Abstract
Epidermal growth factor receptor pathway substrate 8 (EPS8) is critical in the proliferation, progression and metastasis of solid and hematological types of cancer, and thus constitutes an ideal target for cancer immunotherapy. The present study aimed to identify human leukocyte antigen (HLA)-A*1101-restricted cytotoxic T lymphocyte (CTL) epitopes from EPS8 and characterize their immunotherapeutic efficacy in vitro. Two computer-based algorithms were used to predict native EPS8 epitopes with potential high binding affinity to the HLA-A*1101 molecule, which is the HLA-A allele with the highest frequency in the Chinese population. The peptide-induced cytokine production from the CTLs was examined using enzyme-linked immunosorbent spot analysis. The cytotoxic effects on cancer cells by CTLs primed with the identified peptides were examined using flow cytometry. A total of five peptides, designated as P380, P70, P82, P30 and P529, presented with high affinity towards the HLA-A*1101 molecule. In response to stimulation by these five peptides, enhanced secretion of interferon-γ from the CTLs and increased cytolytic capabilities of the CTLs toward cancer cells were noted, with the most potent effects observed from the P380 peptide. Taken together, the present study identified five potential CTL epitopes from EPS8. Among these, P380 presented with the highest therapeutic efficacy in vitro. These peptides may benefit the development of EPS8-based immunotherapy for the treatment of HLA-A*1101-positive hematological malignancies.
Keywords: immunotherapy, epidermal growth factor receptor pathway substrate 8, human leukocyte antigen-A*1101, cytotoxic T lymphocyte, T cell epitope
Introduction
Hematological malignancies (HMs) are clonal malignant disorders originating from cells of the bone marrow or the lymphatic system (1). Despite advances in HM treatment, including combination chemotherapy, targeted therapy and bone marrow transplantation, the disease relapses and the five-year survival rate of patients with HMs remains low (2,3). Therefore, additional therapeutic strategies are required. Previous studies on the involvement of immunogenicity in cancer development have suggested cancer vaccines as a promising and innovative alternative for the treatment of HMs (4–6). The use of cancer vaccines is an approach for active immunotherapy, in which a formulation of specific tumor-associated antigen (TAA) is delivered, together with immunomodulatory agents, to patients with cancer. This activates the host's immune system to mount an immune response, predominantly through cytotoxic T lymphocyte (CTL)-mediated cytolysis, against TAA and eliminates cancer cells (7). Therefore, the key for developing a successful cancer vaccination against solid or hematological types of cancer is to identify specific TAAs, which are selectively presented by tumor cells or dendritic cells (8).
Epidermal growth factor receptor pathway substrate 8 (EPS8) is a 97-kDa protein originally identified as a substrate for kinase activity of epidermal growth factor (EGF) receptor, and augments the mitogenic signaling downstream of EGF (9). The constitutive phosphorylation of EPS8 has been demonstrated to promote the malignant transformation in tumor cells (10). Previous studies have shown that EPS8 is important in several types of solid tumor, including colon, pancreatic, pituitary and cervical cancer (11–14), where the amplification of EPS8 is associated with tumor progression, acquired drug resistance and poor prognosis (15). Aside from solid tumor types, our previous study demonstrated a significant correlation between elevated expression levels of EPS8 and poor overall survival rates in patients with acute myeloid leukemia (AML) (16). Considering the biological significance of EPS8 in cancer, using the full-length EPS8 protein as the cancer vaccine in a murine model of breast carcinoma, He et al (17) showed that the EPS8 vaccine induced a marked CTL response in vitro and effectively prevented tumor growth in vivo (17). These findings suggest that EPS8 is an ideal target antigen for the development of antitumor vaccines.
CTL epitopes are peptides presented by the human leukocyte antigen (HLA) class I molecules and recognized by CTLs. In the present study, the HLA-A*1101 allele was selected for investigation as it is the most common HLA-I allele within the Chinese population (18,19). The present study identified five potential EPS8 peptides, which bind with high affinity to HLA-A*1101. Among these, P380 showed the highest immunogenicity in CTLs, indicating it as a promising immunotherapeutic target for the treatment of HMs.
Materials and methods
Cell lines
The K562 human erythroleukemia cell line, the THP-1 human acute monocytic leukemia cell line and the SW480 colon cancer cell line were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and were routinely preserved in the Department of Hematology laboratory Zhujiang Hospital, Southern Medical University (Guangzhou, China). The HLA-A*1101+ K562 cell line (20) was obtained from the Hematology Institute of Jinan University (Guangzhou, China). The HLA-A*1101+ K562 cell line in which the expression of EPS8 was absent was obtained from the transient transfection of HLA-A*1101+ K562 cells with small interfering RNA (siRNA) specifically targeting EPS8. All cell lines were cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal calf serum, 100 IU/ml penicillin and 100 µg/ml streptomycin (Biological Industries, Beit Haemek, Israel) in a humidified 37°C incubator with 5% CO2.
siRNA transfection
siRNA targeting EPS8 and control siRNA (NC siRNA) (21), which targeted no known human genes, were synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The sequences were as follows: si-h-EPS8101, 5′-GGUGGAUGUUAGAAGUCGA dTdT-3′, si-h-EPS8102, 5′-GGACACAAUUGAUUUCUUA dTdT-3′ and si-h-EPS8103, 5′-GAUCCACCUUAUACUCAUA dTdT-3′.
To knock down the expression of EPS8, the HLA-A*1101+ K562 cells were seeded into 24-well plates at a density of 1×105 cells/well and allowed to grow to ~80% confluence. Subsequently, 50 nM EPS8 siRNA or NC siRNA was transiently transfected into the cells using Lipofectamine® RNAiMAX transfection reagent at room temperature (Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. At 24, 48 and 72 h post-transfection, the cells were harvested and lysed in RIPA buffer containing the proteinase inhibitor, phenylmethanesulfonylfluoride, to extract total proteins.
Western blot analysis
The protein expression levels of EPS8 in the cell lines were detected using western blot analysis. Briefly, the protein concentrations were determined from the cell lysates using a BCA protein assay (Keygen Biotech., Nanjing, China). A 30 µg quantity of total protein from each sample was electrophoresed on a 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane. Following blocking in 5% skim milk for 1 h at room temperature, the membrane was incubated overnight at 4°C in 5% skim milk containing anti-EPS8 primary antibody (cat. no. ab12488; 1:1,000; Abcam, Cambridge, UK) or anti-GAPDH antibody (cat. no. KC-5G5; 1:1,000; Kangcheng Bio-Tech, Shanghai, China) as a loading control. Following three washes with 0.5% TBS-Tween 20, the membrane was incubated in 5% skim milk containing horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. 4030–05; 1:2,000; Southern Biotechnology Associates Inc., Birmingham, AL, USA). The signal was detected using chemiluminescent HRP substrate and blotted onto chemiluminescence-sensitive film (Merck Millipore, Billerica, MA, USA).
HLA phenotyping
The HLA-A phenotypes of the cell lines were determined using flow cytometry with phycoerythrin-conjugated anti-human HLA-A, B and C antibodies, as described previously (20,22).
Epitope prediction and peptide synthesis
The EPS8-derived peptides containing HLA-A*1101-binding motifs were predicted using two computer algorithms: Bioinformatics and Molecular Analysis Section (BIMAS; www.bimas.cit.nih.gov/molbio/hla_bind/) (23) and SYFPEITHI (www.syfpeithi.de/) (24). Peptides among the top 20 peptides from the BIMAS prediction, and with a cut-off score of ≥20 from the SYFPEITHI prediction met the two criteria for selection. The predicted candidate peptides were synthesized using fluorenylmethyloxycarbonyl chemistry (Zhongtai Biological Technology, Hangzhou, China), with >95% purity, as determined using reverse-phase high performance liquid chromatography, and expected molecular weight, as confirmed using mass spectrometry. Reverse-phase high-performance liquid chromatography involves the separation of molecules on the basis of hydrophobicity. The separation depends on the hydrophobic binding of the solute molecule from the mobile phase to the immobilized hydrophobic ligands attached to the stationary phase, i.e. the sorbent. Mass spectrometry is an analytical technique that ionizes chemical species and sorts the ions based on their mass to charge ratio. In simpler terms, a mass spectrum measures the masses within a sample. The lyophilized peptides were dissolved in DMSO at a concentration of 2 mg/ml and stored at −20°C until further use.
Isolation of peripheral blood mononuclear cells (PBMCs) and induction of peptide-specific CTLs
The present study was approved by the Institutional Ethics Committee of Southern Medical University (Guangzhou, China) and informed consent was obtained from all healthy donors. A total of 10 healthy donors (3 female and 7 male, average age 20.5 years) were recruited between April and August 2015 in Zhujiang Hospital, Southern Medical University. PBMCs were purified using standard Ficoll-Hypaque density gradient centrifugation at 800 × g for 30 min at room temperature (Dakewe Biotech Co., Ltd., Shenzhen, China). HLA-A*1101 phenotypic analyses of the donors were performed using a PCR-SBT tying kit (BGI Tech, Shenzhen, China). To generate peptide-specific CTLs, the HLA-A*1101+ PBMCs were incubated with 10 µmol/l candidate peptides (P380, P529, P70, P82, P30) in RPMI-1640 medium at 37°C in a humidified incubator containing 5% CO2. As the negative control, no peptide (P0) was added to the PBMCs. Recombinant human interleukin 2 (rhIL-2) at a concentration of 50 U/ml was added into the medium on the second day. The same quantity of candidate peptides and rhIL-2 were added to the PBMCs every 7 days. On day 3 following the third stimulation, PBMCs were collected as effector cells.
Enzyme-linked immunospot (ELISPOT) assay
The production of interferon-γ (IFN-γ) from the peptide-specific effector cells was analyzed using an ELISPOT assay. Briefly, the effector cells, prepared as described above, were seeded into 96-well assay plates (Dakewe Biotech Co., Ltd.) at a density of 1×105/100 µl/well in serum-containing RPMI-1640 medium. For restimulation, 1×104 HLA-A*1101+ K562 cells, as antigen-presenting cells, were irradiated (5 Gy) to prevent further proliferation, and were added together with different peptides (P380, P70, P82, P30 and P529) or medium alone (negative control). Following overnight culture at 37°C and extensive washing using a washing buffer 5–7 times for 30–60 sec each time, to remove free peptides, the production of IFN-γ was detected using biotinylated anti-IFN-γ antibody (cat nos. DKW22-1000-048/DKW22-1000-096; 1:100; Dakewe Biotech Co., Ltd.), followed by incubation at 37°C for 1 h with streptavidin-HRP solution and 3-amino-9-ethyl carbazole substrate. The numbers of IFN-γ-positive spot-forming cells (SFCs) were counted by Dakota Biotechnology Co., Ltd. (Shenzhen, China). As the positive control, the lymphocyte mitogen, phytohemagglutinin (PHA) stimulation was used. As a background control, X–VIVO15 serum-free medium (Lonza, Basel, Switzerland) was used. Each condition was assessed in triplicate.
Cytotoxicity assay
The cytolytic activity of the effector cells was assessed using flow cytometry. In brief, the target cells were labeled with carboxyfluorescein succinimidyl ester (CFSE; BioLegend, Inc., San Diego, CA, USA) according to the manufacturer's protocol. The effector cells (1×106/ml) were mixed with the target cells at different ‘effector to target’ (E/T) ratios (40:1, 20:1, 10:1) and incubated for 4 h in a 37°C incubator with 5% CO2. The cell mixture was then washed twice with PBS and stained with propidium iodide (PI; 2.5 µg/ml, BioLegend, Inc.) at room temperature in the dark for 15 min. Each condition was assessed in triplicate. Flow cytometric analysis was performed on a Beckman Coulter ACL Elite (Beckman Coulter, Brea, CA, USA), with the results reported as a percentage of CFSE and PI double positive cells.
Statistical analysis
All data are presented as the mean ± standard deviation from at least three independent experiments and were analyzed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). The levels of differences were analyzed using one-way analysis of variance for one independent variable or factorial analysis of variance for more than one independent variable, with LSD post-hoc analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Phenotypic characterization of target cell lines
To characterize the expression of EPS8 and HLA-A phenotypes of the target cell lines used in the present study, western blot analysis was performed to examine the levels of EPS8, and flow cytometry was performed to detect the surface expression of HLA-A*1101. As shown in Fig. 1A and B, the expression of EPS8 was highest in the SW480 human colon cell line, a cancer cell line known to express EPS8 (13), followed by the K562 human erythroleukemia cell line and the HLA-A*1101+K562 cell line. No EPS8 was detected in the THzP-1 human acute monocytic leukemia cell line. Using flow cytometry, it was found that the HLA-A*1101+EPS8+ K562 and HLA-A*1101+EPS8− K562 cells were positive for the expression of HLA-A*1101, whereas the K562 and THP-1 cells were negative (Fig. 2).
Figure 1.

Phenotypic characterization of the expression of EPS8 and human leukocyte antigen-A*1101 in different target cells. The expression levels of EPS8 in the indicated target cells were detected using western blot analysis. GAPDH was detected as the loading control. (A) Representative western blot. (B) Quantification of relative expression levels of EPS8. EPS8, epidermal growth factor receptor pathway substrate 8.
Figure 2.
Detection of the cell-surface expression of HLA-A*1101 in indicated target cells using flow cytometry. The blue histogram represents staining with PE-conjugated isotype-matched IgG; the red histogram represents staining with PE-conjugated HLA-ABC. (A) Representative flow cytometry data showed that K562 and THP-1 were negative for the expression of HLA-A*1101. (B) Representative flow cytometry data showed that HLA-A*1101+EPS8+ K562 and HLA-A*1101+EPS8− K562 were positive for the expression of HLA-A*1101. EPS8, epidermal growth factor receptor pathway substrate 8; HLA, human leukocyte antigen; PE, phycoerythrin.
Generation of HLA-A*1101+EPS8− K562 cells
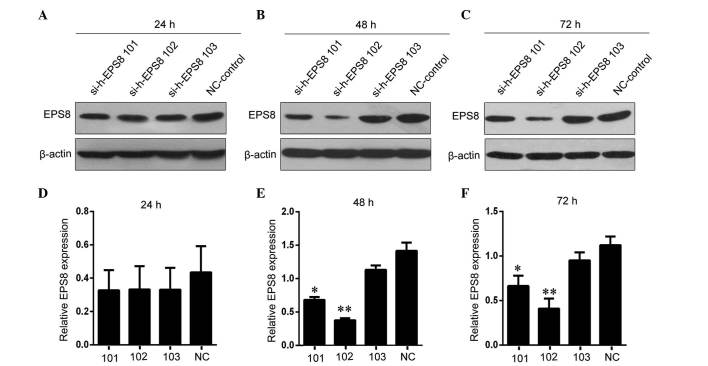
To generate HLA-A*1101+EPS8− K562 target cells, EPS8-specific siRNA was transiently transfected into HLA-A*1101+EPS8+ K562 cells. As shown in Fig. 3, of the three EPS8 siRNA sequences assessed, the si-h-EPS8 102 sequence had the most marked effect on reducing the level of EPS8 at 48 h post-transfection, compared with the levels in the cells transfected with control siRNA. Therefore, this transfection condition was used for the following experiments in the present study.
Figure 3.
Knocking down the expression of EPS8 in HLA-A*110+ K562 cells by siRNA transient transfection. The HLA-A*1101+ K562 cells were transiently transfected with either control siRNA or si-h-EPS8 101–103) At (A) 24, (B) 48 and (C) 72 h post-transfection, the expression levels of EPS8 in the cells were examined using western blot analysis and quantified, as shown in graphs for the (D) 24, (E) 48 and (F) 72 h groups. β-actin was detected as a loading control. *P<0.05 and **P<0.01, compared with the NC group. The transfection effect at 48 h was more marked. EPS8, epidermal growth factor receptor pathway substrate 8; HLA, human leukocyte antigen; siRNA, small interfering RNA; si-h-EPS8 101–103; EPS8-targeting siRNA 101–103; NC, negative control.
Prediction of HLA-A*1101-restricted CTL epitopes of EPS8
To identify peptides on EPS8 with the highest binding affinities to HLA-A*1101, the present study used two algorithms, BIMAS and SYFPEITHI, to scan the complete amino acid sequence of EPS8. A total of five nine-amino-acid peptides meeting the selection criteria from the two algorithms were identified (Table I). Based on the position of the starting amino acid, in order from highest to lowest ranking as predicted by the two algorithms, these five peptides were P380, P529, P82, P30 and P70, respectively.
Table I.
Characteristics of predicted EPS8 CTL epitopes restricted to the HLA-A*1101 allele.
| Position | Length | Sequence | BIMAS rank | SYFEITHI score |
|---|---|---|---|---|
| 380 | 9 | SVLSPLLNK | 1 | 29 |
| 529 | 9 | KTQPKKYAK | 2 | 21 |
| 82 | 9 | ITVDDGIRK | 3 | 26 |
| 30 | 9 | QTDREHGSK | 4 | 20 |
| 70 | 9 | LTTFVLDRK | 5 | 20 |
Length represents the number of amino acids. BIMAS, Bioinformatics and Molecular Analysis Section.
Secretion of IFN-γ from in vitro peptide-primed CTLs
In response to antigen presentation, CTLs secrete cytokines, including IFN-γ, which was used as a functional measurement of the peptide-stimulated CTLs to determine whether EPS8-derived peptides are potent enough to elicit a functional CTL response. PBMCs were isolated from HLA-A*1101+ healthy donors, primed with each of the EPS8-derived peptides, and re-exposed to EPS8 presented by HLA-A*1101+EPS8+ K562 cells, following which the production of INF-γ was examined using an ELISPOT assay, in which the SFCs represented CTLs secreting IFN-γ. As a positive control, the lymphocyte mitogen, PHA, was used, which is known to stimulate the production of IFN-γ from PBMCs (25). As the negative controls, CTL with no priming (P0-CTL) but re-exposed o EPS8 presented by HLA-A*1101+EPS8+ K562 cells (P0-CTL+HLA-A*1101+EPS8+ K562), peptide-primed CTLs without re-exposure to EPS8 presented by HLA-A*1101+EPS8+ K562 cells (P-CTL) or peptide-free non-primed CTLs. For all five peptides examined, PHA led to the highest number of SFCs, suggesting that these peptide-primed lymphocytes were capable of non-specifically responding to lymphocyte mitogens. The second highest number of SFCs was observed in the peptide-primed CTLs with antigen presented by HLA-A*1101+EPS8+ K562 cells, which was significantly higher, compared with the number of SFCs from the P0-CTL+HLA-A*1101+EPS8+ K562 group (P<0.05), indicating that these peptides specifically augmented the CTL responses following antigen presentation. As expected, the P-CTLs and the P0-CTLs exposed to X–VIVO medium presented with minimal, if any, IFN-γ-producing activity (Fig. 4A). When comparing between the five peptides, variability was observed in their capacity to induce the secretion of IFN-γ from CTLs following antigen presentation. P380 presented with significantly higher activity, compared to the other four peptides (P<0.05; Fig. 4B), consistent with its ranking predicted by the BIMAS and SYFPEITHI algorithms.
Figure 4.
Production of IFN-γ by PBMCs primed with different peptides. Peptide-primed PBMCs were exposed to irradiated EPS8-presenting HLA-A*1101+ K562 cells and the production of IFN-γ was detected using an ELISPOT assay. PHA treatment was used as a positive control; P0-CTLs or P-CTLs were used as negative controls. (A) Representative ELISPOT images from each condition is shown. (B) Quantification on the numbers of spot-forming cells is shown. *P<0.05 and **P<0.001, compared with the P380 group. IFN-γ interferon-γ; PBMCs, peripheral blood mononuclear cells; EPS8, epidermal growth factor receptor pathway substrate 8; HLA, human leukocyte antigen; P0, PBMCs with no peptide priming; P-CTL, PBMCs not exposed to EPS8-presenting HLA-A*1101+ K562 cells; ELISPOT, enzyme-linked immunospot; X–VIVO, X–VIVO15 serum-free medium; CTL, control; P, P380.
Cytotoxicity of peptide-primed CTLs
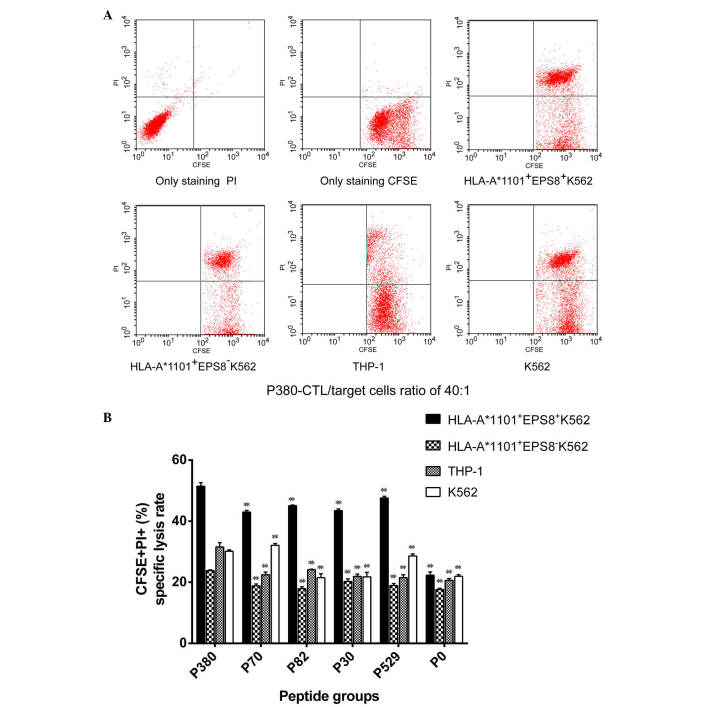
To examine the functional capability of peptide-primed CTLs (effector cells) to cause antigen-bearing tumor cell (target cell) death, the effector and target cells were mixed at different E/T ratios, and the death of the CFSE-labeled target cells was quantified using PI staining. Cytotoxicity was measured as the percentage of CFSE+PI+ cells. For P380, the percentages of cytotoxicity for the E/T ratios of 10:1, 20:1 and 40:1 were 21.57±11.37, 28.68±11.57 and 34.27±10.85%, respectively (P<0.05 between every two groups), with the highest cytotoxicity observed at the E/T ratio of 40:1. When examining the cytotoxicity of the five peptides using this ratio, it was found that all five peptides significantly augmented the cytotoxic activity of the CTLs against HLA-A*1101+EPS8+ K562 cells, but not against the HLA-A*1101+EPS8− K562, THP-1 or K562 cells, compared with the P0-CTLs (Fig. 5A). These results suggested that CTLs primed with these peptides show a high levels of specificity for MHC restriction and tumor antigen presentation. When comparing between the five peptides, P380, P529 and P82 induced similar magnitudes of toxicity in the CTLs (P>0.05), which were significantly higher, compared with those conferred by P70 and P30 (P<0.05; Fig. 5B).
Figure 5.
Cytotoxicity of PBMCs primed with different peptides. Peptide-primed PBMCs (effector cells) were mixed with the indicated CFSE-labeled target cells at an effector to target ratio of 40:1. The cell mixture was stained with PI, and CFSE+PI+ dead target cells were detected using flow cytometry. (A) Representative flow images of cytolysis by the P380-primed PBMCs. (B) Quantification of the percentage of CFSE+PI+ dead cells in the peptide-primed PBMCs on the indicated target cells. **P<0.001, compared with the P380 group. PBMCs, peripheral blood mononuclear cells; EPS8, epidermal growth factor receptor pathway substrate 8; HLA, human leukocyte antigen; CTL, cytotoxic T cell; CFSE, carboxyfluorescein succinimidyl ester; PI, propidium iodide.
Discussion
In the present study, systematic screening for novel immunogenic HLA-A*1101-restricted CTL epitopes derived from EPS8 was performed, and their potential as candidate cancer vaccines targeting HMs were characterized. A total of five nine-amino-acid peptides were identified using two computer programs, BIMAS and SYFPEITH, and these were P380, P529, P82, P30 and P70. Based on the predicted scores, those with a higher score, indicating a higher binding capacity to the HLA*A1101 molecule, were considered ideal antigen peptides. However, as not all peptides binding to HLA molecules are optimal T cell epitopes (26), it is essential to confirm the activities of predicted peptides through in vitro and in vivo experiments.
EPS8 complexes with multiple signaling molecules include Ras/Rac, Src, Src homology 2 domain-containing, son of sevenless homolog-1, E3b1 and focal adhesion kinase to regulate EGF downstream phenotypes (27–32). Biologically, EPS8 modulates various cellular behaviors, including cell proliferation, cell motility/invasiveness, remodeling actin cytoskeleton and membrane protrusion and vesicular trafficking (27). The functional significance of EPS8, together with its aberrant expression and correlation with prognosis in several types of cancer, including colon cancer (13), thyroid cancer (12) and breast cancer (33), suggest that it has potential as a target for cancer therapy (34). Our previous finding on the correlation between EPS8 and AML (16) prompted the investigation of the feasibility of EPS8 as an immunotherapeutic target for HMs. In support of this hypothesis, our previous study identified eight native peptides and generated four modified peptides from EPS8, which show high-affinity binding to HLA-A2.1 molecules. The CTLs primed by these peptides presented with augmented immune activities, as demonstrated by the secretion of IFN-γ and capacity to induce tumor cell death in a variety tissue types, in an HLA-A2.1-restricted and Eps8-specific manner (35).
To the best of our knowledge, no HLA-A*1101-restricted epitopes derived from EPS8 have been identified previously. Therefore, the present study followed the standard procedure already established (36) and started by predicting peptide sequences, which bind to the HLA-A*1101 molecule with high affinity. By combining two different predicting algorithms, five sequences were obtained. Following in vitro synthesis of these peptides to high purity, their immunogenic activity was examined by stimulating PBMCs from healthy donors positive for the HLA-A*1101 allele.
CTLs are a subtype of lymphocytes, which cause death of target cells presenting with specific antigens in a major histocompatibility complex (MHC) I-restricted manner (37). By labeling target tumor cells with CFSE and staining them with PI, it is possible to quantify the death of target cells induced by the peptide-primed CTLs. It was found that, among the five peptides identified, P380, P529 and P80 primed CTLs with similar cytolytic activity against tumor cells positive for HLA-A*1101 and EPS8, but not the tumor cells negative for either or both of these two molecules, suggesting strict specificity on MHC I molecule and tumor antigen. These three peptides also showed higher binding affinity to the HLA-A*1101 molecule, as predicted by the two computer-based algorithms, corroborating the effectiveness of these algorithms in predicting MHC-binding motifs.
Another phenotypic assay commonly used for CTLs is the production and secretion of antitumor cytokines, including IFN-γ, in response to antigen presentation (38). In the present study, when the production of INF-γ by CTLs was analyzed using an ELISPOT assay, it was shown that all five peptides significantly activated the CTLs to produce IFN-γ in response to antigen presentation, and P380 showed the highest immunogenic activity among the five peptides.
As the present study was limited by the blood samples, it was possible to obtain from the donors, it was not possible sort the CD8+ CTLs from the PBMCs or use professional antigen presenting cells, including dendritic cells, for antigen presentation. These limitations may weaken the phenotypes observed, including the number of SFCs observed in the IFN-γ ELISPOT assay or the percentage of CFSE+PI+ cells detected using flow cytometry. However, the data obtained in the present study demonstrated, with statistical significance, that the peptides identified were TAA candidates. In addition, the present study did not identify a cell line positive for HLA-A*1101, but completely negative for EPS8, making it challenging to characterize the binding stability of identified peptides. However, the functional assays of the production of IFN-γ and cytolysis indirectly confirmed that these peptides were able to effectively prime CTLs in the PBMCs. In the present study, all functional characterizations on peptide-primed CTLs were performed in vitro. The immunogenic characteristics of these peptides may vary between in vitro and in vivo conditions, therefore, it is important to further assess their immunogenic activities using a suitable murine model and with blood samples from patients with HMs. Although the present study focused on HMs and used the K562 human erythroleukemia cell line, the peptides identified may also target solid tumors, which requires examination in future investigations.
In conclusion, the present study identified five nine-amino-acid HLA-A*1101-restricted, EPS8-derived CTL epitopes, which started from amino acid positions 380, 529, 82, 30 and 70 on the EPS protein. All five peptides presented with good binding affinity to the HLA-A*1101 molecule. The CTLs primed with these peptides produced increased IFN-γ in response to EPS8 presentation, and specifically recognized and lysed target tumor cells expressing ESP8 and HLA-A*1101. Therefore, these five peptides may become promising TAAs for the development of immunotherapy targeting EPS-expressing HMs and/or solid tumors.
Acknowledgements
The present study was supported by the Natural Science Foundation of China (grant no. 81372249), Science and Technology Planning Project of Guangdong Province, China (grant no. 2013B091500072), Project of Department of Education of Guangdong Province, China (grant no. 2014GKXM029).
Glossary
Abbreviations
- AML
acute myeloid leukemia
- CFSE
carboxyfluorescein succinimidyl ester
- P380-CTL
CTLs induced by P380
- CTLs
cytotoxic T lymphocytes
- E/T
effector to target
- ELISPOT
enzyme-linked immunosorbent spot
- EGF
epidermal growth factor
- EPS8
epidermal growth factor receptor pathway substrate 8
- HM
hematological malignancy
- HLA
human leukocyte antigen
- IFN-γ
interferon-γ
- MW
molecular weight
- PBMCs
peripheral blood mononuclear cells
- PI
propidium iodide
- rhIL-2
recombinant human interleukin 2
- siRNA
small interfering RNA
- SFC
spot forming cells
- SD
standard deviation
- TAAs
tumor-associated antigens
- LAAs
leukemia-associated antigens
References
- 1.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, Durrant IJ, Luger SM, Marks DI, Franklin IM, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 3.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, Stadler M, Kuball J, Cornelissen J, Vorlicek J, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 4.Bocchia M, Defina M, Aprile L, Sicuranza A. Peptide vaccines for hematological malignancies: A missed promise? Int J Hematol. 2014;99:107–116. doi: 10.1007/s12185-013-1497-3. [DOI] [PubMed] [Google Scholar]
- 5.Mohamed YS, Dunnion D, Teobald I, Walewska R, Browning MJ. In vitro evaluation of human hybrid cell lines generated by fusion of B-lymphoblastoid cells and ex vivo tumour cells as candidate vaccines for haematological malignancies. Vaccine. 2012;30:6578–6587. doi: 10.1016/j.vaccine.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Shah GL, Shune L, Purtill D, Devlin S, Lauer E, Lubin M, Bhatt V, McElrath C, Kernan NA, Scaradavou A, et al. Vaccine responses in adult and pediatric cord blood transplantation recipients treated for hematologic malignancies. Biol Blood Marrow Transplant. 2015;21:2160–2166. doi: 10.1016/j.bbmt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H. Therapeutic vaccines for cancer: An overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 8.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazioli F, Minichiello L, Matoska V, Castagnino P, Miki T, Wong WT, Di Fiore PP. Eps8, a substrate for the epidermal growth factor receptor kinase, enhances EGF-dependent mitogenic signals. Embo J. 1993;12:3799–3808. doi: 10.1002/j.1460-2075.1993.tb06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matoskova B, Wong WT, Salcini AE, Pelicci PG, Di Fiore PP. Constitutive phosphorylation of eps8 in tumor cell lines: Relevance to malignant transformation. Mol Cell Biol. 1995;15:3805–3812. doi: 10.1128/MCB.15.7.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YJ, Shen MR, Chen YJ, Maa MC, Leu TH. Eps8 decreases chemosensitivity and affects survival of cervical cancer patients. Mol Cancer Ther. 2008;7:1376–1385. doi: 10.1158/1535-7163.MCT-07-2388. [DOI] [PubMed] [Google Scholar]
- 12.Griffith OL, Melck A, Jones SJ, Wiseman SM. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol. 2006;24:5043–5051. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]
- 13.Maa MC, Lee JC, Chen YJ, Chen YJ, Lee YC, Wang ST, Huang CC, Chow NH, Leu TH. Eps8 facilitates cellular growth and motility of colon cancer cells by increasing the expression and activity of focal adhesion kinase. J Biol Chem. 2007;282:19399–19409. doi: 10.1074/jbc.M610280200. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Patel V, Miyazaki H, Gutkind JS, Yeudall WA. Role for EPS8 in squamous carcinogenesis. Carcinogenesis. 2009;30:165–174. doi: 10.1093/carcin/bgn252. [DOI] [PubMed] [Google Scholar]
- 15.Gorsic LK, Stark AL, Wheeler HE, Wong SS, Im HK, Dolan ME. EPS8 inhibition increases cisplatin sensitivity in lung cancer cells. PLoS One. 2013;8:e82220. doi: 10.1371/journal.pone.0082220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Cai SH, Xiong WY, He YJ, Deng L, Li YH. Real-time quantitative polymerase chain reaction assay for detecting the eps8 gene in acute myeloid leukemia. Clin Lab. 2013;59:1261–1269. doi: 10.7754/clin.lab.2013.121107. [DOI] [PubMed] [Google Scholar]
- 17.He YJ, Zhou J, Zhao TF, Hu LS, Gan JY, Deng L, Li Y. Eps8 vaccine exerts prophylactic antitumor effects in a murine model: A novel vaccine for breast carcinoma. Mol Med Rep. 2013;8:662–668. doi: 10.3892/mmr.2013.1514. [DOI] [PubMed] [Google Scholar]
- 18.Lee TD, Zhao TM, Mickey R, Sun YP, Lee G, Song CX, Cheng DZ, Zhou S, Ding SQ, Cheng DX, et al. The polymorphism of HLA antigens in the Chinese. Tissue Antigens. 1988;32:188–208. doi: 10.1111/j.1399-0039.1988.tb01656.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin M, Chu CC, Chang SL, Lee HL, Loo JH, Akaza T, Juji T, Ohashi J, Tokunaga K. The origin of Minnan and Hakka, the so-called ‘Taiwanese’, inferred by HLA study. Tissue Antigens. 2001;57:192–199. doi: 10.1034/j.1399-0039.2001.057003192.x. [DOI] [PubMed] [Google Scholar]
- 20.Zha XF, Zhou YB, Yang LJ, Chen SH, Li B, Yan XJ, Li YQ. Establishment of stable subline of K562 cells expressing human leucocyte antigen a1101. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:1112–1116. (In Chinese) [PubMed] [Google Scholar]
- 21.Cattaneo MG, Cappellini E, Vicentini LM. Silencing of Eps8 blocks migration and invasion in human glioblastoma cell lines. Exp Cell Res. 2012;318:1901–1912. doi: 10.1016/j.yexcr.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Fu J, Li L, Bouvier M. Adenovirus E3-19K proteins of different serotypes and subgroups have similar, yet distinct, immunomodulatory functions toward major histocompatibility class I molecules. J Biol Chem. 2011;286:17631–17639. doi: 10.1074/jbc.M110.212050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 24.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 25.Deenadayalan A, Maddineni P, Raja A. Comparison of whole blood and PBMC assays for T-cell functional analysis. BMC Res Notes. 2013;6:120. doi: 10.1186/1756-0500-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doytchinova IA, Flower DR. Predicting class I major histocompatibility complex (MHC) binders using multivariate statistics: Comparison of discriminant analysis and multiple linear regression. J Chem Inf Model. 2007;47:234–238. doi: 10.1021/ci600318z. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham DL, Creese AJ, Auciello G, Sweet SM, Tatar T, Rappoport JZ, Grant MM, Heath JK. Novel binding partners and differentially regulated phosphorylation sites clarify Eps8 as a multi-functional adaptor. PLoS One. 2013;8:e61513. doi: 10.1371/journal.pone.0061513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegård M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 29.Scita G, Tenca P, Areces LB, Tocchetti A, Frittoli E, Giardina G, Ponzanelli I, Sini P, Innocenti M, Di Fiore PP. An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J Cell Biol. 2001;154:1031–1044. doi: 10.1083/jcb.200103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenherr C, Serrels B, Proby C, Cunningham DL, Findlay JE, Baillie GS, Heath JK, Frame MC. Eps8 controls Src- and FAK-dependent phenotypes in squamous carcinoma cells. J Cell Sci. 2014;127:5303–5316. doi: 10.1242/jcs.157560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funato Y, Terabayashi T, Suenaga N, Seiki M, Takenawa T, Miki H. IRSp53/Eps8 complex is important for positive regulation of Rac and cancer cell motility/invasiveness. Cancer Res. 2004;64:5237–5244. doi: 10.1158/0008-5472.CAN-04-0327. [DOI] [PubMed] [Google Scholar]
- 32.Innocenti M, Frittoli E, Ponzanelli I, Falck JR, Brachmann SM, Di Fiore PP, Scita G. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1 and Sos-1. J Cell Biol. 2003;160:17–23. doi: 10.1083/jcb.200206079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao J, Weremowicz S, Feng B, Gentleman RC, Marks JR, Gelman R, Brennan C, Polyak K. Combined cDNA array comparative genomic hybridization and serial analysis of gene expression analysis of breast tumor progression. Cancer Res. 2006;66:4065–4078. doi: 10.1158/0008-5472.CAN-05-4083. [DOI] [PubMed] [Google Scholar]
- 34.Yang TP, Chiou HL, Maa MC, Wang CJ. Mithramycin inhibits human epithelial carcinoma cell proliferation and migration involving downregulation of Eps8 expression. Chem Biol Interact. 2010;183:181–186. doi: 10.1016/j.cbi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhou W, Du J, Jiang C, Xie X, Xue T, He Y. Generation of cytotoxic T lymphocytes specific for native or modified peptides derived from the epidermal growth factor receptor pathway substrate 8 antigen. Cancer Immunol Immunother. 2015;64:259–269. doi: 10.1007/s00262-014-1631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuyama R, Aruga A, Hatori T, Takeda K, Yamamoto M. Immunological respons es to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients. Oncoimmunology. 2013;2:e27010. doi: 10.4161/onci.27010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 38.Li R, Qian J, Zhang W, Fu W, Du J, Jiang H, Zhang H, Zhang C, Xi H, Yi Q, Hou J. Human heat shock protein-specific cytotoxic T lymphocytes display potent antitumour immunity in multiple myeloma. Br J Haematol. 2014;166:690–701. doi: 10.1111/bjh.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]