneragan GH. 2016. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 16:144–145. doi: 10.1016/S1473-3099(15)00538-1. [DOI] [PubMed] [Google Scholar]
- 245.Iraz M, Ozad Duzgun A, Sandalli C, Doymaz MZ, Akkoyunlu Y, Saral A, Peleg AY, Ozgumus OB, Beris FS, Karaoglu H, Copur Cicek A. 2015. Distribution of beta-lactamase genes among carbapenem-resistant Klebsiella pneumoniae strains isolated from patients in Turkey. Ann Lab Med 35:595–601. doi: 10.3343/alm.2015.35.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R. 2009. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 30:666–671. doi: 10.1086/598244. [DOI] [PubMed] [Google Scholar]
- 247.Lee JY, Chung ES, Na IY, Kim H, Shin D, Ko KS. 2014. Development of colistin resistance in pmrA-, phoP-, parR- and cprR-inactivated mutants of Pseudomonas aeruginosa. J Antimicrob Chemother 69:2966–2971. doi: 10.1093/jac/dku238. [DOI] [PubMed] [Google Scholar]