Abstract
Vascular endothelial growth factor (VEGF) inhibition has been demonstrated to be an effective strategy in preserving the integrity of the blood-brain barrier (BBB) in patients with acute ischemic stroke. Loss of the BBB is the key event associated with morbidity and mortality in these patients. However, the underlying mechanisms remain poorly understood. In the present study, the effects of VEGF inhibition and the possible mechanism that underlies acute cerebral ischemia in rats was investigated. Following the induction of transient middle cerebral artery occlusion for a 90-min period, either an anti-VEGF neutralizing antibody (RB-222; 5 or 10 µg), or IgG (control), was administered by intracerebroventricular injection at 1 h following reperfusion. Functional outcomes, BBB leakage, brain edema, microvessel numbers and the relative protein levels of VEGF, matrix metalloproteinase (MMP)-2, MMP-9, occludin and collagen-IV were then determined using neurological assessments, Evans Blue staining, brain water content, CD31 staining and western blotting. Treatment with RB-222 at a dose of 5 and 10 µg significantly improved neurological functional outcomes and diminished infarct size, BBB leakage and brain edema compared with the MCAO and IgG groups at 24 h following reperfusion; 10 µg RB-222 was more effective than a 5 µg dose of the antibody. In addition, RB-222 reduced the number of immature microvessels, which subsequently attenuated BBB permeability. RB-222 significantly repressed VEGF expression as well as decreased MMP-2 and MMP-9 expression. However, it enhanced occludin and collagen-IV levels in the ischemic rat brain compared with the MCAO and IgG groups. Taken together, the results indicate that early inhibition of VEGF may have significant potential against cerebral ischemia, partly by regulating the expression of MMPs.
Keywords: cerebral ischemia, vascular endothelial growth factor inhibition, matrix metalloproteinase, blood-brain barrier, brain edema
Introduction
Disruption of the blood-brain barrier (BBB) and the resulting edema are crucial initiating factors in cerebral ischemia/reperfusion injury, as they increase intracranial pressure, impair cerebral perfusion and oxygenation, and contribute to additional ischemic injuries (1–3). A potential therapeutic target in this process is vascular endothelial growth factor (VEGF). As a pleiotropic growth factor, VEGF is associated with angiogenesis, neurogenesis, axonal plasticity, neuronal survival and vascular permeability (4). It has been demonstrated that VEGF expression is upregulated in the peri-infarct regions within 3 h following the onset of ischemia (5). In addition, the expression of VEGF is closely associated with BBB leakage in the acute ischemic brain (6). It has been reported that early administration of VEGF to ischemic rat brains may enhance BBB leakage, induce hemorrhagic transformation and increase infarct size in the brain (6). Therefore, applying an inhibitor of VEGF in the early acute phase of a stroke may reduce the breakdown of the BBB, as well as the subsequent edema.
Matrix metalloproteinases (MMPs) are zinc-containing endopeptidases that digest the components of the extracellular matrix that form the basal lamina surrounding the neurovascular unit (7). Numerous proinflammatory agents that are trigged by ischemia can activate MMP expression immediately after a stroke (8). In acute ischemia, MMP-2 and MMP-9 in particular, mediated degradation of tight junction proteins (TJPs) in the BBB (9). It has been demonstrated that MMP inhibition is an important therapeutic strategy to reduce brain damage during acute stroke (8). Several studies have suggested a strong relationship between the induction of MMP-2 and MMP-9 and brain edema following cerebral ischemic injury (10–12), indicating that MMP-2 and MMP-9 may be important targets to prevent edema complications following cerebral ischemia.
There is growing evidence to demonstrate that early VEGF inhibition has a protective effect against cerebral ischemia (13–16). However, it remains unclear whether VEGF inhibition protects the BBB by regulating the expression of MMPs following acute cerebral ischemia. Therefore, the aim of the present study was to investigate the possible mechanisms underlying early VEGF inhibition in the protection of the BBB following ischemic brain injury, by evaluating ischemic lesions, brain edema, neurological outcomes and MMP levels in a rat stroke model. The results from the current study indicated that the possible mechanisms underlying VEGF inhibition-mediated protection of the BBB involved the MMP pathway.
Materials and methods
Animals
A total of 156 adult male Sprague-Dawley rats (age, 9 weeks; weight, 250–300 g) were purchased from the Animal Experimental Center of Harbin Medical University (Harbin, China). All of the animals were housed in individual cages under a 12-h light/dark cycle at 22–24°C. The rats had free access to food and water and were fasted for 12 h prior to surgery. The Harbin Medical University Animal Supervision Committee approved all experimental procedures. All efforts were made to minimize animal suffering and the number of animals used.
Experimental transient middle cerebral artery occlusion (MCAO) model
All 156 animals were anesthetized with an intraperitoneal injection of 10% chloral hydrate (350 mg/kg; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Body temperature was monitored continuously with a rectal probe and maintained at 37±0.5°C by use of a heat pad. MCAO was induced using the methods described previously (17). Following 90 min of MCAO, reperfusion was initiated by withdrawing the monofilament. Sham rats underwent anesthesia and surgery without the filament insertion. At 1 h following reperfusion, neurological function was evaluated according to Longa's neurological score criteria, with a range from 0 (normal) to 4 (severe symptoms) (17). Rats with neurological scores ≤2, breathing difficulties or serious bleeding (n=6) were excluded from the study.
Experimental groups and in vivo drug treatment
To investigate the role of VEGF in ischemia-reperfusion-induced brain injury, the effects of a neutralizing antibody against VEGF were investigated (RB-222; NeoMarkers, Inc., Portsmouth, NH, USA). The rats were randomly assigned into the following five groups (n=30 per group): Sham, MCAO, IgG (MCAO + IgG), 5 µg RB-222 (MCAO + 5 µg RB-222) and 10 µg RB-222 (MCAO + 10 µg RB-222). At 1 h following induction of reperfusion, 5 or 10 µg/ml RB-222 [1 mg/ml, dissolved in 0.01 M phosphate-buffered saline (PBS)] or an IgG control (anti-gp-120, NeoMarkers, Inc.) was administered intracerebroventricularly. RB-222 or an equal quantitity of IgG was injected into the right lateral ventricle over a period of 5 min using a Hamilton syringe. The location of the injection site was based on the following coordinates: 1.0 mm rostral to bregma, 3.0 mm lateral to middle and 5.0 mm ventral to the skull surface.
Neurological tests
The tests for neurological function (sensory and motor) and behavior (rotarod test and number of left turns) were performed at 24 h after reperfusion as previously described (18) to assess motor coordination and balance changes after brain ischemia. The sums of the neurological severity scores were graded on a scale from 0 (normal) to 42 (severe symptoms). The tests were performed in a blinded manner three times.
Measurement of infarct volume
Following neurological evaluation at 24 h following reperfusion, the rats (n=6 per group) were sacrificed with an overdose of 10% chloral hydrate (400 mg/kg), and the brains were immediately removed. The brain tissue was cut into six 2-mm coronal sections and immersed in 1% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich, Merck Millipore, Darmstadt, Germany) solution at 37°C in the dark for 15 min. The infarction areas of all sections were measured using an image analysis system (Image-Pro Plus 6.0; Media Cybernetics, Inc., Rockville, MD, USA). To compensate for the effect of brain edema, the actual infarct volume was calculated using the following equation: Contralateral hemisphere volume-(ipsilateral hemisphere volume-infarct volume).
Measurement of brain edema
Brain edema was evaluated by calculating the brain water content, which was defined as the difference in weight between wet and dry samples. At 24 h following reperfusion, the rats were sacrificed and their brains were removed, as mentioned above. Subsequently, the brains were separated into the ipsilateral ischemic hemisphere (ipsilateral) and the contralateral hemisphere (contralateral). The brains were weighed to obtain the wet weight (WW), and were weighed again after drying at 110°C for 24 h to determine the dry weight (DW). The percentage brain water content was calculated using the following formula: (WW-DW)/WWx100.
Measurement of BBB permeability
Disruption of the BBB was assessed at 24 h after reperfusion by spectrophotometric measurement of Evans Blue (EB) extravasation. EB dye (2%; 5 ml/kg; Sigma-Aldrich; Merck Millipore) was injected into the right femoral vein over a period of 2 min and allowed to circulate for 60 min. The animals were then anesthetized with an intraperitoneal injection of 10% chloral hydrate (350 mg/kg) and perfused with PBS through the left ventricle until a colorless perfusion fluid was observed from the right atrium. The extravasated EB in the brain was measured by spectrophotometry at an absorbance of 620 nm using a standard solution of EB dye (from 0 to 6.25 µg/ml). EB extravasation in the ischemic penumbra was also observed using a FluoView-1000 Confocal Microscope (Olympus Corporation, Tokyo, Japan).
Measurement of microvessel density
Rats were sacrificed as above and perfused for microvessel counting at 24 h post-reperfusion. The brains were isolated and sliced into 5-mm-thick coronal slices in the peri-bregma region. The brain slices were then fixed in a 4% paraformaldehyde solution overnight at 4°C before they were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek Japan, Co., Ltd., Tokyo, Japan). After the sections were blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck Millipore) at 37°C for 30 min, they were stained at 37°C for 30 min for the endothelial cell marker CD31 (1:50; catalog no. sc-376764; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and VEGF (1:25; catalog no. ab1316; Abcam, Cambridge, MA, USA). Corresponding Alexa 488/594-conjugated secondary antibodies (1:1,000; catalog nos. A-11029 and A-11032; Invitrogen; Thermo Fisher Scientific, Inc.) were used to identify the primary antibodies. Nuclei were counterstained with DAPI (1:1,000; Abcam). The CD31-positive microvessels were observed in six representative sections selected from each animal using a FluoView-1000 Confocal Microscope (Olympus Corporation). Only CD31-positive microvessels with a well-defined linear vessel shape or a clear lumen were counted as one vessel. Single endothelial cells were ignored. Microvessel number was calculated as the mean microvessel count obtained from the six images. Microvessel numbers were determined in a blind manner by two independent investigators.
Western blot analysis
Brain samples were extracted from the ipsilateral cerebral cortex with radioimmunoprecipitation buffer (Beyotime Institute of Biotechnology, Haimen, China) and centrifuged to remove the insoluble material (12,000 × g for 15 min at 4°C). Protein concentrations were determined using a BCA protein assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. A total of 30 µg protein were separated on 10% SDS-PAGE gels. Protein bands were then transferred to polyvinylidene difluoride membranes and incubated for 2 h at 37°C in Tris-buffered saline plus 0.1% Tween 20 (TBST) containing 5% skim milk. Membranes were incubated overnight at 4°C with primary antibodies against VEGF (1:1,000; catalog no. ab1316; Abcam), MMP-2 (1:500; catalog no. sc-13594; Santa Cruz Biotechnology, Inc.), MMP-9 (1:1,000; catalog no. ab76003; Abcam), occludin (1:500; catalog no. sc-271842; Santa Cruz Biotechnology, Inc.) and collagen-IV (1:500; catalog no. sc-11360; Santa Cruz Biotechnology, Inc.). The membranes were then incubated with the corresponding horseradish peroxidase-conjugated secondary antibodies (1:500; catalog nos. ZDR-5306 and ZDR-5307; ZSGB-Bio, Beijing, China) for 1 h at room temperature after washing the membranes three times with TBST. β-actin (1:500; catalog no. TA-09; ZSGB-Bio) expression was determined as a loading control. Labeled proteins were visualized by chemiluminescence using an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology). The intensity of the bands was measured using the ChemiDoc detection system and Quantity One software version 4.6.8 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation. Comparisons between 2 groups were analyzed using an unpaired Student's t-test, and comparisons among >2 groups were analyzed by one-way analysis of variance with a post-hoc Tukey test. Data analysis was performed using SPSS software, (version, 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Neurobehavioral recovery
The neurological scores were 1.2±0, 30.3±3.5, 29.8±2.2, 26.5±3.1 and 18.5±1.9 in the Sham, MCAO, IgG, RB-222 (5 µg) and RB-222 (10 µg) groups, respectively (Fig. 1A). RB-222 treatment at a dose of 10 µg significantly reduced the neurological severity scores at 24 h after reperfusion when compared with the MCAO (P<0.001) or IgG groups (P<0.001); whereas no significant difference between the MCAO group and the RB-222 (5 µg) group was observed (P=0.093). The results of the elevated body swing test and the rotarod test demonstrated no significant differences among the five groups prior to surgery (0 h; Fig. 1B and C). By contrast, neurobehavioral outcomes were significantly improved in the RB-222 (10 µg) group compared with the MCAO (P<0.001 in number of left turns and P=0.017 in rotarod test) and IgG groups (P<0.001 in number of left turns and P=0.014 in rotarod test) at 24 h after surgery (Fig. 1). RB-222 treatment at a dose of 5 µg did not have a significant effect compared with the MCAO (P=0.985 in number of left turns and P=0.961 in rotarod test) or IgG groups (P=0.958 in number of left turns and P=0.944 in rotarod test; Fig. 1B and C).
Figure 1.
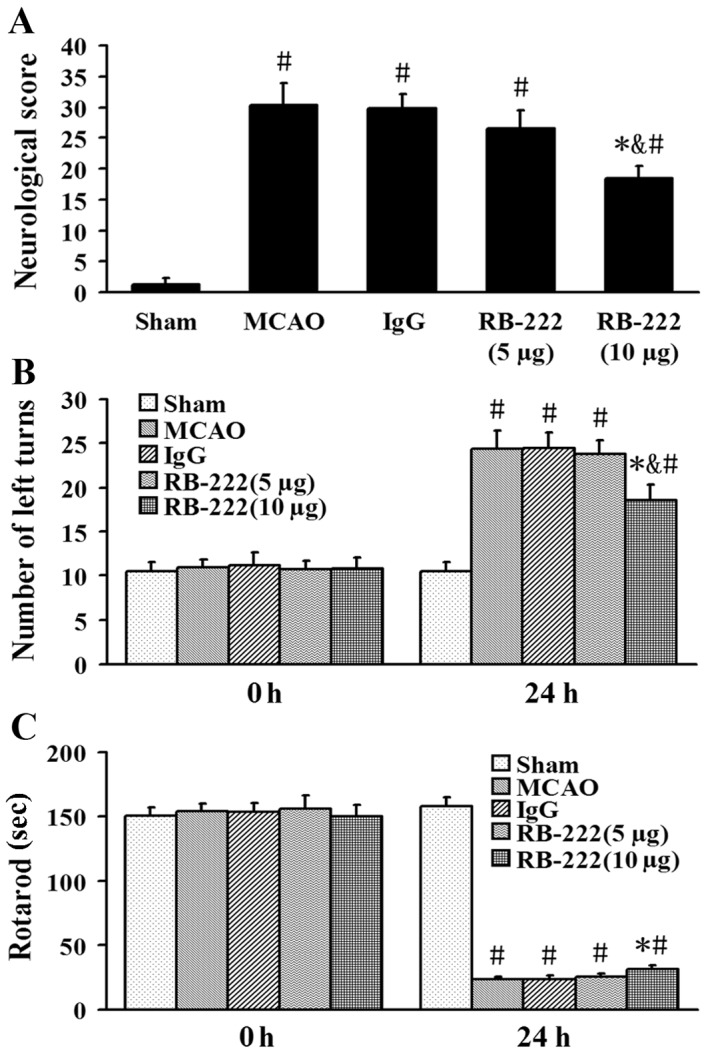
VEGF inhibition improved neurobehavioral recovery in ischemic rats. Functional recovery was evaluated using (A) neurological score, (B) the elevated body swing test and (C) the rotarod test. The elevated body swing test and the rotarod test were performed prior to MCAO and at 24 h following MCAO. RB-222 treatment at a dose of 10 µg significantly improved neurobehavioral outcomes at 24 h after MCAO. Data are presented as the mean ± standard deviation (n=6). #P<0.05 vs. Sham; *P<0.05 vs. MCAO or IgG; &P<0.05 vs. RB-222 (5 µg). VEGF, vascular endothelial growth factor; MCAO, middle cerebral artery occlusion.
Infarct volume
Infarct volume was measured by TTC staining (Fig. 2A). The infarct volume was significantly increased in the MCAO (49.0±4.1%; P<0.001) and IgG-treated (48.1±5.0%; P<0.001) groups compared with the Sham group (Fig. 2). Treatment with RB-222 at doses of 5 µg and 10 µg revealed a significant reduction in infarct volume (34.2±3.3%; P<0.001 and 11.8±2.6%; P<0.001, respectively) compared with the control IgG group. Furthermore, RB-222 administration at a dose of 10 µg was associated with a significant reduction in infarct volume when compared with administration of RB-222 at a dose of 5 µg (P<0.001; Fig. 2).
Figure 2.
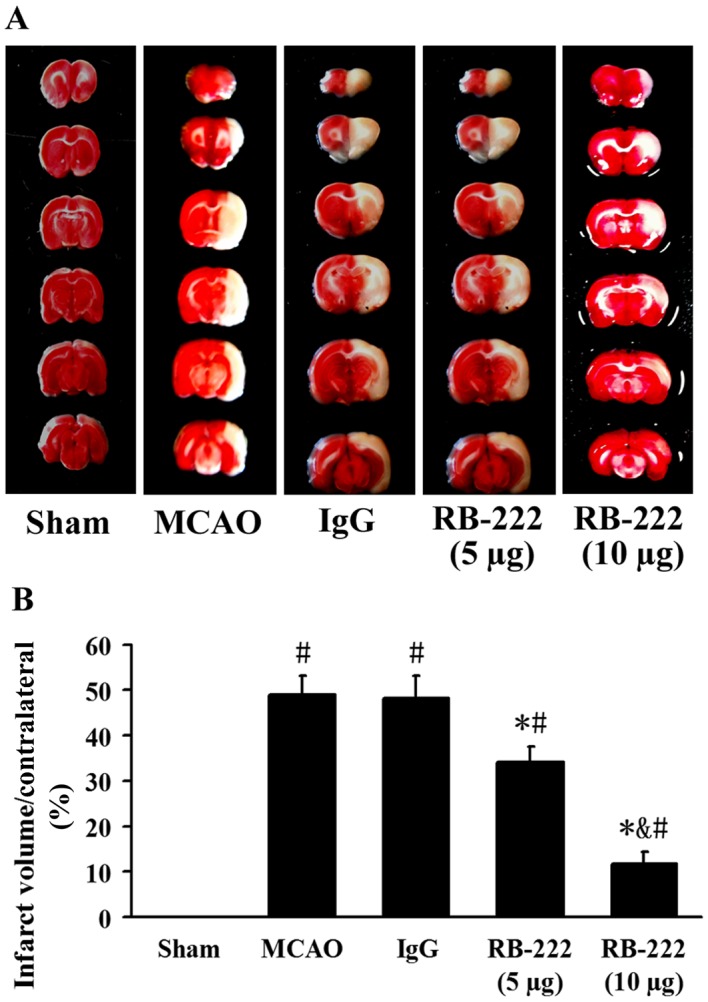
VEGF inhibition reduced infarct volume at 24 h after reperfusion. (A) Representative TTC-stained brain sections following RB-222 treatment (5 or 10 µg) compared with the Sham, MCAO and IgG groups. (B) Statistical analysis of infarct volume. RB-222 treatment significantly reduced the infarct volume compared with the MCAO and IgG groups. Data presented as the mean ± standard deviation (n=6). #P<0.05 vs. Sham; *P<0.05 vs. MCAO or IgG; &P<0.05 vs. RB-222 (5 µg). VEGF, vascular endothelial growth factor; TTC, 2,3,5-triphenyltetrazolium chloride; MCAO, middle cerebral artery occlusion.
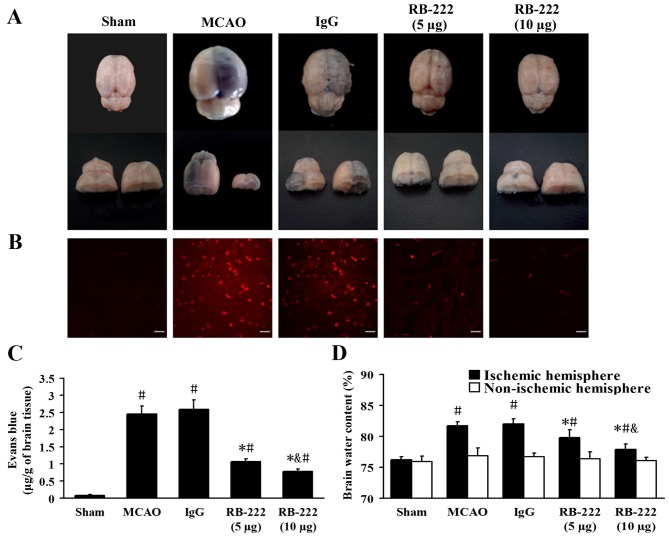
BBB leakage and brain water content
Representative samples of brain sections with EB extravasation are shown in Fig. 3A and B. At 24 h following reperfusion, the EB content of brain tissues from the Sham, MCAO, IgG, RB-222 (5 µg) and RB-222 (10 µg) groups were 0.08±0.02, 2.46±0.23, 2.59±0.28, 1.07±0.08 and 0.78±0.72 µg/g, respectively (Fig. 3C). MCAO induced significant extravasation of EB in the MCAO (P<0.001) and IgG (P<0.001) groups compared with the Sham group. Treatment with 5 and 10 µg RB-222 significantly reduced the EB extravasation (P<0.001 and P<0.001, respectively) compared with the IgG group, with 10 µg RB-222 associated with a greater reduction in EB extravasation (P=0.049 vs. 5 µg RB-222; Fig. 3C).
Figure 3.
VEGF inhibition attenuated EB extravasation and brain edema at 24 h after reperfusion onset. (A) Representative images of EB-stained brains at 24 h following MCAO. (B) Confocal microscopy images showing EB dye (red) in each group (scale bar, 20 µm). (C) Quantitative analysis of EB extravasation (µg/g of brain tissue). (D) Brain water content in the ipsilateral hemisphere was significantly decreased following RB-222 treatment. Data presented as the mean ± standard deviation (n=6). #P<0.05 vs. Sham; *P<0.05 vs. MCAO or IgG; &P<0.05 vs. RB-222 (5 µg). VEGF, vascular endothelial growth factor; EB, Evans Blue; MCAO, middle cerebral artery occlusion.
The mean brain water content in the ischemic and non-ischemic hemispheres was examined and is shown in Fig. 3D. MCAO produced a significant increase in brain water content in the ischemic hemisphere at 24 h following reperfusion in the MCAO (P<0.001) and IgG groups (P<0.001) compared with the Sham group. Treatment with RB-222 at doses of 5 and 10 µg significantly decreased brain water content in the ischemic hemisphere (P=0.001 and P<0.001, respectively) compared with the IgG group, although 10 µg RB-222 was more effective than 5 µg RB-222 in reducing brain water content (P=0.006; Fig. 3D). No significant difference in brain water content was observed among the groups in the non-ischemic hemisphere (Fig. 3D).
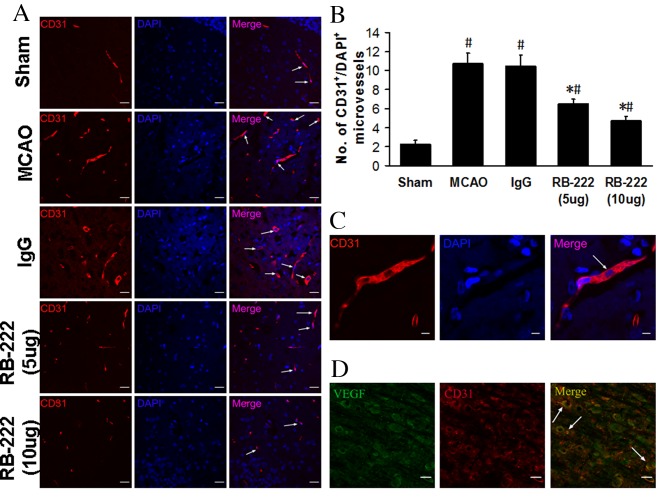
Microvessel counts
The number of microvessels was analyzed in the peri-infarct area at 24 h after reperfusion by CD31 immunostaining (Fig. 4A). Microvessel number was significantly higher in the MCAO (10.75±1.09; P<0.001) and IgG (10.50±1.12; P<0.001) groups compared with the Sham group (2.25±0.43; Fig. 4B). RB-222 treatment at doses of 5 and 10 µg significantly reversed the MCAO-induced increase in microvessel number (6.50±0.50 and 4.75±0.43, respectively; Fig. 4B) when compared with MCAO and IgG groups (P<0.001 for all comparisons). Furthermore, confocal images demonstrated that, in CD31-stained microvessels (Fig. 4C), VEGF colocalized with CD31 in the microvessels (Fig. 4D).
Figure 4.
VEGF inhibition decreased angiogenesis in the ischemic brain within 24 h. (A) Representative fluorescence microscope images of CD31 (red) and DAPI (blue) double-immunostaining in the peri-infarct region of Sham, IgG, MCAO, RB-222 (5 µg) and RB-222 (10 µg) groups. CD31+ cells were significantly decreased in the RB-222-treated groups (scale bar, 20 µm). (B) Statistical analysis of the number of CD31+ microvessels in the peri-infarct area of the rat brains in each group. RB-222 treatment significantly reduced angiogenesis when compared with the MCAO and IgG groups. (C) Magnified confocal images showing CD31 (red) and DAPI (blue) double immunostaining in microvessels (scale bar, 5 µm). (D) Representative image demonstrating the colocalization of VEGF (green) and CD31 (red) in the ischemic rat brains (scale bar, 50 µm). The white arrows in (A) and (C) indicate CD31+ microvessels and in (D) indicate colocalization of VEGF and CD31 in Fig. 4D. Data are presented as the mean ± standard deviation (n=6). #P<0.05 vs. Sham; *P<0.05 vs. MCAO or IgG. VEGF, vascular endothelial growth factor; MCAO, middle cerebral artery occlusion.
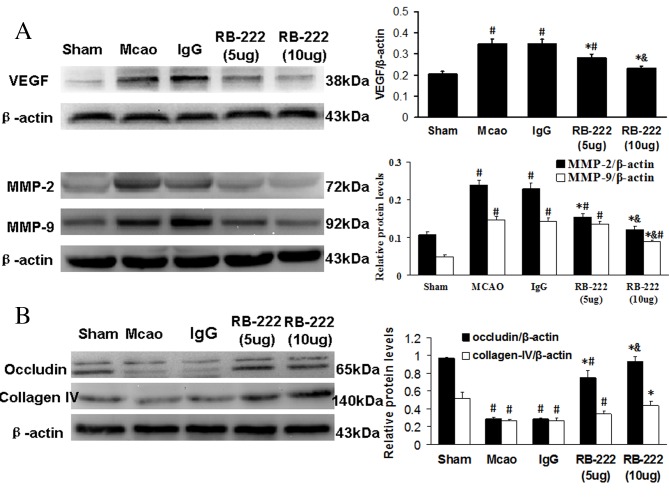
Western blot analysis
The western blotting results demonstrated that cerebral ischemia significantly increased VEGF expression in the MCAO (P<0.001) and IgG groups (P<0.001) at 24 h following reperfusion compared with the Sham group (Fig. 5A). However, RB-222 treatment at doses of 5 and 10 µg significantly attenuated VEGF expression when compared with the MCAO (P=0.001 vs. 5 µg RB-222; P<0.001 vs. 10 µg RB-222) and IgG groups (P=0.001 vs. 5 µg RB-222; P<0.001 vs. 10 µg RB-222). The expression of MMP-2 and MMP-9 was significantly increased in the MCAO and IgG groups when compared with the Sham group, and was significantly decreased following treatment with 5 and 10 µg RB-222 compared with the MCAO and IgG groups (P<0.001 for all comparisons; Fig. 5A). As expected, the expression levels of occludin and collagen-IV were significantly decreased in the MCAO and IgG groups when compared with the Sham group (P<0.001 for all comparisons; Fig. 5B). Treatment with RB-222 at 5 and 10 µg significantly increased the levels of occludin when compared with the MCAO (P<0.001) and IgG (P<0.001) groups. However, a significant increase in collagen-IV was only observed following treatment with 10 µg RB-222 compared with the MCAO (P=0.001) and IgG (P=0.001) groups.
Figure 5.
Western blot analysis of VEGF, MMP-2, MMP-9, occludin and collagen-IV in the ischemic cortical tissue at 24 h following reperfusion. β-actin was used as a loading control. (A) The expression of VEGF was significantly inhibited in the RB-222-treated groups compared with the MCAO and IgG groups. In the RB-222-treated groups, MMP-2 and MMP-9 levels were significantly decreased compared to the levels in the MCAO and IgG groups. (B) A significant reduction in occludin and collagen-IV protein expression levels were observed in the MCAO and IgG groups when compared with the Sham group, while RB-222 treatment markedly reversed the reduction of occludin and collagen-IV expression. Data are presented as the mean ± standard deviation (n=6). #P<0.05 vs. Sham; *P<0.05 vs. MCAO or IgG; &P<0.05 vs. RB-222 (5 µg). VEGF, vascular endothelial growth factor; MCAO, middle cerebral artery occlusion; MMP-2, metalloproteinase-2; MMP-9, metalloproteinase-9.
Discussion
The results of the current study demonstrated the following: i) Ischemia-induced vascular leakage may be partially induced by early VEGF secretion; ii) early VEGF inhibition may alleviate vascular permeability following ischemic stroke; iii) early VEGF inhibition may reduce infarct volume and improve neurobehavioral recovery in the focal cerebral ischemic rats; and iv) this protective effect may have been mediated by a reduction in MMP-2 and MMP-9 expression, together with the upregulation of TJP expression and improved BBB integrity. To the best of our knowledge, this is the first study investigating early VEGF inhibition through intracerebroventricular treatment, and these findings demonstrated a neuroprotective effect, potentially involving the MMP pathway.
Brain infarction and cerebral edema are major life-threatening pathophysiological alterations that may cause neurological deterioration or mortality in the acute phase of ischemic stroke (2,19). The rapid induction of VEGF is a major inducer of the vascular permeability that is involved in BBB disruption and brain edema in the acute phase of ischemic stroke (6). Consistent with previous studies, the current study demonstrated that the upregulation of VEGF expression induces BBB damage and brain edema following acute ischemic stroke. However, the aggravated brain injury was reversed by early administration of RB-222, as demonstrated by decreased EB extravasation and reduced ischemic brain water content. Early inhibition of VEGF has been demonstrated to decrease infarction following stroke in rats (14). In addition, the present study indicated that RB-222, at doses of 5 and 10 µg, reduced infarct size by 30 and 75%, respectively, compared with the MCAO group. Infarct size is widely used to determine the effectiveness of a drug for the treatment of ischemic stroke in most animal studies (20,21). However, the neuroprotective efficacy of RB-222 was determined by measuring neurological function in clinical trials (21). In the present study, treatment with RB-222 at a dose of 10 µg significantly improved neurological outcome, as evidenced by the decrease in severity of neurological response. These results confirmed the neuroprotective effects of VEGF inhibition in a rat model of ischemic stroke.
Angiogenesis provides a natural defense mechanism after stroke by enhancing oxygen and nutrient supply to the ischemic brain tissue (22). Furthermore, angiogenesis is activated in the peri-infarct areas as early as 12–24 h following ischemic stroke, which correlates with longer survival following cerebral ischemia (22,23). VEGF, as a vascular growth factor, serves a critical role in this process, and is primarily secreted in astrocytes, endothelial cells and neural stem cells in ischemic rats (24). However, the neurorestorative process is likely to be effective after the acute phase of stroke (25). Previous studies have indicated that there may be an immediate increase in VEGF expression after stroke; which is a potent vascular permeability factor associated with the formation of brain edema following acute stroke (13,16). Moreover, microvessels formed in response to VEGF at the onset of stroke are more permeable, with an immature endothelial permeability barrier and basal lamina, both of which contribute to edema formation (1,6). Results of the current study demonstrated that treatment with RB-222 significantly reduced microvessel number in the peri-infarct region at 24 h after ischemic/reperfusion injury. In addition, colocalization of VEGF and CD31 in endothelial cells was observed, indicating the critical role of VEGF in angiogenesis. Therefore, early VEGF inhibition decreased focal immature angiogenesis and reduced the BBB permeability and brain edema in ischemic rats.
To investigate the possible molecular mechanisms involved in the protective effects of early VEGF inhibition on acute ischemia-induced brain injury, the expression of MMP-2 and MMP-9 in the cerebral cortex was examined in the present study. A reduction in MMP-2 and MMP-9 expression was observed following RB-222 treatment. It has been reported that the instant elevation of MMPs in acute ischemia-induced brain injury is involved in the digestion of the endothelial basal lamina and the triggering of secondary injury, including brain edema in acute stroke (8). The inhibition of MMPs promotes BBB restoration and neurovascular remodeling following stroke (9). Furthermore, TJPs, which are considered to be a major part of the BBB, were significantly decreased following ischemic stroke (26). This is consistent with prior studies demonstrating that the loss of TJPs following brain ischemia is responsible for BBB disruption by an MMP-dependent step (7,9). Among the TJPs, occludin is a regulatory protein that assembles with claudin-5 between endothelial cells and is associated with endothelial permeability (27–29). In addition, collagen-IV contributes to 90% of the components of the basal lamina and is critically involved in the integrity of the vessel wall (7,10,30). The results of the present study demonstrated that MMP-2 and MMP-9 expression were decreased and occludin and collagen-IV levels were increased following VEGF inhibition. This suggests that, RB-222-mediated inhibition of VEGF expression in the microvasculature at 24 h after cerebral reperfusion, resulted in decreased MMP-2 and MMP-9 levels. This may have led to an increase in occludin levels, thereby maintaining closure of tight junctions, as well as an increase in collagen-IV levels, thus maintaining the structure of the basal lamina.
The present study has limitations. Although MMP-2 and MMP-9 expression levels were significantly decreased following VEGF inhibition, the underlying mechanisms involved remains largely unknown. A previous study indicated that tumor necrosis factor-α (TNF-α) participates in the BBB deterioration that occurs in ischemia/reperfusion injury models (31). Therefore, the role of TNF-α in this process of ischemic injury requires further investigation in the current study. In particular, following translocation to the nucleus, the NF-kB promoter domains present in numerous pro-inflammatory genes, including TNF-α, have been shown to induce the expression of MMP-9 (32). Based on the results presented in the current study, the authors hypothesized that the TNF-α/NF-kB/MMP signaling pathway may be a potential pathway by which RB-222 preserves the BBB following acute ischemic stroke. Further investigation is required in order to clarify this hypothesis. It was observed in the present study, that inhibition of VEGF during the acute phase of stroke attenuates BBB disruption by regulating the expression of MMPs and reducing permeable microvessel formation. This inhibition further decreased infarct size and improved the outcome after ischemia. However, the critical roles of VEGF in the processes of angiogenesis, neurogenesis and functional recovery during the later phase of ischemic injury have been well studied (33). In different phases of ischemic stroke, VEGF serves different roles, therefore it is important to determine whether early administration of RB-222 may affect outcomes in the chronic phase. Further studies are necessary to evaluate the long-term effects of VEGF inhibition and the optimal duration of treatment during the acute phase of stroke.
In conclusion, the results of the present study confirmed that the VEGF signaling pathway serves an important role in BBB damage following acute cerebral ischemic stroke, partially by regulating the expression of occludin and collagen-IV potentially via the MMP signaling pathway. Early inhibition of VEGF may have vast potential for the treatment of ischemic stroke.
Acknowledgements
This study was supported by the Natural Science Foundation of Heilongjiang Province (grant no. D201279), the National High Technology Research and Development Program 863 (grant no. 2012AA02A508), the National Natural Science Foundation of China (grant no. 81372700) and the International Science & Technology Cooperation Program of China (grant no. 2011DFA31470).
References
- 1.Krueger M, Bechmann I, Immig K, Reichenbach A, Härtig W, Michalski D. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:292–303. doi: 10.1038/jcbfm.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayata C, Ropper AH. Ischaemic brain oedema. J Clin Neurosci. 2002;9:113–124. doi: 10.1054/jocn.2001.1031. [DOI] [PubMed] [Google Scholar]
- 3.Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, Davis SM, Donnan GA, Sheth KN, Kimberly WT. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45:3643–3648. doi: 10.1161/STROKEAHA.114.006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70:753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.STR.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen Nv, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 8.Kurzepa J, Kurzepa J, Golab P, Czerska S, Bielewicz J. The significance of matrix metalloproteinase (MMP)-2 and MMP-9 in the ischemic stroke. Int J Neurosci. 2014;124:707–716. doi: 10.3109/00207454.2013.872102. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, Yang Y, Estrada EY, Rosenberg GA. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab. 2013;33:1104–1114. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.STR.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 11.Seo JH, Guo S, Lok J, Navaratna D, Whalen MJ, Kim KW, Lo EH. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr Pharm Des. 2012;18:3645–3648. doi: 10.2174/138161212802002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenglet S, Montecucco F, Mach F. Role of matrix metalloproteinases in animal models of ischemic stroke. Curr Vasc Pharmacol. 2015;13:161–166. doi: 10.2174/15701611113116660161. [DOI] [PubMed] [Google Scholar]
- 13.van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104:1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura R, Nakase H, Tamaki R, Sakaki T. Vascular endothelial growth factor antagonist reduces brain edema formation and venous infarction. Stroke. 2005;36:1259–1263. doi: 10.1161/01.STR.0000165925.20413.14. [DOI] [PubMed] [Google Scholar]
- 15.Chi OZ, Hunter C, Liu X, Weiss HR. Effects of anti-VEGF antibody on blood-brain barrier disruption in focal cerebral ischemia. Exp Neurol. 2007;204:283–287. doi: 10.1016/j.expneurol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Kanazawa M, Igarashi H, Kawamura K, Takahashi T, Kakita A, Takahashi H, Nakada T, Nishizawa M, Shimohata T. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31:1461–1474. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Reglodi D, Tamás A, Lengvári I. Examination of sensorimotor performance following middle cerebral artery occlusion in rats. Brain Res Bull. 2003;59:459–466. doi: 10.1016/S0361-9230(02)00962-0. [DOI] [PubMed] [Google Scholar]
- 19.Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, Schwab S, Smith EE, Tamargo RJ, Wintermark M. American Heart Association Stroke Council: Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1222–1238. doi: 10.1161/01.str.0000441965.15164.d6. [DOI] [PubMed] [Google Scholar]
- 20.Bae ON, Serfozo K, Baek SH, Lee KY, Dorrance A, Rumbeiha W, Fitzgerald SD, Farooq MU, Naravelta B, Bhatt A, Majid A. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke. 2013;44:205–212. doi: 10.1161/STROKEAHA.112.673954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2:13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 23.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.STR.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Wang J, Lin X, Wang L, Shao B, Jin K, Wang Y, Yang GY. Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis. J Cereb Blood Flow Metab. 2014;34:1138–1147. doi: 10.1038/jcbfm.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI200317977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukita S, Furuse M. Pores in the wall: Claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149:13–16. doi: 10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y, Tsukita S, Yokoyama M, Staddon JM. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J Biol Chem. 2001;276:10423–10431. doi: 10.1074/jbc.M007136200. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 30.Sellner J, Leib SL. In bacterial meningitis cortical brain damage is associated with changes in parenchymal MMP-9/TIMP-1 ratio and increased collagen type IV degradation. Neurobiol Dis. 2006;21:647–656. doi: 10.1016/j.nbd.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wiggins-Dohlvik K, Merriman M, Shaji CA, Alluri H, Grimsley M, Davis ML, Smith RW, Tharakan B. Tumor necrosis factor-α disruption of brain endothelial cell barrier is mediated through matrix metalloproteinase-9. Am J Surg. 2014;208:954–960. doi: 10.1016/j.amjsurg.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crafts TD, Jensen AR, Blocher-Smith EC, Markel TA. Vascular endothelial growth factor: Therapeutic possibilities and challenges for the treatment of ischemia. Cytokine. 2015;71:385–393. doi: 10.1016/j.cyto.2014.08.005. [DOI] [PubMed] [Google Scholar]